Abstract
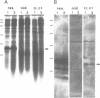
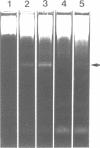
We describe the cloning and characterization of the gene coding for the ribotoxin restrictocin, from Aspergillus restrictus (gene res, EMBL accession Number X56176). This toxin is a potent inhibitor of protein synthesis in eucaryotes and is of potential interest as a component of immunotoxins. To analyze the mechanism of self-protection in the producing organism, the res gene was cloned into the vector pFB39 and introduced into Aspergillus nidulans. The secretion of active restrictocin from transformants suggests that the pro-toxin is not an active nuclease but is activated during the process of secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arruda L. K., Platts-Mills T. A., Fox J. W., Chapman M. D. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1990 Nov 1;172(5):1529–1532. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F. P., Orlandi R., Canevari S., Mezzanzanica D., Ripamonti M., Muñoz S. M., Jorge P., Colnaghi M. I. The Aspergillus toxin restriction is a suitable cytotoxic agent for generation of immunoconjugates with monoclonal antibodies directed against human carcinoma cells. Eur J Biochem. 1989 Jan 2;178(3):795–802. doi: 10.1111/j.1432-1033.1989.tb14511.x. [DOI] [PubMed] [Google Scholar]
- Endo Y., Huber P. W., Wool I. G. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J Biol Chem. 1983 Feb 25;258(4):2662–2667. [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Fando J. L., Alaba I., Escarmis C., Fernandez-Luna J. L., Mendez E., Salinas M. The mode of action of restrictocin and mitogillin on eukaryotic ribosomes. Inhibition of brain protein synthesis, cleavage and sequence of the ribosomal RNA fragment. Eur J Biochem. 1985 May 15;149(1):29–34. doi: 10.1111/j.1432-1033.1985.tb08888.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C. Permeability to alpha sarcin in virus-infected cells. Mol Cell Biochem. 1983;50(2):185–191. doi: 10.1007/BF00285643. [DOI] [PubMed] [Google Scholar]
- Hartley R. W. Barnase and barstar. Expression of its cloned inhibitor permits expression of a cloned ribonuclease. J Mol Biol. 1988 Aug 20;202(4):913–915. doi: 10.1016/0022-2836(88)90568-2. [DOI] [PubMed] [Google Scholar]
- Hartley R. W. Barnase and barstar: two small proteins to fold and fit together. Trends Biochem Sci. 1989 Nov;14(11):450–454. doi: 10.1016/0968-0004(89)90104-7. [DOI] [PubMed] [Google Scholar]
- Huber P. W., Wool I. G. Nuclease protection analysis of ribonucleoprotein complexes: use of the cytotoxic ribonuclease alpha-sarcin to determine the binding sites for Escherichia coli ribosomal proteins L5, L18, and L25 on 5S rRNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):322–326. doi: 10.1073/pnas.81.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNINGS J. C., OLSON B. H., ROGA V., JUNEK A. J., SCHUURMANS D. M. ALPHA SARCIN, A NEW ANTITUMOR AGENT. II. FERMENTATION AND ANTITUMOR SPECTRUM. Appl Microbiol. 1965 May;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A., Vázquez D. Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu Rev Microbiol. 1985;39:649–672. doi: 10.1146/annurev.mi.39.100185.003245. [DOI] [PubMed] [Google Scholar]
- López-Otín C., Barber D., Fernández-Luna J. L., Soriano F., Méndez E. The primary structure of the cytotoxin restrictocin. Eur J Biochem. 1984 Sep 17;143(3):621–634. doi: 10.1111/j.1432-1033.1984.tb08415.x. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Castrillo J. L., Carrasco L. Modification of membrane permeability during Semliki Forest virus infection. Virology. 1985 Oct 30;146(2):203–212. doi: 10.1016/0042-6822(85)90004-2. [DOI] [PubMed] [Google Scholar]
- OLSON B. H., GOERNER G. L. ALPHA SARCIN, A NEW ANTITUMOR AGENT. I. ISOLATION, PURIFICATION, CHEMICAL COMPOSITION, AND THE IDENTITY OF A NEW AMINO ACID. Appl Microbiol. 1965 May;13:314–321. doi: 10.1128/am.13.3.314-321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Natori Y., Tanaka S., Tsurugi K., Endo Y. Complete nucleotide sequence of cDNA for the cytotoxin alpha sarcin. Nucleic Acids Res. 1990 Apr 11;18(7):1897–1897. doi: 10.1093/nar/18.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi R., Canevari S., Conde F. P., Leoni F., Mezzanzanica D., Ripamonti M., Colnaghi M. I. Immunoconjugate generation between the ribosome inactivating protein restrictocin and an anti-human breast carcinoma MAB. Cancer Immunol Immunother. 1988;26(2):114–120. doi: 10.1007/BF00205603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Proteins are cointernalized with virion particles during early infection. Virology. 1987 Sep;160(1):75–80. doi: 10.1016/0042-6822(87)90046-8. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Sacco G., Drickamer K., Wool I. G. The primary structure of the cytotoxin alpha-sarcin. J Biol Chem. 1983 May 10;258(9):5811–5818. [PubMed] [Google Scholar]
- Schindler D. G., Davies J. E. Specific cleavage of ribosomal RNA caused by alpha sarcin. Nucleic Acids Res. 1977 Apr;4(4):1097–1110. doi: 10.1093/nar/4.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Bailey S., Miller S. P., Bodley J. W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988 Feb 25;16(4):1349–1357. doi: 10.1093/nar/16.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Upshall A., Gilbert T., Saari G., O'Hara P. J., Weglenski P., Berse B., Miller K., Timberlake W. E. Molecular analysis of the argB gene of Aspergillus nidulans. Mol Gen Genet. 1986 Aug;204(2):349–354. doi: 10.1007/BF00425521. [DOI] [PubMed] [Google Scholar]
- Veldman G. M., Klootwijk J., de Regt V. C., Planta R. J., Branlant C., Krol A., Ebel J. P. The primary and secondary structure of yeast 26S rRNA. Nucleic Acids Res. 1981 Dec 21;9(24):6935–6952. doi: 10.1093/nar/9.24.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]