Abstract
One may think that plants seem relatively immobile. Nevertheless, plants not only produce movement but these movements can be quite rapid such as the closing traps of carnivorous plants, the folding up of leaflets in some Leguminosae species and the movement of floral organs in order to increase cross pollination. We focus this review on thigmotropic and thigmonastic movements, both in vegetative and reproductive parts of higher plants. Ultrastructural studies revealed that most thigmotropic and thigmonastic movements are caused by differentially changing cell turgor within a given tissue. Auxin has emerged as a key molecule that modulates proton extrusion and thus causing changes in cell turgor by enhancing the activity of H+ATPase in cell membranes. Finding conserved molecules and/or operational molecular modules among diverse types of movements would help us to find universal mechanisms controlling movements in plants and thus improve our understanding about the evolution of such phenomena.
Keywords: carnivorous plants, movement, plant reproduction, pulvinus, thigmonastism, thigmotropism
Introduction
When observing plants, usually it will not be clearly noticeable to see them moving by their own. They seem relatively immobile, stuck to the ground in rigid structures. But for careful watchers as Darwin was in the 19 century,1 it is quite clear that plants do produce movements, and sometimes rapid ones. Fast responses by plants (especially those induced by mechanical stimuli) are generally the product of natural selection exerted by environmental stimuli that require the plants to respond immediately upon the application of stimuli. These reactions are usually called tropic and nastic responses (depending on the influence of stimulus position on the direction of the movement2), and when they are touch-induced, the prefix thigmo is used. Therefore thigmotropic and thigmonastic responses differ from each other as thigmotropisms occur in a direction determined by the position of where the plant was touched, while thigmonastisms are movements that occur independently of the direction of the stimulus.2 An example of thigmotropism is the coiling movement of tendrils in the direction of an object that it touches. On the other hand, the folding movement of the Mimosa pudica leaflets, can be considered as an example of thigmonastism. No matter where the leaf or leaflet is touched, the stimulus is propagated through neighboring leaflets and the folding movement is always in the same way.
However, not all touch-induced responses are fast. Thigmomorphogenesis, for example, is the physiological and morphological adaptation produced by plants in response to environmental mechanical influences generating morphogenetic changes.2,3 The mechanical influences could be natural factors as wind, vibrations and animal rubbing.2,3 Wind, for example, can influence photosynthetic rates, gaseous exchanges, growth, plant architecture and can even contribute to shape the evolutionary history of land plants.4,5
We will focus this review on thigmotropic and thigmonastic movements, both in vegetative and reproductive parts of higher plants, because recent findings pointed to conserved molecules and/or operational molecular modules among diverse types of touch-induced plant movements that could help us to improve our understanding of how the plants transduce mechanical stimuli. These findings are so important and inspiring that they are already priming research with biomimetic material such as the design of electroactive polymers.6,7
Active vegetative parts
Folding up of leaflets in Leguminosae
Some Leguminosae species keep their leaves and/or leaflets unfolded during daytime in order to intercept light and fold them up at night. These movements, known as nyctinasty - the stimulus being the presence or absence of light - are present in many legumes including Mimosa, Albizzia, Samanea, Pterodon, Robinia and Phaseolus, and are regulated by the circadian clock.8-11 Studies concerning nyctinastic movements in Leguminosae, specially in Mimosa has been undertaken since the 1950s and these were already covered by other reviews.9,12,13 However, Mimosa species such as M. pudica Linn., for example, in addition to light induction, also responds to touch stimulus by folding up its leaflets (Fig. 1). This thigmonastic movement is faster than the nyctinastic one,9 and the stimulus can be dispersed from one of the smallest leaflets (called pinnule) to other pinnules in the same pinna (which is the set of pinnules plus the raquis) and even to adjacent pinnae and finally to the hole set of pinnae (the actual leaf) if the stimulus is more intense, like an injury.14 Therefore, when an animal passes near the plant, rubbing it, the thigmonastic movement of the leaves would make the plant appear ¨shrinked¨, thus eventually avoiding predation.
Figure 1.
Thigmonastic movement of leaflets in Mimosa pudica. A: Leaflets open; B: leaflets closing due to touch-induced changes in cell turgor of cells within the pulvinus, a structure located at the base of each leaflet. C: Leaflets closed. The time-lapse between each photograph is about 1 sec.
A specialized motor organ located at the base of the leaflets and leaves, called pulvinus, plays the primary role in the nastic phenomena.8,10,15-17 The pulvinus has two groups of specialized parenchymatous cells, arranged in two opposite zones, the flexor or ventral zone and the extensor or dorsal zone. These specialized cells, called motor cells, are capable of changing their volume and shape very fast due to changes in cell turgor (Fig. 2).8,9 Right after a touch stimulus, a first action potential transmits a signal from the stimulated site to the pulvinus.18 Another action potential triggers the rapid movement in the pulvinus, generating differential flows of K+ and Cl- between the symplasm and apoplasm that are followed by massive water flows, resulting in loss or gain of turgor by the cell (i.e., changes in the vacuole volume).8,9,17,18 As the ions are pumped out of the extensor motor cells the internal water potential increases, leading to water loss and consequently to the shrinkage of these cells.9,15,17 At the same time, the opposite occurs with the flexor zone cells, leading them to a turgid condition and the leaflet folds up.9,15,17 The turgor recovery of the extensor cells occurs when the ions and, concomitantly, water, are pumped back into them.9,15,17 The role of calcium ions during the nastic movements has also been investigated. Ca2+ channels in the tannin vacuole tonoplast would release Ca2+ to the aqueous vacuole and these ions would bind to microfibrillar content during the closing movement of the pulvinus.19 Lately, other studies reinforced Ca2+ as an important regulator of turgor changes in pulvinus, being responsible for the closure of inward-directed K+ channels causing cell shrinking20 and as a part of the signal transduction events (Fig. 2).21
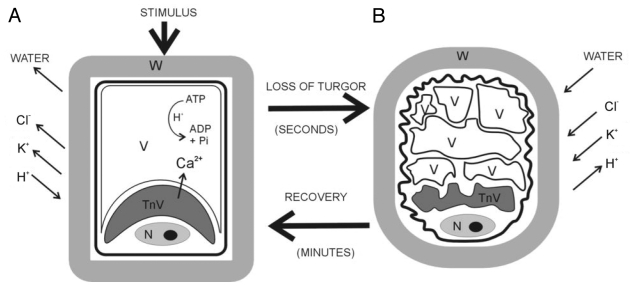
Figure 2.
Schematic representation of the variation in motor cell shape. The cell remains swollen if pulvini movement is inactive (A), whereas it becomes shrunken after pulvini response (B). Motor cells contain two vacuole types, one tannin-rich (TnV) located near the nucleus (N), the other aqueous and central (V). During shrinkage both vacuoles change their shape. K+ and Cl- fluxes mediate movement by triggering osmotic movement of water. In a cell gaining volume the energy-dependent pumping of protons out of the cell drives K+ uptake through specific inward-directed K+ channels. In a cell losing volume the flux of Cl- out of the cell down its concentration gradient drives K+ efflux through specific outward-directed K+ channels. The electrochemical gradient that enables rapid ion transport through plasma membranes is generated by H+-ATPase.
It has become clear that the physiological processes involved in pulvinus movement are highly related to its structural features. Anatomical and ultrastructural analysis has been undertaken in a number of Leguminosae species, showing changes in the vacuole size and shape during the movements.8,10,11,15 The pulvinus motor cells usually have two distinct vacuole types. One is called the tannin vacuole, as it contains great amounts of tannins. These vacuoles are located near the nucleus and they are supposed to function as a potential Ca2+ store.10,15,19 The non-tannin or aqueous vacuoles are electron-transparent, do not contain tannin, are much larger than the tannin vacuoles and occupy a central position in the cells.22 The aqueous vacuole is responsible for most of the changes in the motor cell volume during nastic movements.8,10,11,15,22 When the pulvinus is open, i.e., when the leaflets are unfolded, the aqueous vacuole of the extensor cell is large, occupying almost all the cell volume and restricting the remaining cytoplasm and nucleus to the cell periphery.8,10,11,15 The flexor zone cells are generally multivacuolated with sinuous walls.8,10,11,15 When the pulvinus straightens, the extensor cells often become highly sinuously shaped and the aqueous vacuole gets fragmented.8,10,11,15 On the other hand, the flexor cells become univacuolated and less sinuous, but the changes in size and shape are less obvious when compared with the changes observed in extensor cells.8,10,11,15 This vacuolar reorganization allows the surface area of the tonoplast to remain the same.t.8,23 Changes in the apoplasm, defined by wall thickness and elasticity of cortical cells, are also important for the movement.10,11,15,24 Still, cortical cell walls also behave as cation exchangers and temporary reservoir of ions.25 Features such as the presence of plasmodesmata grouped in pit fields in the motor cells, indicating simplastic continuity ensure the communication among the cells supporting ions transport.23
The electrochemical gradient that enables rapid ion transport through plasma membranes is generated by H+-ATPase, which is present in the motor cells (Fig. 2). In plants, it is known that K+ flows are coupled with inverse H+ flows.12,26 A large amount of plasma membrane H+-ATPase was found in Mimosa pudica phloem parenchyma and companion cells, suggesting that these cells are resting potential maintainers in the sieve element system, a condition required for the transmission of action potentials in response to stimuli, and are fundamental in the recovery of ions in stimulated sieve elements.25 Anatomical studies contributed to confirm the role of the vascular system in the redistribution of ions and in the transmission of stimuli. The occurrence of a sheath of live septate fibers around the vascular tissues, vascular parenchyma cells lacking lignification, with abundant cytoplasm and extensive symplastic connections was observed in some Caesalpinioideae, Faboideae and Mimosoideae species. Taken together, these characterisitics would enable lateral exchanges of ions and stimuli among all pulvinus cells.16 Also, it has been suggested that high water permeability across the vacuolar membrane and energization of the vacuole are crucial for the shrinking of the motor cells. Such features are based on the presence of a great amount of vacuolar H+-ATPase and aquaporins in the tonoplast of the aqueous vacuole in M. pudica.27
It was demonstrated that pulvini movements are likely to be controlled by auxin.28-32 It is known that auxin, in addition to mediate physiological states that involves gene regulation, also affects short-term cellular events and one of these alterations concerns the activity enhancing of H+-ATPase in cell membranes, stimulating proton extrusion.33
Application of IAA to the cut end of excised pinna rachises induced leaflet opening in M. pudica and Cassia fasciculata.28,29,32 Later, experiments applying 2,4-D in C. fasciculata inhibited leaflet folding induced by darkness and promoted the leaflet opening induced by light.32 Similar results were found for Phaseolus vulgaris, using protoplasts isolated from laminar pulvinus.31 The application of IAA induced cell swelling on either extensor and flexor cells and when K+ and Cl- blockers or vanadate, an inhibitor of plasma membrane H+-ATPase, were added to the bathing medium, the IAA-induced swelling response was repressed.31 Taken together, these experiments emphasize that the swelling response depends on K+ and Cl- fluxes and that auxin stimulated the vacuolar H+-ATPase activity, making the permeability of motor cells to these ions to be mainly directed toward an influx of K+ into the cells.30
There is not much information about the effects of ABA on the osmoregulation of pulvinus motor cells. Experiments with P. vulgaris showed that, in opposition to the results obtained with the application of IAA, ABA application causes cell shrinking and its effect is suppressed by an anion-channel inhibitor, suggesting the efflux of Cl- through the activated channels and thus, the charge balance efflux of K+.31
The involvement of aquaporins in order to sustain the rapid water transport in nastic movements was suggested by the observation that plasma membrane-localized aquaporine genes are expressed in pulvinus motor cells of Samanea saman34 and by immuno-localization of aquaporins in M. pudica pulvini.27 The roles of aquaporins in leaf movements were recently reviewed.35
The rearrangement of the actin cytoskeleton is also involved in the pulvinus movement. During bending, the actin filaments undergo fragmentation and are distributed diffusely throughout the cytoplasm playing an important role in the movement.36 The actin present in the M. pudica pulvinus is heavily tyrosine-phosphorylated and changes in the extent of phosphorylation could be correlated with pulvinus movement.37 It has also been demonstrated that M. pudica pulvini can be stimulated by the application of a low voltage to the pulvinus, allowing the analysis of electrical charges in the petiole and pulvinus.38,39
Carnivorous plants
¨Carnivory¨ in plant kingdom is an adaptation to low nutrient habitats and molecular data support multiple, polyphyletic origins of the carnivorous plants (for a review, see ref.40). Many of the carnivorous plants have developed trap mechanisms that involve a highly modified leaves. Some of these modified leaves have “acitve traps,” i.e., exhibit thigmotropic or thigmonastic movements, as in the genera Pinguicula, Drosera, Dionaea, Aldrovandra and Utricularia (Fig. 3).41,42
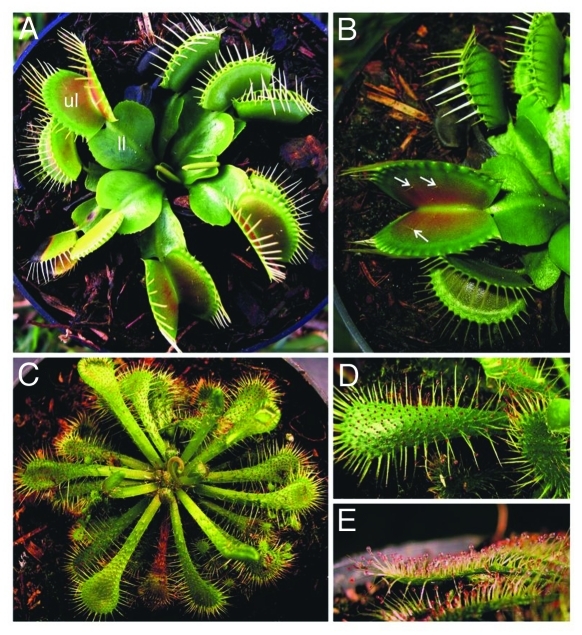
Figure 3.
Dionaea sp, the Venus flytrap (A-B) and Drosera sp (C-E). A: Dionaea modified leaves are divided in two parts, the upper (ul) and the lower leaf (ll). B: The upper leaf has two lobes which center is brightly colored red and contains three sensitive trigger hairs (arrows). The free edge of each lobe is lined with spine-like projections or cilia. C: Drosera have sticky, highly modified colorful leaves that are capable to move acting like small insect traps. D-E: The leaves have several glandular trichomes with mucilage droplets on their apex, commonly called hairs or tentacles. When an insect gets stuck to the tentacles, it acts as a mechanical stimulation which causes the leaf to curl toward and around the prey.
Both Aldrovandra and Dionaea produce traps that rapidly snap-shut.41 Dionaea muscipula Ellis, the Venus flytrap, for example, rapidly reacts to mechanical stimulus. Dionaea modified leaves are divided in two parts, the upper and the lower leaf.41-43 The upper leaf has two lobes attached by a central vein or midrib.41-43 The center of each lobe is brightly colored red due to an anthocyanin pigment and contains three sensitive trigger hairs (Fig. 3B).41-43 Also, the lobes are lined up with UV-reflective glands.41-43 The free edge of each lobe is lined with spine-like projections or cilia. The lower leaf, also known as the footstalk, has an expanded leaf-like structure.41-43
An insect, attracted to the red center of the lobe and to the UV reflective glands, touches the trigger hairs activating mechanical sensitive ion channels that generate an action potential that activates the Dionaea motor cells.44 The action potential, characterized by a large transient depolarization, allows the rapid transmission of information via plasmodesmata or conductive bundles.44 The leaves snap together in a fraction of a second confining the insect, but maintaining the angle between the lobes and the middle of the midrib.45,46 Analyzing the ultrastructure of the trigger hairs, three metabolic active cell regions could be distinguished: indentation, anticlinal and podium.47,48 The cells within these regions contain many plasmodesmata which in turn are largely related to the sensitivity of the trigger hairs.48
As in legume pulvini, the movement of the Venus flytrap lobes is caused by changes in cell volume of opposing tissue groups by differential water flow linked to ion fluxes.45 Nevertheless, despite the fact that researchers have investigated the mechanisms by which the trap closes so fast, only in the past six years, the dynamic of the movement started to become more clear, notably thanks to Forterre et al.49 and Volkov et al.43,44,46,50-53 Forterre et al.49 divided the Venus flytrap movement into two components: an active biochemical component, pertinent to the mechanisms causing microscopic changes, and a passive elastic component, which is the macroscopic closing process determined by the doubly-curved leaf. A difference in turgor pressure constantly maintained between the upper and the lower cell layers of the leaf provides elastic curvature energy storage locked in the leaves.43,54 The transference of fluid from the upper to the lower cell layer after a stimulus in the sensitive trigger hairs, induces the trap to close due to the return to an equilibrium state of the leaf.43,49 The rapid changes in the curvature of each lobe, rather than the movement of the leaf as a whole, are responsible for the closing movement.43 More recently, the total hunting cycle of the Venus flytrap was defined as consisting of five different states: open, closed, locked, constriction/digestion, and semi-open.46 When opened, the trap has a convex shape, and in the other phases, the trap changes its curvature to a concave format.46
D. muscipula can also perceive artificial electrical signals and can be stimulated to close the trap with the same speed as when mechanical stimulation is applied.44,50 Furthermore, it was demonstrated that this plant has a short-term electrical memory by the fact that it can accumulate previously applied small electrical charges, closing the trap when a threshold value is reached.51,52
Species from the carnivorous plant genera Drosera and Pinguicula have sticky, highly modified colorful leaves that are capable to move acting like small insect traps (Fig. 3C-E). The leaves have several glandular trichomes with mucilage droplets on their apex, commonly called hairs or tentacles.41,55 When an insect gets stuck to the tentacles, it acts as a mechanical stimulation which causes the leaf to curl toward and around the prey.41,55 Differently from Dionaea, an action potential causes a rapid cell growth in Drosera and Pinguicula leading do the leaf movement that, in this case, is obviously slower, during minutes to hours.41 In the case of Drosera, the tentacles also bend to retain the prey, and the tentacle movements are usually faster than the leaf curling movement, since the action potential in this case causes rapid cell expansion due to an increase of cell internal pressure.41 In Drosera capensis L., IAA enhances the speed of the movement by a growth stimulus when applied to the leaf. Interestingly, when glass balls are used as artificial preys, the plant does not produce any movement.56 On the other hand, the application of TIBA, which reduces auxin transport between the leaf tip and the prey, inhibits the growth reaction.56
The aquatic plants from the genus Utricularia L., popularly known as bladderworts, represent another example of carnivorous moving plants. In this case, the traps are highly modified leaves in tiny, hollow, thin, oval or spherical capsule-like structures called “bladders” or “utricules.”41,57 The traps bear a single entrance which is sealed by a flat, semicircular and two-cell layered thick valve, also named trapdoor.41,57 Some species have appendages: trigger hairs or ¨antennae¨, on the entrance that attracts the prey and which are sensitive to mechanical contact.41,57 The bladder is under negative hydrostatic pressure by pumping out water. At this state, the outer walls are concave and an elastic instability is maintained.2,58 After a touch stimulus on the trigger hairs, the valve opens within 300 to 700 μs, the walls take on a slightly convex shape and the prey is suctioned inside the bladder along with water by the difference in pressure.2,41,58 This opening movement is the fastest ever recorded for carnivorous plants.58
Tendrils
The climbing habit is widespread throughout plants, since it has evolved in many lineages of ferns, gymnosperms and many angiosperms as Bryonia, Pisum, Vitis and Passiflora, for example (Fig. 4).59 One of the attachment mechanisms by which vines can raise concerns the tendril coiling movements that can be thigmonastic or thigmotropic, depending on the type of the tendril.
Figure 4.
Tendril coiling movement in Passiflora edulis (passionfruit vine). A: The terminal portion of the tendril touches the stem of a neighboring branch. B: About 2h after the photograph shown in A was taken, the same tendril presents touch-induced coiling (contact coiling) movement around the branch. About 4h after the photograph shown in A was taken, the touch-induced coiling process is irreversible and a spiral begins to form due to tendril differential growth.
Jaffe and Galston60 described that tendrils are capable of three types of movement: circumnutation, contact coiling and free coiling. The circumnutation bears on the habitual growth of the tendril, and increases the chances to contact a support. The contact coiling happens when the tendril coils around a support. When the tendril touches an object, the tip grows in length forming a spiral coil to involve it. The reaction time varies from 25 sec to 10 min. Frequently, the maximum sensibility of the tendril is approximately in the one fourth of the length near the tip, so the ability for the coiling movement is greater in this portion.60 If the object is withdrawn, the tendril often uncoils, but the uncoiling movement is substantially slower.60 This reversible early phase is thought to be osmotically driven with contraction of the ventral side and expansion of the dorsal side.60 Maintaining the mechanical stimulus, the coiling process is irreversible and the spiral begins to form due to differential growth.60 Free coiling refers to a spiral shape formation of the tendril due to a coiling movement around its own axis without a touch stimulus that could happen, for example, in the beginning of senescence.60
As in other movements already described here, increase of transmembrane proton and calcium fluxes are related to the beginning of the coiling movement in tendrils61 in addition to the involvement of ATP and light.62 The cytoskeleton seems to mediate the physical stimuli and biochemical responses, in particular the microtubuli, that play a central role on the perception of touch stimuli in tendrils of Pisum and Bryonia.63,64 In Bryonia dioica Jacq. epidermal cells of the tendril tip seem to be related to the perception and transmission of the stimulus, specially the protrusions formed by a differentiation of the outer peripheral cell walls, called tactile bleps.61,64 These sub-cellular zones have a unique arrangement of actin microfilaments and microtubule, and a large amount of membrane-associated calcium, in addition to a thinner cell wall and a peculiar chemical composition when compared with the rest of the cell. All these characteristics are apparently necessary to ensure its mechanoreceptor and transducer function.61,64
Redvine tendrils develop a cylinder of 5–6 cell layers of gelatinous-type fiber between the vascular tissue and the cortex, that appear to be crucial to the ability to elicit the shape changes on the coiling movement and also generating the tensile strength, considering that tendrils that fail to coil also fail to produce the gelatin layer.65 Furthermore, anatomical analysis showed that there is a correlation between the arrangement of the gelatin layer and the tendril ability to coil, that can be in any direction, in a single direction as in Echynocistus lobata, or spontaneously free-coil without touch stimuli.66
Treatments with jasmonates led to an increase of endogenous levels of IAA and 12-oxo-phytodienoic acid (PDA) inducing tendril coiling in B. dioica.67 However, in Pisum, jasmonic acids are not inducers of coiling response, and auxins are more likely related to the mechanical induced reaction.63 The hormonal roles in tendril responses as signal mediators and inducers of differential growth remain uncertain, but auxin is certainly a common player.
Active flowers, the sexual reproductive parts
Despite the fact that there are numerous studies concerning movements of vegetative parts, it is known that many plant species have developed touch-sensitive floral organs and fruits capable of thigmotropic or thigmonastic movements, but there are less precise studies about their physiological and biochemical aspects, being most of them about pollinator visits and the macroscopic description of the movement. These movements are apparently maintained by evolutionary pressure to prevent self-pollination and increase cross-pollination, ensuring the placement of pollen grains on pollinators such as birds and insects.
Portulaca grandiflora Hook, known as moss-rose, has thigmotropic stamens that bend in 0,25s after a mechanical stimulus, returning to its original position in about 5 min.68 The process is ATP dependent and induced by auxin.68 Mechanically stimulated stamen movements were also seen in the genera Opuntia (Cactaceae).69 These flowers have many whorls of stamens that are longer on the outer whorls and shorter in the inner ones.69 When a visiting bee mechanically stimulates the stamen filaments, they instantly bend toward the style (i.e., the center of the flower) and the longer filaments covers the shorter ones, hindering the access of pollen foragers to the source of nectar.69 Interestingly, the bees that effectively pollinate these flowers are the ones capable of crawling down the space between the innermost stamen and the style, having access to the majority of pollen and nectar resources.69
Orchids are known to have developed outstandingly specialized structures in order to increase reproductive success and it is not surprising that some genera belonging to this family present examples of moving floral parts. The orchid genus Catasetum, for example, has sexually dimorphic flowers with significantly differences between male and female flowers, which are pollinated exclusively by male euglossine bees that collect chemical attractants.70,71 When the bee sits in the center of the male flower and touches its sensitive antenna (a exclusively feature of male flowers), the pollinium is ejected and strikes the bee throwing it out of the flower.70,71 The force of the pollen mass that strikes the bee is 2.90 x 10–2 Newtons and the speed of the release varies from 2,65 to 3,03 m/s.70,71 This is not enough to cause injuries to the male euglossine bee, but sufficiently strong to glue the pollinium to the body of the bee, inducing it to avoid further visits to male flowers. The bee would thus prefer the female flowers for its next visit.70,71 Similarly, in the bisexual flowers of Listera cordata (L.) R. Br., the pollinium is released when an insect touches the trigger hairs located at the tip of the floral structure called rostellum.72 Altough, right before releasing the pollinium, the rostellum rapidly shots a droplet of a sticky material that hits the insect and will help to fix on it the pollinium that will be released. The insect with the pollinium glued on it will probably carry the pollen mass to other flowers, pollinating them.72
In the case of the orchid Bulbophylum penicillium E. C. Parish and Rchb the specialized petal called lip looks like a caterpillar, and actually moves like one.73 When a Drosophyla fly, attracted by the flower rotting-fruit odor lands on the lip, the lip starts to move up and down or swings left and right.73 This movement presses the fly toward the column apex, and the fly touches the anther removing the pollinia.73 This fly will visit other flowers carrying the pollinia in its torax, and because of the same moving lip phenomena, the pollinia from the first flower is left on the second one, and cross-pollination probably will occur successfully.73
Passiflora (Passifloraceae) is a genus with a large morphologic diversity of floral structures that defies the explanations given so far to its evolutionary origins.74,75 The movement a floral structure called androgynophore of some Passiflora species from session Xerogona (Fig. 5), is probably related to the pollination of these species, as a mechanism to increase the chances of pollen deposition on the bodies of their effective pollinators, similar to the other examples above (our unpublished observations).
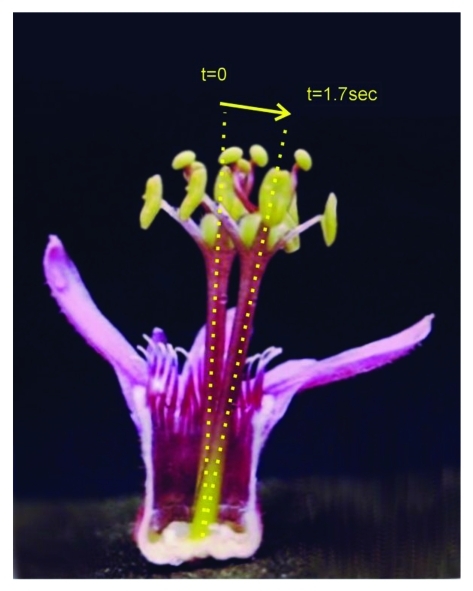
Figure 5.
Androgynophore movement in Passiflora sanguinolenta. The image is the result of the superposition of two photographs of flower partially dissected to show the entire androgynophore column. The photographs were taken before (t = 0) and after (t = 1.7 sec) the tip of the androgynophore was touched. The androgynophore column moves toward the stimulus regaining its original position after about 3min if no further stimulus is applied.
Studying molecular and physiological aspects involved in the mechanisms related to the movement of reproductive parts in plants is of utmost importance in order to clarify the coevolutionary relationships between the different floral morphologies and their pollinators.
Conclusions
Mechanically-induced movement of plant parts evolved as the product of natural selection exerted by environmental stimuli that require the plants to respond immediately upon the application of a given stimulus. These movements might be as diverse as the closing traps of carnivorous plants, the folding up of leaflets in some Leguminosae species and the movement of floral organs in order to increase cross pollination. The nature of these movements might sound diverse but recently, shared key characteristic features of these movements have emerged, indicating that common mechanisms may underlie all of them. Ultrastructural studies revealed that most thigmotropic and thigmonastic movements are caused by differentially changing cell turgor within a given tissue. Auxin has emerged as a key molecule that modulates proton extrusion and thus causing changes in cell turgor by enhancing the activity of H+ATPase in cell membranes. Additionally, the observation of moving reproductive parts, based on similar mechanisms involved in the movement of vegetative parts, indicates a role for pollinating agents as ¨genome tinkerers¨, at least partially responsible for the selection of such characteristics. Finding conserved molecules and/or operational molecular modules among diverse types of movements would help us to find universal mechanisms controlling movements in plants and thus improve our understanding about the evolution of such phenomena.
Acknowledgments
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for funding research on the movement of floral organ parts in our group.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18192
References
- 1.Darwin C. The power of movement in plants. New York: D. Appleton and Company, 1880. [Google Scholar]
- 2.Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–89. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe MJ, Forbes S. Thigmomorphogenesis - the effect of mechanical perturbation on plants. Plant Growth Regul. 1993;12:313–24. doi: 10.1007/BF00027213. [DOI] [PubMed] [Google Scholar]
- 4.de Langre E. Effects of wind on plants. Annu Rev Fluid Mech. 2008;40:141–68. doi: 10.1146/annurev.fluid.40.111406.102135. [DOI] [Google Scholar]
- 5.Niklas KJ. The influence of gravity and wind on land plant evolution. Rev Palaeobot Palynol. 1998;102:1–14. doi: 10.1016/S0034-6667(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 6.Burgert I, Fratzl P. Actuation systems in plants as prototypes for bioinspired devices. Philos Transact A Math Phys Eng Sci. 2009;367:1541–57. doi: 10.1098/rsta.2009.0003. [DOI] [PubMed] [Google Scholar]
- 7.Taya M. Bio-inspired design of intelligent materials. In: Y. BarCohen (ed) Smart structures and materials: electroactive polymer actuators and devices. Proc SPIE 5051 2003:54-65. [Google Scholar]
- 8.Campbell NA, Garber RC. Vacuolar reorganization in the motor cells of Albizzia during leaf movement. Planta. 1980;148:251–5. doi: 10.1007/BF00380035. [DOI] [PubMed] [Google Scholar]
- 9.Satter RL, Galston AW. Mechanisms of control of leaf movements. Annu Rev Plant Physiol. 1981;32:83–110. doi: 10.1146/annurev.pp.32.060181.000503. [DOI] [Google Scholar]
- 10.Moysset L, Simon E. Secondary pulvinus of Robinia pseudoacacia (Leguminosae) - structural and ultrastructural features. Am J Bot. 1991;78:1467–86. doi: 10.2307/2444972. [DOI] [Google Scholar]
- 11.Machado SR, Rodrigues TM. Anatomia e ultraestrutura do pulvino primário de Pterodon pubescens Benth. (Fabaceae – Faboideae) Revista Brasileira de Botanica. 2004;27:135–47. doi: 10.1590/S0100-84042004000100015. [DOI] [Google Scholar]
- 12.Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–47. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Ueda M, Nakamura Y. Chemical basis of plant leaf movement. Plant Cell Physiol. 2007;48:900–7. doi: 10.1093/pcp/pcm060. [DOI] [PubMed] [Google Scholar]
- 14.Malone M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994;128:49–56. doi: 10.1111/j.1469-8137.1994.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleurat-Lessard P. Structural and ultrasturctural features of cortical-cells in motor organs of sensitive plants. Biol Rev Camb Philos Soc. 1988;63:1. doi: 10.1111/j.1469-185X.1988.tb00467.x. [DOI] [Google Scholar]
- 16.Rodrigues TM, Machado SR. The pulvinus endodermal cells and their relation to leaf movement in legumes of the Brazilian cerrado. Plant Biol (Stuttg) 2007;9:469–77. doi: 10.1055/s-2006-955917. [DOI] [PubMed] [Google Scholar]
- 17.Samejima M, Sibaoka T. Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol. 1980;21:467–79. [Google Scholar]
- 18.Sibaoka W. Rapid plant movements triggered by action potentials. Bot Mag Tokyo. 1991;104:73–95. doi: 10.1007/BF02493405. [DOI] [Google Scholar]
- 19.Toriyama H, Jaffe MJ. Migration of calcium and its role in the regulation of seismonasty in the motor cell of Mimosa pudica L. Plant Physiol. 1972;49:72–81. doi: 10.1104/pp.49.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cote GG. Signal-transduction in leaf movement. Plant Physiol. 1995;109:729–34. doi: 10.1104/pp.109.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd AN, Love J, Webb AAR. The plant clock shows its metal: circadian regulation of cytosolic free Ca2+ Trends Plant Sci. 2005;10:15–21. doi: 10.1016/j.tplants.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Marty F. Plant vacuoles. Plant Cell. 1999;11:587–600. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse MJ, Satter RL. Relationships between motor cell ultrastructure and leaf movement in Samanea saman. Physiol Plant. 1979;46:338–46. doi: 10.1111/j.1399-3054.1979.tb02630.x. [DOI] [Google Scholar]
- 24.Rodrigues TM, Machado SR. Anatomia comparada do pulvino primário de leguminosas com diferentes velocidades de movimento foliar. Revista Brasileira de Botanica. 2006;29:709–20. doi: 10.1590/S0100-84042006000400020. [DOI] [Google Scholar]
- 25.Freudling C, Starrach N, Flach D, Gradmann D, Mayer WE. Cell walls as reservoirs of potassium ions for reversible volume changes of pulvinar motor cells during rhythmic leaf movements. Planta. 1988;175:193–203. doi: 10.1007/BF00392427. [DOI] [PubMed] [Google Scholar]
- 26.Ward JM, Maser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain JL, Martinoia E. Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol. 1997;114:827–34. doi: 10.1104/pp.114.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe S, Sibaoka T. Light-Induced and Auxin-Induced Leaflet Opening in Detached Pinnae of Mimosa-Pudica. Plant Cell Physiol. 1983;24:641–7. [Google Scholar]
- 29.Bourbouloux A, Roblin G, Fleuratlessard P. Calcium Involvement in the Iaa-Induced Leaflet Opening of Cassia-Fasciculata. J Exp Bot. 1992;43:63–71. doi: 10.1093/jxb/43.1.63. [DOI] [Google Scholar]
- 30.Bonmort J, Roblin G. Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul. 1996;19:61–5. doi: 10.1007/BF00024403. [DOI] [Google Scholar]
- 31.Iino M, Long C, Wang X. Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol. 2001;42:1219–27. doi: 10.1093/pcp/pce157. [DOI] [PubMed] [Google Scholar]
- 32.Moyen C, Bonmort J, Roblin G. Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudica L. Plant Physiol Biochem. 2007;45:420–6. doi: 10.1016/j.plaphy.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Taiz L, Zeiger E. Auxin, the growth hormone. In: Taiz, L.; Zeiger, E. ed. Plant physiology. Sunderland, Mass.: Sinauer Associates, 2006:487-488. [Google Scholar]
- 34.Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, et al. Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell. 2002;14:727–39. doi: 10.1105/tpc.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehlein N, Kaldenhoff R. Aquaporins and plant leaf movements. Ann Bot (Lond) 2008;101:1–4. doi: 10.1093/aob/mcm278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanzawa N, Hoshino Y, Chiba M, Hoshino D, Kobayashi H, Kamasawa N, et al. Change in the actin cytoskeleton during seismonastic movement of Mimosa pudica. Plant Cell Physiol. 2006;47:531–9. doi: 10.1093/pcp/pcj022. [DOI] [PubMed] [Google Scholar]
- 37.Kameyama K, Kishi Y, Yoshimura M, Kanzawa N, Sameshima M, Tsuchiya T. Tyrosine phosphorylation in plant bending. Nature. 2000;407:37. doi: 10.1038/35024149. [DOI] [PubMed] [Google Scholar]
- 38.Volkov AG, Foster JC, Ashby TA, Walker RK, Johnson JA, Markin VS. Mimosa pudica: electrical and mechanical stimulation of plant movements. Plant Cell Environ. 2010;33:163–73. doi: 10.1111/j.1365-3040.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 39.Volkov AG, Foster JC, Markin VS. Molecular electronics in pinnae of Mimosa pudica. Plant Signal Behav. 2010;5:826–31. doi: 10.4161/psb.5.7.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends Ecol Evol. 2001;16:623–9. doi: 10.1016/S0169-5347(01)02269-8. [DOI] [Google Scholar]
- 41.McPherson S. Carnivorous plants and their habitats. Poole, Dorset, England: Redfern Natural History Productions, 2010. [Google Scholar]
- 42.Slack A, Gate J. Carnivorous plants. Cambridge, Mass.: MIT Press, 2000. [Google Scholar]
- 43.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkov AG, Adesina T, Jovanov E. Closing of venus flytrap by electrical stimulation of motor cells. Plant Signal Behav. 2007;2:139–45. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagerberg WR, Allain D. A quantitative study of tissue dynamics during closure in the traps of Venus flytrap Dionaea muscipula Ellis. Am J Bot. 1991;78:647–57. doi: 10.2307/2445086. [DOI] [Google Scholar]
- 46.Volkov AG, Pinnock MR, Lowe DC, Gay MS, Markin VS. Complete hunting cycle of Dionaea muscipula: consecutive steps and their electrical properties. J Plant Physiol. 2011;168:109–20. doi: 10.1016/j.jplph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Mozingo HN, Klein P, Zeevi Y, Lewis ER. Venuss flytrap observations by scanning electron microscopy. Am J Bot. 1970;57:593. doi: 10.2307/2441058. [DOI] [Google Scholar]
- 48.Williams ME, Mozingo HN. The fine structure of the trigger hair in Venus’s flytrap. Am J Bot. 1971;58:532–9. doi: 10.2307/2441035. [DOI] [Google Scholar]
- 49.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–5. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 50.Volkov AG, Adesina T, Jovanov E. Charge induced closing of Dionaea muscipula Ellis trap. Bioelectrochemistry. 2008;74:16–21. doi: 10.1016/j.bioelechem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Volkov AG, Carrell H, Adesina T, Markin VS, Jovanov E. Plant electrical memory. Plant Signal Behav. 2008;3:490–2. doi: 10.4161/psb.3.7.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochemistry. 2009;75:142–7. doi: 10.1016/j.bioelechem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Volkov AG, Carrell H, Markin VS. Molecular electronics of the Dionaea muscipula trap. Plant Signal Behav. 2009;4:353–4. doi: 10.4161/psb.4.4.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markin VS, Volkov AG, Jovanov E. Active movements in plants: Mechanism of trap closure by Dionaea muscipula Ellis. Plant Signal Behav. 2008;3:778–83. doi: 10.4161/psb.3.10.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson TC, Waller DM. Evolving Darwin's 'most wonderful' plant: ecological steps to a snap-trap. New Phytol. 2009;183:575–87. doi: 10.1111/j.1469-8137.2009.02935.x. [DOI] [PubMed] [Google Scholar]
- 56.Bopp M, Weber I. Hormonal regulation of the leaf blade movement of Drosera capensis. Physiol Plant. 1981;53:491–6. doi: 10.1111/j.1399-3054.1981.tb02738.x. [DOI] [Google Scholar]
- 57.Reifenrath K, Theisen I, Schnitzler J, Porembski S. Trap architecture in carnivorous Utricularia (Lentibulariaceae) Flora. 2006;201:597–605. doi: 10.1016/j.flora.2005.12.004. [DOI] [Google Scholar]
- 58.Singh AK, Prabhakar S, Sane SP. The biomechanics of fast prey capture in aquatic bladderworts. Biol Lett. 2011;7:547–50. doi: 10.1098/rsbl.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putz FE, Mooney HA. The biology of vines. Cambridge; New York: Cambridge University Press, 1991. [Google Scholar]
- 60.Jaffe MJ, Galston AW. The physiology of tendrils. Annu Rev Plant Physiol. 1968;19:417–34. doi: 10.1146/annurev.pp.19.060168.002221. [DOI] [Google Scholar]
- 61.Liss H, Weiler EW. Ion-translocating ATPases in tendrils of Bryonia dioica Jacq. Planta. 1994;194:169–80. doi: 10.1007/BF01101675. [DOI] [Google Scholar]
- 62.Jaffe MJ, Galston AW. Physiological studies on pea tendrils. II. The role of light and ATP in contact coiling. Plant Physiol. 1966;41:1152–8. doi: 10.1104/pp.41.7.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engelberth J. Mechanosensing and signaltransduction in tendrils. Adv Space Res. 2003;32:1611–9. doi: 10.1016/S0273-1177(03)90402-9. [DOI] [PubMed] [Google Scholar]
- 64.Engelberth J, Wanner G, Groth B, Weiler EW. Functional anatomy of the mechanoreceptor cells in tendrils of Bryonia dioica Jacq. Planta. 1995;196:539–50. doi: 10.1007/BF00203654. [DOI] [Google Scholar]
- 65.Meloche CG, Knox JP, Vaughn KC. A cortical band of gelatinous fibers causes the coiling of redvine tendrils: a model based upon cytochemical and immunocytochemical studies. Planta. 2007;225:485–98. doi: 10.1007/s00425-006-0363-4. [DOI] [PubMed] [Google Scholar]
- 66.Bowling AJ, Vaughn KC. Gelatinous fibers are widespread in coiling tendrils and twining vines. Am J Bot. 2009;96:719–27. doi: 10.3732/ajb.0800373. [DOI] [PubMed] [Google Scholar]
- 67.Stelmach BA, Muller A, Weiler EW. 12-oxo-phytodienoic acid and indole-3-acetic acid in jasmonic acid-treated tendrils of Bryonia dioica. Phytochemistry. 1999;51:187–92. doi: 10.1016/S0031-9422(99)00017-5. [DOI] [Google Scholar]
- 68.Jaffe MJ, Gibson C, Biro R. Physiological Studies of Mechanically Stimulated Motor Responses of Flower Parts. 1. Characterization of Thigmotropic Stamens of Portulaca-Grandiflora Hook. Bot Gaz. 1977;138:438–47. doi: 10.1086/336946. [DOI] [Google Scholar]
- 69.Schlindwein C, Wittmann D. Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst Evol. 1997;204:179–93. doi: 10.1007/BF00989204. [DOI] [Google Scholar]
- 70.Romero GA, Nelson CE. Sexual dimorphism in Catasetum orchids - forcible pollen emplacement and male flower competition. Science. 1986;232:1538–40. doi: 10.1126/science.232.4757.1538. [DOI] [PubMed] [Google Scholar]
- 71.Nicholson CC, Bales JW, Palmer-Fortune JE, Nicholson RG. Darwin's bee-trap: the kinetics of Catasetum, a new world orchid. Plant Signal Behav. 2008;3:19–23. doi: 10.4161/psb.3.1.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ackerman JD, Mesler MR. Pollination biology of Listera cordata (Orchidaceae) Am J Bot. 1979;66:820–4. doi: 10.2307/2442469. [DOI] [Google Scholar]
- 73.Liu ZJ, Chen LJ, Liu KW, Li LQ, Rao WH. A floral organ moving like a caterpillar for pollinating. J Syst Evol. 2010;48:102–8. doi: 10.1111/j.1759-6831.2009.00065.x. [DOI] [Google Scholar]
- 74.Dornelas MC, Tsai SM, Rodriguez APM. Expressed sequence tags of genes involved in the flowering process of Passiflora spp. In: Teixeira da Silva JA, ed. Floriculture, Ornamental and Plant Biotechnology. Global Science Books, London, 2006:483-8. [Google Scholar]
- 75.Dornelas MC, Fonseca TC, Rodriguez APM. Brazilian passionflowers and novel passionate tropical flowering gems. In: Teixeira da Silva JA, ed. Floriculture, Ornamental and Plant Biotechnology Global Science Books, London, 2006:629-39. [Google Scholar]