Abstract
Organ-specific changes of iron- and redox-related proteins occur with age in the rat. Ferritin, the major iron storage and detoxifying protein, as well as the proteins of the methionine-centered redox cycle (MCRC) were examined in old and young animals, and showed organ-dependent changes. In spleens and livers of aged rats, ferritin (protein) levels were greater than in young ones, and their iron saturation increased, rendering higher ferritin-bound iron (FtBI). Iron saturation of the ferritin molecule in the tongues and sternohyoids of old rats was lower but ferritin level was higher than in young rats, resulting in increased FtBI with age. Ferritin level in the esophagus of older rats was lower than in young rats but its molecular iron content higher thus the total FtBI remained the same. In the larynx, both ferritin and its iron content were the same in young and old animals. MCRC proteins were measured in livers and spleens only. With aging, methionine sulfoxide reductase A and B (MsrA and MsrB) levels in livers and spleens decreased. Thioredoxin1 (Trx) and Trx-reductase1 were elevated in old spleens, but reduced in livers. Aged spleens showed reduced Msr isozyme activity; but in the liver, its activity increased. mRNA changes with age were monitored and found to be organ specific. These organ-specific changes could reflect the different challenges and the selective pathways of each organ and its resultant capacity to cope with aging.
Keywords: Aging, Organ specific, Iron, Redox state, Ferritin, Methionine-centered redox cycle
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
Changes characteristic for aging closely correlate with oxidation of cellular components and the accumulation of oxidized metabolic products (Friguet 2006; Harman 1956; Kregel and Zhang 2007; Petropoulos and Friguet 2006; Squier 2001). However, a direct causal link between reactive oxygen-derived species (ROS) and aging has yet to be established. ROS have been proposed to function as intracellular signaling molecules as well as activating redox-sensitive transcription factors and regulate gene expression (Sen and Packer 2000; Tappia et al. 2006). Thus, in aging, ROS bring about the accumulation of oxidized products, which are deleterious to the cell, and initiate cellular protective mechanisms, which facilitate an adaptive process.
Labile and redox-active iron can propagate ROS-induced oxidative stress (Ercal et al. 2001). In the liver and spleen, iron concentration is higher and its turnover is faster than in other organs. Therefore, alleviation of the harmful effects of iron in these organs is especially important. In general, 95–98% of non-heme iron is bound to ferritin (Ft), the major iron storage and detoxifying protein; as a result, the intracellular labile iron pool (LIP) is kept below micromolar levels (Liu and Theil 2005). LIP levels determine the extent of synthesis of new Ft subunits or the degradation of existing Ft so that excess iron is scavenged and neutralized within Ft, or iron is released from it to meet current needs (Konijn et al. 1999). This process is regulated via cytoplasmic iron regulatory proteins (IRPs) and iron-responsive elements located on the 5′ end of the Ft subunits mRNA's (Thomson et al. 1999). An increase in Ft (protein and/or mRNA) often indicates events that have resulted in elevated cellular levels of redox-active iron (Levenson and Tassabehji 2004; Hintze and Theil 2006; Cairo et al. 1995).
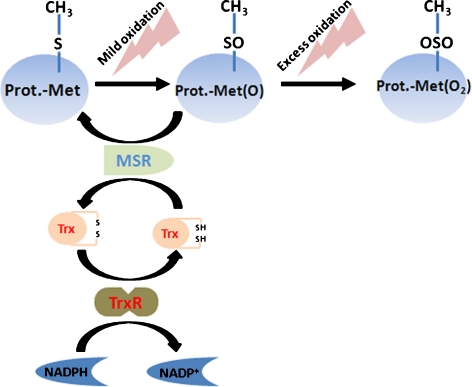
MCRC proteins are implicated in regulating the thiol status and the dithiol–disulfide transition of cellular proteins (Fig. 1). Mild oxidation of methionine (Met) to methionine sulfoxide (MetO) can be reversed by methionine sulfoxide reductases (MsrA and MsrB), thus, preventing the formation of the irreversibly oxidized product—methionine sulfone (MetO2) and, presumably also, the denaturation of MetO2-containing proteins (Moskovitz 2005; Stadtman et al. 2003).
Fig. 1.
The methionine-centered redox cycle (MCRC). Oxidation of methionine (free or protein bound) results in the formation of methionine-sulfone (Met(O2)) basically irreversible in biological systems. In contrast, Met(O) is reduced by methionine sulfoxide reductase (Msr), which receives electrons from thioredoxin (Trx). The oxidized Trx is reduced by Trx-Reductase (TrxR) which is connected to the entire cell redox system via NADPH/NADP+
Thioredoxin (Trx) is another component of the MCRC; it serves as the electron donor for the reduction of MetO via the Msr reaction, while being converted to its oxidized (disulfide) structure, which is re-reduced to Trx by Trx-Reductase (TrxR), using nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor (Fig. 1). In the liver, the Trx system was reported to undergo age-related changes which are regarded as adaptive and protective against oxidative stress (Takeda et al. 1996; Yoshida et al. 2003). Transgenic mice overexpressing human Trx showed an improved control of oxidative stress in spleen and bone marrow cells as well as an extended life span (Mitsui et al. 2002).
Several ROS and iron-related parameters and enzymes of the MCRC were studied previously in the heart (Bulvik et al. 2009) and in aero-digestive organs of rats (Vinokur et al. 2009). In the aging heart, Ft level was increased, but its iron saturation was reduced, and thus, the total Ft-bound iron (total FtBI) in the heart did not change. MsrA and MsrB proteins and MsrA mRNA were elevated as well. In the aero-digestive tract, comparable or decreased levels of the Msr (proteins) were found as well as a reduction in the Msr activity of diverse magnitudes. These findings, in aging organs, were interpreted to represent a protective response to the increased oxidative stress.
In the present study, we concentrated on proteins participating in iron homeostasis and methionine metabolism in aging rat organs. We focused on organs originating from different embryonic origins namely the liver (80% hepatocytes of endodermic origin, 20% reticuloendothelial cells—reticuloendothelial system (RES) from mesoderm origin), the spleen (mesoderm origin), muscle (tongue, sternohyoid from mesoderm origin), and esophagus and larynx (mixed, mostly endodermic). We examined whether MCRC proteins undergo age-related changes in livers and spleens, and Ft and iron in livers, spleens, tongue, sternohyoid, esophagus and larynx.
Materials and methods
Materials
All chemicals were of the highest purity available.
Animals
The study was approved by the Institutional Animal Ethics and Welfare Committee of the Hebrew University-Hadassah Medical School. A total of 15 young (~2 months) and 17 old (~24 months) female ‘Wistar’ rats were purchased from Harlan Laboratories, Jerusalem, Israel, and kept under specific-pathogen-free conditions at room temperature. The diurnal cycle consisted of 12 h of light and 12 h of dark; the animals were fed standard rat chow and water ad libitum. On the day of the experiment, animals were anesthetized by injecting Xylazine (15 mg/kg, intraperitoneal (i.p.)) and Ketamin (100 mg/kg, i.p.). All organs: liver, spleen and the aero-digestive tract organs (tongue, sternohyoid, esophagus and larynx) were rapidly excised, placed in liquid nitrogen and kept until analyzed at −80°C.
Tissues were homogenized with a Cole Parmer Teflon homogenizer. About 100 mg wet tissue was homogenized in 1.6 ml of a special lysis buffer containing 50 mM Tris-HCl, 1 mM cysteine, 1 mM sodium citrate, 0.5 mM MnCl2, 0.25 mM phenyl-methyl-sulfonyl-fluoride, and 0.02% digitonin and adjusted to pH 7.6. Protein concentrations in the lysates were measured using the BCA Protein Assay Kit of Pierce (Rockford, IL, USA), according to the manufacturer's instructions.
Ferritin and iron
Ft was isolated from livers of iron-loaded rats and antiserum prepared in goats as described previously (Konijn and Hershko 1977). Ft from rat hearts was prepared as described for human placenta (Konijn et al. 1985) and antibodies were prepared in New Zealand white rabbits (Vaisman et al. 1999). Goat anti-rabbit immunoglobulin (Ig) G was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA) and conjugated to Escherichia coli beta-galactosidase (Sigma-Aldrich, Israel) as described previously (Konijn et al. 1982).
Tissue Ft levels in the cytosolic fractions of all organs were determined by ELISA as previously described (Berenshtein et al. 2002; Vaisman et al. 2000). Shortly, goat anti-rat liver-Ft was diluted in 0.1 M carbonate–bicarbonate buffer pH 9.6 (Coating buffer) and 0.2 ml/well were used to coat 96-well micro-ELISA plates (Nunc, Roskilde, Denmark). Plates were incubated for 1 h at 37°C followed by overnight incubation at 4°C. After four washings of the coated plates with 0.02 M phosphate-buffered saline (PBS), containing 0.1% (w/v) BSA, 0.05% (v/v) Tween 20, and 0.01% (w/v) NaN3 (washing buffer), the plates were blocked with buffer containing 0.02 M PBS, 0.01% (w/v) NaN3, and 0.5% (w/v) gelatin (blocking buffer) for 1 h at 37°C (0.2 ml/well). After washing of the blocked plates four times with washing buffer, 0.2 ml of samples or standards diluted in PBS containing 0.5% (w/v) BSA and 0.05% (w/w) Tween 20 (dilution buffer) were applied to the wells, followed by 1-h incubation at 37°C. After further washings as above, 0.2 ml/well of Rabbit anti-rat heart Ft in Dilution buffer, was added and incubated for 1 h at 37°C. Next, after further washings, 0.2 ml of goat anti-rabbit γ-globulin-antibodies, conjugated to β-galactosidase in 0.01 M phosphate buffer pH 7.6, 10 mM NaCl, 0.1% BSA, 4% PEG 6000, 2 mM MgCl2, and 0.1% NaN3 (conjugate buffer) was added and incubated for 1 h at 37°C. Then, following washings as above, 0.2 ml/well of chlorophenol-red β-d-galactopyranoside (Roche diagnostic GmbH, Manhiem, Germany) diluted in 0.01 M phosphate buffer, pH 7.2, 10 mM NaCl and 2 mM MgCl2 (substrate buffer) was added and incubated until color was obtained. The developed color was read in a microplate reader (MR 5000 Dynatech Laboratories, Chantilly, VA, USA). A primary filter with a peak transmission at 570 nm and a secondary filter with a transmission at 620 nm were used.
Ft-bound iron was determined spectrophotometrically using the chromogenic chelator bathophenantrolin disulfonic acid (BPS); this reagent is specific for ferrous iron (Nilsson et al. 2002). Aliquots of the tissue homogenate and anti-rat heart Ft antibody were mixed and incubated for 72 h at 4°C. The samples were then centrifuged at 20,000×g for 20 min, the supernatant discarded and the pellet dissolved by overnight incubation in 0.1 ml HNO3 at 37°C. Then, the HNO3 was diluted with an equal volume of a solution containing 1.2 M HCl, 1% TCA and 3.75% thioglycolic acid (TGA) followed by incubation for 15 min at room temperature and subsequent centrifugation for 30 min at 3,000 rpm. Next, an equal volume of a solution composed of 0.25 g BPS/100 ml 2 M Na acetate was added and incubated for 2 min. The color obtained was measured immediately at 535 nm with an Uvikon XL spectrophotometer (Bio-tech instrument, Milano, Italy). A calibration curve was set up with samples that have known concentrations of iron. From the measured concentration of iron and Ft, the degree of Ft saturation by iron was calculated, and presented as the number of iron atoms per Ft molecule as follows:
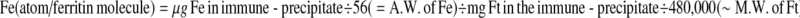 |
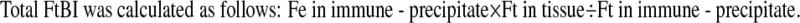 |
MCRC proteins
Msr activity was determined in liver and spleen according to a protocol developed by Moskovitz et al. (1997). The method is based on the enzymatic reduction of dabsyl-methionine-sulfoxide to dabsyl-methionine, which is than measured, following separation by HPLC, at 436 nm. This method measures the total activity of all Msr isoforms present in the organs homogenate.
Quantification of Trx, TrxR1, MsrA and MsrB proteins in livers and spleens was done by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by western blot analysis (Shioji et al. 2000) with some minor modifications. Equal quantities of protein from “young” and “old” samples were heated for 5 min in a boiling water bath. Samples were separated at 160 mV and transferred to a nitrocellulose membrane at 250 mA for 90 min. The membranes were blocked at 4°C, overnight, with 5% dry skim milk powder in 0.05 M Tris-buffered saline pH 7.6, containing 0.05% Tween-20 (TBS-T). The top half of the membrane was incubated for 1 h with anti-β-actin or anti-TrxR1 antibody, and the bottom half was incubated with either rabbit anti-MsrA, anti-MsrB, or anti-Trx antibodies. (The primary antibodies for Trx1 and TrxR1 were generously provided by Dr. S.G. Rhee, (Ewha Womans University, Seoul, Korea) and those for Msrs by J. Moskovitz (School of Pharmacy, University of Kansas, MO, USA)). After washing with TBS-T, the membranes were incubated for 1 h at room temperature with HRP-labeled goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA) in TBS-T containing 5% dry skim milk powder. The membranes were washed with TBS-T and developed with the chemiluminescence detection kit for HRP (EZ-ECL; Biological Industries, Beit-Haemek, Israel) according to manufacturer's instructions. Band intensities were quantified by ImageJ software (http://rsbweb.nih.gov/ij/) and normalized to β-actin. The densitometry data are expressed as their relative change for old rats (young rats = 1).
Quantitative expression of genes encoding for the relevant proteins was measured in livers and spleens by qRT-PCR (Reno et al. 1997). RNA was isolated with a phenol-chloroform extraction solution (Molecular Research Center, Inc, Cincinnati, OH, USA) as suggested by the manufacturer. Total RNA (1 μg) was reverse transcribed using a high capacity cDNA reverse transcription kit (Applied Biosystems, Austin, TX, USA).
The nucleotide sequences, used for primer design, were obtained from the public database GenBank (Table 1) and synthesized by, and purchased from Syntezza (Jerusalem, Israel). Primers for target genes were constructed using the Primer3 software (from: http://frodo.wi.mit.edu/ primer3/), and designed so that the forward primer in each pair was compliment to the exon–exon boundary (e.g., 3–4 in Table 1) in order to avoid genomic DNA amplification. qRT-PCR was carried out with 2-ng cDNA templates in triplicates, in 96-well plates, with power SYBR Green PCR Master Mix (both from Applied Biosystems Pty Ltd, Scoresby, Australia) using the 7500 Fast Real Time-PCR System (Applied Biosystem Carlsbad, CA, USA). Expression of all genes was calculated using the 2(−ΔΔCT) method (Livak and Schmittgen 2001), normalized per the expression of β-actin and presented as arbitrary units according to Reno et al. (1997).
Table 1.
Primers' sequences and the relevant exon numbers used in the PCR analyses
| Gene | Accession no. | Forward primer | Reverse primer | Exon |
|---|---|---|---|---|
| β-Actin | NM031144 | TTCCTTCCTGGGTATGGAATC | CGGATGTCAACGTCACACTT | 3–4:4 |
| Ft-H | NM 012848 | ACGTCTATCTGTCCATGGTCTTG | AAAGTTCTTCAGGGCCACAT | 1–2:2 |
| Ft-L | NM022500 | CCTACCTCTCTCTGGGCTTCT | CTTCTCCTCGGCCAATTC | 1–2:2 |
| MsrA | NM053307 | ATTTGGAATGGGCTGCTTCT | TAGGTGGGATTGCGTGTGTA | 2–3:3 |
| MsrB | NM001031660 | GTGGTCTGCAAACAGTGTGA | CTGTTGATGCAAAACCTTTGTC | 4–5:5 |
| Trx | NM053800 | TGGATGACTGCCAGGATGT | GTCGGCATGCATTTGACTT | 3–4:4 |
| TrxR | NM031614 | TCCTCACAAAATTATGGCAACA | GGAACCGCTCTGCTGAGTAA | 5–6:6 |
The nucleotide sequences used for primer design were obtained from the public database GenBank. Primers for the indicated genes were constructed using the Primer3 software, and designed so that the forward primer in each pair was complimentary to the exon–exon boundary (e.g., 3–4) in order to avoid genomic DNA amplification
RNA quality was evaluated by agarose-gel electrophoresis in 1% gels. Though the aged spleen total RNA was degraded, the quality of total RNA was good in spleen of young and the livers of both aged and young rats. However, the whole RNA extraction process was done at the same time and in the same manner for all experimental groups. The qRT-PCR procedure was performed with several housekeeping genes (hypoxanthine-guanine phosphoribosyltransferase, S18 and Actin) and resulted in an eight Ct difference between the spleens of young and aged animals. These eight Ct differences were used to normalize the targeted genes. Thus, though RNA degradation is a characteristic of aged spleen RNA which is more sensitive than the young one to degradation (Frasca et al. 2005) it could be normalized by the use of the multiple housekeeping genes. No Ct difference was found in total RNA from livers obtained from young and old rats.
Statistical analysis
The data were analyzed using two tails T test, (with α = 0.05). The differences of the means with p ≤ 0.05 were considered statistically significant. The data are presented as means ±SD.
Results
Ft and Ft-bound iron
Spleens from aged rats had more than twice the amount of Ft protein compared to that of young rats (Table 2, p < 0.05). We assume that the total Ft-bound iron (total FtBI) per unit of protein is an indicator for tissue “iron load”. Thus, the total FtBI in old spleens is 2.8-fold higher than in the spleens of young animals. Moreover, the iron core of Ft of “old” spleens contained 5,560 atoms per molecule, 25% more iron atoms than that of the “young” spleens. Since the Ft molecule can harbor up to 4,500 iron atoms (Harrison and Arosio 1996) the impression is that the Ft of old spleens are iron overloaded (Table 2). In the liver, Ft (protein) increased threefold with aging (Table 2) and each molecule of Ft contained 3,280 iron atoms, 33% more iron atoms than the young rat’s livers. As a result, total FtBI concentration in aged rat’s livers was 3.60-fold that in the liver of young rats. Though, the relative increase in Ft saturation, due to aging, was the same in spleens and livers, the total FtBI in spleens of old rats was twice that of the livers. However, spleens of young rats had three times the FtBI concentration than their livers. Thus, in the rat, aging is accompanied by a considerable increase in ‘iron load’ in both, livers and spleens. Accumulation of Ft protein in the aerodigestive tract striated muscles was of the same tendency as that in other organs derived from the mesoderm (e.g. spleen), and the changes are even more pronounced than in the liver and spleen. However, Ft concentration in the aero-digestive tract organs of the striated muscles of the rat and its iron saturation are considerably lower than those in spleen and liver. Ft–iron saturation increased with age in the spleen and liver (Table 2) however, these changes are different in muscles. Iron saturation is unchanged in the aging tongue or reduced in sterohyoid (Table 2), resembling that in the heart muscle (Bulvik et al. 2009). Ft concentrations increased with aging in muscle, liver and spleen tissues, which are mainly of mesoderm origin (spleen and muscle tissues) or from mixed, mesodermic and endodermic origins (liver). Ft levels decreased, or did not change with aging, in organs that are mostly derived from the endoderm (esophagus and larynx).
Table 2.
Quantification of Ft and Total FtBI in different organs of young and old rats
| Young | Old | Ratio | |
|---|---|---|---|
| Spleen | |||
| Ft (μg/mg protein) | 5.4 ± 1.9 | 11.5 ± 1.2* | 2.2 |
| Fe/Ft (atom/molecule) | 4460 ± 280 | 5560 ± 740* | 1.3 |
| FtBI (μg/mg protein) | 2.8 ± 0.8 | 7.6 ± 1.9* | 2.8 |
| Liver | |||
| Ft (μg/mg protein) | 2.9 ± 1.1 | 8.6 ± 1.3* | 2.9 |
| Fe/Ft (atom/molecule) | 2460 ± 160 | 3280 ± 200* | 1.3 |
| FtBI (μg/mg protein) | 0.9 ± 0.4 | 3.3 ± 0.6* | 3.6 |
| Tongue | |||
| Ft (μg/mg protein) | 1.2 ± 0.2 | 5.5 ± 0.6* | 4.6 |
| Fe/Ft (atom/molecule) | 570 ± 210 | 489 ± 100 | 0.9 |
| FtBI (μg/mg protein) | 0.8 ± 0.2 | 2.4 ± 0.4* | 3.0 |
| Sternohyoid | |||
| Ft (μg/mg protein) | 0.4 ± 0.1 | 1.6 ± 0.2* | 4.0 |
| Fe/Ft (atom/molecule) | 1569 ± 380 | 794 ± 280* | 0.5 |
| FtBI (μg/mg protein) | 0.2 ± 0.1 | 0.5 ± 0.1 | 2.3 |
| Esophagus | |||
| Ft (μg/mg protein) | 1.6 ± 0.4 | 0.3 ± 0.1* | 0.2 |
| Fe/Ft (atom/molecule) | 100 ± 29 | 496 ± 178* | 5.0 |
| FtBI (μg/mg protein) | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.0 |
| Larynxes | |||
| Ft (μg/mg protein) | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.9 |
| Fe/Ft (atom/molecule) | 767 ± 411 | 654 ± 174 | 0.9 |
| FtBI (μg/mg protein) | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.7 |
Tissue iron load was calculated by multiplying the amount of Ft by its Fe load. The amount of Ft protein was determined by ELISA and the iron in Ft with the bathophenetroline reagent after immunoprecipitation of the Ft as described in the Methods section. Values represent means ± SD from 15 young and 17 old rats
*p < 0.05
Although Ft synthesis is mainly under translational control (Ponka et al. 1998), in regard to aging, we questioned whether the above changes in liver and spleen are reflected in the transcription level of the Ft subunits. In the aging spleen, mRNA levels for both H and L subunits of Ft were increased 2.5- and 2.8-fold respectively (Table 3), in accord with the increase in Ft protein levels. In contrast, in the livers of aged rats the mRNA level for the H-subunit decreased by 33% and mRNA level for the L-subunit decreased by 22% (Table 3). However, the ratio between Ft-H and Ft-L mRNAs, in both organs, remained unchanged throughout the aging process (Table 3).
Table 3.
Quantification of Ft-H and Ft-L mRNA from spleen and liver tissues of young and old rats
| Young | Old | |
|---|---|---|
| Spleen | n = 8 | n = 5 |
| Ft-H (AU) | 1.00 ± 0.05 | 2.48 ± 0.62* |
| Ft-L (AU) | 1.00 ± 0.08 | 2.82 ± 0.66* |
| Ratio H/L | 1.05 | 0.99 |
| Liver | n = 8 | n = 7 |
| Ft-H (AU) | 1.00 ± 0.05 | 0.67 ± 0.04* |
| Ft-L (AU) | 1.00 ± 0.08 | 0.78 ± 0.06* |
| Ratio H/L | 1.01 | 1.02 |
The results of RT-PCR analyses were normalized against the mRNA of housekeeping genes of the same tissue sample. Values represent means±SD
*p < 0.05
Methionine-centered redox-cycling proteins
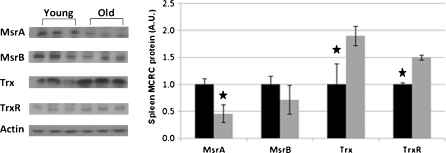
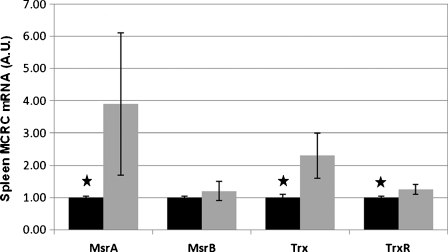
MCRC proteins were analyzed by SDS-PAGE followed by western blot analyses (Figs. 2 and 3), as well as by measuring the enzymatic activity of methionine sulfoxide reductase (Msr). Aging in the spleen is characterized by a 40% decrease (p < 0.001) in total Msr activity (Table 4). This change correlated well with a concomitant decrease in the amount of MsrA (p < 0.05) and a slight, non-significant decrease in MsrB (p > 0.05) proteins, in the spleen. The results suggest that the observed decrease in total Msr activity, in the spleen, was due to a reduction in the levels of both isoforms of this enzyme with aging, but that of MsrA was more pronounced. The levels of Thioredoxin (Trx) and its reductase—TrxR, increased with age, probably as a compensatory response for the loss of Msr proteins. Conversely, with aging, a trend toward higher levels of mRNA encoding the MCRC proteins was found (Fig. 4). Thus, while Msr proteins are lost during the aging process, the expression of their mRNAs increased.
Fig. 2.
Spleen MCRC protein components. Spleens of young (~2 months) and old (~24 months) rats were homogenized and equal quantities of protein of the cytosolic fraction separated by SDS-PAGE and analyzed by western blots with specific antibodies against the target proteins. In total, spleens of 15 young (black bars) and 17 old (gray bars) rats were analyzed. Left panel: representative western blots samples from spleens of three young and three old rats. Right panel: relative densitometry readings. The densitometry data are expressed in arbitrary units (A.U.), young equals 1. Values represent means±SD; star p < 0.05
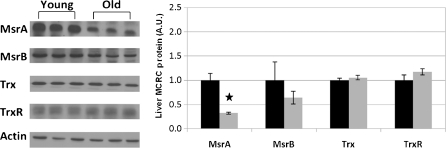
Fig. 3.
Liver MCRC protein components. Livers of young (~2 months) and old (~24 months) rats were homogenized and equal quantities of proteins of the cytosolic fraction were separated by SDS-PAGE and analyzed by Western blots with specific antibodies against the target proteins. In total, livers of 15 young (black bars) and 17 old (gray bars) rats were analyzed. Left panel: representative western blots samples from livers of three young and three old rats. Right panel: relative densitometry readings. The densitometry data are expressed in arbitrary units (A.U.), young equals 1. Values represent means±SD; star p < 0.05
Table 4.
Msr activity (picomole of methionine per milligram of protein per minute) in livers and spleens of 15 young and 17 old rats
| Young | Old | Ratio | |
|---|---|---|---|
| Spleen | 58.5 ± 4.4 | 36.1 ± 4.6* | 0.62 |
| Liver | 63.3 ± 4.8 | 96.5 ± 2.8* | 1.52 |
Values represent means±SD
*p < 0.05
Fig. 4.
Spleen MCRC genes expression. One-microgram quantities of spleen RNA extracts from young (~2 months) and old (~24 months) rats were used for the reverse transcription procedure. Real Time-PCR was performed using primers specific for the target genes. In total, spleens from four young and seven old rats were analyzed. The PCR data were normalized to the amplification of the β-actin, hypoxanthine-guanine phosphoribosyltransferase and the S18 housekeeping genes and then expressed as the relative change in spleens of old rats (young rats, black bars = 1). Values represent means±SD; star p < 0.05
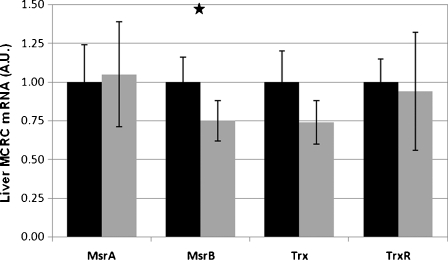
In contrast to the spleen, in the livers, we observed with aging a 1.5-fold increase (p < 0.0001) in the total activity of Msr enzymes (Table 4). However, the amount of Msr proteins decreased considerably with age (Fig. 3). The levels of other proteins of the MCRC in the liver—Trx and TrxR—remained unchanged with aging. No significant age-related changes in gene expression encoding MsrA, Trx1 and TrxR1 were demonstrated; MsrB mRNA expression was slightly decreased (p < 0.05, Fig. 5). In the liver, Msr proteins decreased with age, even more than in the spleen, but the gene transcription did not change.
Fig. 5.
Liver MCRC genes expression. One-microgram quantities of liver RNA extracts from young (~2 months) and old (~24 months) rats were used for the reverse transcription procedure. Real Time-PCR was performed using primers specific for the target genes. In total, livers from four young and seven old rats were analyzed. The PCR data were normalized to the amplification of the β-actin housekeeping gene and then expressed as the relative change in livers of old rats (young rats, black bars = 1). Values represent means±SD; star p < 0.05
These results demonstrate tissue-specific patterns of change with age, of the repertoire of defense proteins.
Discussion
Though the definition for the terms of ‘old’ and ‘young’ rats is undecided, in this paper, we defined rats just before sexual maturation as young. Female Wistar rats have a median lifespan of 900 days (Koolhaas 2010), taken this into account the old rats we used paralleled to humans at the brink of retirement, about 65 years of age.
Due to an imbalance between free-radical generation and their elimination, ROS accumulates and causes buildup of oxidative-modified proteins and a decline in cellular antioxidant defense (Harman 1981; Friguet 2006; Squier 2001). ROS formation is closely coupled to redox-active iron availability through the Haber–Weiss reaction (Leibovitz and Siegel 1980). Thus, redox-active iron enhances ROS-induced damage and turns ROS-mediated signals from pro-survival to cell death processes (Bandyopadhyay et al. 1999). Therefore, the storage of cellular iron in a redox-inactive form is critical for the prevention of undesirable oxidation of cellular components, especially during aging.
We showed that the capacity of cellular iron storage and, thus, the extent of cellular defense against iron-induced free-radical damage is organ specific, and is related to the embryonic origin of the organ. Organs originating from the mesoderm are more protected than those derived from the endoderm are.
It was suggested that ROS participate in cell signaling and are involved in gene expression via redox-sensitive transcription factors (Kregel and Zhang 2007; Sen and Packer 2000). Thus, age-associated oxidative changes could indicate not only the deterioration of cellular defense pathways but also of cell adaptation to aging.
The level of the iron storage protein—Ft—was significantly higher in spleens, livers and in muscles of the aero-digestive tract of aged rats as compared to that in young ones. However, in tissues of endodermic origin (esophagus and larynx) Ft levels did not change or even decreased, causing in the esophagus an increase in iron level within the Ft molecule. In the spleen, Ft buildup was accompanied with an increase in both Ft-H and Ft-L gene expression, pointing to enhanced transcription of the mRNA for these proteins. However, decreased turnover cannot be ruled out since it was found previously that hemosiderin, a poorly soluble degradation product of Ft (Hoy and Jacobs 1981), accumulates in the red and white pulps of the spleen in aged rats (Masuda et al. 1993).
In the liver of aging rats, Ft accumulation increased and was accompanied by a decrease in Ft mRNAs levels and a marked increase in FtBI (3.60-fold). This was in spite of just a moderate increase of 33% in Ft saturation, which can be indicative of higher levels of the cellular LIP. Consequently, this higher level would have led to low levels of active IRPs (Konijn et al. 1999) in the liver, allowing for more extensive synthesis of the Ft protein (Hintze and Theil 2006). A marked accumulation of Ft in livers of aged rats was also reported (Rikans et al. 1997). L-subunits of Ft are highly expressed in iron storage organs, including the liver (Levenson and Tassabehji 2004) where Ft mRNA translation is responsive to iron (Ponka et al. 1998). Ft synthesis was significantly stimulated in the liver of rats subjected to oxidative stress generated by treatment with phorone, consequently, increased accumulation of H- and L-subunit mRNAs was reported. In addition, nuclear run-on experiments provided evidence of stimulation of Ft transcription in the liver by oxidative stress and expansion of the free iron pool (Cairo et al. 1995).This could prove true for the aging process, as well.
The increase of total FtBI in the spleen and liver of rats upon aging (Table 2) probably stems from accelerated breakdown of heme from red blood cells (Takeda et al. 1996). Our recently published results for the aging rat hearts showed that FtBI decreased by half in aged hearts while the Ft protein, itself, doubled, attaining the same levels of total FtBI in old and young rat hearts (Bulvik et al. 2009).
Unlike the liver and spleen, the heart is not an ‘iron storage organ’. In the heart, Ft-H is the dominant subunit. The liver and spleen are both capable of storing iron and as such, their Ft proteins are rich in L-subunits. Other organs we monitored, which are associated with the aero-digestive tract, are mainly of the mesoderm or the endoderm origin. In the gastrocnemius, Ft, transferrin, and tissue iron levels increased with age (Altun et al. 2007). We did not measure total tissue iron levels, as such, in the mesoderm-originating tongue and sternohyoid muscles. The greater part of non-heme iron in muscles is Ft which was elevated in the tongue and sternohyoid of the aged rats, as expected. The mean iron concentration in the Ft molecule decreased or did not change with age, because of the increased Ft level, the final FtBI in the tissue increased in the aging muscles. Likewise, previous research revealed that, though the heart is not the classical striated skeletal muscle, advanced age caused a marked increase in its total Ft as well as the mRNA for the L-Ft subunit (Bulvik et al. 2009). The relative Ft level in the esophagus decreased with age but Ft–iron content was elevated; thus, the total tissue iron burden remained stable. With advancing age, in the larynx there are no changes in the Ft and iron levels.
Spleen and liver, which are parts of the RES, and liver being 80% parenchymal tissue, show resemblance in Ft accumulation and Ft–iron saturation. Spleen Ft seems to be saturated to its capacity and more, probably because of increased erythroid breakdown supported by age-related conditions. The esophagus and larynx are different from the heart, liver, spleen and muscle, in their ‘physiological body assignment’, their anatomic structure, embryonic origin, and thus show different patterns of Ft level and its iron load.
Apparently, the accumulation of Ft is in response to an expansion of the intracellular labile iron pool, provoked by ROS that activate multiple molecular mechanisms to reconstitute Ft content, thus limiting the pro-oxidant challenge of iron (Cairo et al. 1995). This Ft accumulation can be viewed as an adaptive protective response of the spleen and liver against iron released from Ft or heme, by ROS that may well initiate iron-mediated oxidative damage, in an organ-dependent mode. The heart kept a constant Ft-bound-iron level with aging accompanied by increased Ft-L-subunit expression, but decreased FtBI content (Bulvik et al. 2009).The spleen and liver responded both to aging by an increase in Ft accumulation and Ft–iron saturation.
The role of MCRC proteins in maintaining cellular redox status is well documented; longer life spans, induced by caloric restriction were accompanied by elevated levels of MsrA and MsrB (Moskovitz 2005; Rohrbach et al. 2006). Over-expression of human Trx resulted in increased lifespan in mice (Mitsui et al. 2002) and mice devoid of the MsrA gene (msra −/− mouse) had a shorter life span and high sensitivity toward oxidative stress (Moskovitz et al. 2001).
Adequately functioning MCRC proteins is essential for cell viability. Due to the presence of functional –S–CH3 groups, the methionyl-containing proteins are especially sensitive to oxidative stress, thus, non enzymatic oxidation of the methionine sulfur and formation of methylsulfoxide (–CH2-SO-CH3) can lead to reduction or elimination of biological activity (Brot and Weissbach 1991).
Comparing spleens from aged and young rats showed that in the aged rats, protein levels of both MsrA and MsrB, the major components of the MCRC complex, were significantly decreased, and consequently, Msr enzymatic activity was significantly lowered. The elevated levels of mRNAs of the Msr isozymes, in particular of MsrA, in the aged spleens did not correlate with the protein levels and activities (Fig. 4), indicating an enhanced need for maintaining higher levels of MCRC activities. Decreased level of MCRC with increased expression of the MsrA gene, could point toward rapid catabolism, by oxidation and proteolysis of these proteins in the aged spleen. In addition, the assumption that there is a metabolic need for increased MCRC activities is supported by the increased Trx and TrxR transcription and expression (Figs. 4 and 2) assuring ample supply of substrate for methionine sulfoxide reductase (Fig. 1).
In the liver, Msr isozymes levels (A and B) decreased with age, however, Msr activity increased, in the spleen. In this study, the Trx protein level in the spleen of old rats was twice that of young rats, thus, emphasizing the importance of the MCRC system in the repair of age-related methionine oxidation. Upregulation of Trx can induce cell proliferation (Gallegos et al. 1996) and inhibit apoptosis (Chen et al. 2002).
Aging in rats was found to be associated with a reduction in the number of cells in the spleen (Cheung and Nadakavukaren 1983) which makes it difficult to hypothesize on the mechanisms involved in the increase in Trx observed in our study.
In previous research, decreased Msr activity was found in hearts and tissues of the aero-digestive track of aging rats (Bulvik et al. 2009; Vinokur et al. 2009). In this study too, we found that the MsrA protein decreased in spleen and in the liver by aging, but the amount of other ROS related enzymes—MsrB, Trx and TrxR—either decreased marginally, or increased (in the spleen), or did not change at all.
The significantly increased expression of genes encoding for MsrA, Trx and TrxR in the spleen of aged rats indicates, on the one hand, a higher de novo synthesis of the MCRC proteins but on the other, probably also increased turnover as reflected by the low level of tissue proteins. A possible mechanism is that ROS induced the activation of gene transcription (Sen and Packer 1996), and the excess of ROS might result from impaired antioxidant activity of MCRC in the aged spleen. The liver seems to be more resistant to aging and changes are less pronounced in old rats.
Correspondingly, the increase in Ft-H and Ft-L mRNAs in the spleen, with aging, reflects excessive level of iron and/or ROS in this organ.
In general, the organ-specific alterations with aging can be seen as an adaptation to old age. Thus, oxidation of critical Methionine residues within selected proteins may serve to down-regulate energy metabolism and the generation of ROS, and may be regarded as an adaptive response to age-related oxidative stress (Squier 2001).
In conclusion, aged rat livers and spleens express increased levels of Ft protein as well as Ft-H and Ft-L mRNAs. This Ft buildup correlates with a marked increase in the amounts of iron that accumulate in these two organs with age. Various elements of MCRC manifest different trends upon aging. The age-related changes are more prominent in the spleen than in the liver suggesting the latter is more resistant to oxidative damage, including during aging.
Acknowledgments
We are grateful to Dr. A. Reznick, of the Technion-Israel Institute of Technology, for the supply the old animals. We also thank Dr. Sue Goo Rhee (Ewha Womans University, Seoul, Korea) and Dr. Jackob Moskovitz (School of Pharmacy, University of Kansas) for generously providing antibodies.
Glossary
- Ft
Ferritin
- Ft-H and Ft
L—the H and L subunits of Ft
- FtBI
Ft-bound iron
- MCRC
Methionine-centered redox cycle
- Met
Methionine
- MetO
Methionine sulfoxide
- MetO2
Methionine sulfone
- Msr
Methionine sulfoxide reductase
- ROS
Reactive oxygen-derived species
- Trx
Thioredoxin 1
- TrxR
Thioredoxin reductase 1
References
- Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36(2):223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U, Dipak Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Science. 1999;77:658–666. [Google Scholar]
- Berenshtein E, Vaisman B, Goldberg-Langerman C, Kitrossky N, Konijn AM, Chevion M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol Cell Biochem. 2002;234–235(1–2):283–292. doi: 10.1023/A:1015923202082. [DOI] [PubMed] [Google Scholar]
- Brot N, Weissbach H. Biochemistry of methionine sulfoxide residues in proteins. Biofactors. 1991;3(2):91–96. [PubMed] [Google Scholar]
- Bulvik B, Grinberg L, Eliashar R, Berenshtein E, Chevion MM. Iron, ferritin and proteins of the methionine-centered redox cycle in young and old rat hearts. Mech Ageing Dev. 2009;130(3):139–144. doi: 10.1016/j.mad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Cairo G, Tacchini L, Pogliaghi G, Anzon E, Tomasi A, Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J Biol Chem. 1995;270(2):700–703. doi: 10.1074/jbc.270.2.700. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277(36):33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- Cheung HT, Nadakavukaren MJ. Age-dependent changes in the cellularity and ultrastructure of the spleen of Fischer F344 rats. Mech Ageing Dev. 1983;22(1):23–33. doi: 10.1016/0047-6374(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1(6):529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Frasca D, Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175(10):6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580(12):2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Gallegos A, Gasdaska JR, Taylor CW, Paine-Murrieta GD, Goodman D, Gasdaska PY, Berggren M, Briehl MM, Powis G. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res. 1996;56(24):5765–5770. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hintze KJ, Theil EC. Cellular regulation and molecular interactions of the ferritins. Cell Mol Life Sci. 2006;63(5):591–600. doi: 10.1007/s00018-005-5285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy TG, Jacobs A. Ferritin polymers and the formation of haemosiderin. Br J Haematol. 1981;49(4):593–602. doi: 10.1111/j.1365-2141.1981.tb07269.x. [DOI] [PubMed] [Google Scholar]
- Konijn AM, Hershko C. Ferritin synthesis in inflammation I. Pathogenesis of impaired iron release. Br J Haematol. 1977;37(1):7–16. [PubMed] [Google Scholar]
- Konijn AM, Levy R, Link G, Hershko C. A rapid and sensitive ELISA for serum ferritin employing a fluorogenic substrate. J Immunol Methods. 1982;54(3):297–307. doi: 10.1016/0022-1759(82)90314-3. [DOI] [PubMed] [Google Scholar]
- Konijn AM, Tal R, Levy R, Matzner Y. Isolation and fractionation of ferritin from human term placenta–a source for human isoferritins. Anal Biochem. 1985;144(2):423–428. doi: 10.1016/0003-2697(85)90135-6. [DOI] [PubMed] [Google Scholar]
- Konijn AM, Glickstein H, Vaisman B, Meyron-Holtz EG, Slotki IN, Cabantchik ZI. The cellular labile iron pool and intracellular ferritin in K562 cells. Blood. 1999;94(6):2128–2134. [PubMed] [Google Scholar]
- Koolhaas JM. The Laboratory rat. In: Hubrecht R, Kirkwood J, editors. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8. West Sussex: Wiley-Blackwell; 2010. [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Leibovitz BE, Siegel BV. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980;35(1):45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- Levenson CW, Tassabehji NM. Iron and ageing: an introduction to iron regulatory mechanisms. Ageing Res Rev. 2004;3(3):251–263. doi: 10.1016/j.arr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Liu X, Theil EC. Ferritin as an iron concentrator and chelator target. Ann N Y Acad Sci. 2005;1054:136–140. doi: 10.1196/annals.1345.016. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Masuda T, Satodate R, Tsuruga K, Kasai T. Quantitative assessment of a change of hemosiderin deposition with age in splenic compartments of rats. Tohoku J Exp Med. 1993;170(3):169–179. doi: 10.1620/tjem.170.169. [DOI] [PubMed] [Google Scholar]
- Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4(4):693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703(2):213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci U S A. 1997;94(18):9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson UA, Bassen M, Savman K, Kjellmer I. A simple and rapid method for the determination of “free” iron in biological fluids. Free Radic Res. 2002;36(6):677–684. doi: 10.1080/10715760290029128. [DOI] [PubMed] [Google Scholar]
- Petropoulos I, Friguet B. Maintenance of proteins and aging: the role of oxidized protein repair. Free Radic Res. 2006;40(12):1269–1276. doi: 10.1080/10715760600917144. [DOI] [PubMed] [Google Scholar]
- Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35(1):35–54. [PubMed] [Google Scholar]
- Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22(6):1082–1086. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Ardinska V, Hornbrook KR. Age-associated increase in ferritin content of male rat liver: implication for diquat-mediated oxidative injury. Arch Biochem Biophys. 1997;344(1):85–93. doi: 10.1006/abbi.1997.0172. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Gruenler S, Teschner M, Holtz J. The thioredoxin system in aging muscle: key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R927–R935. doi: 10.1152/ajpregu.00890.2005. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. Faseb J. 1996;10(7):709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72(2 Suppl):653S–669S. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- Shioji K, Kishimoto C, Nakamura H, Toyokuni S, Nakayama Y, Yodoi J, Sasayama S. Upregulation of thioredoxin (TRX) expression in giant cell myocarditis in rats. FEBS Lett. 2000;472(1):109–113. doi: 10.1016/S0014-5793(00)01446-0. [DOI] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36(9):1539–1550. doi: 10.1016/S0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Moskovitz J, Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal. 2003;5(5):577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- Takeda T, Kimura M, Yokoi K, Itokawa Y. Effect of age and dietary protein level on tissue mineral levels in female rats. Biol Trace Elem Res. 1996;54(1):55–74. doi: 10.1007/BF02785320. [DOI] [PubMed] [Google Scholar]
- Tappia PS, Dent MR, Dhalla NS. Oxidative stress and redox regulation of phospholipase D in myocardial disease. Free Radic Biol Med. 2006;41(3):349–361. doi: 10.1016/j.freeradbiomed.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int J Biochem Cell Biol. 1999;31(10):1139–1152. doi: 10.1016/S1357-2725(99)00080-1. [DOI] [PubMed] [Google Scholar]
- Vaisman B, Santambrogio P, Arosio P, Fibach E, Konijn AM. An ELISA for the H-subunit of human ferritin which employs a combination of rabbit poly- and mice monoclonal antibodies and an enzyme labeled anti-mouse-IgG. Clin Chem Lab Med. 1999;37(2):121–125. doi: 10.1515/CCLM.1999.022. [DOI] [PubMed] [Google Scholar]
- Vaisman B, Meyron-Holtz EG, Fibach E, Krichevsky AM, Konijn AM. Ferritin expression in maturing normal human erythroid precursors. Br J Haematol. 2000;110(2):394–401. doi: 10.1046/j.1365-2141.2000.02167.x. [DOI] [PubMed] [Google Scholar]
- Vinokur V, Grinberg L, Berenshtein E, Gross M, Moskovitz J, Reznick AZ, Chevion M, Eliashar R. Methionine-centered redox cycle in organs of the aero-digestive tract of young and old rats. Biogerontology. 2009;10(1):43–52. doi: 10.1007/s10522-008-9152-8. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Oka S, Masutani H, Nakamura H, Yodoi J. The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxid Redox Signal. 2003;5(5):563–570. doi: 10.1089/152308603770310211. [DOI] [PubMed] [Google Scholar]