Abstract
Background
Kawasaki disease (KD) is the most common cause of acute vasculitis and acquired cardiac disease in US children. Untreated, children may develop coronary artery aneurysms, myocardial infarction and sudden death as a result of the illness. Up to a third of KD patients fail to respond to intravenous gammaglobulin (IVIG), the standard therapy, and alternative treatments are being investigated. Genetic studies have indicated a possible role for IL-1β in KD. We therefore explored the role of IL-1β in a murine model of KD.
Methods and Results
Using an established mouse model of KD that involves injection of Lactobacillus casei cell wall extract (LCWE), we investigated the role of IL- 1β and caspase-1 (activated by the inflammasome and required for IL-1β maturation) in coronary arteritis, and evaluated the efficacy of IL-1 receptor antagonist (IL-1Ra) as a potential treatment. LCWE-induced IL-1β maturation and secretion was dependent on the NLRP3 inflammasome in macrophages. Both caspase1-deficient and IL-1R-deficient mice were protected from LCWE-induced coronary lesions. Injection of recombinant IL-1β to caspase-1-deficient mice restored the ability of LCWE to cause coronary lesions in response to LCWE. Furthermore, daily injections of the IL-1Ra prevented LCWE-mediated coronary lesions, up to three days after LCWE injection.
Conclusions
Our results strongly suggest that caspase-1 and IL-1β play critical roles in the development of coronary lesions in this KD mouse model, blocked by IL-1Ra. Therefore, anti-IL-1β treatment strategies may constitute an effective, more targeted treatment of KD to prevent coronary lesions.
Keywords: Kawasaki disease
Kawasaki disease (KD) is an acute febrile illness and systemic vasculitis of unknown etiology1 that predominantly afflicts children <5 yrs of age. 25% of untreated patients with KD will develop acute coronary arteritis often with the development of coronary artery aneurysms, and accordingly, KD represents the leading cause of acquired heart disease among children.2–4 Though the etiology is unclear, KD involves systemic inflammation with a distinct predilection for the coronary arteries. The resulting coronary arteritis in KD is characterized histologically by inflammatory cell infiltration and destruction of extracellular matrix, especially elastic tissue in vascular media, with resultant coronary artery aneurysm formation.5Mortality in KD virtually always results from ischemic myocardial disease. The risk is highest in the first year after KD, but myocardial infarction has been increasingly reported in young adults with missed KD.6 Long term cardiovascular complications among survivors of childhood KD are reported with increasing frequency.7–9 The limited understanding of the etiologic agent(s) and cellular and molecular pathology of vasculitis, continues to thwart development of more efficacious treatments or cures.10–12
Currently, treatment with a single dose of IVIG is effective in reducing the incidence of coronary artery aneurysms and has been the gold-standard treatment to prevent coronary lesions in children with KD.13 However, up to a third of children will have persistent or recrudescent fever after initial IVIG treatment (IVIG non-responders) and are at increased risk for development of coronary abnormalities.14–16The optimal therapy for these IVIG non-responders remains controversial, and agents used for secondary or “rescue” therapy vary among centers. Anti-TNF mAbs, including infliximab, have potent anti-inflammatory effects and are currently undergoing evaluation in large-scale clinical trials. When re-treatment of IVIG-resistant KD patients is required, infliximab resolve fever more quickly and decrease the number of days of hospitalization compared to IVIG treatment.17
To further explore the pathogenesis of vasculitis in Kawasaki Disease, we used theLactobacillus casei cell wall extract (LCWE)-induced mouse model of coronary arteritis, a well established model that histopathologically mimics the coronary arteritis of KD.18–20Most importantly, this experimental mouse model has proven to be useful in duplicating or predicting human treatment responses, as IVIG and anti-TNF mAb were found to be effective in preventing coronary lesions in LCWE-induced mouse model. 19, 21
Kawasaki Disease is an inflammatory disease that leads to generalized vasculitis. IL-1β is one of the prototypic pro-inflammatory cytokines that is considered as the gatekeeper of inflammation and its induction and release is independent of TNF-α. IL-1β has been shown to be upregulated in patients who have failed standard therapy with IVIG. Pro-IL-1β is biologically inactive until it is enzymatically cleaved by the caspase-1 complex (inflammasome) to generate the bioactive IL-1β protein, which is then secreted.22 IL-1β signaling is mediated through the type I IL-1 receptor (IL-1RI). Additionally, the IL-1β receptor antagonist (IL-1Ra), an endogenous molecule, can bind the IL-1β receptor and prevent normal IL-1 signaling.23 Recombinant IL-1Ra (Anakinra) has been approved for the treatment of many inflammatory diseases, such as rheumatoid arthritis.24It has been suggested that IL-1β plays a critical role in chronic inflammatory diseases such as atherosclerosis, gout, diabetes, and more recently possibly linked to Kawasaki Disease.24–26
Several clinical clues exist to suggest that IL-1β may play an important role in KD. Maury et al. reported that serum level of IL-1β was significantly increased in KD patients compare to age- matched healthy control.27 Popper et al. reported gene expression patterns of KD patients, demonstrating that acute KD was characterized by increased relative abundance of gene transcripts associated with innate immune and proinflammatory response, including the IL-1β gene.28Furthermore, several reports now show that IVIG non-responder patients have increased IL-1β gene expression and diminished IL-1Ra expression.29 Furthermore, while the exact mechanism by which IVIG is effective in preventing coronary artery lesions in KD patients is unknown, several studies have determined that IVIG is associated with reduction in IL-1β secretion in KD patients (in-vivo),30, 31 and IVIG has been shown to down regulate IL-1 and upregulate IL-1Ra production in-vitro.32, 33 Collectively, these observations strongly suggest that IL-1β may play an important role in KD.
We previously used MyD88 and TLR2 knockout mice to show that toll like receptor (TLR) signaling is critically involved in LCWE-induced coronary lesions in the KD mouse model.34 In addition to being the adaptor molecule for TLR2 signaling, MyD88 is required for both the formation of pro-IL-1β (via NF-κB activation) and for IL-1β signaling. Based on all these clinical and experimental observations, we hypothesized that IL-1β plays a key role in KD patients. Accordingly, we investigated the specific role of IL-1β and the effectiveness of an anti-IL-1 therapeutic agent, IL-1R antagonist, in the LCWE induced mouse model of KD. Here we report that LCWE does not induce coronary arteritis in caspase-1-deficient and IL-1R-deficient mice, indicative ofthe key role IL-1β plays in the pathogenesis of coronary lesions in the KD mouse model. We also observed that IL-1Ra effectively blocks LCWE-induced vasculitis and coronary lesions in this model, suggesting that novel treatments using inhibitors of IL-1β could provide effective and more targeted therapies and prevent the cardiac complications in human KD.
Methods
Mice
Wild-type C57BL/6, Type I IL-1R (Il1r1)−/− and Ifn-γ−/− mice (all on C57BL/6 background) were purchased from Jackson Laboratory (Bar Harbor, ME). Casp1−/− mice were obtained from Dr. R. A. Flavell (Yale University, New Haven, CT). Il17a−/− mice were obtained from Dr. Y. Iwakura (University of Tokyo, Tokyo, Japan). Nlrp3 −/− and Asc−/− mice were obtained from Dr. K. A. Fitzgerald (University of Massachusetts, Worcester, MA). All animals were housed under specific pathogen-free conditions at the animal center of the Cedars-Sinai Medical Center. Experiments were conducted under approved IACUC protocols. Number of animals used in various experiments ranged between 5–12 in each group as specified in the legends of each figure.
Reagents
LPS from E. coli (InvivoGen, San Diego, CA), Recombinant human IL-1 receptor antagonist (IL-1Ra) (Anakinra-Kineret, Amgen), recombinant mouse IL-1β (Sigma,St. Louis, MO), human TNF-α mAb (Infliximab, Merck), and adenosine 5-triphosphate (Sigma,St. Louis, MO) were used in these studies. IL-1Ra was used at 25 mg/kg or 500 μg/mouse given i.p. The dose was based on several published studies and pilot dose-dependent studies that we have done. Human TNF-α mAb was used at 10 mg/kg or 200 μg/mouse given i.p., a dose that was based on other published studies.
Preparation of LCWE
L. casei (ATCC 11578) cell wall extract was prepared as previously described.34 In brief, L. casei were grown in Lactobacillus MRS broth (Difco) for 48 hrs, harvested and washed with PBS. The harvested bacteria were disrupted by two packed volume of 4% SDS/PBS during overnight. Cell wall fragment were washed 8 times with PBS to remove any residual SDS. The SDS treated cell wall fragment was sonicated for 2 hrs with three-quarter-inch horn and a garnet tip at maximum power. During sonication, the cell wall fragments were maintained by cooling in dry ice/ethanol bath. After sonication, the cell wall fragments were spun for 20 min at 12,000 rpm at 4 °C. The supernatant was centrifuged for 1 hour at 38,000 rpm at 4 °C and the pellet discarded. The total rhamnose content of the cell wall extract was determined by colorimetric phenol-sulfuric assay as described earlier.18
KD Mouse Model and Inflammation Scores for Coronary Artritis, Aortitis and Myocarditis
Mice aged 4–5 weeks old were i.p. injected with 250 μg of LCWE (total rhamnose amount as determined above) or PBS. Mice were sacrificed and hearts were removed at day 7 or 14 and embedded in OCT compound for histological examination. Following a cut through the aortic root, coronary artery lesions, aortic root vasculitic lesions (aortitis) and myocardial inflammation were identified in serial sections (7 μm) stained with hematoxylin and eosin or elastin/collagen staining. Only sections that showed the 2nd coronary artery branch separating from aorta were analyzed. Histopathological examination and inflammation severity scoring of the coronary arteritis, aortic root vasculitis and myocarditis was performed by a coronary pathologist who was blinded to the genotypes or experimental groups (MF). KD lesions were assessed using the following scoring system: Score 0 = no inflammation, 1 = rare inflammatory cells, 2 = scattered inflammatory cells, 3 = diffuse infiltrate of inflammatory cells, 4 = dense clusters of inflammatory cells. Multi-nuclear cells were indicative of acute inflammation while mono-nuclear cells reflected chronic inflammation. Aortic root was evaluated for severity of aortitis, and cross sections of coronary artery for severity of coronary artery inflammation and combined the two scores to generate a severity score that we called “vessel inflammation score”. Myocardial inflammation score was described as follows; score 0 = no myocardial fibrosis, 1 = very minimal focal subepicardial interstitial fibrosis just infiltrating beneath epicardial fat, 2 = mild subepicardial interstitial fibrosis infiltrating deeper into subepicardial myocardium, 3 = multifocal subepicardial interstitial fibrosis, 4 = replacement fibrosis.Incidence rate was evaluated by the presence of any coronary, aortic or myocardial inflammation score of equal or greater to 1.
Measurement of body temperature
The rectal body temperature of each individual mouse was measured three times a day at the same times of the day by a digital thermometer (PRT-03, Pavia inc. MN, US), and the average value was calculated.
Preparation of Bone marrow derived macrophages
Femurs were flushed and bone marrow cells were cultured in RPMI-1640 medium containing 10% FBS and 20% L929 cell-cultured medium for 7 days. Cells were washed with PBS and non-adherent cells were removed, then adherent cells were collected, seeded in 96 well plate one day prior stimulation. Cells were treated with LCWE for 12hrs and the culture supernatants were collected for measurement of various cytokines.
Cytokine measurement
The cytokine concentrations in the plasma or culture supernatants of IL-1β, TNF-α (eBioscience, CA, USA), PGE2 and pentraxin 3 (R&D systems, MN, US) were quantified by ELISA. The assays were performed as described in manufacturers' protocols.
Statistical Analysis
Results are reported as mean ± SE. All data were analyzed using Prism 4.03 Statistical Program. To compare differences in serum cytokine levels, the two-tailed Student's t-test (at 95% confidence interval) was used to compare unpaired samples between experimental groups. We used Fisher's exact Test to compare incidence of coronary lesion formation. For experiments involving three groups we used one-way ANOVA with Tukey's post-hoc test. When the data analyzed was not distributed normally, we used the Mann-Whitney test (to compare unpaired samples between experimental groups) or the Kruskal-Wallis with Dunn's post-hoc test (for experiments involving three groups).Alternatively, non-normally distributed data was transformed using a square root transformation.For experiments involving repeated measurements, we used the repeated measurements two-way ANOVA with Bonferroni's post hoc test. A probability value of p<0.05 was considered statistically significant. Asterisk marks means * :p<0.05, ** : p<0.01, *** : p<0.001.
Results
Primarybone marrow derived macrophages secrete TNF-α, IL-1β and PGE2 in response to LCWE stimulation
To investigate the LCWE-induced inflammatory responses in-vitro from macrophages, we isolated primary bone marrowmacrophages (BMM) from WT mice and stimulated them with 10 μg/ml of LCWE for 12hours. We measured the levels of TNF-α, IL-1β, and PGE2 in the supernatants by ELISA as all of these cytokines have been associated with KD.17, 27, 35As expected, LCWE induced production of TNF-α in BMM (Figure 1A).Interestingly IL-1β and PGE2 were also induced in BMM by LCWE (Figure 1A), indicating a possible role for them in the LCWE KD mouse model.
Figure 1.
LCWE induces IL-1β in a NLRP3 and ASC dependent manner. (A)Bone marrow derived macrophages were stimulated with 10 μg/ml of LCWE for 12hrs and the level of IL-1β, TNF-α, or PGE2 in the supernatants were determined by ELISA. (B, C) Bone marrow macrophages derived from Nlrp3−/−, Asc−/− or WT mice were stimulated with 10 μg/ml LCWE for 12hrs and IL-1β (B) and TNF-α (C) levels in the supernatants were determined by ELISA. Experiments were performed in triplicate. Data shown are mean±SE and were compared by use of Student's t test or one-way ANOVA with Tukey's post-hoc test (B and C). A probability value of P<0.05 was considered statistically significant. N.D. abbreviates `not detectable'.
LCWE induces IL-1β release in macrophages via NLRP3 - and ASC-dependent inflammasome
To investigate how LCWE induces IL-1β in macrophages, BMM were isolated from WT, Nlrp3−/− and Asc−/− mice and stimulated with 10μg/ml of LCWE. In order for Caspase-1 to process pro-IL-1β into mature IL-1β, Casapse-1 needs to be activated by one of the multimeric protein complexes known as inflammasomes. Inflammasomes require two signals for activation: signal 1 (NF-κB driven-typically though TLR signaling) induces the production of pro-IL-1β, and signal 2, which activates the inflammasome complex. One such inflammasome, the NLRP3 inflammasome, is activated by many diverse stimuli (second signal) such as extracellular ATP, bacterial infections, and various danger signals. We therefore investigated if LCWE induced IL-1β secretion also utilized this pathway (NLRP3). We observed that LCWE induced IL-1β secretion in an NLRP3- and ASC-dependent manner (Figure 1B) (p<0.001) whereas TNF-α secretion was not affected by NLRP3- or ASC-deficiency (Figure 1C). ASC is another component of the inflammasome complex that is required for activation of Casapse-1. These data indicate that LCWE activates the NLRP3 inflammasome to induce IL-1β secretion and that LCWE provides both signal 1 and signal 2 for inflammasome activation.
Caspase-1-deficiency protects mice from LCWE- induced vasculitis and coronary lesions
To test the functional role of inflammasome activation and IL-1β secretion in LCWE- induced coronary artery inflammation,Caspase-1−/− (Casp1−/−) mice were injected with LCWE and the hearts harvested at day 14. WT mice displayed pronounced vasculitis with acute and chronic cellular infiltration, elastin disrupture in the aorta, and intense concentric inflammation around the coronary arties approaching occlusion. In contrast, Casp1−/− mice showed clean and open coronary arteries with no vasculitis and intact elastin structure in the aorta (Figures 2A and 2B). The total vessels inflammation score was significantly lower in Casp1−/− mice compared to WT mice injected with LCWE (Figure 2C) (p<0.001). The incidence of vascular lesion (Figure 2D) was significantly diminished in Casp1−/− mice compared to WT mice (p<0.001). We also assessed the effects of caspase-1 activity on LCWE induced myocardial inflammation and found that Casp1−/−mice had significantly reduced scores compared to WT mice (Figure 2E, p<0.05). These results demonstrate that caspase-1 activity is required for LCWE-induced coronary artery inflammation and suggests that the NLRP3 inflammasome is required for this process.
Figure 2.
Caspase-1 mice are protected from LCWE-induced vasculitis and coronary arteritis. C57BL6/J or Casp1−/− mice were injected i.p. with 250 μg of LCWE and their hearts were harvested on day 14, (n=9). (A) Representative Hematoxylin and eosin (H&E)-stained heart sections and elastin/collagen-stained sections (B) are shown. The scale bar indicates 250 μm. Heart vessels inflammation score (C) and incidence (D) were evaluated as described in Material and Methods. Myocardial inflammation (E) was evaluated as described in Material and Methods. Data shown are mean±SE and were compared using the Mann-Whitney test (C and E) and Fisher's exact test for incidence (D). A probability value of P<0.05 was considered statistically significant.
Recombinant IL-1β reconstitutes the development of coronary lesions in Caspase-1 deficient mice
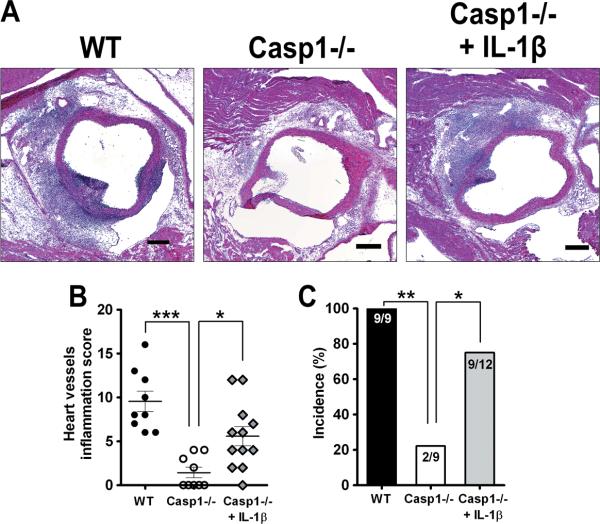
To verify that caspase-1 activation exerted its protective role via activating IL-1β secretion (as opposed to IL-18, another inflammasome induced cytokine), Casp1−/−mice were injected with recombinant IL-1β (10ng) or PBS from day 0 to day 5 following LCWE injection. IL-1β injection restored LCWE-induced coronary lesions in Casp1−/− mice (Figure 3A, B, C).Vessel inflammation score was significantly increased in Casp1−/− mice that received IL-1β compared to PBS treated Casp1−/− mice (Figure 3B, p<0.05). IL-1β daily injection alone was not sufficient to induce the coronary lesion in Casp1−/− mice without LCWE (data not shown). These results indicate that IL-1β activation via Caspase-1 plays a key role in LCWE-induced coronary artery inflammation and suggests that IL-18 plays little to no role in the lesion development.
Figure 3.
Exogenous recombinant IL-1β reconstitutes LCWE-induced vasculitis and coronary arteritis in Casp1−/− mice. C57BL6/J or casp-1−/− mice were injected i.p. with LCWE and then treated with 10 ng of mouse recombinant IL-1β (i.p.) daily from day 0 to day 5. On day 7, hearts were harvested and analyzed. Representative H&E-stained sections are shown (A). The scale bar indicates 250 μm. Heart vessels inflammation score (B) and incidence (C) were evaluated (n=9 or12 per group). Data shown are mean±SE and were compared by one-way ANOVA with Tukey's post-hoc test and Fisher's exact test for incidence. A probability value of P<0.05 was considered statistically significant.
IL-1R-deficient mice are protected from LCWE induced coronary lesions
To define the role of IL-1β in the LCWE-mediated KD mouse model, we next investigated IL-1R−/− mice. IL-1R−/− mice were significantly protected form LCWE-induced coronary lesions (Figure 4A and 4B), as no lesions were detected in IL-1R−/− mice compared to WT mice (Figure 4C, p<0.001). These results demonstrate that IL-1β signaling plays a critical role in the development of LCWE-mediated coronary arteritis.
Figure 4.
IL-1R deficient mice are protected from LCWE induced vasculitis and coronary arteritis. WT (A), Il1r1−/− mice (B) were administrated LCWE (i.p.) and their hearts were harvested on day 14. H&E staining was performed and representative sections are shown (A and B). The scale bar indicates 250 μm. (C) Incidence was evaluated by use of Fisher's exact test (n=5 or 9). A probability value of P<0.05 was considered statistically significant.
IL-1 Receptor antagonist (IL-1Ra) blocks LCWE-induced coronary lesions
We next investigated whether IL-1Ra (Anakinra) could block LCWE induced coronary lesions. IL-1Ra was injected (500μg) (i.p.) daily to C57BL/6 mice from 1 day prior to LCWE injection to day 5. The mice were sacrificed on day 7 with the hearts harvested for analysis. We observed that IL-1Ra significantly blocked LCWE-induced coronary lesions (Figure 5A–C) and elastin disrupture (data not shown). The overall vascular inflammation score was significantly decreased in IL-1Ra treated group compared to PBS treated control group (p<0.001, Figure 5B). The incidence of vasculitis was significantly decreased in IL-1Ra-treated mice, compared to PBS treated controls (9/9 v.s. 1/9, p<0.01, Figure 5C). Myocardium inflammation score was also significantly reduced in IL-1Ra treated mice compared to control mice (p<0.001) (Figure 5D). To compare these results with efficacy of anti-TNFα mAb treatment, we injected a different group of mice with 200 μg human anti-TNF-α mAb (Infliximab), i.p. once on they same day (day 0) of LCWE injection and harvested the hearts on day 7 as above. Anti-TNF-α mAb was also able to inhibit LCWE-induced vasculitis as measured by vascular inflammation score (Figure 5A, 5B), and incidence of coronary lesions (Figure 5C), but not myocarditis (Figure 5D) compared to PBS control, as well as rat IgG control. IL-1Ra treatment showed a strong trend towards more effective inhibition (89% inhibition, 8/9 mice protected) for the incidence of coronary lesions compared to anti-TNF mAb (56% inhibition, 5/9 mice protected) (Figure 5C). IL-1Ra also provided a strong trend towards more effective protection for LCWE-induced myocarditis, compared to anti-TNF mAb group (Figure 5D). Since IL-1Ra was able to almost completely prevent coronary lesion formation when given at the same time as the LCWE injection and throughout the LCWE protocol, we next investigated whether IL-1Ra could still inhibit coronary arteritis when given after LCWE administration. IL-1Ra treatment was administered as before except with varying starting point relative to LCWE injection as described in Figure 6A. We observed that IL-1Ra significantly inhibited LCWE-mediated coronary lesions even if treatment was delayed up to 3 days following LCWE injection (Figures 6B and 6C). These results suggest that inhibition of IL-1β signaling even several days following the injection of the LCWE, is effective in preventing coronary artery vasculitis in this mouse model of KD.
Figure 5.
IL-1 receptor antagonist (IL-1Ra) protects against LCWE induced vasculitis, coronary arteritis and myocarditis. Following LCWE injection, WT mice were administrated i.p daily with 500 μg IL-1Ra (from day -1 to day 5), 200 μg human TNF-α mAb (once on day 0) or same volume of PBS for control and hearts were harvested at day 7 for analysis. Representative H&E-stained sections are shown (A). The scale bar indicates 250 μm. Heart vessels inflammation score (B), incidence (C) and myocardium inflammation score (D) were evaluated for each group as mentioned in Methods. Data shown are mean±SE and were compared by the Kruskal-Wallis with Dunn's post-hoc test (B and D) and Fisher's exact test for incidence (C), (n=9). A probability value of P<0.05 was considered statistically significant.
Figure 6.
IL-1Ra treatment can prevent LCWE induced vasculitis and coronary arteritis up to three days after LCWE injections. Following LCWE injection, groups ofWT mice were administrated daily (i.p.) with 500 μg IL-1Ra from different starting time points. Experimental schematic is shown (A). The hearts were collected at day 7 and analyzed H&E-staining (B). The scale bar indicates 250 μm. (C) The lesion size was measured to evaluate the effective inhibition by IL-1Ra administration. Data shown are mean±SE and were compared using one-way ANOVA with Dunnett's post-hoc test (n=5). The data was transformed using square root transformation prior to the analysis. All the groups were compared against the control group (no IL-1Ra). A probability value of P<0.05 was considered statistically significant. *P<0.05, ** : P<0.01, *** : P<0.001.
LCWE-induced KD mouse model is associated with body temperature elevation and increased circulating PGE2, and Pentraxin 3 levels
IL-1β is an important pyrogenic cytokine that may be associated with clinical symptoms of KD patient. One hallmark of KD is the presence of high fever for many days. Therefore, we measured daily rectal body temperature following LCWE injection in WT mice. LCWE-injected mice displayed significantly increased body temperature compared to PBS-injected control group (p<0.05 to p<0.001) (Figure 7A). Additionally, we measured serum level of pentraxin 3 (a markerof systemic inflammation) and PGE2 (known to be elevated in KD patients)35 during LCWE induced coronary arteritis. Similar to patients with KD, serum PGE2, and pentraxin 3 levels at day 7 or day 14 were significantly increased in LCWE-injected WT mice compared to PBS treated or naïve WT mice (p<0.001, Figure 7B–E). Consistent with the inhibition of coronary lesions, circulating levels of PGE2 and pentraxin 3 were significantly decreased in Caspase-1−/− mice (day 14) (p<0.05 and p<0.001, Figure 7B and D) and IL-1Ra treated mice (day 7) (p<0.05 and p<0.01, Figure 7C and E) compared to extract injected WT mice. These results suggest that similar to the systemic inflammation seen in KD patients, the LCWE-mediated KD mouse model, in addition to focal coronary lesions, also induces systemic inflammatory changes.
Figure 7.
LCWE induces increased body temperature and inflammatory biological markers in the serum of mice. C57BL/6 WT mice were injected (i.p.) with 250μg of LCWE or same volume of PBS. (A) Rectal body temperature was measured as described in methods, (n=9). (B) Plasma PGE2 levels were quantified by ELISA. Plasma from age matched C57BL/6J mice was used as control. (C) Plasma pentraxin 3 level were measured. Data shown are mean±SE and were compared using the repeated measures two-way ANOVA with Bonferroni's post-hoc test (A) or one-way ANOVA with Tukey's post-hoc test (B–E). A probability value of P<0.05 was considered statistically significant.
Discussion
KD is now recognized as the leading cause of acquired heart disease in children in the United States and developed world.2–4 The underlying etiology and mechanisms leading to vessel inflammation, coronary artery lesions, and aneurysms that are the hallmarks of KD remain largely unknown. We show in this study that IL-1β signaling is critically required for the development of LCWE induced vasculitis. While the caspase-1 deficient mice were clearly defective in their response to LCWE, casapse-1 is known to cleave and activate multiple cytokines, including IL-1β and IL-18. However, given the total lack of lesions found in IL-1R1 deficient mice, and the ability of rIL-1β to reconstitute lesion development in Casp1−/−, mice, it is clear that IL-1β plays a critical role in LCWE induced coronary arteritis or KD mouse model. We have previously shown that LCWE-induced KD mouse model is dependent on both innate34and adaptive36 immunity and that both TLR2/MyD88 signaling pathway and presence of T cells are required for coronary lesions to develop. In this study we now show that IL-1β is critically important and required in the KD mouse model and that IL-1Ra treatment can effectively prevent LCWE-induced coronary lesions even if treatment is started up to three days following the extract injection. Given that T cells are required for this mouse model, and that a previous study found that INF-γ deficient mice are not protected from developing coronary lesions following LCWE injection,37 we hypothesized that IL-17A may play a role in this model. Additionally, several studies have shown that IL-1β signaling can drive Th17 skewing.38, 39However, to our surprise, we found that IL-17A-deficient mice developed robust coronary lesions in response to LCWE (Supplemental Figure 1). One possibility is that T cells accumulating in the coronary lesions20induce a strong chemokine induction recruiting large numbers of monocytes, macrophages, and DC that secretes large amounts of inflammatory cytokines such as IL-1β.
It is important to acknowledge that while LCWE-induced coronary arteritis mouse is a model for KD, it cannot be considered as exactly similar to human disease as the etiologic agent for KD is yet to be discovered. However, there are striking similarities in the histopathology and kinetics of lesions between human KD and this LCWE-induced coronary arteritis mouse model of KD.18, 19, 21, 40–42 The LCWE-induced mouse model of coronary arteritis appears to be unique in demonstrating not only acute myocarditis and coronary arteritis with aneurysm formation, but also chronic scarring of the coronary arteries with the formation of stenotic segments, luminal obstruction and evidence of coronary artery thrombosis18, 40, 41. The LCWE-mediated KD mouse model has been studied for over 35 years, and it has reliably predicted treatment responses to agents such as IVIG in in humans with KD. The current gold standard treatment of IVIG for KD has been shown efficacious in preventing LCWE-induced coronary lesions in the KD murine model.21In that study, Myones et al reported 40–67% inhibition in the incidence of coronary lesions when human IVIG was given to LWCE-injected mice between day 3 and 5.21 This is very similar to the 55% protection in the incidence of coronary arteritis that we have observed in mice treated with human IVIG given i.p.at the same time as LCWE (data not shown).Further, the mouse model was also used to show that polyclonal rabbit antibody againstmurine TNF-α was able to suppress LCWE-mediated coronary lesions.19This finding among others has been the basis for using anti-TNF mAb in treating KD in a number of KD patients,17 and resulted in a larger ongoing clinical trial.43In the current study,since we saw a very significant protection of coronary lesion formation and myocarditis with human IL-1Ra in this mouse model, we also wished to compare the efficacy of IL-1Ra with that of human anti-TNFα mAb. Consistent with the earlier published data with polyclonal anti-murine TNF Ab, we also observed a significant protection by human anti-TNFα mAb in the LCWE-induced coronary arteritis in this KD model. There was a trend toward a more effective inhibition in the incidence of coronary lesion formation and inflammation severity score as well as myocarditis scorein the IL-1Ra treated group compared to anti-TNF mAb group.
Symptoms of KD include high fever for many days and systemic inflammation. We also show that the LCWE mouse model is associated with systemic inflammatory findings, including increased body temperature. Circulating levels of PGE2, another pyrogen, in addition to IL-1β was also elevated in the mouse model. We also observed that the KD mice had substantially elevated circulating levels of pentraxin 3, a molecule that is a local marker for a vascular disease and coronary vasculitis.44, 45
Our findings suggest that the LCWE induced mouse model of KD provides translational value to KD and posits the question; what role doe IL-1β play in human KD? Several studies have suggested that immune activation and the secretion of cytokines may contribute to the pathogenesis of KD. In particular, IL-1β has been shown to increase significantly in patients during acute KD.30, 31 In addition, there are several clinical and experimental clues that strongly implicate the role of IL-1β in KD. Previous studies have shown that IVIG influences the production and release of IL-1β in KD patients,30, 31 and that IL-1β polymorphisms26associated with increased IL-1 β production are associated with IVIG-resistance. Collectively, our findings and the emerging clinical and genetic data in KD patients and in IVIG-non-responders, suggest that IL-1β may also play an important role in human disease and perhaps may provide a potential treatment in KD patients or certain subsets of IVIG non-responders. This type of approach may fit well with the NIH's goal towards personalized medicine.
Although treatment with intravenous gammaglobulin is an effective therapy for KD, its mechanism of action is unknown, and not all children respond and optimal treatment of IVIG refractory KD remains unclear.13, 15, 16, 46–48 Identification of the etiology of KD10 and better understanding of the pathology of coronary lesions would greatly enhance efforts to improve targeted therapy and prevent the cardiac complications of KD. In a large multicenter study, nearly 15% of KD patients required retreatment with IVIG because of failure to respond to initial treatment.48 Resistance to IVIG in children with KD has been reported to range between 7.8–38.2% and is associated with increased risk for coronary aneurysms.13–16, 46–48Therefore, there is an urgent need for alternative therapeutic modalities. Alternative treatments for patients with IVIG resistance are controversial. Additional IVIG treatments,14 steroids,49 anti-TNF mAb17, 50 or other treatments have been used for IVIG-resistant patients with mixed results. While anti-TNF mAb treatment in IVIG-non-responders have led to faster resolution of fever, and fewer days of hospitalization, a retrospective study suggested that it did not reduce coronary aneurysms in patients.17Therefore, our findings that IL-1β plays a significant role in LCWE-induced coronary lesions and that the blocking of its signaling by the IL-1Ra prevents lesion development support an innovative therapeutic category of anti-IL-1β immunomodulatory agents that may prevent coronary disease in KD patients. These observations warrant future prospective clinical trials to determine the efficacy of IL-1Ra or other monoclonal anti IL-1β Ab in children with KD. In summary, we observed that IL-1β is critically involved in LCWE-mediated coronary arteritis seen in the KD mouse model and that these lesions can efficiently be prevented by IL-1Ra treatment. These observations provide innovative mechanistic insights into the cellular and molecular understanding of the vasculitis and coronary arteritis in the LCWE-induced KD mouse model and may provide novel therapeutic strategies, including anti-IL-1β agents, to prevent the development of coronary lesions in KD patients.
Supplementary Material
Acknowledgments
Funding Sources: Supported by grants from the National Institutes of Health (AI072726 to M.A and AI1070162 to DS.)
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (mlns) prevailing in japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 2.Burns JC. Kawasaki disease update. Indian J Pediatr. 2009;76:71–76. doi: 10.1007/s12098-009-0031-3. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA, Committee on Rheumatic Fever EdaKD. Young CoCDit. Association AH. Pediatrics AAo Diagnosis, treatment, and long-term management of kawasaki disease: A statement for health professionals from the committee on rheumatic fever, endocarditis and kawasaki disease, council on cardiovascular disease in the young, american heart association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 6.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J. Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 7.Gordon JB, Kahn AM, Burns JC. When children with Kawasaki Disease grow up. Myocardial and vascular complications in adulthood. J.Am Coll Cardiol. 2009;54:1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukazawa R. Long-term prognosis of kawasaki disease: Increased cardiovascular risk? Curr Opin Pediatr. 2010;22:587–592. doi: 10.1097/MOP.0b013e32833e12f7. [DOI] [PubMed] [Google Scholar]
- 9.Senzaki H. Long-term outcomes of Kawasaki Disease. Circulation. 2008;118:2763–2772. doi: 10.1161/CIRCULATIONAHA.107.749515. [DOI] [PubMed] [Google Scholar]
- 10.Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of kawasaki disease--cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley AH, Baker SC, Shulman ST, Garcia FL, Fox LM, Kos IM, Crawford SE, Russo PA, Hammadeh R, Takahashi K, Orenstein JM. Rna-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute kawasaki disease. PLoS One. 2008;3:e1582. doi: 10.1371/journal.pone.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowley AH, Shulman ST, Garcia FL, Guzman-Cottrill JA, Miura M, Lee HL, Baker SC. Cloning the arterial iga antibody response during acute kawasaki disease. J Immunol. 2005;175:8386–8391. doi: 10.4049/jimmunol.175.12.8386. [DOI] [PubMed] [Google Scholar]
- 13.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, Mason WH, Meissner HC, Rowley AH, Shulman ST, Reddy V, Sundel RP, Wiggins JW, Colton T, Melish ME, Rosen FS. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 14.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in kawasaki disease. J Pediatr. 1993;123:657–659. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 15.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in kawasaki disease. US/canadian kawasaki syndrome study group. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Tremoulet A, Best B, Song S, Wang S, Corinaldesi E, Eichenfield J, Martin D, Newburger J, Burns J. Resistance to intravenous immunoglobulin in children with kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson Ve, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for Intravenous Immunoglobulin Resistance in Kawasaki Disease: A Retrospective Study. J Pediatr. 2011;158:644–649. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Lehman T, Walker S, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group b lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1985;28:652–659. doi: 10.1002/art.1780280609. [DOI] [PubMed] [Google Scholar]
- 19.Lehman TJA, Sherry B, Gietl DM, Nguyen HT, Cerami A. Suppression of lactobacillus casei cell wall induced coronary arteritis in mice by antibody to murine tumor necrosis factor. Proceedings of the Third International conference on Kawasaki Disease; Tokyo. 1988. pp. 203–206. [Google Scholar]
- 20.Yilmaz A, Rowley A, Schulte DJ, Doherty TM, Schroder NW, Fishbein MC, Kalelkar M, Cicha I, Schubert K, Daniel WG, Garlichs CD, Arditi M. Activated myeloid dendritic cells accumulate and co-localize with cd3+ t cells in coronary artery lesions in patients with kawasaki disease. Exp Mol Pathol. 2007;83:93–103. doi: 10.1016/j.yexmp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Myones BL, Bathoria JM, Lehman TJA, Shulman ST. Human IVIG inhibits Lactobacillus casei-inducible coronary arteritis in a murine model. In: Kato H, editor. Elsevier Science; Kawaski Disease.: Proceedings of the 5th International Kawasaki Disease Symposium; Fukuoka, Japan. 1995. pp. 252–256. [Google Scholar]
- 22.Thornberry N, Bull H, Calaycay J, Chapman K, Howard A, Kostura M, Miller D, Molineaux S, Weidner J, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 23.Bujak M, Frangogiannis N. The role of il-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: A systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 25.Mitroulis I, Skendros P, Ritis K. Targeting il-1beta in disease; the expanding role of nlrp3 inflammasome. Eur J Intern Med. 2010;21:157–163. doi: 10.1016/j.ejim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Weng K, Hsieh K, Ho T, Huang S, Lai C, Chiu Y, Huang S, Lin C, Hwang Y, Ger L. Il-1b polymorphism in association with initial intravenous immunoglobulin treatment failure in taiwanese children with kawasaki disease. Circ J. 2010;74:544–551. doi: 10.1253/circj.cj-09-0664. [DOI] [PubMed] [Google Scholar]
- 27.Maury C, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with kawasaki disease. N Engl J Med. 1988;319:1670–1671. doi: 10.1056/NEJM198812223192515. [DOI] [PubMed] [Google Scholar]
- 28.Popper S, Shimizu C, Shike H, Kanegaye J, Newburger J, Sundel R, Brown P, Burns J, Relman D. Gene-expression patterns reveal underlying biological processes in kawasaki disease. Genome Biol. 2007;8:R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fury W, Tremoulet A, Watson V, Best B, Shimizu C, Hamilton J, Kanegaye J, Wei Y, Kao C, Mellis S, Lin C, Burns J. Transcript abundance patterns in kawasaki disease patients with intravenous immunoglobulin resistance. Hum Immunol. 2010;71:865–873. doi: 10.1016/j.humimm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute kawasaki disease. Lancet. 1989;2:1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Uemura S, Tone S, Iizuka T, Koike M, Hirayama K, Maeda J. Effects of immunoglobulin and gamma-interferon on the production of tumour necrosis factor-alpha and interleukin-1 beta by peripheral blood monocytes in the acute phase of kawasaki disease. Eur J Pediatr. 1996;155:291–296. doi: 10.1007/BF02002715. [DOI] [PubMed] [Google Scholar]
- 32.Arend WP, Leung DY. Igg induction of il-1 receptor antagonist production by human monocytes. Immunol Rev. 1994;139:71–78. doi: 10.1111/j.1600-065x.1994.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 33.Okitsu-Negishi S, Furusawa S, Kawa Y, Hashira S, Ito S, Hiruma F, Mizoguchi M, Yoshino K, Abe T. Suppressive effect of intravenous immunoglobulins on the activity of interleukin-1. Immunol Res. 1994;13:49–55. doi: 10.1007/BF02918224. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz M, Schulte D, Agle L, Wong M, Zhang W, Ivashkiv L, Doherty T, Fishbein M, Lehman T, Michelsen K, Arditi M. Tlr2 and myd88 contribute to lactobacillus casei extract-induced focal coronary arteritis in a mouse model of kawasaki disease. Circulation. 2005;112:2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530. [DOI] [PubMed] [Google Scholar]
- 35.Lee T, Furukawa S, Fukuda Y, Yabuta K, Kato H. Plasma prostaglandin e2 level in kawasaki disease. Prostaglandins Leukot Essent Fatty Acids. 1988;31:53–57. doi: 10.1016/0952-3278(88)90076-2. [DOI] [PubMed] [Google Scholar]
- 36.Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, Wong M, Doherty TM, Lehman T, Crother TR, Sorrentino R, Arditi M. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking kawasaki disease. J Immunol. 2009;183:5311–5318. doi: 10.4049/jimmunol.0901395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan W, Duong T, Yeung R. Presence of ifn-gamma does not indicate its necessity for induction of coronary arteritis in an animal model of kawasaki disease. J Immunol. 2004;173:3492–3503. doi: 10.4049/jimmunol.173.5.3492. [DOI] [PubMed] [Google Scholar]
- 38.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasigliè D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, Chiesa S, Penco F, Martini A, Gattorno M. Role of il-1 beta in the development of human t(h)17 cells: Lesson from nlpr3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehman TJ. Can we prevent long term cardiac damage in kawasaki disease? Lessons from lactobacillus casei cell wall-induced arteritis in mice. Clin Exp Rheumatol. 1993;11(Suppl 9):S3–6. [PubMed] [Google Scholar]
- 41.Lehman TJ, Mahnovski V. Animal models of vasculitis. Lessons we can learn to improve our understanding of kawasaki disease. Rheum Dis Clin North Am. 1988;14:479–487. [PubMed] [Google Scholar]
- 42.Hui-Yuen J, Duong T, Yeung R. Tnf-alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of kawasaki disease. J Immunol. 2006;176:6294–6301. doi: 10.4049/jimmunol.176.10.6294. [DOI] [PubMed] [Google Scholar]
- 43.Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for intravenous immunoglobulin resistance in kawasaki disease: A retrospective study. J Pediatr. 2011;158:644–649. e641. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: Lessons from ptx3. Cell Tissue Res. 2010;343:237–249. doi: 10.1007/s00441-010-1018-0. [DOI] [PubMed] [Google Scholar]
- 45.Norata G, Garlanda C, Catapano A. The long pentraxin ptx3: A modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20:35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP. The treatment of kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 47.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 48.Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, Newburger JW. Treatment of kawasaki disease: Analysis of 27 us pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 49.Shulman ST. Is there a role for corticosteroids in kawasaki disease? J Pediatr. 2003;142:601–603. doi: 10.1067/mpd.2003.258. [DOI] [PubMed] [Google Scholar]
- 50.Oishi T, Fujieda M, Shiraishi T, Ono M, Inoue K, Takahashi A, Ogura H, Wakiguchi H. Infliximab treatment for refractory kawasaki disease with coronary artery aneurysm. Circ J. 2008;72:850–852. doi: 10.1253/circj.72.850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.