Abstract
On basis of fruit differential respiration and ethylene effects, climacteric and non-climacteric fruits have been classically defined. Over the past decades, the molecular mechanisms of climacteric fruit ripening were abundantly described and found to focus on ethylene perception and signaling transduction. In contrast, until our most recent breakthroughs, much progress has been made toward understanding the signaling perception and transduction mechanisms for abscisic acid (ABA) in strawberry, a model for non-climacteric fruit ripening. Our reports not only have provided several lines of strong evidences for ABA-regulated ripening of strawberry fruit, but also have demonstrated that homology proteins of Arabidopsis ABA receptors, including PYR/PYL/RCAR and ABAR/CHLH, act as positive regulators of ripening in response to ABA. These receptors also trigger a set of ABA downstream signaling components, and determine significant changes in the expression levels of both sugar and pigment metabolism-related genes that are closely associated with ripening. Soluble sugars, especially sucrose, may act as a signal molecular to trigger ABA accumulation through an enzymatic action of 9-cis-epoxycarotenoid dioxygenase 1 (FaNCED1). This mini-review offers an overview of these processes and also outlines the possible, molecular mechanisms for ABA in the regulation of strawberry fruit ripening through the ABA receptors.
Keywords: ABA receptor, abscisic acid (ABA), fruit ripening, signal perception and transduction, strawberry
Fruits can be classified into two types including climacteric and non-climacteric fruits according as the patterns of respiration and ethylene production during ripening. Ripening of a climacteric fruit is predominately dependent on ethylene biosynthesis and action.1 Over the past decade, much progress has been made toward understanding the signal perception and transduction mechanisms for ethylene-regulated ripening of climacteric fruits in model plant tomato.2 In contrast, although the molecular mechanisms underlying the development and ripening of non-climacteric fruits, have been extensively studied and many ABA signaling components have been identified, including: hexose transporter and ASR protein,3,4 a calcium-dependent protein kinase ACPK1,5 sugar-inducible protein kinase VvSK1,6 and calcineurin B-like calcium sensor 1-protein kinase CIPK23,7 as well as G-protein signaling components GPA1, PP2C protein phosphatases, sugar-related WRKY transcription factors, ABRE-binding factor (ABF), and AP2 transcription factors,8 nevertheless, the molecular mechanisms of ABA perception and signaling transduction in the ripening of non-climacteric fruits remain largely unclear.
Our recent reports have provided strong evidences that ABA plays a crucial role in the regulation of ripening related-gene expression through ABA perception and signaling transduction in strawberry fruit.9,10 These and previous studies provide several lines of evidences that the interaction between sugar and ABA may be a core mechanism in regulation of the ripening of non-climacteric fruits.
ABA is Required for Strawberry Fruit Ripening
As a plant hormone, ABA not only plays a central role in the adaptation of plants to adverse conditions, but also regulates various aspects of plant growth and development, such as seed dormancy, seedling growth, and fruit development.11-13 Notably, ABA is considered to play even more important roles than that of ethylene in fruit maturation and senescence.14 Ripening of strawberry (Fragaria x ananassa), a model for non-climacteric fruits, is previously reported to be promoted by ABA.15 In accordance with this notion, our recent reports also showed that exogenous ABA can significantly accelerate strawberry fruit ripening, and a remarkable decrease in ABA content that results from the downregulation of the transcripts of a 9-cis-epoxycarotenoid dioxygenase gene (FaNCED1) by virus-induced gene silencing (VIGS), can significantly retard the ripening process.9 To our knowledge, this is the first direct molecular evidence on the requirement of ABA for strawberry fruit ripening.
It is interesting to note that several previous reports show that NCEDs is a small gene family in plants, for example, at least seven AtNCEDs genes (1, 2, 3, 4, 5, 6, and 9) in Arabidopsis,16,17 five OsNCEDs genes (1, 2, 3, 4, and 5) in rice,18 and three PaNCEDs genes (1, 2, and 3) in avocado fruit, in which PaNCED1 and PaNCED3 were strongly induced at ripening.19 Although FaNCED1 is a predominant contributor to ABA accumulation in the ripening fruit,9 characterization of the nature of FaNCEDs gene family in strawberry fruit is underway.
ABA Perception in Strawberry Fruit
The most outstanding progress in recent-year studies of the ABA signaling pathway is the identification of several ABA-binding components in Arabidopsis, including G protein-related receptors, the Mg-chelatase H subunit, and START-domain PYR/PYL/RCAR.20 Although the specific ABA-binding sites in grape berries were detected ten years ago,21 little progress has been made toward understanding the ABA perception mechanism in non-climacteric fruits, until our recent breakthroughs in strawberry fruits, in which at least two proteins homologous to the Arabidopsis ABA receptor PYR/PYL/RCAR or ABAR/CHLH were identified.9,10
Using a newly-established tobacco rattle virus (TRV)-induced gene silencing (VIGS) technique in strawberry fruit, downregulation of the expression levels of the putative ABA receptor FaPYR1 or FaCHLH/ABAR leads to similar, ABA-insensitive, various chimeric fruits that are consistent with the RNA-interference extent of the receptor gene, demonstrating that these potential receptors act as positive regulators of ripening in response to ABA.9,10 Interestingly, the FaCHLH/ABAR gene is only one member in the strawberry genome,9 in which FaPYRs contains 11 members of the gene family.10 Subsequently determination of the ABA binding ability of the FaPYR1 and FaCHLH/ABAR proteins will provide us with more information on the regulated mechanism by ABA in strawberry fruit development.
ABA Downstream Signaling Components in Strawberry Fruit
In response to environmental and developmental cues, a series of ABA signal cascades in plant cells should be relayed from hormone perception to gene expression and regulation. In the past years, in addition to ABA-binding proteins, numerous cellular signaling components including second messengers, protein kinases/phosphatases, transcription factors that modulate ABA responses have been identified in Arabidopsis.12,22
In our recent reports have also shown that downregulation of the FaPYR1or FaCHLH/ABAR gene both can alter several classical ABA-responsive genes including ABI1, ABI3, ABI4,ABI5, SnRK2, and the later also include both sugar and pigment metabolism-related genes, such as SigE, AMY, and CHS.9,10 Notably, some node proteins includingABI3, ABI4, ABI5, and SnRK2, play an important role in the interaction between sugar and ABA during many aspects of plant growth and development in Arabidopsis,23,24 for example, ABI3/VP1 functions as a repressor of α-amylase genes in maize seed development,25,26 and SnRK2 mediates the regulation of sucrose metabolism in Arabidopsis growth.24 More notably, several recent-year reports have shown that CHLH/ABAR not only interacts with the SigE sigma factor to inhibit transcription activity of SigE that is a positive regulator of sugar catabolism,27 it also interacts with a group of WRKY transcription factors that function as negative regulators of ABA signals.28 In addition, G-protein signaling components GPA1 and RGS1, PP2C protein phosphatases, sugar-related WRKY transcription factors, ABRE-binding factor (ABF), and AP2 transcription factors are regulated by sugar and ABA in another non-climacteric fruit (grape berry).8 In consistent with early reports that sugar can act as a signal to induce anthocyanin biosynthesis in fruit,29,30 we also exhibit that soluble sugars, especially sucrose, may act as a promoter to trigger ABA accumulation by the action of 9-cis-epoxycarotenoid dioxygenase 1(FaNCED1).9 In conclusion, our and previous reports involved in ABA downstream-signaling-component studies in fleshy fruits suggest that the interaction between sugar and ABA may be a core mechanism in the regulation of non-climacteric fruit ripening.
Possible Mechanisms for ABA in the Regulation of Strawberry Fruit Ripening
Fruit ripening is closely associated with changes in sugar metabolism, softening, and color development. As described above, a group of sugar- and pigment-related genes (such as SigE, AMY, and CHS) are regulated through ABA signal cascades, in which are involved in ABA receptors (such as FaPYR1 and FaABAR/CHLH), a set of ABA-responsive modulators (such as ABI1, ABI3, ABI4, ABI5, and SnRK2 in ripening strawberry fruit;9,10 or GPA1, PP2C protein phosphatases, WRKY transcription factors, ABF transcription factors in ripening grape berry8). Based on the data available, especially two models proposed for ABA signaling pathways including ‘PYR1-PP2C-SnRK2’ and ‘ABAR-WRKY40-ABI5′ core signaling networks in Arabidopsis,28,31 we attempt to picture the possible mechanisms for ABA in the regulation of strawberry fruit at molecular level.
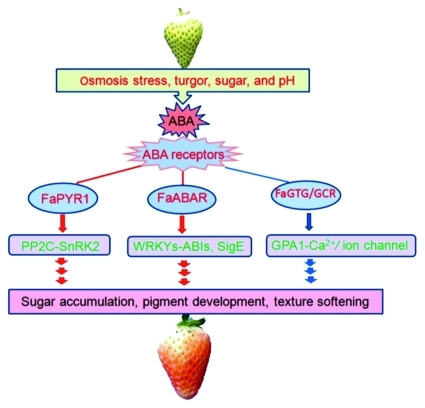
As showed in Figure 1, with the advent of strawberry fruit ripening, the accumulated sugar-, declined acid-, and enlarged cell-derived physiological changes such as osmosis stress, turgor, sugar and pH, might act as early signals to promote ABA accumulation. The ABA signal is received by (at least) two putative ABA receptors: FaABAR/CHLH and FaPYR1, or by (maybe) strawberry homologs of G protein-related receptors GTGs/GCR220 (the upstream of GPA1, here named FaGTG/GCR). These receptors may convey its signal respectively as follows: FaABAR/CHLH through SigE or WRKY transcription factors, FaPYR1 through PP2C (ABI1), and FaGTG/GCR presumably through GPA1. In the ABA signaling pathway, second messengers, protein kinases, protein phosphatase 2Cs, and transcription factors form integrated signaling pathways. Finally, the FaABAR/CHLH and FaPYR1 signaling pathways might regulate fruit sugar and pigment metabolism through ABF and SigE transcription factors; whereas the FaGTGs/GCR2 signaling pathways might regulate the fruit softening through Ca2+signals or ion channels.
Figure 1.
A model for abscisic acid (ABA) receptors-mediated signaling pathways in the regulation of strawberry fruit ripening. At the onset of fruit ripening, some physiological parameters such as osmosis stress, turgor, sugar and pH, might act as early signals to trigger ABA biosynthesis. The ABA signal is received by three putative ABA receptors, including FaABAR, FaPYR1 or FaGTG/GCR, then the signal is relayed respectively by their interaction complexes, namely FaPYR1-PP2C-SnRK2, FaABAR-WARKs-ABIs or FaABAR-SigE, and FaGTGs/GCR2-GPA1-Ca2+/ion channel. In ABA cascades, receptors, second messengers, protein phosphatase 2Cs protein kinases, and various transcription factors form signaling pathways, finally regulating ripening-related events including sugar and pigment metabolism and texture softening. Red lines/arrows indicate demonstrated interactions; blue lines/arrows indicate predicted interactions.
Concluding Remarks and Future Directions
Our and previous results have provided substantial evidences to demonstrate that the expression and regulation of the ripening-related genes of non-climacteric fruit, such as sugar- and pigment-metabolism-related genes, are required for both ABA accumulation and a series of ABA signal cascades, in which include ABA receptors, second messengers, protein kinases, protein phosphatase 2Cs, and transcription factors. Given that all ABA receptors are gained from Arabidopsis and that fleshy fruit pulp is a special plant tissue, it will be necessary to search for additional ABA receptors in fleshy fruit. Second, the detailed information on how ABA perception is transduced to the regulation of their downstream events needs to be elucidated in future studies. Third, the complexity of cross-talks between ABA signaling pathway and other hormone signaling pathways (such as ethylene or IAA) will be a challenge to test. Fourth, determination of the ABA binding ability of the strawberry putative ABA receptors is an important task for future studies. Prospectively, although the pivotal role of ABA in mediating the response to non-climacteric fruit ripening has been currently established, the way from understanding and utilizing the knowledge of ABA action to guide genetic engineering for manipulation of fruit ripening is to be long way to go in coming years.
Acknowledgments
This work was supported by the China National Natural Science Foundation (grant no. 30971977), the Beijing Natural Science Foundation and Scientific Research Key Program of Beijing Commission of Education (grant no. KZ200910020001) and the Beijing Natural Science Foundation (grant no. 6082005).
Glossary
Abbreviations:
- FaABAR
ABA putative receptor/ Mg-chelatase H subunit
- FaPYR1
START-domain PYR/PYL/RCAR
- FaGTG/GCR
GPCR-type G proteins/G protein-coupled receptor
- PP2C
protein phosphatase 2C
- SnRK2
Snf1-related kinases
- WRKYs
WRKY transcription factors
- ABIs
abscisic acid-insensitive proteins
- SigE
sigma factor
- GAP1
G-protein α subunits
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18024
References
- 1.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–55. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- 2.Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends Plant Sci. 2004;9:331–8. doi: 10.1016/j.tplants.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, et al. Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol. 1999;120:1083–94. doi: 10.1104/pp.120.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell. 2003;15:2165–80. doi: 10.1105/tpc.013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, et al. Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol. 2006;140:558–79. doi: 10.1104/pp.105.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lecourieux F, Lecourieux D, Vignault C, Delrot S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010;152:1096–106. doi: 10.1104/pp.109.149138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuéllar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, et al. A grapevine Shaker inward K(+) channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J. 2010;61:58–69. doi: 10.1111/j.1365-313X.2009.04029.x. [DOI] [PubMed] [Google Scholar]
- 8.Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta. 2010;232:219–34. doi: 10.1007/s00425-010-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, et al. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157:188–99. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai YM, Jia HF, Li CL, Dong QH, Shen YY. FaPYR1 is involved in strawberry fruit ripening. J Exp Bot. 2011;62:5079–89. doi: 10.1093/jxb/err207. [DOI] [PubMed] [Google Scholar]
- 11.Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(Suppl):S15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan QH, Li MJ, Peng CC, Zhang N, Zou X, Zou KQ, et al. Abscisic acid activates acid invertases in developing grape berry. Physiol Plant. 2005;125:157–70. doi: 10.1111/j.1399-3054.2005.00552.x. [DOI] [Google Scholar]
- 14.Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009;60:1579–88. doi: 10.1093/jxb/erp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003;39:171–4. doi: 10.1023/A:1022539901044. [DOI] [Google Scholar]
- 16.Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–33. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 17.Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313X.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G, Ye N, Zhang J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009;50:644–51. doi: 10.1093/pcp/pcp022. [DOI] [PubMed] [Google Scholar]
- 19.Chernys JT, Zeevaart JA. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000;124:343–53. doi: 10.1104/pp.124.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Yang X, Weston DJ, Chen JG. Abscisic acid receptors: past, present and future. J Integr Plant Biol. 2011;53:469–79. doi: 10.1111/j.1744-7909.2011.01044.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang DP, Zhang ZL, Chen J, Jia WS. Specific abscisic acid-binding sites in mesocarp of grape berry: properties and subcellular localization. J Plant Physiol. 1999;155:324–31. doi: 10.1016/S0176-1617(99)80112-6. [DOI] [Google Scholar]
- 22.Hirayama T, Shinozaki K. Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007;12:343–51. doi: 10.1016/j.tplants.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Dekkers BJW, Schuurmans JAMJ, Smeekens SCM. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol Biol. 2008;67:151–67. doi: 10.1007/s11103-008-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010;153:99–113. doi: 10.1104/pp.109.150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoecker U, Vasil IK, McCarty DR. Integrated control of seed maturation and germination programs by activator and repressor functions of Viviparous-1 of maize. Genes Dev. 1995;9:2459–69. doi: 10.1101/gad.9.20.2459. [DOI] [PubMed] [Google Scholar]
- 26.Hoecker U, Vasil IK, McCarty DR. Signaling from the embryo conditions Vp1-mediated repression of alpha-amylase genes in the aleurone of developing maize seeds. Plant J. 1999;19:371–7. doi: 10.1046/j.1365-313X.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- 27.Osanai T, Imashimizu M, Seki A, Sato S, Tabata S, Imamura S, et al. ChlH, the H subunit of the Mg-chelatase, is an anti-sigma factor for SigE in Synechocystis sp. PCC 6803. Proc Natl Acad Sci U S A. 2009;106:6860–5. doi: 10.1073/pnas.0810040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22:1909–35. doi: 10.1105/tpc.110.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gollop R, Farhi S, Perl A. Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci. 2001;16:579–88. doi: 10.1016/S0168-9452(01)00445-9. [DOI] [Google Scholar]
- 30.Gollop R, Even S, Colova-Tsolova V, Perl A. Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J Exp Bot. 2002;53:1397–409. doi: 10.1093/jexbot/53.373.1397. [DOI] [PubMed] [Google Scholar]
- 31.Fujii H, Zhu JK. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci U S A. 2009;106:8380–5. doi: 10.1073/pnas.0903144106. [DOI] [PMC free article] [PubMed] [Google Scholar]