Abstract
Mice homozygous for targeted deletion of the interleukin 10 gene (Il-10) have been partially characterized as a model for human frailty. These mice have increased serum interleukin (IL)-6 in midlife, skeletal muscle weakness, and an altered skeletal muscle gene expression profile compared to age and sex-matched C57BL/6 (B6) control mice. In order to further characterize for use as a frailty model, we evaluated the evolution of inflammatory pathway activation, endocrine change, and mortality in these mice. Serum was collected in groups of age- and sex-matched B6.129P2-Il10tm1Cgn/J (IL-10tm/tm) mice and B6 control mice at age 12, 24, 48, 72, and 90 weeks. Cytokines including IL-6, interleukin 1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), chemokine (C-X-C motif) ligand 1 (KC), IL-12, and IL-10 were measured using electro-chemiluminescent multiplex immunoassay and insulin-like growth factor 1 (IGF-1) was measured using solid-phase enzyme-linked immunosorbent assay. A separate longitudinal cohort was monitored from age 35 weeks to approximately 100 weeks. Survival was evaluated by Kaplan–Meier survival estimates and detailed necropsy information was gathered in a subset of mice that died or were sacrificed. In IL-10tm/tm mice compared to B6 controls, serum IL-6, IL-1β, TNF-α, IFN-γ, KC levels were significantly elevated across the age groups, serum mean IGF-1 levels were higher in the 48-week-old groups, and overall mortality rate was significantly higher. The quadratic relationship between IGF-1 and age was significantly different between the two strains of mice. Serum IL-6 was positively associated with IGF-1 but the effect was significantly larger in IL-10tm/tm mice. These findings provide additional rationale for the use of the IL-10tm/tm mouse as a model for frailty and for low-grade inflammatory pathway activation.
Keywords: IGF-1, IL-6, IL-10, C57BL/6, Inflammation, Mortality, Frailty
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
The frailty syndrome in older adults likely results from cumulative decline in multiple physiological systems, leading to poor tolerance to stressors and increased vulnerability to adverse health outcomes (Fried et al. 2001; Walston et al. 2006). Multiple population-based studies have demonstrated that increased interleukin (IL)-6 and other markers of inflammation are important physiological correlates of frailty, disability, and mortality (Cesari et al. 2004; Cohen et al. 2003; Ferrucci et al. 1999; Fried et al. 2001; Harris et al. 1999; Leng et al. 2002; Walston et al. 2002). Because of the inherent challenges in human studies, genetically modified animal models can greatly facilitate the testing of biological hypotheses related to frailty, low-grade inflammation, health span, and late-life decline (Miller 2009; Tatar 2009). A mouse homozygous for a targeted deletion in the interleukin 10 (Il-10) gene (IL-10tm/tm) was recently proposed as a mouse model for frailty and low-grade inflammation (Walston et al. 2008). The lack of the anti-inflammatory cytokine IL-10 in this mouse leads to increased expression of nuclear factor-kappa B-induced inflammatory mediators (Hanada and Yoshimura 2002; Kuhn et al. 1993; Rennick et al. 1995). This mouse has previously been developed and characterized as a model for Crohn’s disease, which it develops if it is not kept in barrier conditions. Like frail older humans, older IL-10tm/tm mice have increased serum IL-6 and accelerated onset of muscle weakness with increasing age as compared to C57BL/6 (B6) control mice (Walston et al. 2008). However, more complete inflammatory profiles, endocrine changes, and the mortality profile of this mouse compared to control strain remain unknown. In order to fully characterize this mouse model of frailty, we developed a cross-sectional comparison and longitudinal survival study to more comprehensively study its inflammatory mediators, endocrine change, and mortality.
Materials and methods
Animals
IL-10 deficient B6.129P2-Il10tm1Cgn/J (IL-10tm/tm) mice and age- and sex-matched B6 background mice were used for this study. IL-10tm/tm mice were homozygous for the Il10tm1Cgn targeted mutation and were fully backcrossed on B6 background (Kuhn et al. 1993). All mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) or the National Institute on Aging (Bethesda, MD, USA) and were housed in Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facilities and were maintained under specific pathogen-free (SPF) barrier conditions to prevent pathogen contact. Prior to introduction to the barrier, mice were quarantined for at least 1 month and tested to confirm SPF status. The barrier was sentinel tested quarterly for excluded agents including ecto- and endoparasites, mouse hepatitis virus, epizootic diarrhea of infant mice, Theiler's murine encephalomyelitis virus, mouse parvovirus I, mouse minute virus, mouse adenovirus type 2, ectromelia virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus, Sendai virus, and mouse cytomegalovirus and Helicobacter spp. Mice were housed in 75 sq in, autoclave sterilized, high temperature polycarbonate shoebox cages in ventilated racks (Allentown Inc., Allentown, NJ, USA) containing autoclaved corncob bedding (Harlan Teklad, Indianapolis, IN, USA), autoclaved mouse chow 2018SX (Harlan Teklad), and reverse osmosis-filtered hyperchlorinated water dispensed through an in-cage automatic watering system (Edstrom Industries, Waterford, WI, USA). Rooms were maintained at 72 ± 2°F on a 14-hr light/10-h dark cycle with automated monitoring by Siemens Building Technologies, Inc. (Zurich, Switzerland). Cages were changed every 2 weeks in laminar airflow change stations (The Baker Co., Sanford, ME, USA) with surface cleaning and disinfection with MB-10 disinfectant (Quip Laboratories, Inc., Wilmington, DE, USA). All caging was sanitized by automatic cage washing systems and autoclaved prior to use. All mice received a unique ear marking to ensure correct longitudinal data collection.
Study design
A cross-sectional study design was utilized to compare serum interferon-gamma (IFN-γ), interleukin 1 beta (IL-1β), IL-6, IL-10, interleukin 12 (IL-12p70), chemokine (C-X-C motif) ligand 1 (KC), tumor necrosis factor-alpha (TNF-α), and insulin-like growth factor 1 (IGF-1) levels between IL-10tm/tm and B6 mice. For the cross-sectional study, 20 female IL-10tm/tm and 20 female B6 mice were sacrificed by CO2 at age 12, 24, 48, and 72 weeks (80 mice per strain). Blood was aspirated from the heart and serum was separated by centrifugation and stored at −80°C.
A longitudinal (survival) study was used to assess the differences in mortality rates between the two mouse strains. In this study, retired breeder IL-10tm/tm (male, n = 25; female, n = 25) and B6 mice (male, n = 25; female, n = 25) entered the study at approximately 35 weeks of age and were observed until death or a moribund condition occurred. Complete necropsy and histopathology evaluations were performed on mice that died or became moribund before scheduled sacrifice and on a proportion of sacrificed mice whose tissues were not utilized for other testing. Mice that survived 65 weeks of observation (90 week old) were sacrificed by CO2, blood was collected by cardiac puncture and serum was stored at −80°C for subsequent IGF-1 and cytokine measurements. Complete necropsy and histopathology evaluations of tissues were completed on a subset of these mice. All mice in the survival study had blood samples taken from facial vein at age 48 weeks for IGF-1 and cytokine measurements as per the cross-sectional study.
Serum cytokine and IGF-1 measurements
Serum cytokines were measured in 25 μL singlicates from stored serum samples by 96-well plate-based multiplex sandwich immunoassays (Meso Scale Discovery (MSD), Gaithersburg, MD, USA). All MSD cytokine multiplex immunoassays were performed by the Johns Hopkins Bayview Institute for Clinical and Translational Research Core Chemistry Laboratory per manufacture’s protocol. Each well of the MSD multi-array platform was precoated with antibodies to mouse IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, KC, and TNF-α in a patterned array. Capture of serum cytokine was identified by detection antibodies coupled to electro-chemiluminescent labels read by a charge-coupled device (MSD Sector Imager). Sample cytokine concentrations were determined using curve fit models (sigmoidal dose–response or four-parameter logistic). The lower limit of detection was calculated as the signal that was 2.5 standard deviations over the zero calibrator and these values were: IFN-γ (0.38 pg/ml), IL-1β (0.75 pg/ml), IL-6 (4.5 pg/ml), IL-10 (11 pg/ml), IL-12p70 (35 pg/ml), KC (3.3 pg/ml), and TNF-α (0.85 pg/ml). Serum IGF-1 level was quantified from stored serum samples of the cross-sectional and survival studies using a Quantikine mouse solid-phase enzyme-linked immunosorbent assay (ELISA) IGF-1 kit with the mean minimum detectable dose of 3.5 pg/ml, per manufacturer's protocol (R&D Systems, Inc., Minneapolis, MN, USA). Duplicate measurements were made for each sample and the average IGF-1 value was used for statistical analysis.
Statistics
Group means of cytokines and IGF-1 levels were calculated for 12, 24, 48, and 72 week-old IL-10tm/tm and B6 mice from the cross-sectional study and for 48- and 90-week-old mice from the survival study. For group comparisons, a two-tailed t test was used to compare IGF-1 levels and the Wilcoxon rank-sum test was used to compare cytokine levels due to skewed distribution. Furthermore, an overall p value by mouse strain for each cytokine was calculated by using log-transformed cytokines in a linear regression model that was adjusted by age. Multiple testing correction was not performed because of the small data set and statistical tests were only used when strong biological basis for differences in results were expected.
Regression modeling is commonly used in biological sciences to describe relationships between multiple variables and could effectively demonstrate the relationship between a dependent variable (e.g., IGF-1) and one or more independent variable (e.g., age, genotype). Therefore, the trajectories of IGF-1 change with age in female IL-10tm/tm and B6 mice from the cross-sectional study (12, 24, 48, and 72 week old) and the survival study (90 week old) were fitted by linear regression analyses with both linear and quadratic time effects (model A) and the difference between the two mouse strains was tested based on corresponding coefficient estimates and F tests. Independent variables in model A include age (weeks), age2, genotype (B6 or IL-10tm/tm), age × genotype, and age2 × genotype. In analyzing relationship among multiple variables, an interaction, or the simultaneous influence of two independent variables (e.g., IL-6, genotype) on the dependent variable (e.g., IGF-1) may not be additive. This difference can be assessed by adding an interaction term (e.g., IL-6 × genotype) to a regression model. Therefore, the effect of serum IL-6 on IGF-1 level was tested by introducing IL-6 measurement and interaction terms (IL-6 × independent variables in model A) to quadratic regression models (model B). Survival functions of IL-10tm/tm and B6 mice by genotype or by sex and genotype were derived from Kaplan–Meier estimates. Incident mortality rate ratio was expressed as the hazard ratio of death of IL-10tm/tm to B6 mice. Statistical differences between Kaplan–Meier curves were tested by the log-rank test.
Results
In each age and strain group utilized for the cross-sectional cytokine measurements, one to seven of the total number of samples used for cytokine measurement failed on initial and repeated measurements, and therefore was not included in the statistical analyses. In addition, some mice died over the period of observation resulting in the sample sizes of between eight and 19 for each measured group (Table 1). Mean serum levels of IFN-γ, IL-1β, IL-6, KC, and TNF-α were significantly higher in the IL-10tm/tm compared to B6 mice at most of the five discrete ages (p′) measured and significantly higher across the entire life span (P″) for all cytokines (Table 1). Because IL-10 was not detected in the IL-10tm/tm mice, and because IL12p70 was detected in only two samples using this multiplex platform, no comparisons were made for these cytokines between the two strains. Similar cytokine measurements were detected in 48-week-old female B6 and IL-10tm/tm mice from the longitudinal study (data not shown).
Table 1.
Mouse cytokines results by age and genotype
| Genotype (n) | Age (week) | IL-1B | p′ | IL-6 | p′ | TNF-α | p′ | IFN-γ | p′ | KC | p′ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| B6 (16) | 12 | 1.20 (0.73) | 0.403 | 6.25 (1.36) | <0.001 | ND | − | 1.81 (0.50) | <0.001 | 60.94 (22.44) | 0.010 |
| IL-10tm/tm (19) | 12 | 1.37 (0.64) | 12.7 (7.34) | 4.21 (2.67) | 6.75 (4.48) | 108.72 (84.05) | |||||
| B6 (18) | 24 | 1.18 (0.55) | 0.012 | 7.25 (2.00) | 0.289 | 2.03 (1.76) | 0.012 | 2.24 (0.93) | <0.001 | 67.87 (15.32) | 0.056 |
| IL-10tm/tm (13) | 24 | 2.32 (1.32) | 19.32 (21.98) | 4.10 (2.92) | 4.09 (1.27) | 118.94 (82.45) | |||||
| B6 (16) | 48 | 1.24 (1.44) | 0.002 | 8.48 (6.12) | 0.004 | ND | − | 1.80 (0.71) | <0.001 | 65.83 (25.90) | 0.006 |
| IL-10tm/tm (14) | 48 | 2.35 (1.20) | 15.68 (11.84) | 3.82 (2.27) | 6.24 (3.78) | 94.22 (32.14) | |||||
| B6 (16) | 72 | 1.99 (1.61) | <0.001 | 8.78 (3.09) | 0.168 | 1.46 (1.76) | 0.018 | 2.18 (0.68) | <0.001 | 75.82 (15.79) | 0.94 |
| IL-10tm/tm (17) | 72 | 3.73 (2.22) | 20.63 (29.43) | 3.22 (2.32) | 3.41 (1.27) | 100.8 (46.51) | |||||
| B6 (15) | 90 | 1.62 (1.39) | 0.003 | 13.77 (24.36) | <0.001 | ND | − | 1.52 (0.43) | <0.001 | 80.19 (53.07) | 0.003 |
| IL-10tm/tm (8) | 90 | 2.63 (0.85) | 30.36 (28.34) | 3.65 (1.90) | 18.02 (20.02) | 149.27 (58.27) | |||||
| Overall P″ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
All serum cytokine levels are measured in picograms per milliliter
P′ = P value by strains in each age group, rank sum test
P″ = P value by strain adjusted by age, linear regression based on log transformed cytokines.
ND not detectable
IGF-1 was measured in female mice in the cross-sectional study at four discrete ages (12, 24, 48, and 72 weeks), and in the longitudinal (survival) study at 48 and 90 weeks in both males and females. In the five discrete ages involving only females, 48-week-old IL-10tm/tm mice (n = 20) had significantly higher levels of mean serum IGF-1 than age-matched B6 mice (n = 20) (391 ± 63 vs. 327 ± 39 ng/ml, p value of <0.001; Table 2). No significant differences were observed in the group mean IGF-1 levels between IL-10tm/tm and B6 mice at 12, 24, 72, and 90 weeks of age (Table 2). As in the cross-sectional study, serum levels of IGF-1 and IL-6 at age 48 weeks in both the male and female groups in the longitudinal study group were also significantly higher in the IL-10tm/tm compared to B6 control mice (Tables 3 and 4).
Table 2.
Difference in mean serum IGF-1 levels between female B6 and IL-10tm/tm mice by age
| Age (week) | Mean IGF-1 ± SD (ng/ml) | ||
|---|---|---|---|
| B6 (n) | IL-10tm/tm (n) | p Value | |
| 12 | 237 ± 56 (20) | 237 ± 43 (20) | 0.998 |
| 24 | 304 ± 55 (20) | 281 ± 40 (19) | 0.142 |
| 48 | 327 ± 39 (20) | 391 ± 63 (20) | <0.001* |
| 72 | 272 ± 37 (20) | 277 ± 37 (20) | 0.687 |
| 90 | 283 ± 43 (19) | 278 ± 44 (9) | 0.782 |
SD standard deviation
*p = 0.05 significance level
Table 3.
Difference in mean serum IGF-1 levels between 48-week-old B6 and IL-10tm/tm mice by sex and genotype in the survival study
| Comparison p value | |||||
|---|---|---|---|---|---|
| Genotype and sex (n) | Mean IGF-1 ± SD (ng/ml) | Male B6 | Male IL-10tm/tm | Female B6 | Female IL-10tm/tm |
| Male B6 (25) | 243 ± 40 | – | |||
| Male IL-10tm/tm (24) | 282 ± 46 | 0.002* | – | ||
| Female B6 (25) | 259 ± 45 | 0.168 | 0.089 | – | |
| Female IL-10tm/tm (25) | 285 ± 35 | <0.001* | 0.782 | 0.028* | – |
SD standard deviation
*p = 0.05 significance level
Table 4.
Difference in mean serum IL-6 Levels between 48-week-old B6 and IL-10tm/tm mice by sex and genotype in the survival study
| Comparison p value | |||||
|---|---|---|---|---|---|
| Genotype and sex (n) | Mean IL-6 ± SD (pg/ml) | Male B6 | Male IL-10tm/tm | Female B6 | Female IL-10tm/tm |
| Male B6 (21) | 7.23 ± 2.88 | – | |||
| Male IL-10tm/tm (22) | 15.24 ± 13.29 | <0.001* | – | ||
| Female B6 (18) | 9.41 ± 5.01 | 0.027* | 0.071 | – | |
| Female IL-10tm/tm (22) | 14.00 ± 11.62 | < 0.001* | 0.760 | 0.026* | – |
SD standard deviation
*p = 0.05 significance level
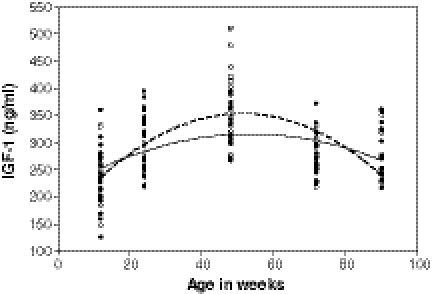
A trajectory of change in serum IGF-1 level with age was derived from pooled data in the 12, 24, 48, 72, and 90-week-old female mice. Both the poor goodness-of-fit of the linear regression model (R2 = 0.03) and a scatter plot (Fig. 1) suggested a quadratic relationship between IGF-1 level and age. A quadratic regression model was therefore fitted between serum IGF-1 and age for both strains of mice (Table 5, model A). The IL-10tm/tm mice had an estimated peak IGF-1 level of 353 ng/ml at 51.6 weeks of age, which was higher than the estimated peak IGF-1 level of 316 ng/ml at 53.5 weeks of age in B6 mice as calculated by model A. The difference in the rate of change of IGF-1 with age between IL-10tm/tm and B6 mice was statistically significant (p value of 0.004; Table 5, model A).
Fig. 1.
Scatter plot of serum IGF-1 level by age in weeks for female IL-10tm/tm (open circle) and B6 mice (enclosed circle). Fitted lines represent the quadratic relationship between IGF-1 and age in female IL-10tm/tm (dash line) and B6 mice (solid line). The quadratic distribution of IGF-1 with age is significantly different between IL-10tm/tm and B6 mice (p value of 0.047)
Table 5.
Association between IGF-1 and age, controlling for genotype in model A and for IL-6 and genotype in model B by quadratic regression
| Variable | Model A | Model B | ||||
|---|---|---|---|---|---|---|
| ß coefficient | SE | p Value | ß coefficient | SE | p Value | |
| Age | 3.902 | 0.927 | <0.0001* | 4.734 | 1.385 | 0.001* |
| Age2 | −0.037 | 0.0090 | <0.0001* | −0.037 | 0.013 | 0.007* |
| Genotype | −59.01 | 26.85 | 0.029* | – | – | – |
| Age × genotype | 3.920 | 1.360 | 0.004* | 4.215 | 1.227 | 0.001* |
| Age2 × genotype | −0.040 | 0.014 | 0.004* | −0.052 | 0.016 | 0.001* |
| IL-6 | – | – | – | 10.599 | 5.209 | 0.044* |
| IL-6 × genotype | – | – | – | −11.115 | 4.484 | 0.014* |
| Age × IL-6 | – | – | – | −0.214 | 0.189 | 0.259 |
| Age2 × IL-6 | – | – | – | 0.001 | 0.002 | 0.527 |
| Age × genotype × IL-6 | – | – | – | 0.191 | 0.164 | 0.246 |
| Age2 × genotype × IL-6 | — | — | — | −0.0007 | 0.001 | 0.627 |
SE standard error
*p = 0.05 significance level
The effect of serum IL-6 on IGF-1 levels in female IL-10tm/tm and B6 mice was determined by adding IL-6 and IL-6 × genotype interaction term to the regression model in model A (Table 5, model B). Higher levels of serum IL-6 was significantly associated with higher levels of IGF-1 in B6 mice (p value of 0.044) at baseline. In addition, the extent of IL-6 effect on IGF-1 was significantly greater in female IL-10tm/tm than B6 mice (p value of 0.014). Other factors including interaction terms between IL-6 and age, and IL-6, age and genotype were not significant in the model.
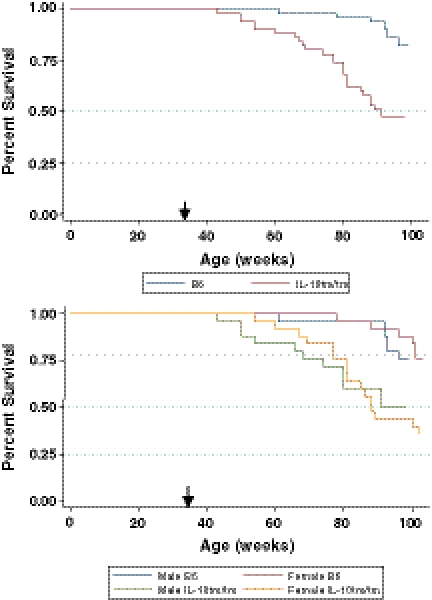
The first IL-10tm/tm mouse died at 43 weeks of age compared to 61 weeks in B6 mice. Twenty-seven of 50 IL-10tm/tm mice died by the conclusion of the survival study compared to 12 of 50 in the B6 group (Fig. 2, top panel). This resulted in an incident mortality rate ratio of 2.64 (95% CI: 1.29, 5.71) comparing IL-10tm/tm to B6 mice. The Kaplan–Meier survival estimates demonstrated better overall survival in B6 mice by log-rank test (p value of <0.001). Subgroup analysis of survival by sex showed a significant incident mortality rate ratio of 3.08 (95% CI: 1.13, 9.52) comparing female IL-10tm/tm mice to female B6 mice. The incident mortality rate ratio of male IL-10tm/tm mice to male B6 mice failed to reach statistical significance (2.20; 95% CI: 0.75, 7.25). The Kaplan–Meier survival curves demonstrated better overall survival in B6 mice by log-rank test regardless of sex (p value of <0.01; Fig. 2, bottom panel).
Fig. 2.
Kaplan–Meier survival curves by strain (top panel) and by strain and sex (bottom panel). Black arrows indicate the age at which mice entered the survival study. Better overall survival is observed in B6 control mice (top panel, blue line) than IL-10tm/tm mice (top panel, red line) by log-rank test (p value of <0.001). Subgroup analyses of survival by sex show better overall survival in female B6 mice (bottom panel, red line) than female IL-10tm/tm mice (bottom panel, orange line), and in male B6 mice (bottom panel, blue line) than male IL-10tm/tm mice (bottom panel, green line) by log-rank test (p value of <0.01)
Complete necropsy and histopathology evaluations were performed in 15 IL-10tm/tm mice that died in the survival study. Respiratory disease (upper respiratory infection with or without bronchopneumonia) was considered to be a significant cause or contributor to illness or death in 13 of these 15 IL-10tm/tm mice. Upper respiratory infection (URI) in 13 IL-10tm/tm mice had mild to marked chronic and suppurative (necrotizing) inflammation and/or exudates in at least two sites (nose, middle ear, larynx, or trachea). Of these 13 IL-10tm/tm mice with URI, 12 also had bronchopneumonia. Hematopoietic neoplasia (lymphoma or histiocytic sarcoma) was considered to be a significant cause or contributor to illness or death in seven of 15 IL-10tm/tm mice. Five IL-10tm/tm mice had both respiratory disease and neoplasia that were considered to be a significant cause or contributor to illness or death.
Complete necropsy and histopathology evaluations were performed in five B6 mice. Four of five B6 mice had neoplastic disease, including two hematopoietic neoplasms (one lymphoma, one histiocytic sarcoma), one sarcoma in the skin and subcutis, one thyroid adenoma and carcinoma, one pulmonary adenoma, and one mammary adenocarcinoma. The only respiratory tract-related lesion in the B6 mice was a mild histiocytic otitis in one mouse that was readily distinguished from the chronic, necrotizing, and suppurative lesions in the IL-10tm/tm mice. This mouse also had perivascular neoplastic infiltrates in the lung and no other respiratory tract lesions. Because otitis media is not uncommon in unmanipulated mice of various strains, this was interpreted as an incidental finding.
Discussion
Frailty in older adults is strongly associated with elevated IL-6, adverse health outcomes, and increased mortality (Cesari et al. 2004; Ferrucci et al. 1999; Fried et al. 2001; Leng et al. 2002; Walston et al. 2002). Although the underlying mechanism for frailty is likely multisystemic, the activation of inflammatory pathways is likely to play a central role in its development and its related vulnerability to adverse health outcomes (Walston et al. 2006). In this study, we confirmed and extended initial findings by demonstrating that IL-10tm/tm mice have significantly increased levels of several inflammatory cytokines, higher levels of IGF-1 at midlife, and found that they had higher mortality rates when compared to age and sex-matched B6 control mice.
The broad increase in inflammatory cytokines was as expected as IL-10 is an important negative regulator of cell-mediated immunity, specifically Th1 function (Moore, et al. 1993). It is secreted by a variety of cells including T helper cells, monocytes, macrophages, B cells, and keratinocytes (Moore et al. 1993). In vitro studies demonstrate that IL-10 limits production of cytokines such as IL-1, IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor, and IL-12, the generation of reactive oxygen intermediates, and the expression of surface MHC class II and co-stimulatory molecules (Tripp et al. 1993; Fiorentino et al. 1991; D’Andrea et al. 1993). Furthermore, IL-10 directly inhibits IFN-γ production and activity in the innate and adaptive immune system via its action on NK and T cells (Chomarat et al. 1993; Moore et al. 1993). Therefore, IL-10 exerts its inhibitory effects on multiple pro-inflammatory cytokines at various steps of the inflammatory cascade. In the absence of IL-10, these pro-inflammatory cytokines are unregulated and therefore induce a low-grade inflammatory state observed in the IL-10tm/tm frail mouse model. Of all the cytokines measured, IFN-γ was among the most elevated across all ages in IL-10tm/tm mice. IFN-γ plays a crucial role in activating macrophages and resulting in antigen presentation, microbial killing, and cytokine production and yields a pro-inflammatory effect by mediating and potentiating TNF-α and IL-1β synthesis (Chomarat et al. 1993). Therefore, elevated IFN-γ may play an important role in the development of low-grade inflammation in the IL-10tm/tm frail mouse. Although IL-10 and IFN-γ levels have not been extensively studied in human population studies on aging and frailty, in part because of limitations in measuring serum samples that have been stored for long periods of time, investigators have reported significant elevations in macrophages and other inflammatory cells in frail compared to non-frail humans (Leng et al. 2009; Yao et al. 2011). The chronic inflammatory pathway activation observed in frail, older adults is likely multifactorial and stems from chronic disease states, altered body composition, and increased production of free radicals rather than a deficit in IL-10 (Walston et al. 2006). Aging mice of any strain likely have similar age-related inflammatory stimulation, but the IL-10tm/tm frail mouse has a genetic disposition to increased inflammatory reactivity to any stimulation. Whether or not frail humans have a similar disposition remains to be determined. Despite this etiological difference in the vulnerability to frailty between this mouse model and humans, it is clear that the IL-10tm/tm frail mouse develops and maintains a broad pro-inflammatory profile across age groups that will likely be useful for the study of inflammation-related changes in frailty and aging.
Similar to observations of accelerated mortality in older humans with low-grade inflammation and frailty, the IL-10tm/tm mice have a higher mortality rate than the age- and sex-matched control mice. Our necropsy data indicates that respiratory infections were more common contributory causes of death in the IL-10tm/tm mice; however, cancer-related death were similar in both strains of mice. Respiratory diseases including both upper respiratory infection and bronchopneumonia with chronic and necrotizing lesions were common in IL-10tm/tm mice but non-existent in B6 mice. Although the cause of this high incidence of respiratory diseases in IL-10tm/tm mice is unknown, it is probably associated with these mice’s underlying low-grade inflammatory state and its altered adaptive immunity (Salminen and Kaarniranta 2010). Many prior studies have demonstrated that these mice are more susceptible to infectious organisms. Indeed, the IL-10tm/tm mice require barrier conditions and sterilized chow in order to prevent the development of a Crohn’s disease like gut syndrome (Kuhn et al. 1993). Interestingly, the mice do not appear to die from these conditions at younger ages as observed in our longitudinal and cross-sectional study. Hence, the differential mortality rate accelerates after 1 year of age, providing further support for the use of the IL-10tm/tm mouse as a model to study the biology of frailty and low-grade inflammation. Further study of immune system changes in humans and in mouse models is needed in order to identify mechanisms of late-life vulnerability to infections, including potential effects of IL-10 suppression, gene variation, the impact of low-grade inflammation on immune system remodulation, and the interactive decline in multiple biological systems (Varadhan et al. 2008).
In addition to the cytokine and mortality findings, we identified that the IL-10tm/tm mouse has higher levels of IGF-1 at midlife than matched controls and that an interaction between IL-6 and IGF-1 may be operant in this mouse model. This provides another potential clue to the premature mortality observed in these mice. Prior studies have cited high rates of IGF-1 in midlife as strong correlates of earlier mortality (Yuan et al. 2009). IGF-1 is a circulating hormone of the somatotropic axis (growth hormone (GH)-IGF-1) that increases protein synthesis, accelerates cell cycle, and blocks initiation of apoptosis (Mourkioti and Rosenthal 2005). Because the insulin/IGF-1 signaling (IIS) pathway has been linked to lifespan in numerous model organisms including Caenorhabditis elegans, Drosophila melanogaster, and mice (Bartke 2008; Clancy et al. 2001; Guarente and Kenyon 2000; Tatar et al. 2001), it has long been of interest to gerontologists. For example, lower level of IGF-1 in mice is associated with an 18–60% increase in lifespan (Brown-Borg et al. 1996; Chiba et al. 2007). Caloric restriction, the only intervention known to increase mammalian lifespan, also reduces insulin/IGF-1 expression (Masternak et al. 2005). In contrast, GH transgenic mice have elevated IGF-1 signaling and a significantly reduced lifespan of ∼50% compared to control strains (Wolf et al. 1993). This inverse correlation between median lifespan and plasma IGF-1 at 6, 12, and 18 months of age has been validated in 31 inbred mouse strains in the Aging Phenome Project (Yuan et al. 2009). In the current study, the IL-10tm/tm mice had higher serum IGF-1 levels by regression analyses and most notably at midlife; however, these mice had shorter lifespan than B6 controls. Therefore, this inverse relationship between lifespan and serum IGF-1 observed in many other longevity studies in mice persists in this mouse model of low-grade inflammation.
The presence of a positive IL-6 and IGF-1 interaction suggests a potential role for inflammation–neuroendocrine interaction in the IL-10tm/tm mouse. Mediators of the immune system are known to modulate the GH/IGF-1 axis. For example, TNF-α and IL-6 induce IGF-1 resistance in neurons and muscle cells (Kelley 2004; Kelley et al. 2007). While the molecular mechanism mediating inflammation–neuroendocrine interaction is not known, the activation of the IL-6 modulated JAK-STAT-SOCS pathway may be involved (Bodell et al. 2009; Haddad et al. 2005). Whether IL-6 and IGF-1 interaction and IGF-1 midlife elevation have a direct effect on frailty and mortality remains to be determined. To our knowledge, no longitudinal human population study has simultaneously collected data on mortality, IGF-1 and IL-6 making comparisons with our mouse data difficult. Although higher serum IGF-1 and IL-6 at midlife correlate with accelerated mortality in the IL-10tm/tm frail mouse, lower serum IGF-1 and higher IL-6 have been observed in frail compared to non-frail older women with ages ranging from 70 to 90 (Cappola et al. 2003; Leng et al. 2004). There were suggestions in our data that this inverse correlation in late life may also hold true in this mouse model as the downward trajectory of serum IGF-1 in late life was accelerated in IL-10tm/tm mice compared to B6 controls. However, given that many of the older, frail mice died before the 90-week mark, small sample size likely impacted our ability to determine any statistically significant differences in IGF-1 in late life between the two mouse strains. Further studies in older mice would be necessary in order to determine if IL-10tm/tm mice, like humans, develop significantly lower IGF-1 levels compared to B6 mice late in life.
There are several cautions regarding the data collected in this study. First, the IL-10tm/tm mice are genetically altered to not produce IL-10. Hence, the genetic manipulation and the absence of IL-10 may directly contribute to changes in longevity and other biological parameters measured in this study rather than indirectly through broad inflammatory pathway activation. However, this particular caution in the use of genetically modified animals is also inherent for most if not all of the genetically altered mouse models used for longevity and other aging related studies (Miller 2009; Tatar 2009). Another potential limitation comes from the use of a multiplex platform for the measurement of cytokines (Leng et al. 2008). The choice to use a multiplex platform was necessary because of the prohibitive cost and large sample volume necessary to perform conventional ELISA measurements on individual cytokines. Multiple samples failed for unclear reasons, which may be related to sample collection and not the assay itself. IL-12p70 was for the most part undetectable using this assay, which also may or may not be related to the assay itself. The variability between age groups in some cytokine levels may also be related to this, or to the drop out of sicker mice in the oldest group. Despite this limitation, the assay performed very well on the vast majority of samples collected and enabled us to demonstrate clear and reliable differences between the groups of mice. Next, the IGF-1 data must be interpreted with caution, given that IGF binding proteins (IGFBPs), IGFBP proteases, and other markers of IGF-1 bioavailability were not measured in this study. In addition, the IGF-1 measurements at 48 weeks of age were consistently 20–25% lower in the survival study compared to the cross-sectional study, likely due to changes in manufacture supply of ELISA kits. Despite this inconsistency, the relative difference in IGF-1 between IL-10tm/tm and B6 mice at 48 weeks of age were similar between the cross-sectional and the survival study populations. As for mortality, although the Kaplan–Meier estimate provided substantial amounts of data on survival, we were not able to derive the median lifespan of the IL-10tm/tm and B6 mice because not all animals were followed until death. Furthermore, we lacked sufficient longitudinal IGF-1 and IL-6 data to perform COX proportional hazard model to assess for the impact of IGF-1 and IL-6 on survival. Lastly, there may be a possible selection bias because mice entered the survival study at 35 weeks of age rather than at the time of birth. This bias was not expected to be significant since both strains of mice housed in SPF condition had low mortality rates before age 48 weeks.
Despite these limitations, there are many potential implications to our findings. To our knowledge, the IL-10tm/tm mouse is one of the first mouse models characterized for low-grade inflammation that survives well into adulthood and that demonstrates a positive in vivo association between serum levels of IGF-1 and IL-6. This could have important implications on the study of late-life decline and decreased health span related to low-grade inflammation. Such a model could play important roles in biological discovery related to the impact of inflammation on a wide variety of tissues and disease states, and on the role of low-grade elevation of inflammatory cytokines and the vulnerability to infections. In addition, the IGF-1 observation in the IL-10tm/tm frail mouse supports the notion of communication between IL-6 and the IIS signaling pathway and potentially provides an important tool to further study interactions between low-grade inflammation, the somatotropic axis of aging, and the development of frailty. Taken together with previous findings of accelerated muscle weakness and skeletal muscle gene expression differences identified in this mouse (Walston et al. 2008), the present findings provide support for the further characterization, development, and utilization of the IL-10tm/tm mouse strain for the study of frailty and for the study of the biological effects of low-grade inflammation on aging, multisystemic decline, and late-life vulnerability.
Acknowledgments
This research was supported by the National Institute on Aging, Claude D. Pepper Older Americans Independence Centers, grant P30 AG021334, National Institute on Aging, grant R21-AG025143, and Beeson AFAR award.
References
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7(3):285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol. 2009;106(2):443–453. doi: 10.1152/japplphysiol.90831.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.M242. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaza H, Shimokawa I. Role of insulin and growth hormone/insulin-like growth factor-I signaling in lifespan extension: rodent longevity models for studying aging and calorie restriction. Curr Genomics. 2007;8(7):423–428. doi: 10.2174/138920207783591726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. doi: 10.1016/S0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- Chomarat P, Rissoan MC, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. P J Exp Med. 1993;177(2):523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815. [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408(6809):255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13(4–5):413–421. doi: 10.1016/S1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Kelley KW. From hormones to immunity: the physiology of immunology. Brain Behav Immun. 2004;18(2):95–113. doi: 10.1016/j.bbi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21(4):384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the Women's Health and Aging Studies I. Exp Gerontol. 2009;44:511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40(8–9):679–684. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 2009;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Moore KW, O’Garra A, Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26(10):535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76(3 Pt 2):S174–S178. doi: 10.1016/S0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22(4):573–577. doi: 10.1016/j.cellsig.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tatar M. Can we develop genetically tractable models to assess healthspan (rather than life span) in animal models? J Gerontol A Biol Sci Med Sci. 2009;64:161–163. doi: 10.1093/gerona/gln067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129:666–670. doi: 10.1016/j.mad.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63(4):391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68(1–3):71–87. doi: 10.1016/0047-6374(93)90141-D. [DOI] [PubMed] [Google Scholar]
- Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27:79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Evsikova CM, Xing S, Marion MA, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8(3):277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]