Abstract
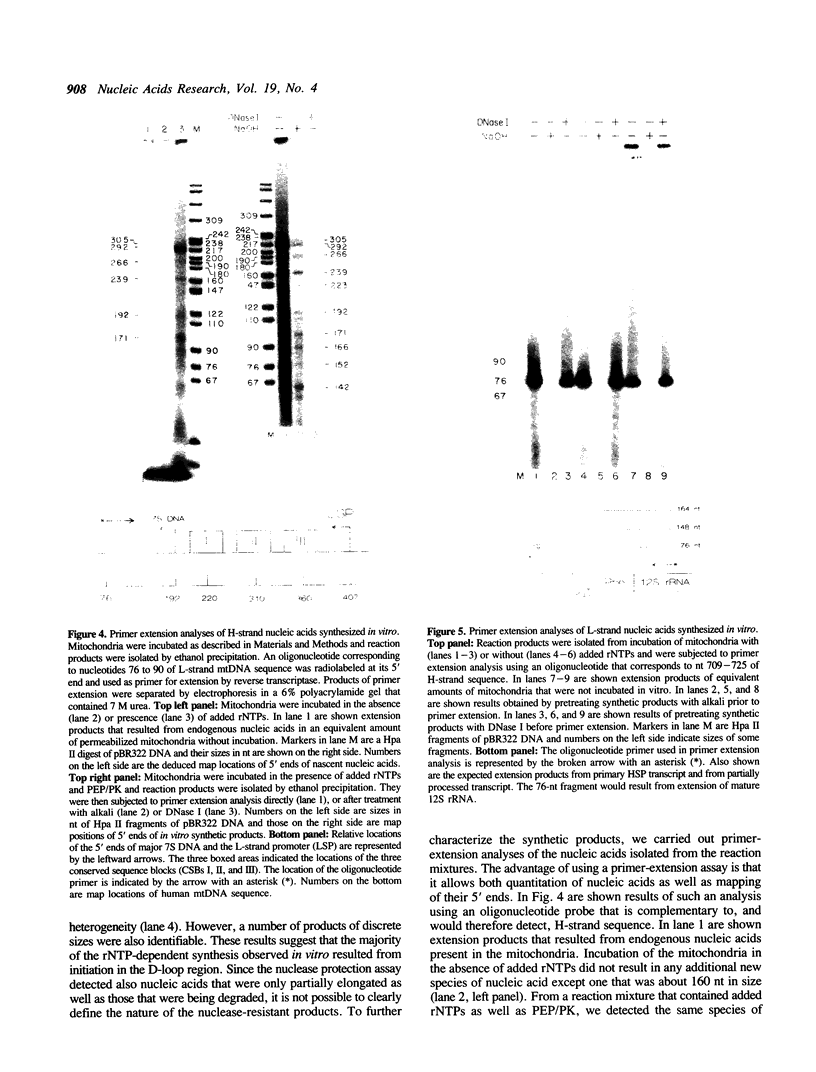
We have characterized some of the experimental conditions that are essential for initiation of human mitochondrial DNA synthesis. Mitochondria were purified from HeLa cells and were permeabilized with Triton X-100. When supplied with rNTPs and dNTPs, the permeabilized mitochondria synthesized nucleic acids that ranged in size from about 600 to 2000 nucleotides. In vitro DNA synthesis occurred on endogenous DNA templates and required a continuous supply of ATP. Analyses of the synthetic products revealed that almost all of them were of heavy-strand sequence and included authentic 7S DNA. Most of the synthetic products had 5' ends that mapped to similar locations as those previously identified for nascent heavy-strand DNA. Identification of these parameters should facilitate our efforts to achieve in vitro replication of heavy-strand mitochondrial DNA.
Full text
PDF
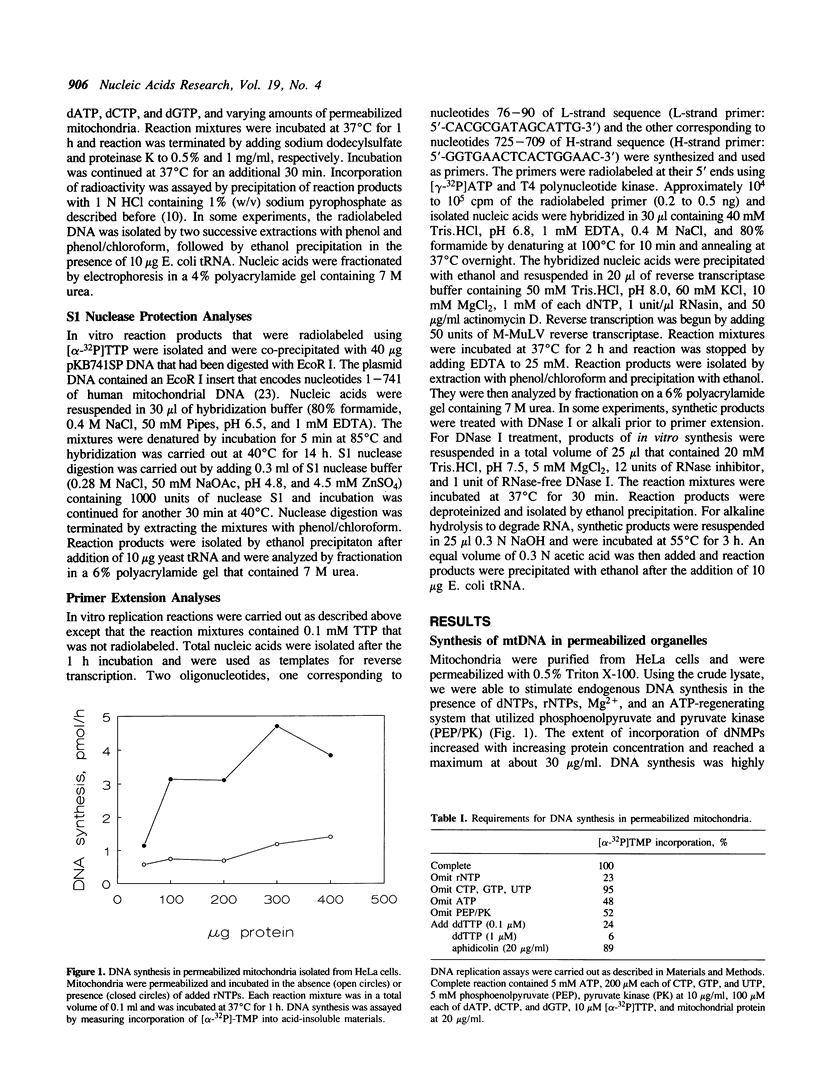
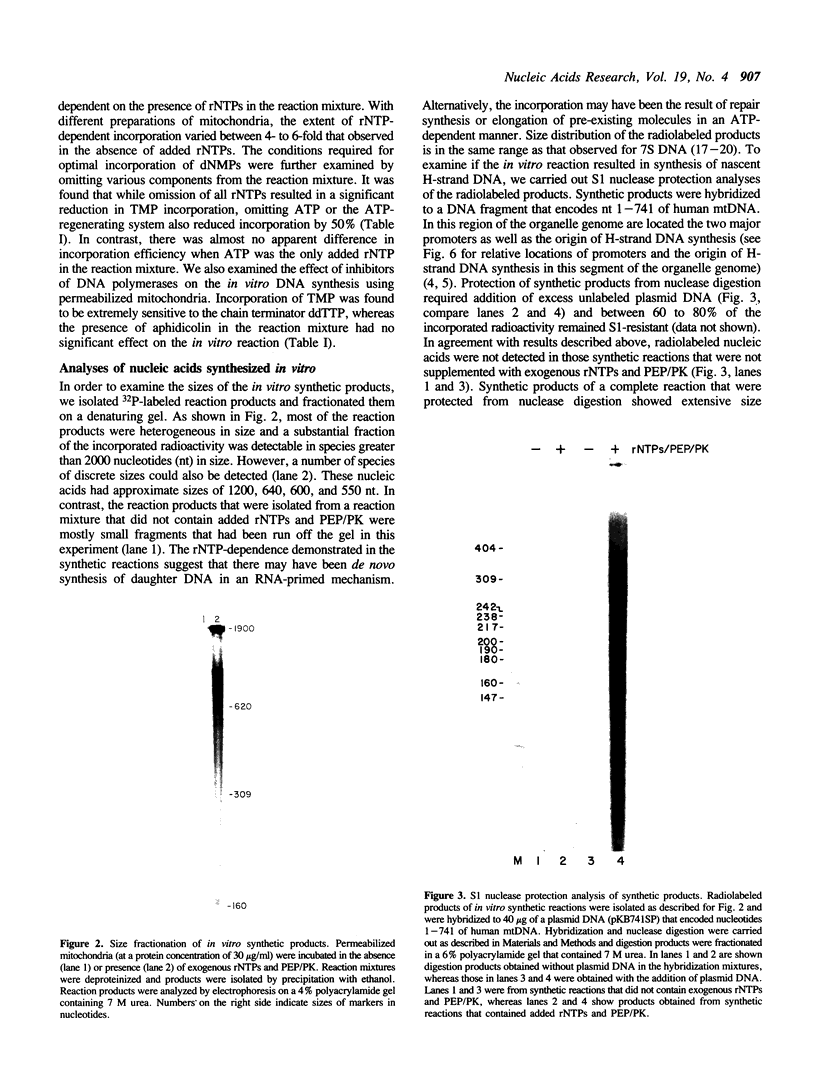



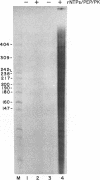
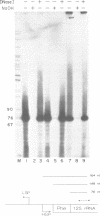
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnberg A., van Bruggen E. F., Borst P. The presence of DNA molecules with a displacement loop in standard mitochondrial DNA preparations. Biochim Biophys Acta. 1971 Aug 26;246(2):353–357. doi: 10.1016/0005-2787(71)90147-x. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: kinetics of synthesis and turnover of the initiation sequence. J Mol Biol. 1978 Feb 15;119(1):49–68. doi: 10.1016/0022-2836(78)90269-3. [DOI] [PubMed] [Google Scholar]
- Brennicke A., Clayton D. A. Nucleotide assignment of alkali-sensitive sites in mouse mitochondrial DNA. J Biol Chem. 1981 Oct 25;256(20):10613–10617. [PubMed] [Google Scholar]
- Brown W. M., Shine J., Goodman H. M. Human mitochondrial DNA: analysis of 7S DNA from the origin of replication. Proc Natl Acad Sci U S A. 1978 Feb;75(2):735–739. doi: 10.1073/pnas.75.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell. 1984 Mar;36(3):635–643. doi: 10.1016/0092-8674(84)90343-x. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Crews S., Ojala D., Posakony J., Nishiguchi J., Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979 Jan 18;277(5693):192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- Doda J. N., Wright C. T., Clayton D. A. Elongation of displacement-loop strands in human and mouse mitochondrial DNA is arrested near specific template sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6116–6120. doi: 10.1073/pnas.78.10.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon-Bluteau D., Cordonnier A., Brun G. DNA synthesis in a mitochondrial lysate of Xenopus laevis oocytes. H strand replication in vitro. J Mol Biol. 1987 Sep 20;197(2):175–185. doi: 10.1016/0022-2836(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Clayton D. A. A transcription factor required for promoter recognition by human mitochondrial RNA polymerase. Accurate initiation at the heavy- and light-strand promoters dissected and reconstituted in vitro. J Biol Chem. 1985 Sep 15;260(20):11330–11338. [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Displacement-loop replication initiation sequence in animal mitochondrial DNA exists as a family of discrete lengths. Proc Natl Acad Sci U S A. 1978 Feb;75(2):677–681. doi: 10.1073/pnas.75.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson J. E., Wong T. W., Clayton D. A. Both the conserved stem-loop and divergent 5'-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem. 1986 Feb 15;261(5):2384–2390. [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Low R. L. Mapping of control elements in the displacement loop region of bovine mitochondrial DNA. J Biol Chem. 1987 May 5;262(13):6204–6213. [PubMed] [Google Scholar]
- Koike K., Kobayashi M. Synthesis of mitochondrial DNA in vitro: two classes of nascent DNAs. Biochim Biophys Acta. 1973 Nov 14;324(4):452–460. doi: 10.1016/0005-2787(73)90204-9. [DOI] [PubMed] [Google Scholar]
- Parsons P., Simpson M. V. Deoxyribonucleic acid biosynthesis in mitochondria. Studies on the incorporation of labeled precursors into mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Mar 25;248(6):1912–1919. [PubMed] [Google Scholar]
- Tapper D. P., Clayton D. A. Mechanism of replication of human mitochondrial DNA. Localization of the 5' ends of nascent daughter strands. J Biol Chem. 1981 May 25;256(10):5109–5115. [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. In vitro replication of human mitochondrial DNA: accurate initiation at the origin of light-strand synthesis. Cell. 1985 Oct;42(3):951–958. doi: 10.1016/0092-8674(85)90291-0. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. Isolation and characterization of a DNA primase from human mitochondria. J Biol Chem. 1985 Sep 25;260(21):11530–11535. [PubMed] [Google Scholar]
- ter Schegget J., Borst P. DNA synthesis by isolated mitochondria. I. Effect of inhibitors and characterization of the product. Biochim Biophys Acta. 1971 Aug 26;246(2):239–248. [PubMed] [Google Scholar]
- ter Schegget J., Borst P. DNA synthesis by isolated mitochondria. II. Detection of product DNA hydrogen-bonded to closed duplex circles. Biochim Biophys Acta. 1971 Aug 26;246(2):249–257. [PubMed] [Google Scholar]