Abstract
Nonalcoholic fatty liver disease (NAFLD) and alterations in hepatic lipoprotein kinetics are common metabolic complications associated with obesity. Lifestyle modification involving diet-induced weight loss and regular exercise decreases intrahepatic triglyceride (IHTG) content and very low density lipoprotein (VLDL) triglyceride (TG) secretion rate. The aim of this study was to evaluate the weight loss-independent effect of following the physical activity guidelines recommended by the Department of Health and Human Services on IHTG content and VLDL kinetics in obese persons with NAFLD. Eighteen obese people (BMI: 38.1 ± 4.6 kg/m2) with NAFLD were randomized to 16 weeks of exercise training (45-55% V̇O2peak, 30-60 min × 5 days/week; n = 12) or observation (control; n = 6). Magnetic resonance spectroscopy and stable isotope tracer infusions in conjunction with compartmental modeling were used to evaluate IHTG content and hepatic VLDL-TG and apolipoprotein B-100 (apoB-100) secretion rates. Exercise training resulted in a 10.3 ± 4.6 % decrease in IHTG content (p<0.05), but did not change total body weight (103.1 ± 4.2 kg before and 102.9 ± 4.2 kg after training) or percent body fat (38.9 ± 2.1 % before and 39.2 ± 2.1 % after training). Exercise training did not change the hepatic VLDL-TG secretion rate (17.7 ± 3.9 μmol/min before and 16.8 ± 5.4 μmol/min after training) or VLDL-apoB-100 secretion rate (1.5 ± 0.5 nmol/min before and 1.6 ± 0.6 nmol/min after training). Conclusions: Following the Department of Health and Human Services recommended physical activity guidelines has small but beneficial effects on IHTG content, but does not improve hepatic lipoprotein kinetics, in obese persons with NAFLD.
Keywords: obesity, free fatty acids, dyslipidemia, alanine transaminase
Nonalcoholic fatty liver disease (NAFLD) is a common complication of obesity, and is associated with abnormalities in hepatic very-low-density lipoprotein (VLDL) metabolism and increased serum triglyceride (TG) concentration (1, 2). Lifestyle modification involving diet-induced weight loss and regular physical activity reduces intrahepatic triglyceride (IHTG) content and improves the metabolic derangements associated with NAFLD (3-17). Very small amounts of weight loss can cause marked decreases (20%-60%) in IHTG content (18-20). In fact, we have found that even 48 h of calorie restriction caused a 20% decrease in IHTG content in obese subjects (21). However, few studies have evaluated the effect of regular physical activity, independent of weight loss, on NAFLD.
Current guidelines from the Department of Health and Human Services, the American College of Sports Medicine, and the American Heart Association, recommend that adults perform at least 150 min, but preferably 300 min of moderate-intensity physical activity per week; or at least 75 minutes, but preferably 150 minutes, of vigorous physical activity per week to achieve health benefits (22, 23). Data from two studies have recently shown that 4-12 weeks of vigorous intensity exercise training caused a small decrease in IHTG content (24, 25). However, obese people are more likely to adhere to a moderate intensity than a vigorous intensity exercise program (26), in part, because obese persons have lower levels of pleasure after high intensity exercise (27), and exercise intensity is perceived to be higher in obese than lean subjects (28). The potential benefits of regular moderate intensity exercise on IHTG content and hepatic metabolic function in obese people with NAFLD has not been carefully studied.
The purpose of this study was to investigate the effect of the recommended exercise guidelines for moderate intensity endurance exercise training (150-300 min per week) on IHTG content and VLDL kinetics in obese subjects with NAFLD. We hypothesized that exercise training would result in decreased IHTG content and hepatic VLDL-TG and VLDL-apolipoprotein B-100 (apoB-100) secretion rates. Magnetic resonance spectroscopy (MRS) was used to assess IHTG content and stable isotopically labeled tracer techniques were used to assess VLDL-TG and VLDL-apoB-100 kinetics in our study subjects before and after 16 weeks of supervised exercise training.
Methods
Trial Design
This was a single-center, randomized, controlled trial conducted at Washington University School of Medicine in St. Louis, MO, approved by the local Human Research Protection Office and in compliance with the ethical guidelines of the 1975 Declaration of Helsinki. All subjects provided written informed consent before participating in the study.
Participants
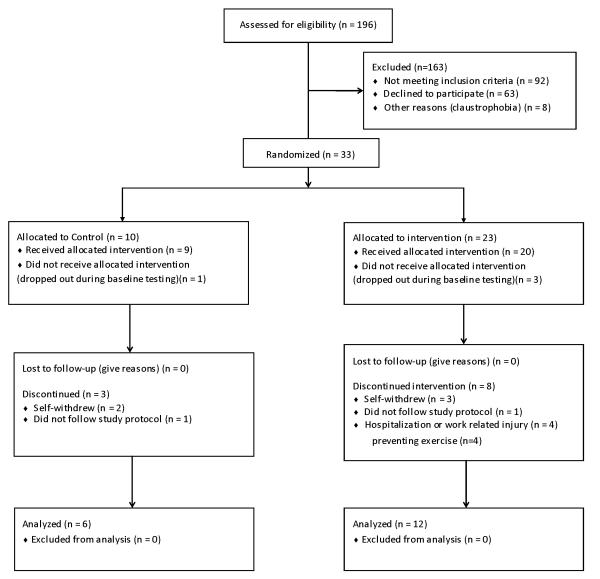
Eighteen obese subjects (5 men; 13 women) with NAFLD (IHTG content >10 %), who were recruited between June 2006 and July 2010, participated in this study. The flow of study participants is shown in Figure 1. All subjects completed a medical evaluation, which included a history and physical examination, blood tests, the Michigan Alcohol Screening Test (29), and an oral glucose tolerance test. Subjects were excluded from the study if they had chronic liver disease other than NAFLD, a Michigan Alcohol Screening Test score >4, diabetes, or plasma TG concentration >400 mg/dl. All subjects were weight stable (<3 % change in self-reported weight for at least 3 months before the study) and sedentary (<1 h of self-reported exercise per week).
Figure 1.
Diagram of study subject flow.
Experimental Protocol
Body composition, aerobic fitness, and hepatic VLDL-TG and VLDL-apoB-100 secretion rates were assessed before and after 16 weeks of exercise training or the control period.
Body composition analyses
Body fat mass and fat-free mass (FFM) were determined by using dual-energy-X-ray absorptiometry (Hologic QDR 4500, Waltham, MA)(30). Intrahepatic TG content was determined by using MRS (Magnetom Trio/3T, Siemens, Erlanger, Germany) (31).
Aerobic fitness
Peak aerobic capacity (V̇O2 peak) was determined by measuring peak oxygen consumption while walking on a motor-driven treadmill at a constant speed of 3.0 mph while increasing the grade by 2.5% every 2 minutes (32). Respiratory gas exchange rates were measured throughout the duration of the test by using indirect calorimetry (Parvo Medics’ TrueOne® 2400, Sandy, UT).
VLDL-TG and VLDL-apoB-100 kinetics
Subjects were admitted to the Clinical Research Unit in the evening before the study and consumed a standard meal at 1900 h. Subjects then fasted (except for water) until the completion of the study the next day. At 0500 h the next morning, one catheter was inserted into a forearm vein for tracer infusions and a second catheter was inserted into a vein on the opposite hand, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples. At 0600 h, a bolus of [1,1,2,3,3-2H5]glycerol (75 μmol/kg) and 12-h constant infusions of [5,5,5-2H3]leucine (0.06 μmol·kg−1·min−1; priming dose: 4.2 μmol/kg;) and [2H2]palmitate (0.03 μmol·kg−1·min−1) were administered through the catheter in the forearm vein. Blood samples were collected before the bolus tracer injection and at 5, 15, 30, 60, 90 and 120 min and then hourly for 10 h after the tracer infusions were started. An aliquot of each plasma sample was kept in the refrigerator to isolate VLDL by ultracentrifugation (33); the remainder of each sample was frozen and stored at −80°C until final analyses were performed.
Randomization and Study Intervention
Subjects who passed screening were randomized into the control or the exercise group by an independent statistician who did not participate in subject enrollment by using a 2:1 computer-generated randomization scheme. Subjects in the control group were instructed to continue their current activities of daily living; they were contacted once a week to review compliance with the study protocol and reported to the research center once a month to obtain accurate body weight measurements. Subjects in the exercise group were instructed to exercise for 30-60 min, 5 times per week at 45%-55% of their V̇O2 peak (i.e., brisk walk). Subjects initiated their exercise program by walking on a treadmill for 15-30 minutes at a heart rate equivalent to 45-55% of their pre-training V̇O2 peak, and progressively increased the duration of exercise during the initial 4 weeks until 30-60 min of moderate intensity exercise 5 times a week was achieved. Subjects exercised under direct supervision once a week in our exercise facility; the remaining sessions were completed at home. Compliance with home exercises was assessed by recording heart rate (Polar Electro heart rate monitor, Kempele, Finland) during all exercise sessions. Subjects were required to complete 16 weeks of exercise training; those who completed <3 exercise sessions in any week were required to extend their training period by an additional week. Subjects in the exercise group were weighed weekly on the same scale and without shoes before supervised exercise. The post-intervention lipid metabolism study was performed on the day after the last bout of exercise was performed.
Sample analyses
Total cholesterol, HDL-cholesterol (HDL-C), and LDL-cholesterol (LDL-C) concentrations in plasma were measured enzymatically by using a Hitachi 917 autoanalyzer (Hitachi, Tokyo, Japan). Plasma alanine transaminase (ALT) concentration was measured enzymatically by using a Cobas c501 autoanalyzer (Roche, Indianapolis, USA). Plasma free fatty acid (FFA) concentrations were determined by gas chromatography (Hewlett-Packard 5890-II; Hewlett-Packard, Palo Alto, CA) after adding hepadecanoic acid to plasma as an internal standard (34). Total TG and VLDL-TG concentrations in plasma were determined by using a colorimetric enzymatic kit (Sigma Chemicals, St. Louis, MO). VLDL-apoB-100 concentration was determined by using a commercially available immunoturbidimetric kit (Wako Chemicals, Richmond, VA). Plasma glycerol, palmitate, and leucine tracer-to-tracee ratios (TTR) were measured by using gas chromatography/mass spectrometry (MSD 5973; Hewlett-Packard) (35).
Calculations
Palmitate rate of appearance (Ra) in plasma was calculated between 60 and 180 minutes of tracer infusion by using Steele’s equation for steady-state conditions (36). The fractional turnover rates (FTR) of VLDL-TG and VLDL-apoB-100 were determined by fitting the TTR time-courses of free glycerol in plasma and glycerol in VLDL-TG and the TTR time-courses of free leucine in plasma and leucine in VLDL-apoB-100, respectively to a compartmental model (35, 37). The secretion rates of VLDL-TG and VLDL-apoB-100 were calculated by multiplying the FTR of VLDL-TG and VLDL-apoB-100 by the steady-state plasma VLDL-TG and VLDL-apoB-100 concentrations and the plasma volume, respectively (35, 37). The molar ratio of VLDL-TG and VLDL-apB-100 secretion rates was calculated by dividing the VLDL-TG secretion rate by the VLDL-apoB 100 secretion rate and provides an index of the TG content of newly secreted VLDL particles (38). The relative contribution of systemic plasma FFA to VLDL-TG production, which is defined as FFA taken up by the liver from the systemic circulation and directly incorporated into VLDL-TG, was determined by the degree of isotopic dilution of the palmitate enrichment in VLDL-TG vs. plasma (35, 39). The remaining fatty acids in VLDL-TG are considered “non-systemic”, and include fatty acids derived from lipolysis of intrahepatic and visceral adipose tissue TG, lipolysis of plasma lipoproteins taken up by the liver, and hepatic de novo lipogensis (40).
Statistical analysis
Power Calculation
The primary study endpoint was the change in IHTG content in the exercise vs. the control group. Assuming an average (± SD) IHTG content of 23 ± 7 % in obese subjects with NAFLD, which is the average value observed in our previous studies, and no change in IHTG content in the control group, we estimated that a total of 6 subjects in the control group and 12 subjects in the exercise group would give us a power of 95% to detect a 30% change and a power of 80% to detect a 20% change in IHGT content in the exercise group. Power calculations were performed by using G*Power 3.1.2 software, (Franz Faul, Universität Kiel, Germany).
Data Analysis
The Student’s t-test for independent samples (normally distributed data sets) or the Mann Whitney U test (skewed data sets) were used to compare outcome measures in the two groups at baseline. Analysis of covariance (ANCOVA) was used to evaluate the effect of exercise (vs. control) on the outcome measures after adjusting for differences in baseline plasma TG concentrations between groups; variables that were not normally distributed were log-transformed for analysis. Pearson’s product moment correlation coefficient was used to evaluate the relationship between plasma ALT concentration and IHTG content. All results are expressed as mean ± SEM or median [quartiles]. A two-sided p-value of ≤0.05 was considered statistically significant. Statistical analyses were performed by using SPSS (Windows) v17.0 for Windows (IBM, New York).
Results
Compliance with the intervention
Eighteen subjects completed all study testing; n = 6 in the control group (1 man; 5 women, age: 47.5 ± 3.1 years) and n = 12 in the exercise group (4 men; 8 women, age: 48.6 ± 2.2 years). The 12 subjects who completed study testing in the exercise group performed 94 ± 1.5% of the scheduled exercise sessions; 8 subjects completed the exercise program within 16 weeks and 4 within 18 weeks. On average, subjects exercised for 224 ± 22 min per week. No study related adverse events occurred.
Body composition and peak aerobic capacity
Values for V̇O2 peak, body weight, body mass index (BMI), body fat mass, FFM, and IHTG content at baseline were similar in the exercise and control groups (Table 1). V̇O2 peak increased by 9.0 ± 2.5 % after exercise training, but the difference between the exercise and the control groups was not statistically significant, possible due to a type 2 statistical error because of the small number of subjects. Body weight, body fat mass and FFM values at the end of the study were not different from the corresponding values obtained at the beginning of the study. There was a 10.3 ± 4.6% relative decrease in IHTG content in the exercise group compared with the control group (p = 0.044), (Figure 2).
Table 1.
Body composition and peak aerobic capacity before and after intervention in the exercise and the control groups.
| Control Group (n = 6) |
Exercise Group (n = 12) |
||||
|---|---|---|---|---|---|
| Before | After | Before | After | P value for Interaction |
|
| BMI (kgm-2) | 40.0 ± 2.2 | 40.1 ± 2.1 | 37.1 ± 1.1 | 37.1 ± 1.1 | 0.51 |
| Body mass (kg) | 113.7 ± 6.0 | 113.9 ± 5.7 | 103.1 ± 4.2 | 102.9 ± 4.2 | 0.48 |
| Body fat mass (%) | 42.5 ± 3.6 | 42.3 ± 3.4 | 38.9 ± 2.1 | 39.2 ± 2.1 | 0.42 |
| Fat free mass (kg) | 63.8 ± 4.9 | 65.0 ± 4.9 | 62.1 ± 3.9 | 62.0 ± 3.7 | 0.128 |
| V̇O2 peak (ml/kg/min) | 18.5 ± 2.9 | 19.9 ± 2.5 | 22.8 ± 1.3 | 24.8 ± 1.5 | 0.22 |
BMI: body mass index. IHTG: intrahepatic triglyceride. V̇O2 peak: peak aerobic capacity.
Values are means ± SEM. No between group differences for all Before values were detected by using a Student's t-test.
Figure 2.
Intrahepatic triglyceride content in the Control (n = 6) and Exercise (n = 11) groups before and after the 16 week intervention. Data are means ± SEM. ANCOVA revealed a significant group x time interaction (p = 0.044).
Plasma lipids and ALT concentrations
There were no differences in total TG, VLDL-TG, VLDL-apoB-100, and FFA concentrations in plasma between the control and exercise groups at baseline (all p > 0.12), (Table 2). Exercise training did not affect plasma total TG, VLDL-TG, VLDL-apoB-100, and FFA concentrations. Plasma ALT concentration decreased by 12.8 ± 3.1% in the exercise group compared with the control group (p = 0.04), (Table 2). The exercise-induced change in plasma ALT concentration correlated directly with the exercise-induced change in IHTG content (R2 = 0.60, p < 0.001).
Table 2.
Plasma lipids and ALT concentrations.
| Control Group (n = 6) |
Exercise Group (n = 12) |
||||
|---|---|---|---|---|---|
| Before | After | Before | After | P-value Interaction | |
| Total cholesterol (mg/dL) | 163.7 ± 8.5 | 167.5 ± 9.1 | 174.7 ± 7.8 | 165.8 ± 4.5 | 0.20 |
| HDL- cholesterol (mg/dL) | 38.3 ± 2.1 | 38.5 ± 3.1 | 41.1 ± 2.9 | 39.9 ± 2.5 | 0.57 |
| LDL– cholesterol (mg/dL) | 91.5 [75.5,97.8] | 96.5 [61.0,106.8] | 98.0 [92.0,122.8] | 97.0 [91.3,104.8] | 0.94 |
| Total TG (mg/dL) | 187.0 [100.3,262.5] | 213.5 [79.8,303.8] | 126.0 [105.8,183.5] | 141.5[111.5,165.5] | 0.30 |
| VLDL-TG (mg/dL) | 81.9 [41.3,162.9] | 73.9 [40.1,135.3] | 41.8 [35.6,58.7] | 40.9 [32.9,59.6] | 0.54 |
| VLDL-apoB-100 (mg/dL) | 5.2 [3.4,13.3] | 6.1 [3.4,11.3] | 3.9 [3.1,6.6] | 4.4 [3.8,5.3] | 0.19 |
| FFA (mmol/L) | 0.430 ± 0.050 | 0.480 ± 0.050 | 0.517 ± 0.060 | 0.530 ± 0.050 | 0.86 |
| ALT (IU/L) | 33.7 ± 6.0 | 39.3 ± 9.2 | 45.6 ± 8.6 | 39.3 ± 7.4 | 0.04 |
ALT: Alanine transaminase. Apo-B-100: apolipoprotein B-100. FFA: Free fatty acid. TG: Triglyceride. VLDL: Very low density lipoprotein.
Values are means ± SEM or medians [quartiles]. No differences in between group Before values were detected using a Student’s ttest or a Mann Whitney U test for non-normally distributed values
Lipid Kinetics
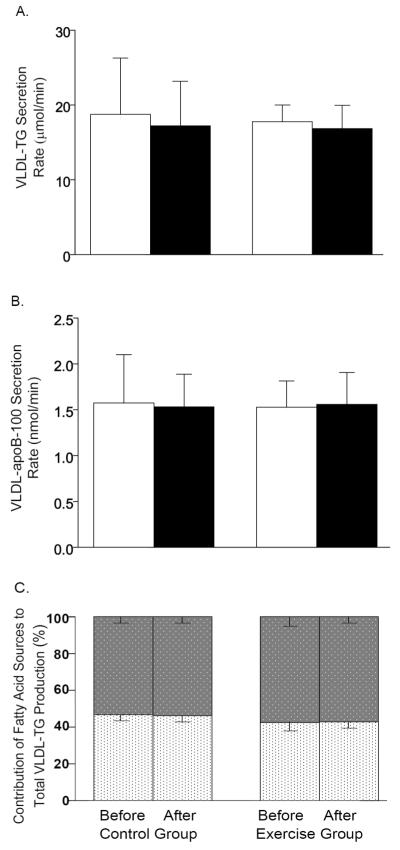
FFA Ra into plasma was not different between the exercise (3.8 ± 1.1 μmol/kg/min) and control (3.6 ± 1.1 μmol/kg/min) groups at baseline and did not change after either exercise training or control intervention (4.0 ± 1.1 μmol/min and 4.0 ± 1.2 μmol/kg/min, respectively) (all p >0.6). There were also no differences between the exercise and control groups in baseline VLDL-TG and VLDL-apoB-100 secretion rates (all p >0.18) and exercise had no effect on these metabolic outcome measures (all p >0.5; Figure 3 A and B). The proportion of fatty acids in VLDL-TG derived from systemic plasma FFA and non-systemic fatty acid sources were not different between the exercise and control groups at the beginning of the study, and were not affected by the exercise program (Figure 3C). The molar ratio of VLDL-TG and VLDL-apoB-100 secretion rates did not differ between the exercise and control groups at baseline (13,136 ± 1,737 and 11,576 ± 774, respectively) and was not affected by the interventions (12,293 ± 1,913 and 10,990 ± 1,223 respectively, p= 0.932).
Figure 3.
VLDL-TG secretion rate (A) and VLDL-ApoB-100 secretion rate (B) before (white bars) and after (black bars) intervention in the Control and Exercise groups. Relative contribution of non-systemic fatty acids (gray speckled bars) and systemic fatty acids (white speckled bars) to secreted VLDL-TG (C). Data are means ± SEM.
Discussion
We investigated the effects of the current physical activity guidelines recommended by the Department of Health and Human Services, the American College of Sports Medicine, and the American Heart Association on IHTG content and VLDL kinetics in obese people with NAFLD. Our data demonstrate that this moderate intensity exercise program causes a small decrease in IHTG content, even when body weight and total body fat mass are maintained. Moreover, the change in IHTG content was directly associated with a change in plasma ALT concentration. In contrast, the exercise program did not improve the abnormalities in lipoprotein metabolism associated with NAFLD, specifically hepatic VLDL-TG or VLDL-apoB-100 secretion rates, or plasma VLDL-TG and VLDL-apoB-100 concentrations (1, 2). Therefore, these data demonstrate that the current physical activity recommendations have modest beneficial effects on IHTG content, and suggest that the marked improvement in steatosis and lipoprotein metabolism associated with lifestyle intervention (3-17) is largely due to weight loss rather than increased physical activity.
A series of studies have evaluated the effect of lifestyle-induced weight loss on IHTG content in subjects with NAFLD (3-17). Mean weight loss varied among studies, ranging from 1.5% to 14% of initial body weight, and the effect of weight loss on IHTG content also varied, ranging fromã20% to a ~60% relative reduction. The magnitude of weight loss-induced effects on IHTG content depends on the initial IHTG content; the reduction is greater in subjects who have more than those who have less IHTG for the same amount of weight loss (4, 20). In addition, even short term negative energy balance with minimal weight loss has a marked effect on IHTG content (4, 18, 20, 21). These data, in conjunction with results from the present study, demonstrate that lifestyle therapy that results in even minimal weight loss, without moderate physical activity, is an effective therapy for NAFLD.
Several studies have evaluated the effect of endurance exercise alone, without concomitant weight loss, on IHTG content (24, 25, 41, 42). Two studies found no change in IHTG content with endurance exercise without weight loss (41, 42). However, these studies may not reflect the true effect of endurance exercise in patients with NALFD for a number of reasons; one study was conducted in subjects with a normal amount of liver fat (42), and the other study used computed tomography, which does not provide a quantitative assessment of IHTG content, to determine changes in IHTG content (41). The two other studies (24, 25) were conducted in obese adults and adolescents who had NAFLD and found that vigorous intensity exercise training (70% V̇O2 peak for 90-135 min/week for 4-12 weeks) caused a small decline in total IHTG content, similar to the effect of the moderate intensity exercise program in our study. However, it is more likely that obese people will adhere to a moderate-intensity than a vigorous-intensity endurance exercise program (26-28).
We did not detect an effect of 16 weeks of moderate intensity exercise training on plasma FFA or VLDL-TG and VLDL-apoB 100 concentrations or kinetics. These data extend the observations from Johnson et al (24), who found that vigorous intensity exercise training without weight loss did not affect plasma TG concentrations in obese subjects with NAFLD, and are consistent with the conclusion from several systematic reviews, which found a minimal effect of moderate or vigorous-intensity exercise on plasma TG concentrations (43-46). Our findings suggest that the reduction in IHTG content is due to an increase in hepatic fatty acid oxidation, not an increase in TG export out of the liver or a decrease in FFA delivery to the liver. This notion is consistent with data from studies conducted in rodent models that found chronic exercise increases hepatic fatty acid oxidation and prevents steatosis (47) (48).
We found that the decrease in IHTG content after exercise training correlated with a decrease in plasma ALT concentrations. Although increased plasma ALT concentration is considered a marker of hepatocellular injury (49), changes in plasma ALT concentrations do not necessarily correlate with changes in liver histology (50, 51) and most people with NAFLD have plasma ALT concentrations that are within the laboratory normal range (52). In fact, 14 of our 18 subjects had baseline plasma ALT concentrations that were within our laboratory’s normal range; although, only 2 subjects had normal plasma ALT concentrations when the proposed cut-off values of 30 IU/l for men and 19 IU/l for women were used (53, 54). Nevertheless, data from a recent meta-analysis found that the hazard ratio for diabetes was 1.83 for each 1 unit increase in log transformed ALT concentration (55). Therefore, although the change in plasma ALT observed in our study is small, it is possible this change has beneficial medical implications.
Our study has several limitations. First, we studied a small number of subjects, which decreased our ability to detect differences between the control and exercise groups. Nonetheless, we were able to detect an effect of exercise on IHTG content. We should have also been able to detect a 15%-20% difference in VLDL-TG or VLDL-apoB-100 metabolism before and after training with the number of subjects enrolled in our study, based on the known reproducibility of our stable isotope-labeled tracer methods (35). In addition, the variability in baseline values for IHTG and VLDL kinetics were similar in the exercise and control groups. Therefore the ability to detect significant effects of exercise training was the same for the VLDL kinetics as it was for IHTG content. Furthermore, VLDL-TG or VLDL-apoB-100 kinetics after exercise training were nearly identical to those obtained before training, so adding hundreds of additional subjects would not have changed our conclusions, provided the effects in these additional subjects would be similar to those already studied. Second, although we speculate that the reduction in IHTG content was due to an increase in hepatic fatty acid oxidation, not an increase in TG export out of the liver or a decrease in FFA delivery to the liver, it is possible that we missed diurnal changes in FFA and VLDL-TG kinetics that contributed to the improvement in steatosis.
In conclusion, we found that following the physical activity guidelines recommended by the Department of Health and Human Services causes a small decrease in IHTG content and plasma ALT concentrations, but do not affect hepatic VLDL-TG or VLDL-apoB-100 secretion rates or plasma lipid concentrations, in obese persons with NAFLD. These results demonstrate that moderate intensity endurance exercise training alone has only modest therapeutic effects on hepatic TG and lipoprotein metabolism. Further studies are needed to evaluate the mechanism through which moderate-intensity exercise training decreases IHTG content.
Acknowledgments
Grant support: This research was supported by National Institutes of Health grants UL1 RR24992 KL2 RR024994 (Washington University Clinical and Translational Science Award), DK052574 (Digestive Disease Research Core Center), DK56341 (Nutrition Obesity Research Center), DK78738, DK37948, and HD57796.
Abbreviations
- ALT
alanine transaminase
- apoB-100
apolipoprotein B-100
- FFA
free fatty acids
- HDL
high density lipoprotein
- IHTG
intrahepatic triglyceride
- LDL
low density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- TG
triglyceride
- VLDL
very low density lipoprotein
Footnotes
Disclosure Statement: No conflicts of interest exist for any of the contributing authors.
This study is registered at ClinicalTrials.gov, number NCT00771108.
References
- 1.Adiels M, Taskinen MR, Packard C, Caslake M, Soro-Paavonen A, Westerbacka J, Vehkavaara S, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Haring HU, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]
- 4.Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- 5.Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, Rijcken TH, Korevaar JC, van Aalderen WM, Jansen PL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96:669–674. doi: 10.1136/adc.2010.199760. [DOI] [PubMed] [Google Scholar]
- 6.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 7.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oza N, Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Horie H, Ushirogawa M, et al. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol. 2009;44:1203–1208. doi: 10.1007/s00535-009-0115-x. [DOI] [PubMed] [Google Scholar]
- 10.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer S, Kantartzis K, Machann J, Venter C, Niess A, Schick F, Machicao F, et al. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest. 2007;37:535–543. doi: 10.1111/j.1365-2362.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- 12.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 14.Thamer C, Machann J, Stefan N, Haap M, Schafer S, Brenner S, Kantartzis K, et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007;15:531–538. doi: 10.1038/oby.2007.568. [DOI] [PubMed] [Google Scholar]
- 15.Thamer C, Machann J, Stefan N, Schafer SA, Machicao F, Staiger H, Laakso M, et al. Variations in PPARD determine the change in body composition during lifestyle intervention: a whole-body magnetic resonance study. J Clin Endocrinol Metab. 2008;93:1497–1500. doi: 10.1210/jc.2007-1209. [DOI] [PubMed] [Google Scholar]
- 16.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–5819. doi: 10.3748/wjg.v12.i36.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 18.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048–1052. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 21.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2008 Physical Activity Guidelines for Americans. Department of Health and Human Services; Hyattsville, MD: 2008. Retrieved April 12, 2011 from http://www.health.gov/paguidelines/guidelines/default.aspx. [Google Scholar]
- 23.ACSM, AHA Support Federal Physical Activity Guidelines Indianapolis. American College of Sports Medicine. 2011 Retrieved December 6, 2011 from http://www.acsm.org/about-acsm/media-room/acsm-in-the-news/2011/08/01/acsm-aha-support-federal-physical-activity-guidelines.
- 24.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijden G-J, Wang ZJ, Chu ZD, Sauer PJJ, Haymond MW, Rodriguez LM, Sunehag AL. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity. 2009;18:384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 26.Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE, Kanasky WF, Jr., et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452–458. [PubMed] [Google Scholar]
- 27.Ekkekakis P, Lind E, Vazou S. Affective Responses to Increasing Levels of Exercise Intensity in Normal-weight, Overweight, and Obese Middle-aged Women. Obesity. 2009;18:79–85. doi: 10.1038/oby.2009.204. [DOI] [PubMed] [Google Scholar]
- 28.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes. 2005;30:652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- 29.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 30.Genton L, Hans D, Kyle UG, Pichard C. Dual-Energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 31.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 32.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 33.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 35.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. Journal of Lipid Research. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 37.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 38.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women Produce Fewer but Triglyceride-Richer Very Low-Density Lipoproteins than Men. Journal of Clinical Endocrinology & Metabolism. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 39.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 40.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 2008;16:2281–2288. doi: 10.1038/oby.2008.358. [DOI] [PubMed] [Google Scholar]
- 42.Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–413. doi: 10.1007/s00125-006-0498-7. [DOI] [PubMed] [Google Scholar]
- 43.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2004;13:1148–1164. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley GA, Kelley KS, Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 2005;29:881–893. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 46.Tambalis K, Panagiotakos DB, Kavouras SA, Sidossis LS. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology. 2009;60:614–632. doi: 10.1177/0003319708324927. [DOI] [PubMed] [Google Scholar]
- 47.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 48.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300:G874–883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic Fatty Liver Disease: Predictors of Nonalcoholic Steatohepatitis and Liver Fibrosis in the Severely Obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 50.Wong VW-S, Wong GL-H, Choi PC-L, Chan AW-H, Li MK-P, Chan H-Y, Chim AM-L, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 51.Dixon J, Bhathal P, O’Brien P. Weight Loss and Non-alcoholic Fatty Liver Disease: Falls In Gamma-Glutamyl Transferase Concentrations are Associated with Histologic Improvement. Obesity Surgery. 2006;16:1278–1286. doi: 10.1381/096089206778663805. [DOI] [PubMed] [Google Scholar]
- 52.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 53.Prati D, Taioli E, Zanella A, Torre ED, Butelli S, Del Vecchio E, Vianello L, et al. Updated Definitions of Healthy Ranges for Serum Alanine Aminotransferase Levels. Annals of Internal Medicine. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 54.Omagari K, Takamura R, Matsutake S, Ichimura M, Kato S, Morikawa S, Nagaoka S, et al. Serum alanine aminotransferase concentration as a predictive factor for the development or regression of fatty liver. J Clin Biochem Nutr. 2011;49:200–206. doi: 10.3164/jcbn.11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine Aminotransferase, γ-Glutamyltransferase, and Incident Diabetes. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]