Abstract
Background
Rheb is a GTP-binding protein that promotes cell survival and mediates the cellular response to energy deprivation (ED). The role of Rheb in the regulation of cell survival during ED has not been investigated in the heart.
Methods and Results
Rheb is inactivated during cardiomyocyte (CM) glucose deprivation (GD) in vitro, and during acute myocardial ischemia in vivo. Rheb inhibition causes mTORC1 inhibition, because forced activation of Rheb, through Rheb overexpression in vitro and through inducible cardiac-specific Rheb overexpression in vivo, restored mTORC1 activity. Restoration of mTORC1 activity reduced CM survival during GD and increased infarct size after ischemia, both of which were accompanied by inhibition of autophagy, whereas Rheb knockdown increased autophagy and CM survival. Rheb inhibits autophagy mostly through Atg7 depletion. Restoration of autophagy, through Atg7 re-expression and inhibition of mTORC1, increased cellular ATP content and reduced endoplasmic reticulum stress, thereby reducing CM death induced by Rheb activation. Mice with high fat diet-induced obesity and metabolic syndrome (HFD mice) exhibited deregulated cardiac activation of Rheb and mTORC1, particularly during ischemia. HFD mice presented inhibition of cardiac autophagy and displayed increased ischemic injury. Pharmacological and genetic inhibition of mTORC1 restored autophagy and abrogated the increase in infarct size observed in HFD mice, but they failed to protect HFD mice in the presence of genetic disruption of autophagy.
Conclusions
Inactivation of Rheb protects CMs during ED through activation of autophagy. Rheb and mTORC1 may represent therapeutic targets to reduce myocardial damage during ischemia, particularly in obese patients.
Keywords: Rheb, mTORC1, autophagy, myocardial ischemia
Introduction
Heart failure is viewed as one of the major health care problems worldwide, with acute myocardial infarction (MI) as the most common predisposing cause 1. It is fundamental to clarify the mechanisms regulating cardiomyocyte (CM) death and survival during ischemic injury, in order to find new therapies to reduce the amount of myocardial loss after a sudden coronary occlusion.
Ras homology enriched in brain (Rheb) is a small GTP-binding protein that has been shown to regulate the cellular stress response, both in lower organisms and in mammalian cell lines. In particular, Rheb appears to be a critical sensor of energy stress, being inactivated under this condition. Inhibition of Rheb during cellular stress promotes the upregulation of adaptive mechanisms, such as cell cycle arrest and growth inhibition, which may save energy, favor DNA repair, and thus be protective 2–4. On the contrary, Rheb is hyperactivated in cancer cells, where it promotes stress resistance and survival, and Rheb activation was found to directly inhibit apoptotic pathways induced by amino acid deprivation and genotoxic stress 5, 6. Therefore, it is not clear whether Rheb activity is protective or detrimental during cellular stress. Remarkably, the role of Rheb in response to acute energy deprivation, and in regulation of cell death and survival, has never been investigated in the heart.
Rheb activity is regulated by upstream kinases, such as Akt, AMPK and GSK-3β, which control Rheb through direct modulation of the heterodimer composed of the tuberous sclerosis complex proteins 1 (TSC1) and 2 (TSC2). The TSC1/TSC2 complex inhibits Rheb by exerting a strong GTPase activity toward it 2, 7. Rheb directly binds and selectively activates the multiprotein complex 1 of mammalian target of rapamycin (mTORC1), which in turn mediates many cellular functions, such as protein translation 8. mTORC1 is also inhibited in response to energy stress, and its inactivation reduces protein synthesis and upregulates autophagy 8. However, mTORC1 activation also promotes cell survival and inhibits apoptosis in several stress conditions, and therefore, whether mTORC1 inhibition is detrimental or protective during cellular stress is stimulus-dependent 5, 8.
The role of mTORC1 in mediating survival and death of CMs has only been investigated in models of chronic cardiac remodeling, with discordant results 9–11. Importantly, the effect of direct and selective mTORC1 versus mTORC2 modulation during CM acute energy deprivation, such as myocardial ischemia, remains to be elucidated. It is also unclear how mTORC1 is modulated during CM energy deprivation and whether Rheb, an immediate upstream regulator of mTORC1, is critically involved in such regulation in CMs.
In our study, we investigated the role of Rheb in the regulation of cell death and survival during CM starvation and ischemia, and the underlying molecular mechanisms. In particular, we studied whether a direct and selective modulation of mTORC1 induced by Rheb is involved in the effects exerted by Rheb on CM survival during energy stress. Recent reports have shown that obesity and metabolic syndrome, which are characterized by an increased risk of cardiovascular mortality 12 and increased myocardial susceptibility to ischemic injury 13–16, are associated with a hyperactivation of tissue mTORC1 17, 18. Therefore, we also evaluated whether cardiac mTORC1 is activated in obesity and metabolic syndrome, whether Rheb is involved in such phenomena, and whether a deregulated activation of Rheb and mTORC1 may be responsible for the increased susceptibility to ischemia associated with these conditions.
Methods
Experimental Procedures
Experimental procedures and animal models are described in the expanded Methods section in the online-only Data Supplement. Experimental procedures, heterozygous GFP-LC3 transgenic mice, beclin-1 knock-out mice and conditional mTOR knock-out mice have also been described elsewhere 9, 19, 20. All experimental procedures with animals were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
Statistics
Data are expressed as (mean ± SEM). When specified in the figure legends, presentation of bar charts was standardized by control mean × 100, so that the presented bars represent the mean percentage of variation ± SEM, with respect to the control mean. The difference in means between 2 groups was evaluated using the t-test when sample size was appropriate and the population was normally distributed; otherwise the Mann-Whitney U test was adopted. When differences among 3 or more groups were evaluated, the One-way ANOVA or the Kruskal-Wallis test was used. The post hoc comparisons were performed using the Bonferroni post-hoc test or the Mann-Whitney U test with Bonferroni correction. The shown statistical significance of differences between groups was always calculated by post-hoc comparisons when multiple groups were compared. Statistical analyses were performed with the use of SPSS 15.0 (SPSS Inc, Chicago, Ill) and GraphPad-Prism 5.00 (GraphPad-Software, San Diego, Ca). P values of <0.05 were considered statistically significant.
Results
Rheb mediates mTORC1 inhibition during starvation in CMs
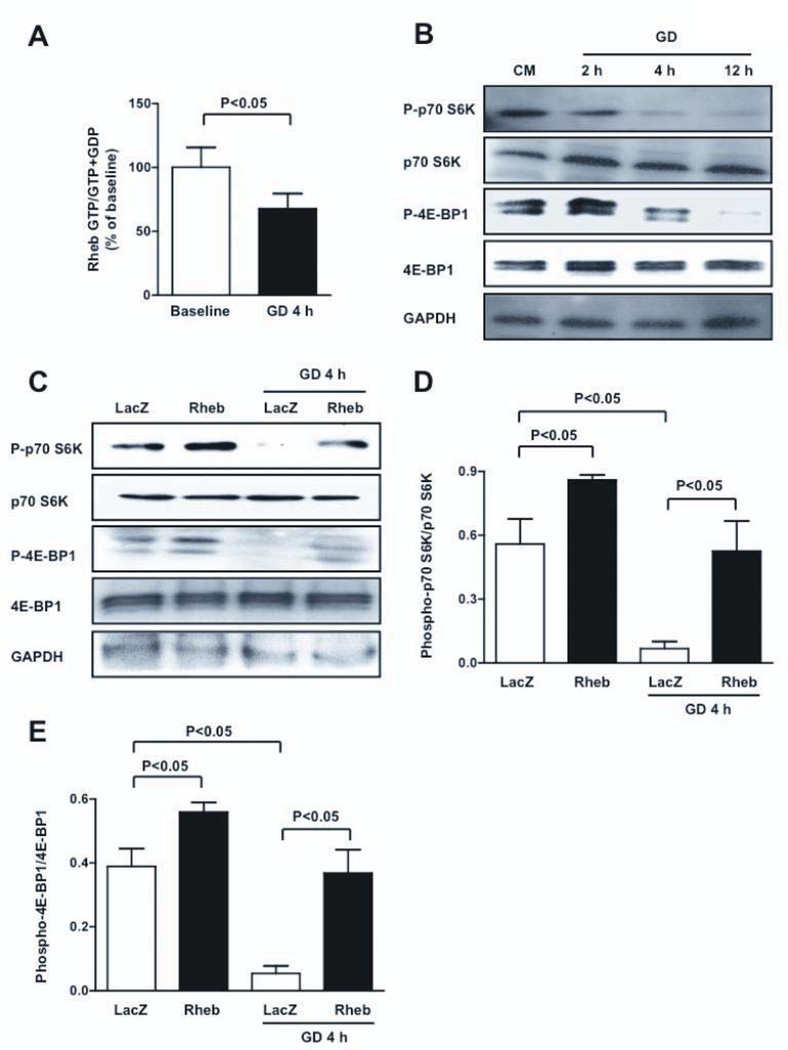
In order to investigate whether Rheb acts as a sensor of energy deprivation in CMs, neonatal rat ventricular CMs were subjected to glucose deprivation (GD). GTP binding of Rheb was decreased significantly in response to GD (Figure 1A), indicating that Rheb is inactivated by GD. During GD, phosphorylation of p70S6K and 4E-BP1 was progressively reduced, indicating that mTORC1 was inhibited (Figure 1B). Knock-down of Rheb, with adenovirus harboring shRNA-Rheb, inhibited phosphorylation of p70S6K at baseline, suggesting that inactivation of Rheb is sufficient to inactivate mTORC1 (Figure IA in the online only Data Supplement). Transduction of CMs with adenovirus harboring wild-type Rheb abolished the GD-induced decreases in phosphorylation of p70S6K and 4E-BP1 (Figure 1C–E and Figure IB), suggesting that Rheb inactivation is required for GD-induced suppression of mTORC1. In addition, Rheb physically interacts with mTOR both at baseline and during GD (Figure IC), thus indicating that Rheb directly regulates mTORC1 in CMs.
Figure 1. mTORC1 is downregulated during GD through Rheb inactivation.
A, Rheb activity was assessed by the ratio of Rheb-bound GTP to Rheb-bound GTP+GDP levels at baseline and during GD; N=5. B, CMs were subjected to GD for different periods of time. Phosphorylation statuses of p70S6K (Thr 389) and 4E-BP1 (Thr 37/46) were evaluated. C–E, CMs were transduced with adenovirus (Ad) harboring wild-type Rheb or LacZ. After 48 hours, phosphorylation statuses of p70S6K and 4E-BP1 were evaluated at baseline and after GD. Immunoblots and densitometric analyses are presented. N=5.
Rheb is negatively regulated by the GAP activity of the TSC1/TSC2 complex 7. Downregulation of TSC2, with adenovirus harboring shRNA-TSC2 (Figure ID), induced phosphorylation of p70S6K, suggesting that endogenous TSC2 negatively regulates mTORC1 in CMs (Figure IE–F). Activation of mTORC1 by downregulation of TSC2 was abolished in the presence of Rheb knockdown, suggesting that TSC2 regulates mTORC1 through Rheb (Figure IE–F). On the other hand, as indicated by the phosphorylation status of Akt, the activity of mTORC2, another branch of the mTOR pathway, was unaffected by GD, and overexpression of Rheb failed to activate Akt (Figure IG–H). These results suggest that GD inhibits mTORC1, but not mTORC2, by inactivating Rheb.
Activation of Rheb sensitizes CMs to cell death during GD, while inhibition is protective
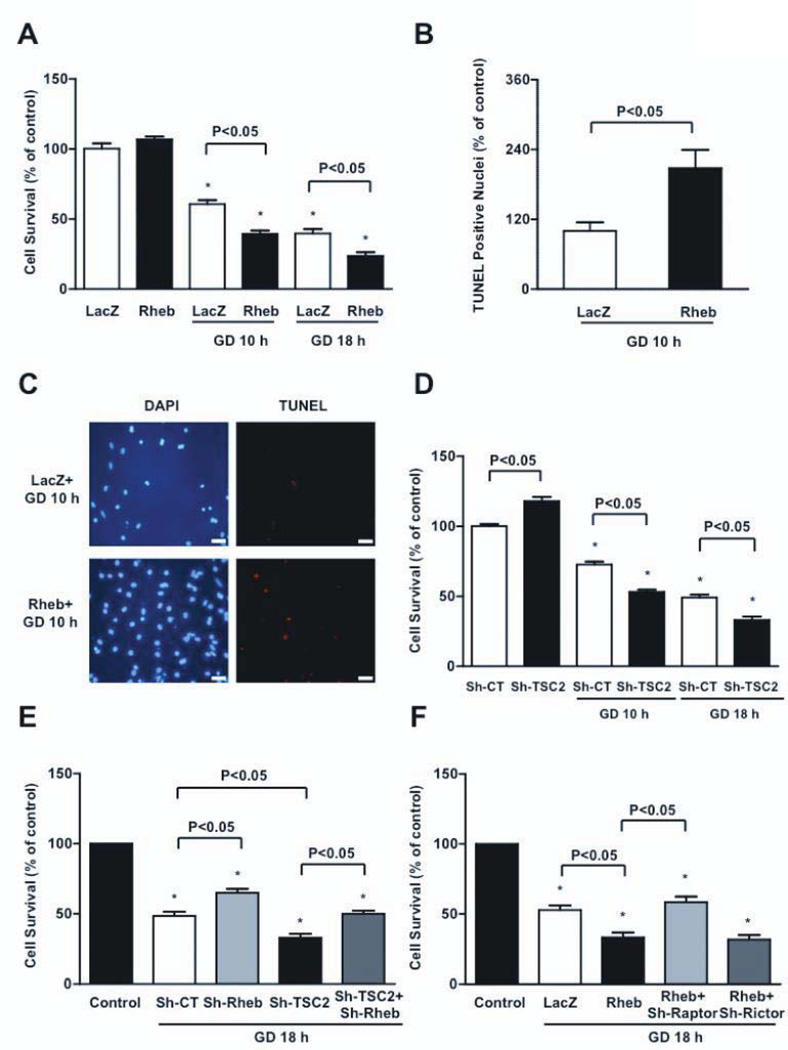
We then investigated the role of Rheb in regulating CM survival during GD. Although Rheb is cell-protective in other cell types, CMs in which the activity of mTORC1 was normalized with overexpressed Rheb displayed decreased survival after 10 and 18 hours of GD, compared to control virus-treated CMs (Figure 2A). CMs overexpressed with Rheb displayed significantly more apoptosis and necrosis, as assessed by TUNEL assays and propidium iodide staining, respectively (Figure 2B–C, Figure IIA). TSC2 knockdown also decreased survival and increased apoptosis of CMs in response to GD (Figure 2D and Figure IIB). Conversely, downregulation of endogenous Rheb increased the survival of CMs during GD and rescued the decrease in cell survival in the presence of TSC2 knockdown during GD (Figure 2E). Furthermore, selective inhibition of mTORC1, through Raptor downregulation, with adenovirus harboring shRNA-Raptor, significantly increased CM survival during GD in Rheb-overexpressing CMs. In contrast, selective mTORC2 inhibition, through Rictor depletion, did not increase survival in Rheb-overexpressing CMs during GD (Figure 2F). Collectively, this data suggests that Rheb negatively regulates CM survival during GD through mTORC1 activation. Thus, inactivation of endogenous Rheb during GD is an adaptive mechanism that promotes survival of CMs.
Figure 2. Rheb activation during GD increases CM death and apoptosis.
A, Cell viability was evaluated in CMs transduced with Ad-Rheb or Ad-LacZ at baseline and during GD. N=4. B–C, Percentage of TUNEL positive cells was also evaluated after GD. N=4, bar=50 µm. D, CMs were transduced with Ad harboring short hairpin-RNA (sh)-TSC2 or with sh-scramble. After 96 hours, cell viability was evaluated at baseline and after GD. N=5. E, Cell viability was evaluated in CMs transduced with sh-scramble (sh-CT), sh-Rheb, sh-TSC2, or sh-Rheb plus sh-TSC2 at baseline and after GD. N=5. F, Cell viability was evaluated in CMs transduced with Ad-LacZ, Ad-Rheb, or Ad-Rheb plus sh-Raptor or sh-Rictor, at baseline and after GD. N=3. Cell viability was assessed by Cell Titer Blue assay. Data is presented as a percentage of the relevant control (regular medium), with the control being set at 100%. * p<0.05 with respect to relevant control (regular medium).
Rheb regulates CM autophagy
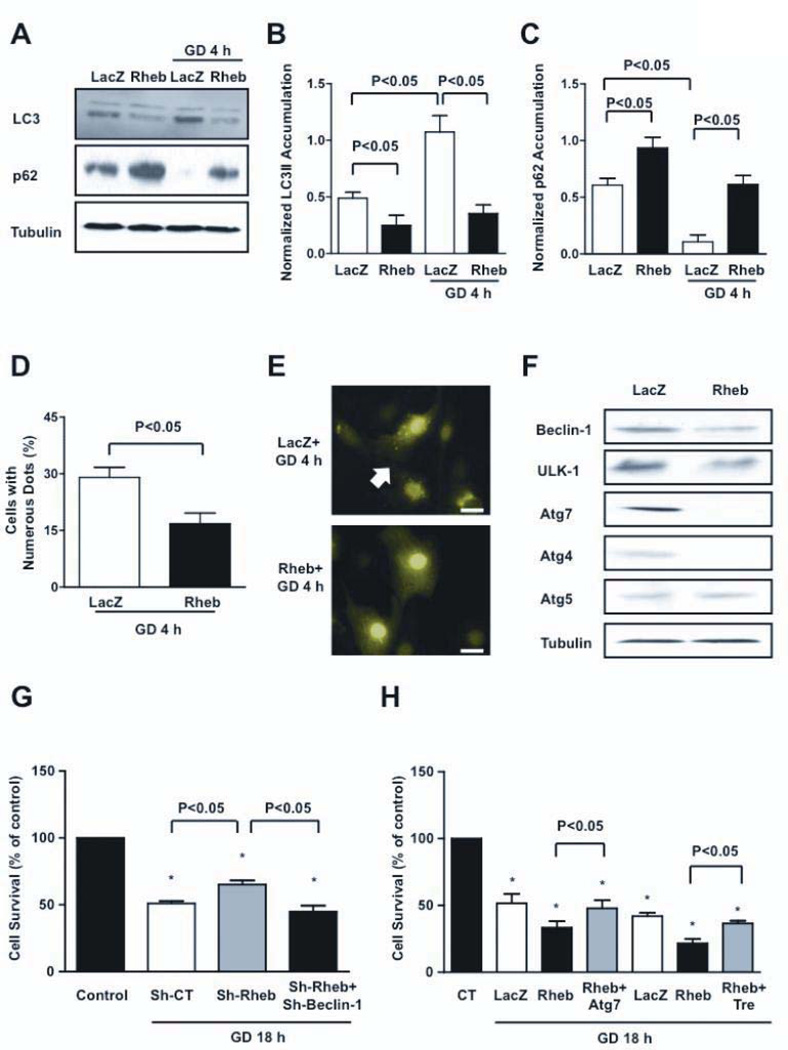
We investigated the molecular mechanism through which inactivation of Rheb protects CMs during GD. Since Rheb inactivation causes mTORC1 inhibition during GD, and because mTOR is a negative regulator of autophagy 21, we hypothesized that downregulation of Rheb during GD is required for stimulation of autophagy, which may be protective in this context 19. As shown previously, GD increased LC3-II and decreased p62, a protein degraded by autophagy 21, suggesting that GD activates autophagy in CMs. However, in Rheb-overexpressing CMs, LC3-II expression was lower and expression of p62 was greater, both at baseline and during GD (Figure 3A–C). The number of GFP-LC3 dots, an indicator of autophagosome accumulation, during GD was significantly smaller in Rheb-overexpressing CMs than in control CMs (Figure 3D–E). Rheb overexpression induced significant downregulation of autophagy genes, including beclin-1, ulk-1, atg4 and atg7 (Figure 3F). Conversely, downregulation of endogenous Rheb significantly increased autophagy at baseline and during GD (Figure III). These results suggest that endogenous Rheb negatively regulates autophagy and that inactivation of Rheb is necessary and sufficient for stimulation of autophagy in CMs during GD.
Figure 3. Rheb is a negative regulator of autophagy.
A–C, CMs were transduced with Ad-LacZ or Ad-Rheb for 48 hours. LC3 isoforms and p62 accumulation were evaluated at baseline or after 4 hours of GD. A representative immunoblot is shown (A), together with densitometric analyses of LC3-II (B) and p62 (C). N=5. D–E, CMs were transduced with Ad-GFP-LC3 together with Ad-LacZ or Ad-Rheb. GFP-LC3 puncta were evaluated after 4 hours of GD (bar=10 µm). N=3. F, Expression of autophagic genes was evaluated in CMs transduced with Ad-LacZ or Ad-Rheb. G, Cell viability was evaluated in CMs transduced with sh-scramble, sh-Rheb, or with sh-Rheb plus sh-Beclin-1. N=4. Data is presented as a percentage of the relevant control (CT, baseline), with CT being set at 100%. H, Cell viability was evaluated in CMs transduced with Ad-LacZ, Ad-Rheb, and with Ad-Rheb together with Ad-Atg7 or trehalose. Sucrose treatment (100 mM) was used as control treatment for trehalose. N=3.Atg7= Ad-Atg7; Tre= trehalose. * p<0.05 with respect to cells cultured with a normal medium.
We then asked if autophagy mediates the cell-protective effect of Rheb inactivation. The protective effect of Rheb downregulation during GD was completely abrogated when Beclin-1 was downregulated with adenovirus harboring shRNA-beclin1 (Figure 3G). These results suggest that autophagy plays an important role in mediating the protective effect of Rheb inactivation during GD. Conversely, in order to restore autophagy during GD in Rheb-overexpressing CMs, we expressed Atg7 with adenovirus transduction (Figure IVA). We took this approach since Atg7 is a crucial protein for autophagosome formation, because Atg7 is markedly downregulated in Rheb-overexpressing CMs, and because overexpression of Atg7 is sufficient to re-induce autophagy when autophagy is inhibited 21, 22. Atg7 overexpression significantly restored autophagy in Rheb-overexpressing CMs during GD (Figure IVB–D). We also used trehalose, which induces autophagy without affecting the mTORC1 pathway 23. Trehalose restored Atg7 and Beclin-1 expression in Rheb-overexpressing CMs without affecting the mTORC1 pathway, thereby restoring autophagy during GD (Figure IVE–F). Importantly, both Atg7 expression and trehalose pretreatment significantly increased the survival of Rheb-overexpressing CMs during GD (Figure 3H). Collectively, these results suggest that Rheb regulates the survival and death of CMs during GD through regulation of autophagy in vitro.
Rheb overexpression increases energy stress and ER stress during GD
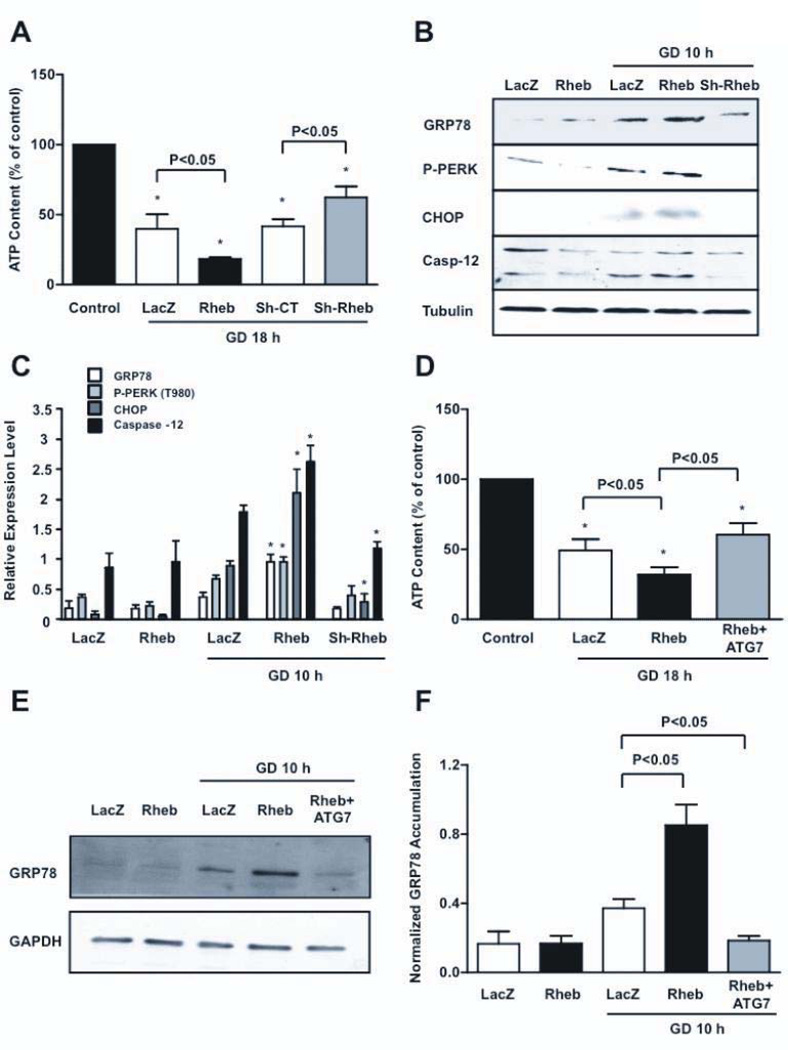
Important consequences of autophagy include restoration of ATP contents and protein quality control. Rheb overexpression during GD significantly enhanced ATP depletion, whereas Rheb disruption significantly increased ATP content (Figure 4A). Overexpression of Rheb increased GRP78, phosphorylation of PERK and upregulation of C/EBP Homologous Protein (CHOP), and Caspase-12 (fragment), markers of ER stress, in CMs during GD (Figure 4B–C). Conversely, upregulation of the ER stress markers during GD was significantly attenuated in CMs in which Rheb was knocked-down (Fig. 4B–C). Restoration of autophagy in Rheb-overexpressing CMs, through Atg7 overexpression, significantly attenuated ATP depletion (Figure 4D) and ER stress during GD, indicating that autophagy inhibition is responsible for these derangements (Figure 4E–F).
Figure 4. Rheb activation increases ATP depletion and ER stress during GD.
A, CM ATP content at baseline and after 18 hours of GD was evaluated in CMs with Rheb overexpression or depletion. Data is presented as the fluorescence of each sample as a percentage of the control. * p<0.05 vs. control (regular medium). N=4. B–C, Unfolded protein response markers were evaluated at baseline and after GD. * p<0.05 vs. LacZ after GD. N=5. D–F, ATP content (D, N=5) and GRP78 accumulation (E–F, N=3) were evaluated in Rheb-overexpressing CMs with or without Ad-Atg7, after GD.
Inhibition of Rheb is protective during prolonged myocardial ischemia
In order to investigate the role of Rheb in regulating CM survival and death in response to energy deprivation in vivo, we used a mouse model of prolonged ischemia, in which the left descending coronary artery was ligated for 3 hours. During ischemia, the GTP-bound form of Rheb was significantly decreased, while the total expression of Rheb was not altered, suggesting that Rheb is inactivated by prolonged ischemia in vivo (Figure 5A–B). The activity of mTORC1, as evaluated with p70S6K phosphorylation, was also decreased during ischemia (Figure 5C–D).
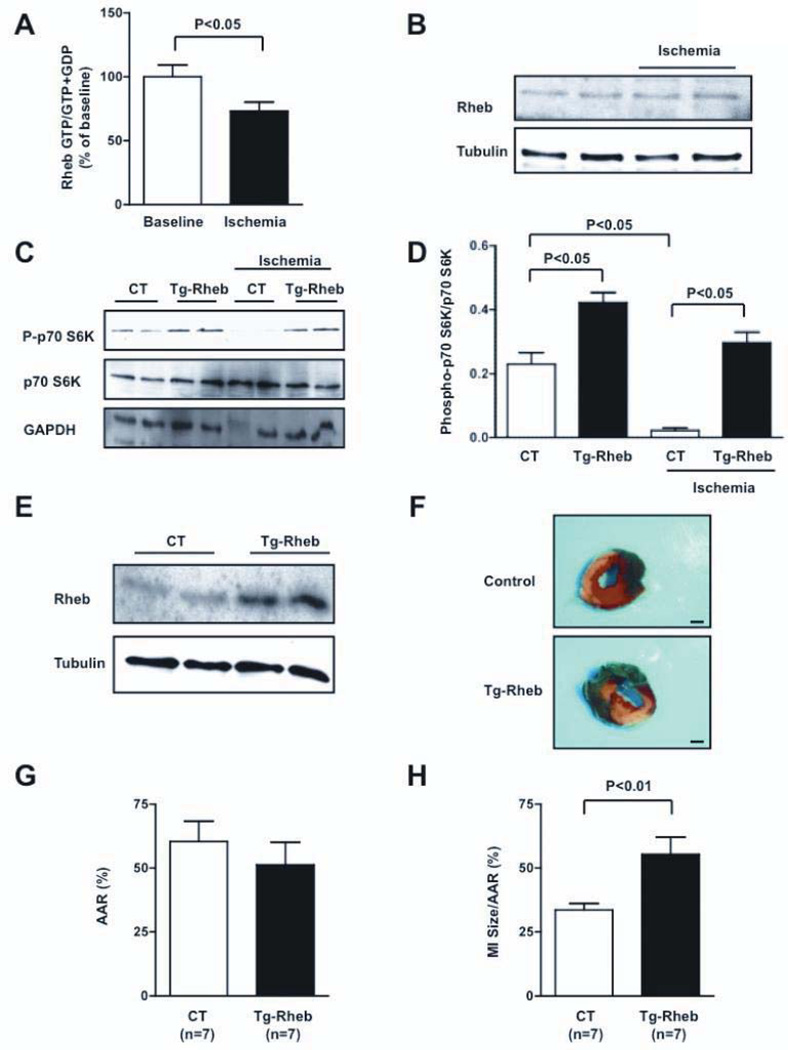
Figure 5. Rheb activation is detrimental during prolonged myocardial ischemia in vivo.
A, Rheb-bound GTP levels were evaluated at baseline and after 30 minutes of ischemia. N=7. B, The amount of Rheb expression was evaluated at baseline and during ischemia. C–D, Cardiac p70S6K phosphorylation was evaluated in Tg-Rheb mice and controls, both at baseline and after 30 minutes of ischemia. N=4 for each group. E, Cardiac Rheb expression was evaluated in Tg-Rheb mice and control mice (FVB background). F–H, Tg-Rheb and control mice (Rheb+/tTA− and Rheb−/tTA+) were subjected to 3 hours of ischemia. LV myocardial sections after Alcian blue and triphenyltetrazolium chloride staining is shown (F; bar=1 mm), as well as the area at risk (AAR, G) and myocardial infarct (MI) size/AAR (H) quantification.
In order to evaluate the significance of Rheb inhibition during ischemia in vivo, we generated transgenic mice with cardiac-specific overexpression of Rheb (Tg-Rheb), using a Tet-off system. In these mice, expression of the Rheb transgene in the heart was induced in the absence of doxycycline. Doxycycline (Dox) was administered to the mice during the gestational period and for the first 3–4 weeks of life to avoid the effect of transgene expression during cardiac development in Tg-Rheb (Figure 5E and Figure VA–B). Dox was terminated 6–8 weeks before the experiment in order to allow full transgene expression and eliminate possible actions of Dox upon cell death/survival. In this protocol, expression of Rheb was 2.3-fold greater in Tg-Rheb than in control littermates (Rheb+/tTA−mice). Rheb exhibited diffuse cytoplasmic distribution in CMs of both control mice and Tg-Rheb (Figure VC–D). Tg-Rheb presented a normal cardiac phenotype at 3 months of age (Table 1 in the online-only Data Supplement). In Tg-Rheb mice, mTORC1 activity was significantly increased, both at baseline and during prolonged ischemia, compared to control mice (Figure 5C–D), suggesting that Rheb inactivation is required for mTORC1 inhibition during prolonged ischemia. After 3 hours of ischemia, Tg-Rheb mice exhibited a significantly greater MI size than control mice (Figure 5F–H). The extent of CM apoptosis and necrosis after prolonged ischemia was also greater in Tg-Rheb than in controls, as evaluated with TUNEL and Hairpin-2 staining, respectively (Figure VI). Tg-Rheb presented increased ischemic injury even after a brief period of ischemia (30 minutes), as evaluated with Hairpin-2 staining. Tg-Rheb also exhibited significantly enhanced myocardial damage even after a longer coronary occlusion (6 hours; Figure VII).
There was less induction of autophagy in Tg-Rheb than in control mice at baseline and during ischemia, as indicated by reduced LC3-II and increased p62 accumulation (Figure VIIIA–C). Expression of p62 did not differ at the mRNA level between controls and Tg-Rheb, indicating that increased p62 accumulation was due to reduced degradation (mRNA expression in Tg-Rheb 0.96-fold vs. controls, P=NS). The level of myocardial ATP in the ischemic area after ischemia was significantly lower in Tg-Rheb than in controls (Figure VIIID). The level of CHOP, an indicator of ER stress, after ischemia was also significantly greater in Tg-Rheb than in control mice (Figure VIIIE–F). These results indicate that inhibition of endogenous Rheb is protective during prolonged ischemia in vivo.
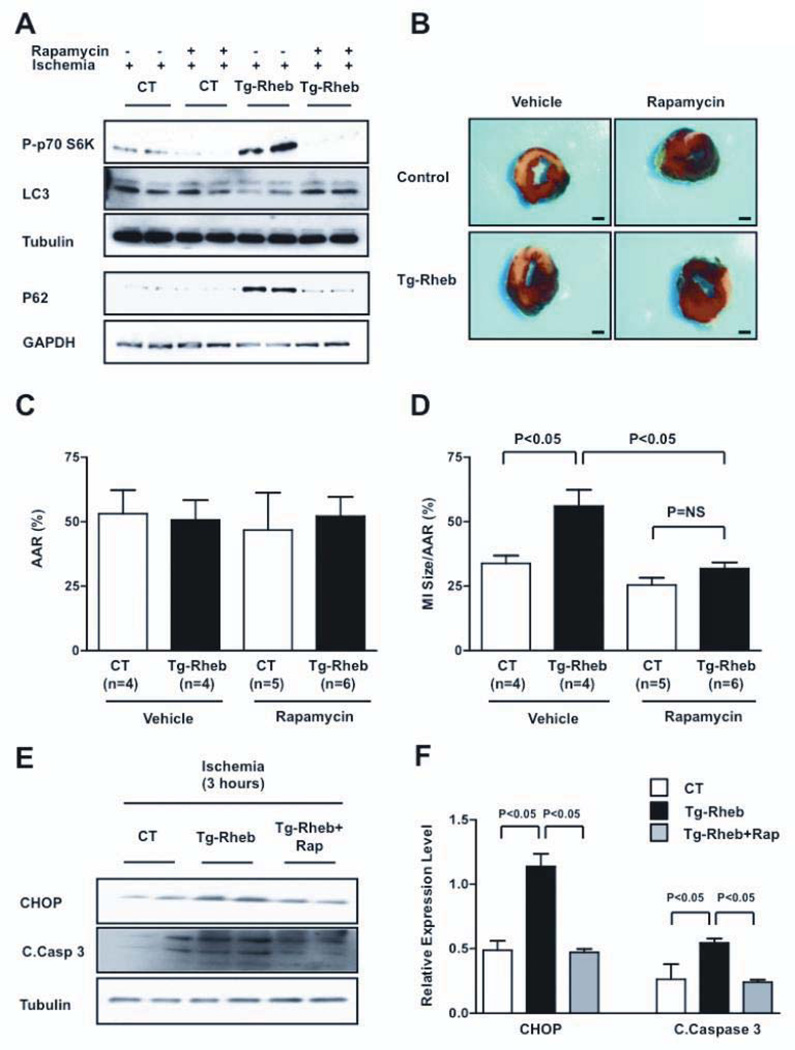
In order to evaluate whether the deleterious effect of Rheb overexpression during prolonged ischemia is due to the lack of mTORC1 inactivation and activation of autophagy, rapamycin, a selective inhibitor of mTORC1 and stimulator of autophagy, was administered to Tg-Rheb and control mice just before the prolonged ischemia. Rapamycin inhibited mTORC1 activity and stimulated autophagy in Tg-Rheb mice after prolonged ischemia (Figure 6A). Rapamycin significantly reduced the size of MI in response to prolonged ischemia in Tg-Rheb mice compared to vehicle administration (Figure 6B–D). Rapamycin treatment also significantly reduced CHOP accumulation and caspase-3 cleavage in Tg-Rheb hearts after prolonged ischemia (Figure 6E–F). These results suggest that Rheb promotes myocardial injury during prolonged ischemia by stimulating mTORC1, inhibiting autophagy and stimulating ER stress.
Figure 6. Rapamycin-induced autophagy limits myocardial damage in Rheb-overexpressing mice.
A, Rapamycin (1 mg/kg) was administered intraperitoneally to Tg-Rheb and control mice (Rheb+/tTA−) 60 minutes before coronary ligation. p70S6K phosphorylation and LC3 expression levels were evaluated after 30 minutes of ischemia, whereas p62 expression levels were evaluated after 3 hours of ischemia. B–D, The MI /AAR ratio in Tg-Rheb and controls treated, or not treated, with rapamycin was evaluated. Bar=1 mm. E–F, CHOP expression and caspase-3 cleavage were also evaluated after 3 hours of ischemia. N=3 for each group.
High fat diet (HFD)-induced obesity is associated with deregulation of Rheb and increased myocardial susceptibility to prolonged ischemia
Obesity and metabolic syndrome are associated with high cardiovascular mortality and reduced cardiac function after MI 12–16. Complications of obesity are associated with deregulated mTORC1 activation and inhibition of autophagy in other organs 17, 18, 22. We therefore investigated whether obesity is associated with deregulated Rheb activation, which in turn mediates an increased susceptibility to myocardial ischemia.
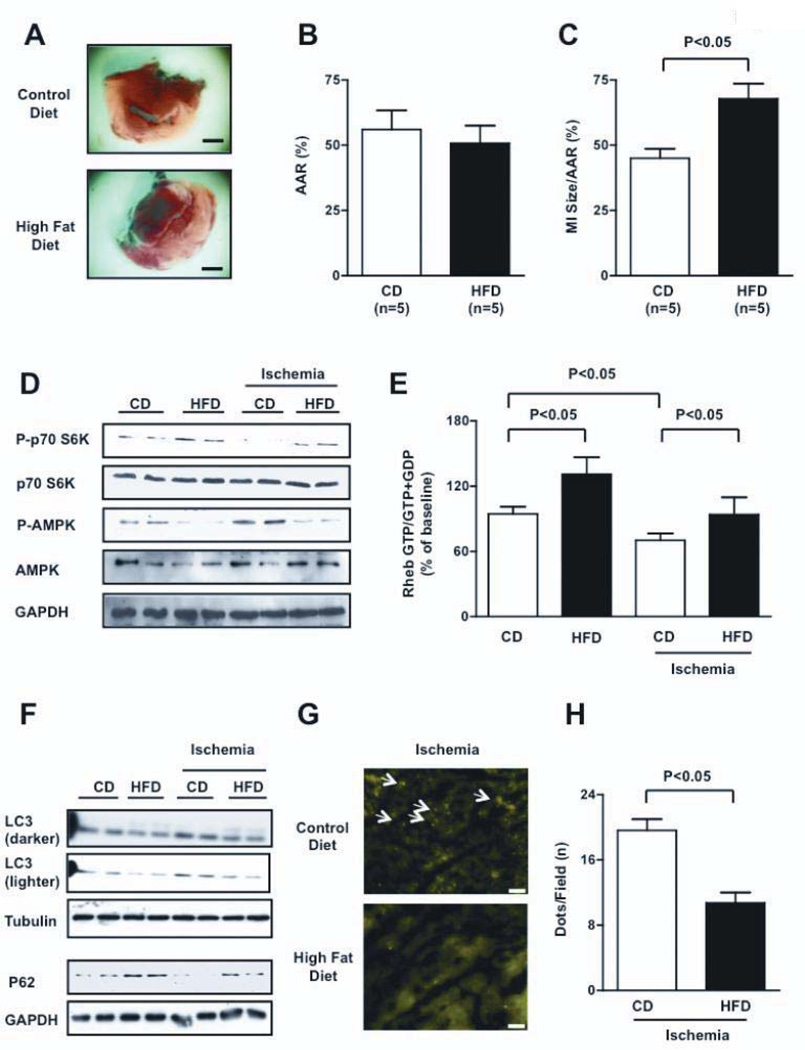
In order to induce obesity, C57BL/6J mice were fed with high fat diet (HFD mice) for 18–20 weeks. HFD mice developed obesity and exhibited a significant increase in serum levels of glucose, cholesterol, triglycerides and non-esterified fatty acid compared to mice fed with control diet (CD mice), suggesting that HFD mice develop metabolic syndrome (Table II in the online-only Data Supplement). Insulin levels and the HOMA index were significantly elevated in HFD mice, consistent with the notion that these mice develop insulin resistance. HFD mice presented increases in mTOR-dependent IRS-1 phosphorylation (serine 636), which is a marker of decreased insulin sensitivity 18 at baseline (Figure IXA). HFD mice showed a significant increase in LV mass and LV wall thickness but preserved LV systolic function (Table III). Both cell size and expression of atrial natriuretic factor (ANF), a fetal-type gene, were increased, suggesting that HFD mice develop cardiac hypertrophy (Figure IXB–D). After prolonged (3 hours) ischemia, HFD mice exhibited a significantly greater MI size than CD mice (Figure 7A–C), which was accompanied by greater numbers of TUNEL-positive and Hairpin-2-positive cells (Figure X), signifying that HFD increases myocardial susceptibility to ischemic injury. HFD mice also presented a greater percentage of hairpin-2 positive cells with respect to control mice after 30 minutes of ischemia (11.5 ± 1.3% vs. 4.2 ± 1.0%, p<0.05).
Figure 7. HFD-induced obesity is associated with greater myocardial injury and deregulated Rheb/mTORC1 activation.
A–C, MI/AAR was evaluated in CD and HFD mice after ischemia. Bar=1 mm. D, Phosphorylation statuses of p70S6K and AMPK (Thr 172) were evaluated both at baseline and after 30 minutes of ischemia (densitometric analysis is shown in Figure XIA). E, Myocardial Rheb-bound GTP content was evaluated in CD and HFD mice, both at baseline and after 30 minutes of ischemia. N=5 for each group. F, Myocardial autophagy in HFD mice was significantly inhibited compared with control mice, both at baseline and after 30 minutes (LC3-II levels) or 3 hours of ischemia (p62 levels). Representative immunoblots are presented, and densitometric analysis is reported in Supplemental Figure XIB–C. G–H, Tg-GFP-LC3 mice fed with control or high fat diet were subjected to ischemia. Representative heart sections are shown. Bar=50 µm. Arrows indicate autophagosomes (G). The number of autophagosomes per microscopic field in the two groups after 30 minutes of ischemia is reported (H). N=4 each group.
In HFD mice, the activity of mTORC1 was greater at baseline and remained elevated during prolonged ischemia (Figure 7D and Figure XIA). Thus, the suppression of mTORC1 in response to prolonged ischemia observed in CD mice was attenuated in HFD mice. Although the GTP-bound form of Rheb was significantly reduced during prolonged ischemia in CD mice, it was increased at baseline and not significantly diminished during prolonged ischemia in HFD mice (Figure 7E), suggesting that the activity of Rheb and mTORC1 is elevated at baseline and remains greater in HFD mice than in CD mice during prolonged ischemia. Intriguingly, the activity of AMPK, a negative regulator of the Rheb/mTORC1 pathway, was reduced in HFD mice both at baseline and during ischemia, as indicated by a reduction in its phosphorylation status. Conversely, it was activated in CD mice during ischemia (Figure 7D and Figure XIA).
Consistent with activation of the mTORC1 pathway, autophagy in the heart was significantly suppressed in HFD mice both at baseline and during ischemia, as indicated by decreased LC3-II and increased p62 accumulation (Figure 7F, Figure XIB–C). p62 mRNA expression was unchanged (0.84-fold vs. CD mice, P=NS). Accumulation of autophagosomes, as evaluated using GFP-LC3 dots, was significantly less in HFD mice than in CD mice (Figure 7G–H). The number of GFP-LC3 dots was significantly reduced in HFD mice during ischemia, also after administration of chloroquine which inhibits lysosomal enzyme activity. This data indicates reduced autophagosome formation in HFD mice (Figure XID–E).
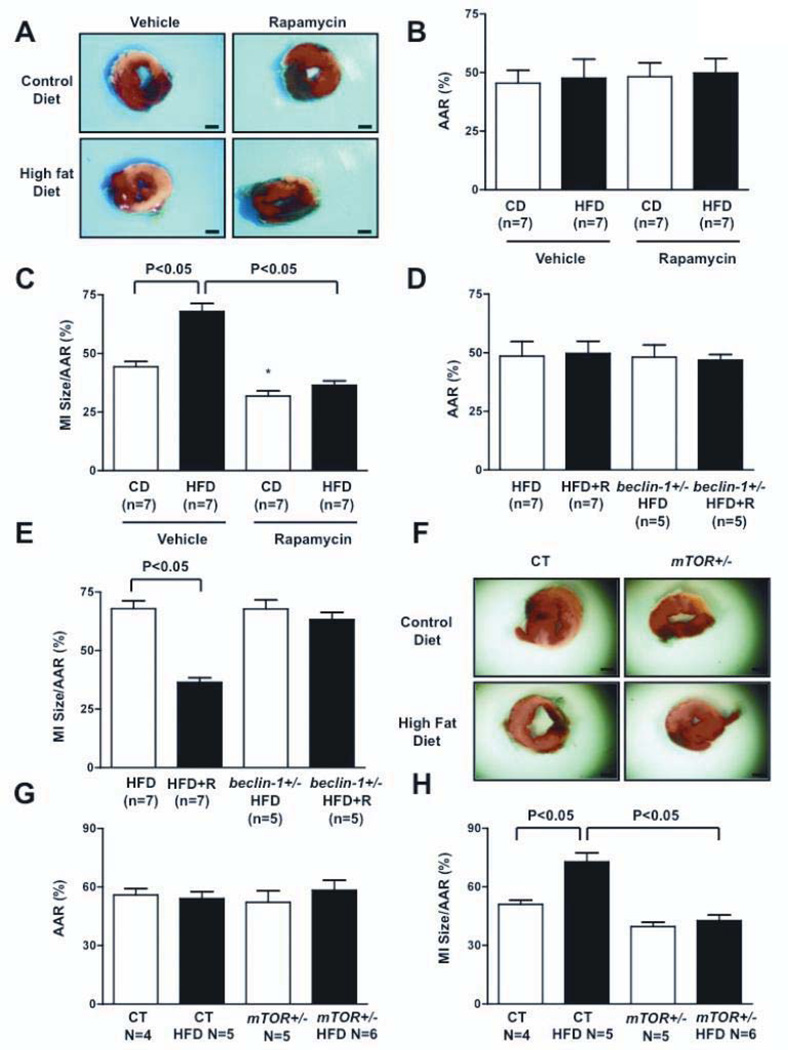
In order to investigate if deregulated activation of the mTORC1 pathway is responsible for the reduced tolerance to prolonged ischemia of HFD mice, we administered rapamycin to these animals and evaluated its effect on ischemic injury. As we observed with Tg-Rheb, rapamycin treatment increased autophagy (Figure XIF) and significantly reduced the MI size of both HFD mice and CD mice (Figure 8A–C). Rapamycin administration failed to reduce ischemic injury in heterozygous beclin-1 knock-out mice (beclin-1+/−), in which autophagy cannot be activated, when fed with HFD (Figure 8D–E). These results indicate that autophagy re-activation mediates the beneficial effect of mTORC1 inhibition in HFD mice.
Figure 8. mTORC1 inhibition is protective in HFD mice through autophagy activation.
A–C, Rapamycin (1 mg/kg) was administered intraperitoneally to HFD and CD mice 60 minutes before coronary ligation. Mice were subjected to 3 hours of ischemia. The MI/AAR ratio was evaluated. Bar=1 mm. * p<0.05 vs. CD. D–E, Either control or beclin-1+/− mice fed with HFD were subjected to 3 hours of ischemia. The MI/AAR ratio was evaluated. R=rapamycin. F–H, After feeding with HFD or CD, tamoxifen (30 mg/kg) was administered to α-MHC-MerCreMer-mTOR flox/+ mice (mTOR+/−) and α-MHC-MerCreMer-mTOR +/+ mice (controls) for 7 days. The mice were subjected to 3 hours of ischemia. The MI/AAR ratio was evaluated.
In order to further demonstrate that deregulated mTORC1 activation increases the ischemic susceptibility of HFD mice, we subjected mice, with inducible cardiac-specific heterozygous mTOR knockout, that were fed with HFD, to prolonged ischemia. This strategy allowed us to partially inhibit the mTOR pathway and to normalize the increased activation of mTORC1 observed in HFD mice. Tamoxifen was administered to α-MHC-MerCreMer-mTOR flox/+ mice (mTOR+/−) for 7 days. Cardiac mTOR protein levels were reduced in mTOR+/− mice 3 weeks after tamoxifen administration (Figure XIIA). Cardiac mTOR deletion reduced mTORC1 activity and increased autophagy in mTOR+/− mice fed with control diet or HFD (Figure XIIB–D). Remarkably, cardiac mTOR deletion reduced MI size in mice fed with control diet and HFD with respect to controls (Figure 8F–H). In summary, cardiac activation of the Rheb-mTORC1 pathway in HFD-induced obesity is detrimental during prolonged ischemia due to inhibition of autophagy.
Discussion
We have demonstrated that Rheb is inhibited in response to GD and prolonged ischemia, and inhibition of Rheb in turn inhibits mTORC1 in CMs. Forced activation of Rheb in such conditions stimulates ATP depletion and ER stress by suppression of autophagy, thereby inducing cell death. Thus, Rheb acts as a sensor of energy stress and as a critical regulator of CM survival in response to energy starvation.
We have shown previously that both GD and ischemia in CMs and in the heart, respectively, induce suppression of mTOR 19, 20. It should be noted that mTOR is inhibited by both Rheb-dependent and independent mechanisms 19, 20. For example, several upstream kinases, including AMPK and GSK-3β, which indirectly regulate mTOR, inhibit Rheb through phosphorylation and consequent activation of GAP activity in TSC2 2, 3, 8. It should be noted, however, that mTOR is also inhibited through Rheb-independent mechanisms, such as Akt-dependent phosphorylation of mTOR and PRAS40, and AMPK-dependent Raptor phosphorylation 8. Our results indicate that Rheb interacts with mTOR, that Rheb is inhibited during GD and myocardial ischemia, and that its inactivation is required for mTORC1 inhibition. Conversely, neither overexpression of Rheb nor GD affected the activity of mTORC2. We therefore propose that Rheb acts as a central and direct regulator of mTORC1 during energy starvation in CMs.
Our results suggest that forced Rheb activation exacerbates cell death and apoptosis during GD and prolonged ischemia, but it does not affect cell survival at baseline. On the other hand, downregulation of Rheb increased survival of CMs during GD, intimating the involvement of endogenous Rheb in the regulation of survival/death during GD and prolonged ischemia. Previous studies have indicated that Rheb promotes cell survival and inhibits apoptotic cell death in response to stress in several cancer cells 5, 6. Activation of Rheb in unstressed conditions also induces hypertrophy without cell death in CMs (not shown). Thus, the function of Rheb in cells appears to be context-dependent.
Importantly, we found that downregulation of mTORC1 mediates the protective effects of Rheb inhibition during energy deprivation, since depletion of Raptor but not of Rictor, which are the adaptor proteins of complex 1 and complex 2 of mTOR, respectively, increased survival of Rheb-overexpressing CMs during GD in vitro. In addition, pharmacological and genetic inhibition of mTORC1 reduced the susceptibility to ischemic myocardial damage of Rheb-overexpressing and obese mice, and a protective effect was also observed in control animals in vivo. These results suggest that Rheb is an obvious therapeutic surrogate of mTORC1, to achieve increased CM survival during energy deprivation.
The role of mTOR in regulating the stress response is poorly understood in terminally differentiated cell types such as CMs. In particular, the role of mTOR in the regulation of CM survival has been primarily investigated through indirect means, e.g., the use of pharmacological inhibitors, which may have mTOR-independent effects 19, 20. In addition, the role of mTOR in cardiac stress has been mostly studied in animal models of chronic ventricular remodeling in which mTORC1 is activated, while we observed mTORC1 inhibition during CM energy deprivation 9–11. Interestingly, mTORC1 activation has been indicated as protective during cardiac mechanical overload 9, 11. On the other hand, in our study, we demonstrated that selective and direct mTORC1 activation is detrimental during acute cardiac energy deprivation, whereas both pharmacological and genetic mTORC1 inhibition are protective. In particular, we provided the first evidence that genetic mTOR inhibition is protective during myocardial ischemia. Thus, the function of mTORC1 in CMs appears to be context-dependent. mTORC1 activation might be required for cell growth in response to mechanical overload, whereas mTORC1 inhibition is important for preservation of energy status in response to energy deprivation.
Rheb inhibition during energy deprivation is required for autophagy activation, which is protective in this condition. In fact, suppression of autophagy by knockdown of Beclin-1 completely abrogated the protective effect of Rheb knockdown in CMs during GD. Restoration of autophagy through treatment with trehalose or overexpression of Atg7, which stimulates autophagy through mTOR-independent mechanisms, significantly reduced CM death induced by forced Rheb activation. Therefore, although it is still debated whether autophagy is protective or detrimental during cardiac stress 24, we have demonstrated that Rheb-regulated autophagy is protective during CM nutrient starvation and ischemia. In particular, we showed that Rheb-regulated autophagy is protective through the preservation of ATP content and reduction of misfolded protein accumulation, namely ER stress.
Rheb-induced inhibition of autophagy was accompanied by downregulation of Atg7 protein levels. Overexpression of Atg7 was sufficient to restore autophagy and to suppress Rheb-induced cell death during GD, suggesting that Rheb regulates autophagy in part through Atg7. mTORC1 was suggested to modulate autophagy through Ulk1/2 regulation 21. The role of Ulk1/2 in mediating expression of Atg7 remains to be elucidated.
Interestingly, inadvertent activation of the Rheb/mTORC1 pathway is observed in HFD-induced obesity. Obesity is characterized by glucose intolerance and dyslipidemia, and it is associated with an increased susceptibility to myocardial ischemia 13–16. We demonstrated that autophagy is reduced in the hearts of mice with HFD-induced obesity. These mice exhibited exacerbated myocardial injury in response to prolonged ischemia, which was normalized by rapamycin treatment or genetic mTOR inhibition, suggesting that increased mTORC1 activity may be responsible for the increased susceptibility. Remarkably, inhibition of Beclin-1 was associated with the failure of pharmacological mTORC1 inhibition to reduce ischemic injury in HFD mice, indicating that re-activation of autophagy is the crucial mechanism mediating the beneficial effects of mTORC1 inhibition in HFD-induced obesity.
Severe obesity and metabolic syndrome are associated with increased cardiovascular risk events and a poor prognosis in patients after acute MI 12, 14, 15, 25, 26. If our results hold true in humans, it may be helpful to treat patients with obesity and metabolic syndrome using pharmacological inhibitors of Rheb or mTORC1, to stimulate autophagy during an acute episode of myocardial ischemia. Our results are also supported by an interesting previous study which showed that obesity increases vascular senescence and vascular dysfunction in response to mTOR activation27.
Other previous studies showed increased basal mTORC1 activity in the liver 17, 28, adipose tissue 29, vasculature 27, skeletal muscle 17, 28, 30, 31 and cardiac muscle 32–34 in both genetic and diet-induced models of obesity and dysmetabolic conditions. AMPK inhibition has been proposed as the main intracellular mechanism leading to mTORC1 activation 18, 30–34. Our study extends this previous evidence, suggesting that Rheb is involved in the activation of mTORC1 induced by AMPK downregulation.
Several stimuli may enhance the activity of the Rheb/mTORC1 pathway in the tissues of obese and dysmetabolic animals. High caloric intake may represent one possible cause. High levels of circulating and cardiac lipids may also represent potential mechanisms. In addition, increases in circulating insulin, amino acids, cytokines and adipokines may contribute to the increased Rheb/mTORC1 activity in HFD mice 17, 18, 28, 31, 32, 35.
In summary, our study demonstrates that inactivation of Rheb protects CMs during energy deprivation through activation of autophagy, reduction of energy expenditure and attenuation of ER stress (Figure XIIE). Rheb and mTORC1 may represent therapeutic targets to reduce myocardial damage during acute myocardial ischemia, particularly in patients with obesity and metabolic syndrome.
Clinical perspective.
The incidence of heart failure after acute myocardial infarction (MI) remains very high in patients. This highlights the necessity to clarify the mechanism regulating the survival and death of cardiomyocytes in response to ischemia and to find new cardioprotective therapies reducing ischemic injury. We discovered that Rheb, a small GTP-binding protein, plays a pivotal role in regulating the survival of cardiomyocytes during prolonged myocardial ischemia. Rheb activity is reduced in the ischemic heart, thereby causing the suppression of the mTORC1 pathway. Inhibition of the Rheb/mTORC1 pathway is an adaptive response during ischemia, because forced restoration of cardiac Rheb activity is detrimental under this condition. Rheb inhibition is required for the activation of autophagy, an intracellular degradation process for proteins and organelles, which is protective during energy stress through preservation of cellular energy and relief of ER stress. We discovered that obesity and metabolic syndrome (Ob/MS) are associated with cardiac activation of Rheb/mTORC1 at baseline and during ischemia. In obese mice, autophagy in the heart was suppressed and ischemic injury was exacerbated. Remarkably, inhibition of mTORC1 restores autophagy and reduces infarct size in these animals after prolonged ischemia. Thus, our results suggest that Rheb and mTORC1 may be promising therapeutic targets to reduce myocardial damage after prolonged ischemia in patients with Ob/MS who display deregulated activation of the Rheb/TORC1 pathway and consequent inhibition of autophagy.
Supplementary Material
Acknowledgments
The authors wish to thank Christopher D. Brady and Daniela Zablocki for their critical revisions of the manuscript.
Funding sources
Dr. Sciarretta is supported by the American Heart Association Founders Affiliate FDA Spring 2010 Post-Doctoral Fellowship (10POST4260019). This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, AG27211 and the Foundation of Leducq Transatlantic Network of Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 3.Aspuria PJ, Sato T, Tamanoi F. The TSC/Rheb/TOR signaling pathway in fission yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR. Cell Cycle. 2007;6:1692–1695. doi: 10.4161/cc.6.14.4478. [DOI] [PubMed] [Google Scholar]
- 4.Karassek S, Berghaus C, Schwarten M, Goemans CG, Ohse N, Kock G, Jockers K, Neumann S, Gottfried S, Herrmann C, Heumann R, Stoll R. Ras homolog enriched in brain (Rheb) enhances apoptotic signaling. J Biol Chem. 2010;285:33979–33991. doi: 10.1074/jbc.M109.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babcock JT, Quilliam LA. Rheb/mTOR Activation and Regulation in Cancer: Novel Treatment Strategies Beyond Rapamycin. Curr Drug Targets. 2011;12:1223–1231. doi: 10.2174/138945011795906589. [DOI] [PubMed] [Google Scholar]
- 6.Ma D, Bai X, Zou H, Lai Y, Jiang Y. Rheb GTPase controls apoptosis by regulating interaction of FKBP38 with Bcl-2 and Bcl-XL. J Biol Chem. 2010;285:8621–8627. doi: 10.1074/jbc.M109.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–C1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 13.Clavijo LC, Pinto TL, Kuchulakanti PK, Torguson R, Chu WW, Satler LF, Kent KM, Suddath WO, Pichard AD, Waksman R. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in-hospital complications. Cardiovasc Revasc Med. 2006;7:7–11. doi: 10.1016/j.carrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Zeller M, Steg PG, Ravisy J, Laurent Y, Janin-Manificat L, L'Huillier I, Beer JC, Oudot A, Rioufol G, Makki H, Farnier M, Rochette L, Verges B, Cottin Y. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med. 2005;165:1192–1198. doi: 10.1001/archinte.165.10.1192. [DOI] [PubMed] [Google Scholar]
- 15.Abdulla J, Kober L, Abildstrom SZ, Christensen E, James WP, Torp-Pedersen C. Impact of obesity as a mortality predictor in high-risk patients with myocardial infarction or chronic heart failure: a pooled analysis of five registries. Eur Heart J. 2008;29:594–601. doi: 10.1093/eurheartj/ehn010. [DOI] [PubMed] [Google Scholar]
- 16.du Toit EF, Smith W, Muller C, Strijdom H, Stouthammer B, Woodiwiss AJ, Norton GR, Lochner A. Myocardial susceptibility to ischemic-reperfusion injury in a prediabetic model of dietary-induced obesity. Am J Physiol Heart Circ Physiol. 2008;294:H2336–H2343. doi: 10.1152/ajpheart.00481.2007. [DOI] [PubMed] [Google Scholar]
- 17.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 18.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 20.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential Roles of GSK-3{beta} During Myocardial Ischemia and Ischemia/Reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Narayanan U, Renna M, Jimenez-Sanchez M, Sarkar S, Underwood B, Winslow A, Rubinsztein DC. Mammalian macroautophagy at a glance. J Cell Sci. 2009;122:1707–1711. doi: 10.1242/jcs.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 24.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 26.Aronson D, Nassar M, Goldberg T, Kapeliovich M, Hammerman H, Azzam ZS. The impact of body mass index on clinical outcomes after acute myocardial infarction. Int J Cardiol. 2010;145:476–480. doi: 10.1016/j.ijcard.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 29.Ranieri SC, Fusco S, Panieri E, Labate V, Mele M, Tesori V, Ferrara AM, Maulucci G, De Spirito M, Martorana GE, Galeotti T, Pani G. Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2010;107:13420–13425. doi: 10.1073/pnas.1008647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake JC, Alway SE, Hollander JM, Williamson DL. AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1546–R1554. doi: 10.1152/ajpregu.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivas DA, Yaspelkis BB, 3rd, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5'-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Endocrinol. 2009;202:441–451. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8:1738–1746. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung MM, Koonen DP, Soltys CL, Jacobs RL, Febbraio M, Dyck JR. Increased CD36 expression in middle-aged mice contributes to obesity-related cardiac hypertrophy in the absence of cardiac dysfunction. J Mol Med (Berl) 2011;89:459–469. doi: 10.1007/s00109-010-0720-4. [DOI] [PubMed] [Google Scholar]
- 34.Turdi S, Kandadi MR, Zhao J, Huff AF, Du M, Ren J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2011;50:712–722. doi: 10.1016/j.yjmcc.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.