Abstract
Horizontal gene transfer (HGT), the transmission of a gene from one species to another by means other than direct vertical descent from a common ancestor, has been recognized as an important phenomenon in the evolutionary biology of prokaryotes. In eukaryotes, in contrast, the importance of HGT has long been overlooked and its evolutionary significance has been considered to be mostly negligible. However, a series of genome analyses has now shown that HGT not only do probably occur at a higher frequency than originally thought in eukaryotes but recent examples have also shown that they have been subject to natural selection, thus suggesting a significant role in the evolutionary history of the receiver species. Surprisingly, these examples are not from protists in which integration and fixation of foreign genes intuitively appear relatively straightforward, because there is no clear distinction between the germline and the somatic genome. Instead, these examples are from nematodes, multicellular animals that do have distinct cells and tissues and do possess a separate germline. Hence, the mechanisms of gene transfer appears in this case much more complicated. In this commentary, I will further discuss two recent publications that describe HGT in nematodes, one that highlights the importance of HGT in the emergence of plant parasitism and another one that probably represents the most convincing example of a potential transfer between two different metazoan animals, an insect and a nematode.
Keywords: bacteria, gene, horizontal, lateral, nematode, plant, transfer
Horizontal Gene Transfer and its Evolutionary Significance for Evolutionary Genomics
Horizontal gene transfer (HGT) can be defined as the movement of a gene from one donor species to a receiver species by means other than vertical inheritance from a direct common ancestor. It is now clearly established and accepted that HGT has been an important phenomenon in the evolutionary history of bacterial genomes.1 The amplitude of gene transfers between different bacteria has even suggested that phylogenetic trees are too limited by nature to correctly represent the suite of evolutionary events that has led to current genomes.2 Far from being neutral events, HGT in bacteria are known to be involved in acquisition of antibiotic resistance (through plasmids and transposable elements) and suspected to contribute to the making of pathogenicity islands.3 More recently, striking examples of whole chromosome horizontal transfers that can provide pathogenicity to non-pathogenic strain in fungi of the fusarium genus have been revealed.4 These examples argue for importance and functional significance of HGT in these eukaryotes too. However, the contribution of HGT to current animal genomes has long been overlooked and neglected. Yet, in the initial analysis of the human genome, as much as 223 genes were originally described as acquired via HGT.5 As this initial claim was soon after refuted,6 it may have put a stop to early efforts aiming at identifying HGT events in the first few animal genome sequenced. Later on, though, several convincing examples of HGT in eukaryotes, including multicellular animals, and supported by phylogenetic analysis have been published (reviewed in ref. 7 for eukaryotes and in ref. 8 for animals). Another possible reason to explain why HGT in animals has experienced some difficulties to be recognized as an important phenomenon in their evolutionary history is that, in contrast to cases in bacteria or fungi, the vast majority of animal examples lacked a clear link between the function of the transferred genes (when known) and the biology of the receiver animal. This has led to even question whether HGT in animals could lead to functional genes in receiver genomes and whether it was evolutionary significant.9 Recently a few examples of HGT in animals have been published in which not only the function of the transferred gene was known but could also clearly be linked to the biology of the receiver species. For instance, in aphids functional genes involved in the biosynhtesis of carotenoid, an important pigment in the ecology of these species were shown to have been acquired from fungi via HGT.10 Here, I will discuss two recently published analyses of HGT cases in nematode genomes. One that highlights the crucial role that multiple HGT events have apparently played in the emergence of plant parasitism11 and another one that reports insect to nematode HGT,12 the first clear example of potential gene transfer between animals.
Multiple HGT Events Have Favored Emergence of Plant Parasitism in Nematodes
Plant parasitism is thought to have emerged at least three times independently during the evolutionary history of nematodes because plant parasitic species are found in phylogenetically distant and distinct clades of the nematode tree of life.13 Interestingly, in every clade of plant-parasitic nematode with available biosequence data, HGT events have been identified.11 Though, systematic association of HGT events with plant-parasitism in nematodes does not necessarily entail a role in the emergence of this life style. Interestingly, in the case of plant-parasitic nematodes, the genes acquired by horizontal transfer are not only transcribed and translated in nematode genomes but several of these genes of bacterial and fungal origin clearly play crucial roles in the parasitism process. The clearest example consists in a set of genes encoding plant cell wall-degrading enzymes. These enzymes encompass cellulases, pectate lyases, xylanases, polygalacturonases and are usually absent from animal genomes. They are involved in the degradation, modification and softening of the plant cell wall, a process essential in several steps of the parasitism interaction, including penetration and navigation in plant tissue. The first cellulases from a plant-parasitic nematode were identified in 1998 and also constituted the first report of such an enzyme in animals.14 Resemblance with bacterial cellulases and otherwise absence from other animals suggested that they may have been acquired via HGT. More recently, a systematic phylogenetic analysis of genes encoding plant cell wall-degrading enzymes confirmed that multiple independent HGT events from different bacterial sources was the most likely hypothesis to explain the presence of these genes in the genomes of plant-parasitic nematodes.15 The same analysis showed that after acquisition via HGT, some of the genes underwent massive duplications, suggesting that individuals with multiple copies of these genes had a selective advantage. Another interesting result was the fact that genes acquired via HGT and coding for these degrading enzymes were indistinguishable from the rest of the nematode genes based on codon usage and GC content. This feature suggested that they had been acquired a long time ago and have since been domesticated by nematode genomes. Hence, a series of genes encoding cell wall-degrading enzymes were successfully transferred to the genomes of plant-parasitic nematodes in which they are transcribed and translated and fulfill crucial function in the parasitic interaction with plants. Further reinforcing the importance of HGT in emergence of plant parasitism in nematodes, a series of other genes have most probably been horizontally transferred from bacteria and fungi and their putative functions support different parasitic processes. These processes encompass modulation of plant defense pathways, establishment of the nematode's feeding site and processing of nutrients absorbed from the plant.11 Although, in these cases, the link between the exact biochemical function of the gene product and the parasitic process is less straightforward than for cell wall-degrading enzymes, they also show long-term evolutionary retention in different clades of plant-parasitic nematodes. Furthermore, their closest homologies are also to genes present in fungi and bacteria that do have parasitic or symbiotic interaction with plants. These characteristics strongly suggest a role in plant-parasitism for these genes. Their otherwise absence from fully sequenced nematode genomes that do not present parasitic interactions with plants, including the model nematode C. elegans, further reinforces this idea. Another interesting fact reported in the review discussed in this commentary,11 is that, apparently, different genes have been acquired at different time points of the nematodes evolutionary history and from different bacterial and fungal sources. Multiple successful transfers from different sources suggest that this event might be much more frequent than usually considered. I speculate that in most cases transfers of genes, regardless of their frequency, simply fail to be fixed in receiver genomes. Because of the divergence between transcriptional and translational mechanisms of donor and receiver species, it appears likely that most transferred genes fail to be expressed and eventually become pseudogenes. In the rare cases compatible with expression in the receiver species, the probability for an acquired gene to be fixed in the population and a species appears very low if it does not provide any selective advantage. Far from being neutral, genes acquired by plant-parasitic nematodes from bacteria and fungi have allowed them to develop the ability to penetrate and navigate into plant tissue and thus to access a new ecological niche preserved from their usual predators and competitors. Fixation of these genes of foreign origin in various different plant-parasitic nematode clades was thus probably driven by this evolutionary novelty providing a clear advantage. In plant-parasitic nematodes, besides these genes, a series of others, encoding hypothetical proteins of as yet unknown function also present a patchy distribution or absence in other metazoan and closest homology to bacterial or fungal genes.16-18 These genes are thus also likely to have been horizontally transferred in nematode genomes and would deserve more detailed phylogenetic analysis. At the moment, it cannot be stated whether they play any role in plant parasitism because neither their biochemical function nor the possible biological process in which they might be involved are known. These genes nevertheless represent potential new parasitism genes and would deserve more characterization.
Necromenic Nematodes of the Pristionchus Genus Have Acquired Multiple Genes via HGT, Including from Insect Donor
Nematodes of the Pristionchus genus have a necromenic life style. They live in specific association with a beetle host that they do not directly parasitize. Instead, they feed on microorganisms that decompose the insect once it is dead. For that reason they have been considered as intermediates between free living nematodes and those that are parasitic on insects.19 Sequencing and analysis of the genome of Pristionchus pacificus revealed the presence of genes encoding cellulases whose biochemical functions was experimentally confirmed.20 As these genes present closest homology to amoebozoa then archaea and bacteria they have been considered as acquired by HGT from microbial genes. Presence of functional cellulase genes in a nematode that is not parasitic on plants appeared to be very intriguing. Currently, the role of these cellulases in the biology of Pristionchus is not completely determined but they could be involved in the degradation of cellulose present in the biofilm synthesized by some bacteria that they feed on. Whatever the role of these cellulases their origin is clearly distinct from those present in plant-parasitic nematodes as they show only low sequence similarity and each match completely different sets of microbial species. Hence, cellulases have been acquired at least two times independently from different microbial sources in the Pristionchus genus and in plant-parasitic nematodes.
These cellulase genes were further analyzed in the transcriptomes of ten additional nematodes of the Pristionchus genus. Following demonstration of transcription of these cellulase genes, the authors experimentally showed that the encoded enzymes actually degrade cellulose. Furthermore, phylogenetic trees based on these cellulase genes perfectly matched the Pristionchus species phylogeny, supporting an acquisition in their last common ancestor and longevity of these transferred genes in Pristionchus genomes.21 Detailed analysis of three of these cellulase genes in 24 different isolates of Pristionchus revealed that a high rate of gene duplications and losses occurred since their acquisition, suggesting possible positive selection acting on copy numbers. A phenomenon also observed in several genes encoding cell wall-degrading enzymes in plant-parasitic nematodes in which they form multigene families.15
Besides cellulase genes, initial analysis of the genome of Pristionchus revealed the presence of Diapausin genes. Diapausins encode antifungal peptides specifically produced during diapause. These genes are absent from the genomes of other nematodes and are otherwise found in insects, including the beetles they live in association with. This finding could well represent the first example of a successful gene transfer between the genomes of two different animals. An alternative hypothesis is that a Diapausin gene was present in the last common ancestor of nematodes and insects and that those observed in extant genomes derive from this ancestral gene through vertical transmission. However, this would imply an improbably high number of independent gene losses to explain their absence from all the other currently sequenced nematode genomes and transcriptomes. Their otherwise presence in the only insect-associated nematode with a fully sequenced genome further argues in favor of an HGT event from insects. A recent detailed analysis of the gene content of Pristionchus pacificus revealed that a subset of the genes lacking homology to other nematodes had a peculiar codon usage distinct from that of the core genes conserved with other nematodes.12 Interestingly, some of these genes featured a codon usage more similar to that of insects than to that of nematodes. A total of 509 genes with insect-like characterisitics, including the Diapausin genes were thus considered to have been acquired via HGT of insect origin in this nematode. Most of these genes consisted of non-LTR retrotransposons. As the Pristionchus genes lacking similarity with other nematode genes were particularly frequent in the vicinity of retrotransposons, the authors suggested that these mobile genetic elements could have played a role in the transfer of genetic material of foreign origin in the Pristionchus genome. Co-distribution of transposable elements and genes putatively acquired by HGT had also been reported in bdelloid rotifers,22 small aquatic animals that reproduce asexually and resist desiccation. In contrast, in plant-parasitic nematodes, genes acquired from bacteria and fungi showed no evidence for co-distribution with transposable elements.15 Another contrasting feature is that genes acquired via HGT in plant-parasitic nematodes, discussed in the previous section, cannot be distinguished from the rest of the genes based on their GC content and codon usage.15 This suggested ancient acquisition and erosion of original donor GC content and codon usage. The similarity in codon-usage between insects and Pristionchus genes putatively acquired via HGT from the beetles suggest that the event might be much more recent. It would be interesting to check whether diapausin genes are present in other Pristionchus species and whether they underwent gene duplications and losses that could be compared with those observed for the cellulase genes.21
How Were the Genes Transferred in Nematode Genomes?
Both for plant-parasitic and necromenic nematodes, the question of the mechanisms that have allowed successful gene transfer in their genomes is intriguing and remains undetermined. Intuitively, gene transfer in the genome of an animal appears very challenging. Indeed, even if we assume that the gene is transferred in a genomic location compatible with its transcription and translation, several barriers have to be passed before a gene can be integrated in the genome of an animal. Animals have a separate germline and HGT must reach cells in the germline to have a chance to be transmitted to the offspring, otherwise they will remain individual-specific. In contrast to prokaryotes, the genetic material is protected in the nucleus, thus foreign DNA fragments have to pass this protection. Animal cells also have a series of defense mechanisms that aim at degrading foreign DNA, in particular from viruses. This ensemble of features can be seen as obstacles to HGT, yet several examples show that they can be individually eluded. In spite of defense mechanisms many viruses successfully transfer their genetic material into the genome of animal cells and endogenous retroviruses, including in the human genome, echo ancient successful transfers of viral genetic material in the DNA of germinal cells. Similarly, the bacterium Agrobacterium tumefaciens is able to transfer a fragment of DNA of its Ti plasmid into nuclear DNA of plant cells. The two papers discussed in this commentary propose a series of routes for HGT in nematodes. Concerning plant-parasitic nematodes, the authors suggest several non-mutually exclusive hypotheses (summarized in Figure 1), including acquisition from the diet by bacterivorous ancestors of plant parasites or acquisition from ancient endosymbionts.11 The role of transposable elements in the horizontal transmission of genetic material is also briefly evoked but not further detailed because no evidence of association of genes of foreign origin and transposable elements was found so far in the genomes of plant-parasitic nematodes. However the events might have been so ancient that traces are now eroded exactly as for codon usage and GC content. The possibility that viruses or plasmids may have served of intermediates is also discussed.
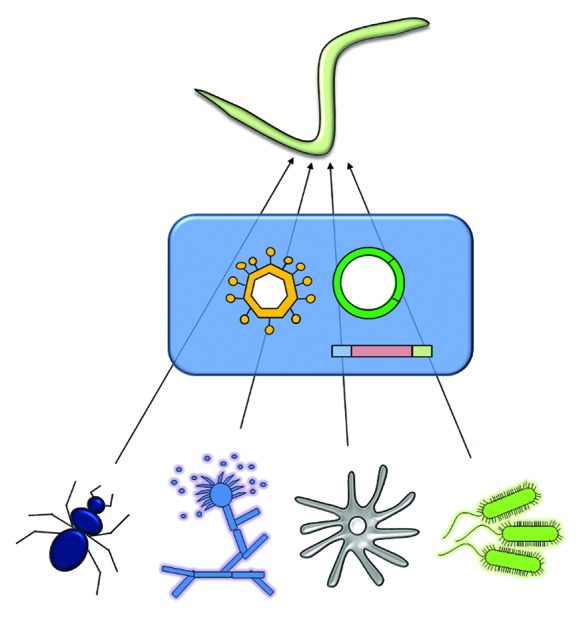
Figure 1. Putative donor species that have contributed genetic material via HGT to current nematode genomes discussed in this commentary, according to published phylogenetic analyses. An insect, a fungus, an amoeba and bacteria are represented on the bottom part. The possible vectors that may have acted as intermediate to facilitate HGT are represented within the blue rectangle. These vectors consist of transposable elements, plasmids and viruses. In the case of bacterial donor it is also hypothesized that genes may have been directly transferred without vector by mechanisms similar to type IV secretion system.11
In the case of Diapausin genes of insect origin in the genome of the necromenic nematode Pritsionchus pacificus, the authors present viruses as obvious candidate vectors for horizontal transfer, in particular because fragments of the same Diapausin gene are found in iridoviruses.23 An alternative scenario is the role of transposable elements as carriers of genetic material of foreign origin (Fig. 1). Indeed, in their analyses the authors showed a clear co-localization of HGT candidates near transposable elements and, interestingly, some transposable elements in the genome of P. pacificus show highest similarities to those of insects. Hence it is highly plausible that transposable elements have played an active role in the transfer of genes from insect donors to the nematode genome.
Conclusion
As more animal genomes are being sequenced we can expect new cases of HGT to be reported. Usually, candidate HGT in animal genomes are identified by searching genes that are more similar to bacterial or fungal genes than to genes of closer relatives like other animals. However these approaches will not allow identifying HGT events between different animals. The fact that such events can happen, as reported in the recent analysis of Pristionchus genome12 suggests that they should now be investigated at a larger scale in the various animal genomes publicly released. Thus, the current view of the amplitude of HGT in nematodes and more generally in animals is probably an underestimate of the true importance of the phenomenon. Whether HGT are evolutionary significant and have driven the emergence of new functions in nematodes now becomes clearer and clearer, as it appears that HGT have at least facilitated the emergence of plant-parasitism in nematodes.11 Presence of genes of foreign origin as multigene families, both in plant-parasitc and necromenic nematodes also support the idea that individuals with multiple copies of these genes may have been favored by natural selection. Evolutionary and functionally significant HGT have also been revealed in other animal phyla and there is a very convincing case in aphids.10 We can anticipate that new clear examples will emerge when the functions of the genes identified as cases of HGT in animals are being functionally characterized. Assessing the contribution of HGT to animal genome evolution at a large scale will probably constitute one of the next challenges in evolutionary biology, as it is now clear that HGT has irrevocably changed the way the tree of life and species concepts are perceived in microbiology.
Acknowledgments
The author would like to thank Ralf J. Sommer, Lisa N. Schuster and Christian Rödelsperger for interesting discussions on HGT in the Pristionchus genus.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/18776
References
- 1.Gogarten JP, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol. 2005;3:679–87. doi: 10.1038/nrmicro1204. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D. The post-Darwinist rhizome of life. Lancet. 2010;375:104–5. doi: 10.1016/S0140-6736(09)61958-9. [DOI] [PubMed] [Google Scholar]
- 3.Kado CI. Horizontal gene transfer: sustaining pathogenicity and optimizing host-pathogen interactions. Mol Plant Pathol. 2009;10:143–50. doi: 10.1111/j.1364-3703.2008.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–73. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 6.Salzberg SL, White O, Peterson J, Eisen JA. Microbial genes in the human genome: lateral transfer or gene loss? Science. 2001;292:1903–6. doi: 10.1126/science.1061036. [DOI] [PubMed] [Google Scholar]
- 7.Andersson JO. Lateral gene transfer in eukaryotes. Cell Mol Life Sci. 2005;62:1182–97. doi: 10.1007/s00018-005-4539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunning Hotopp JC. Horizontal gene transfer between bacteria and animals. Trends Genet 2011 [DOI] [PMC free article] [PubMed]
- 9.Blaxter M. Symbiont genes in host genomes: fragments with a future? Cell Host Microbe. 2007;2:211–3. doi: 10.1016/j.chom.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Moran NA, Jarvik T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 2010;328:624–7. doi: 10.1126/science.1187113. [DOI] [PubMed] [Google Scholar]
- 11.Haegeman A, Jones JT, Danchin EG. Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Mol Plant Microbe Interact. 2011;24:879–87. doi: 10.1094/MPMI-03-11-0055. [DOI] [PubMed] [Google Scholar]
- 12.Rödelsperger C, Sommer RJ. Computational archaeology of the Pristionchus pacificus genome reveals evidence of horizontal gene transfers from insects. BMC Evol Biol. 2011;11:239. doi: 10.1186/1471-2148-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–5. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 14.Smant G, Stokkermans JP, Yan Y, de Boer JM, Baum TJ, Wang X, et al. Endogenous cellulases in animals: isolation of beta-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc Natl Acad Sci USA. 1998;95:4906–11. doi: 10.1073/pnas.95.9.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danchin EG, Rosso MN, Vieira P, de Almeida-Engler J, Coutinho PM, Henrissat B, et al. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc Natl Acad Sci USA. 2010;107:17651–6. doi: 10.1073/pnas.1008486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elling AA, Mitreva M, Gai X, Martin J, Recknor J, Davis EL, et al. Sequence mining and transcript profiling to explore cyst nematode parasitism. BMC Genomics. 2009;10:58. doi: 10.1186/1471-2164-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl EH, Thorne JL, McCarter JP, Bird DM. Horizontally transferred genes in plant-parasitic nematodes: a high-throughput genomic approach. Genome Biol. 2003;4:R39. doi: 10.1186/gb-2003-4-6-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasmuth J, Schmid R, Hedley A, Blaxter M. On the extent and origins of genic novelty in the phylum nematoda. PLoS Negl Trop Dis. 2008;2:e258. doi: 10.1371/journal.pntd.0000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieterich C, Sommer RJ. How to become a parasite - lessons from the genomes of nematodes. Trends Genet. 2009;25:203–9. doi: 10.1016/j.tig.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Dieterich C, Clifton SW, Schuster LN, Chinwalla A, Delehaunty K, Dinkelacker I, et al. The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet. 2008;40:1193–8. doi: 10.1038/ng.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer WE, Schuster LN, Bartelmes G, Dieterich C, Sommer RJ. Horizontal gene transfer of microbial cellulases into nematode genomes is associated with functional assimilation and gene turnover. BMC Evol Biol. 2011;11:13. doi: 10.1186/1471-2148-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladyshev EA, Meselson M, Arkhipova IR. Massive horizontal gene transfer in bdelloid rotifers. Science. 2008;320:1210–3. doi: 10.1126/science.1156407. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Sato K, Saito Y, Yamashita T, Agoh M, Okunishi J, et al. Insect diapause-specific peptide from the leaf beetle has consensus with a putative iridovirus peptide. Peptides. 2003;24:1327–33. doi: 10.1016/j.peptides.2003.07.021. [DOI] [PubMed] [Google Scholar]


