Abstract
Astrocytes are key players in brain function; they are intimately involved in neuronal signalling processes and their metabolism is tightly coupled to that of neurons. In the present review, we will be concerned with a discussion of aspects of astrocyte metabolism, including energy-generating pathways and amino acid homoeostasis. A discussion of the impact that uptake of neurotransmitter glutamate may have on these pathways is included along with a section on metabolic compartmentation.
Keywords: amino acid, astrocyte, compartmentation, energy, metabolism
Abbreviations: α-KG, α-ketoglutarate; AAT, aspartate aminotransferase; CFP, cyan fluorescence protein; DAB, diaminobenzidine; FRET, fluorescence resonance energy transfer; [glc]i, intracellular glucose concentration; GABA, γ-aminobutyric acid; GABA-T, GABA aminotransferase; GDH, glutamate dehydrogenase; GLUT, glucose transporter; GP, glycogen phosphorylase; GS, glutamine synthetase; GSK3, GS kinase 3; PAG, phosphate-activated glutaminase; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; TCA, tricarboxylic acid; YFP, yellow fluorescence protein
ASTROCYTE ENERGY METABOLISM
Glucose entry, phosphorylation and generation of glycogen in astrocytes
Astrocytes are ideally positioned to sense synaptic activity in the brain (Kacem et al., 1998), they control blood flow (Gordon et al., 2008; Allen and Barres, 2009), interact with neurons and endothelial cells (Vesce et al., 1999) and likely act as signalling integrators at different temporal and spatial domains (Parpura and Zorec, 2010; Parpura et al., 2010). Astrocytic endfeet lie between all brain capillaries and neuronal terminals (Tsacopoulos and Magistretti, 1996; Mathiisen et al., 2010) and during neuronal activity astrocytes may exhibit an increased glucose uptake and possibly have a key role in coupling synaptic activity to glucose utilization (Magistretti, 2006) and provision of glucose for neuronal metabolism (DiNuzzo et al., 2010, 2011). Sodium-coupled uptake of glutamate by astrocytes and the ensuing activation of the Na+/K+-ATPase may trigger glucose uptake and glycolytic processing (Fox et al., 1988, Magistretti, 2006), the latter process providing energy in the form of ATP to fuel glutamate uptake (Schousboe et al., 2011).
The [glc]i (intracellular glucose concentration) is dependent on the [glc]e (external glucose concentration), but is limited to a maximal value of ∼0.4 mM (Bittner et al., 2010; Prebil et al., 2011a). This upper limit is likely due to the plasma membrane permeability for glucose, which is in balance with the cytosolic glucose utilization. The rate of glucose flux through GLUT1 (glucose transporter 1), the major GLUT in astrocytes (Loaiza et al., 2003), is thus in balance with the rate of utilization by hexokinase activity. Interestingly, the level of [glc]i in astrocytes (0.4 mM) is much lower than that measured in 3T3-L1 fibroblasts (10.2 mM) and preadipocytes (2.6 mM), but comparable with adipocytes (0.6 mM) (Kovacic et al., 2011), determined at similar extracellular glucose levels.
In astrocytes, the cytosolic glucose concentration declines to a new lower steady-state value in approximately 20 s, when extracellular glucose changes from 0.5 to 0.0 mM. At higher initial extracellular glucose level (i.e. 5 mM; higher load for the metabolism), the decline in cytosolic glucose concentration is slower (60 s), which is likely due to the rate-limited cytosolic consumption of glucose (Prebil et al., 2011a).
GLUT1 (45-kDa isoform) is located in the astrocytic endfeet around blood vessels (Morgello et al., 1995) and in astrocytic cell bodies and processes (Leino et al., 1997). The GLUT2 was also found in astrocytes (Leloup et al., 1994; Leino et al., 1997; Arluison et al., 2004). On the other hand, the 55-kDa isoform of GLUT1 is located in endothelial cells which form the blood–brain barrier. Glucose enters neurons trans-cellularly through astrocytes via the 45-kDa isoform of GLUT1 or directly via GLUT3, a neuronal GLUT (Maher et al., 1994). It may be noted that GLUT1 is stimulated by glutamate in vitro and by neuronal activity in vivo (Loaiza et al., 2003; Porras et al., 2008; Chuquet et al., 2010).
After glucose entry, glucose is phosphorylated by type I hexokinase (Needels and Wilson, 1983; Griffin et al., 1992). In astrocytes, most of the type I hexokinase is associated with mitochondria (Lynch et al., 1991), and the activity of hexokinase bound to mitochondria is greater than the cytosolic hexokinase (Nagamatsu et al., 1996). However, inhibition of gap junctions promotes the translocation of type I hexokinase from mitochondria towards microtubules, and induces a significant expression of type II hexokinase and GLUT3, which are normally not present in astrocytes; all these changes aid to sustain a higher rate of cell proliferation (Sánchez-Alvarez et al., 2004). It was shown that levels of glucose-1,6-bisphosphate higher than 0.2 mM inhibit astroglial hexokinase in a concentration-dependent manner; at 1.2 mM, the hexokinase activity is almost completely inhibited (Lai et al., 1999). Endothelin-1, a vasoconstricting agent (Nie and Olsson, 1996), stimulates the translocation and up-regulation of both GLUT1 transporter and type 1 hexokinase in astrocytes (Sánchez-Alvarez et al., 2004). Type II hexokinase is induced by deprivation of glucose (Niitsu et al., 1999). It was recently shown that ischaemic stress increases the expression of GLUT3, which is responsible for the enhanced storage of intracellular glucose during reperfusion (Iwabuchi and Kawahara, 2011).
After glucose phosphorylation, glucose-6-phosphate is the intermediate from which either glycolysis, the pentose–phosphate pathway or the generation of glycogen may start (Figure 1). The amount of glucose metabolized in the pentose–phosphate pathway is less than one-tenth compared with glycolysis in cultured astrocytes (Leo et al., 1993; Ben-Yoseph et al., 1994), where lactate is a major metabolic product (Wiesinger et al., 1997). Several toxic agents, such as chlorinated acetates, fluoxetine, ethanol and copper, were shown to disturb glucose uptake or metabolism in astrocytes (Abdul Muneer et al., 2011; Allaman et al., 2011; Scheiber and Dringen, 2011; Schmidt et al., 2011).
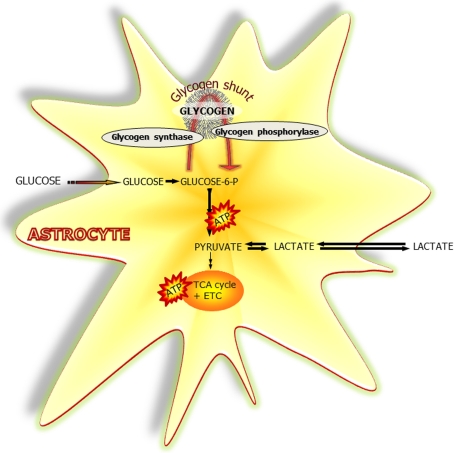
Figure 1. Glucose and glycogen metabolism in astrocytes.
Simplified schematic representation of glucose metabolism via glycolysis or via the ‘glycogen shunt’ illustrating how glucose units may be metabolized via incorporation into and subsequent hydrolysis from the branched glycogen molecule preceding metabolism to pyruvate and lactate, i.e. glycogenolysis. Glucose-6-P, glucose-6-phosphate; ETC, electron transport chain.
It was reported that in rat brain the extracellular concentration of glucose, measured by a microdialysis technique, is as low as 0.47 mM (Fellows et al., 1992). Moreover, in ischaemic conditions, the extracellular glucose concentration, measured with micro-electrodes, is less than 0.05 mM, and in hypoglycaemia less than 0.19 mM (Silver and Erecińska, 1994), which may be the milieu where glucose may leak out of the astrocyte, since the resting cytosolic glucose concentration was measured to be 0.4 mM, especially if noradrenalin is present (Prebil et al., 2011a).
Emerging evidence suggests that metabolic substrates other than glucose (e.g. glycogen, lactate and glutamate) provide significant amounts of energy in the brain (Brown et al., 2001; Dienel and Cruz, 2006). In the brain, glycogen (Cataldo and Broadwell, 1986; Wender et al., 2000) as well as GP (glycogen phosphorylase) predominantly reside in astrocytes (Ibrahim, 1975). The term glycogen shunt (Figure 1) represents the fraction of metabolized glucose that passes through glycogen molecules, prior to entering the glycolytic pathway, and may be as large as 40% of overall glucose metabolism (Walls et al., 2009). The glycogen reservoir can provide fuel for energy production during hypoglycaemia (Swanson and Choi, 1993; Brown and Ransom, 2007), as well as during normal brain metabolism (Swanson, 1992; Fillenz et al., 1999; Walls et al., 2009). Glycogen content appears to be dependent on insulin signalling in astrocytes (Heni et al., 2011).
Glycolysis and glycogenolysis appear to provide most of the energy required during an abrupt energy demand (Hertz et al., 2007). Glycogen might serve as a source of lactate which may be transferred to neurons (Wender et al., 2000; DiNuzzo et al., 2011), or converted to pyruvate, which enters the TCA (tricarboxylic acid) cycle (Sickmann et al., 2005). Lactate and ketone bodies have been shown to fuel a substantial portion of brain-energy metabolism in prolonged starvation, diabetes and under hypoglycaemia (reviewed by Pellerin and Magistretti, 2004). In addition, lactate may act as a signalling molecule (reviewed by Barros and Deitmer, 2010). On the other hand, astrocytic networks can also effectively remove lactate from activated glycolytic domains, and the lactate can be dispersed throughout the syncytium to the endfeet for release to blood (Gandhi et al., 2009). Finally, channelling of blood-borne glucose to the extracellular space for use in neurons has been suggested by DiNuzzo et al. (2010, 2011) based on the idea that breakdown of glycogen inhibits phosphorylation by hexokinase.
Effects of glutamate entry on energy metabolism
A FRET (fluorescence resonance energy transfer)-based approach employing nanosensors (Fehr et al., 2003) may be reliably used to monitor dynamic activity-induced changes in cytosolic glucose levels in astrocytes (Bittner et al., 2010, 2011; Prebil et al., 2011a, 2011b). In the first generation of such nanosensors, such as FLIPglu-600μ, a decrease in the FRET signal was observed upon glucose binding (Takanaga et al., 2008). The second generation of nanosensors, such as FLII12PGLU-700μΔ6, have an improved signal-to-noise ratio and a higher dynamic measuring range in vivo, from 0.05 to 9.6 mM (Takanaga et al., 2008). Phosphorylated sugars have no effect on the FRET ratio (Fehr et al., 2003). To dynamically monitor the cytosolic glucose concentration ([glc]i), the CFP (cyan fluorescence protein) fluorescence is excited and the fluorescence of CFP and YFP (yellow fluorescence protein) is monitored. The ratio between YFP and CFP is calculated over a defined region of the imaged cell. Cells superfused with extracellular medium, containing a high glucose concentration, display high intensity of CFP fluorescence and low intensity of YFP fluorescence. Thus, the high YFP/CFP ratio (ΔR) indicates an elevated cytosolic glucose concentration. The exchange of glucose-rich external solution with the one devoid of glucose results in the FRET ratio decline, indicating glucose depletion from the cell (Takanaga et al., 2008).
The sensor may be calibrated in situ by measuring the difference between the FRET ratio during superfusion with the increasing extracellular glucose concentration and intermittent superfusion with a solution devoid of glucose (ΔR). To calibrate the sensor, the plasma membrane should be permeabilized (e.g. by using β-escin) to allow fast and unhindered access of glucose to the sensor in the cell interior. The saturation level of the sensor is first calculated (S = ΔR/ΔRmax), and then by using the binding constant Kd of the sensor the intracellular concentration of glucose is estimated ([glc]i = {Kd×S}/{1−S}; Prebil et al., 2011a). It is important to note that since the sensor measures the level of unphosphorylated glucose, it is assumed that a decrease in [glc]i reflects increased utilization of glucose, i.e. increased uptake and glycolytic breakdown of extracellular glucose.
The effect of glutamate as a neurotransmitter in the synapse is strongly dependent on astrocytic metabolism (Hertz, 2006). Since glutamate does not readily cross the blood–brain barrier, glucose serves as a precursor for glutamate synthesis (Hertz and Dienel, 2002). Neurons lack the enzyme pyruvate carboxylase (Yu et al., 1983) and therefore depend on astrocytes for de novo synthesis of glutamate (Danbolt, 2001; Hertz and Zielke, 2004). Rapid glutamate uptake by glial transporters located near release sites (Chaudhry et al., 1995; Bergles et al., 1997; Clark and Barbour, 1997; Dzubay and Jahr, 1999) removes the transmitter and thus terminates the excitatory postsynaptic potential. In physiological conditions, glutamate uptake into astrocytes is driven by the electrochemical gradient of sodium (O'Kane et al., 1999) and mediated mainly through two glial glutamate transporters: GLAST (EAAT1) and GLT1 (EAAT2) (Rothstein et al., 1994; Danbolt, 2001).
Trafficking of glutamate transporters to the plasma membrane has been studied (Robinson, 2002; Fournier et al., 2004; Stenovec et al., 2008) and it is likely that glutamate transporters get incorporated into the plasma membrane by exocytosis (Cheng et al., 2002; Stenovec et al., 2008). Consistent with the presence of a regulated exocytotic pathway in astrocytes (Kreft et al., 2004; Pangrsic et al., 2006, 2007; Parpura and Zorec, 2010), a calcium-dependent increase in cumulative exocytosis increases the glutamate transporter density (Stenovec et al., 2008), which is important for maintaining a low extracellular glutamate concentration, essential for the prevention of chronic glutamate neurotoxicity (Rothstein et al., 1996).
After glutamate uptake into astrocytes, it is either converted to glutamine (Figure 2) by the astrocyte-specific GS (glutamine synthetase; Derouiche, 2004) or at high glutamate concentration is oxidatively degraded after conversion to α-KG (α-ketoglutarate; Yu et al., 1982; McKenna et al., 1996, 2000). The latter pathway (Figure 3) clearly shows that besides being an excitatory transmitter, glutamate is an important metabolic fuel, which is oxidatively metabolized in astrocytes (Miller et al., 1975; Hertz et al., 1988; Swanson et al., 1990; Zielke et al., 1998; Dienel and Cruz, 2006; Hawkins, 2009). This aspect is discussed in further detail in a subsequent section.
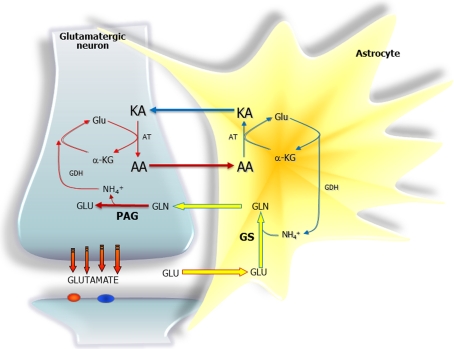
Figure 2. Schematic representations of the proposed amino acid (AA) shuttles at the glutamatergic synapse involved in the return of ammonia generated in neurons when the glutamate-glutamine cycle is running.
In the lactate–alanine shuttle the amino acid (AA) is alanine and the ammonia produced in neurons is fixed into α-KG by the GDH reaction to form glutamate, then transaminated by alanine aminotransferase into the KA (keto-acid) pyruvate to form alanine which is exported to astrocytes. In the astrocytes this process is then reversed, and pyruvate is transported in the other direction (it is likely that pyruvate may be converted to lactate for the transfer process to occur). In the branched-chain AA shuttle the AA is a branched-chain AA such as leucine. Here, the ammonia fixed in the GDH reaction in the neurons is transaminated into the KA (keto-acid) α-ketoisocaproate to form leucine, which is exported to astrocytes. In the astrocytes, the process is reversed, i.e. α-ketoisocaproate is transported in the other direction. Abbreviations: Glc, glucose; Gln, glutamine; Glu, glutamate.
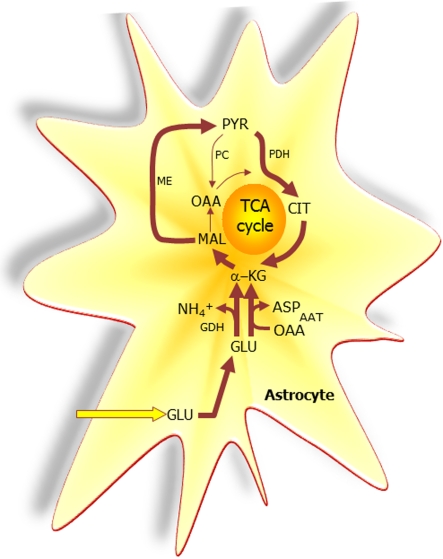
Figure 3. The extent to which glutamate (GLU) is oxidized in astrocytes seems to increase particularly during higher GLU concentrations.
A net synthesis of TCA cycle intermediates occurs when the initial reaction is catalysed by GDH which paves the way for the complete oxidation of the carbon skeleton of GLU. This requires pyruvate recycling via the concerted action of malic enzyme (ME) and pyruvate dehydrogenase (PDH) converting malate into acetyl-CoA producing NAD(P)H. Acetyl-CoA is oxidized completely in one turn of the TCA cycle. A partial oxidation of GLU is acquired when pyruvate (PYR) is reduced to lactate (LAC) instead of being oxidized to acetyl-CoA. The redox state of the cell is likely important in the regulation of the destiny of the GLU molecule. Alternative to the activity of GDH, AAT facilitates the formation of α-KG from GLU at the expense of OAA (oxaloacetate); thus no net synthesis of TCA cycle intermediates is obtained. In contrast to the complete oxidation initiated by the activity of GDH, AAT enables the truncated TCA cycle which refers to the net synthesis of aspartate from GLU, a pathway shown to accelerate during hypoglycaemic conditions. PC, pyruvate carboxylase; CIT, citrate.
Astrocytes respond to glutamate by enhancing both glucose utilization and lactate production and release (Pellerin and Magistretti, 1994, 2003; Fray et al., 1996), which has been suggested to lead to the increase in extracellular lactate that follows cortical activation (Hu and Wilson, 1997); however, the exact sources and sinks of extracellular lactate during activation are still elusive, as discussed recently by Kasischke (2011), and neurons might indeed contribute to extracellular lactate (Caesar et al., 2008; Bak et al., 2009; Contreras and Satrustegui, 2009) as well as consume at least half of the available extracellular glucose (Zielke et al., 2007). Glutamate may induce glycolysis in astrocytes (Pellerin et al., 2007) which is mediated by the activation of a Na+-dependent uptake system rather than the activation of extracellular glutamate receptors (Pellerin and Magistretti, 1994); however, others have not been able to show this coupling between glutamate uptake and stimulation of glycolysis, probably indicating astrocytic diversity (Hertz et al., 1998, 2007). Using FRET nanosensors for glucose, it was recently confirmed that glutamate stimulates glycolysis in cultured astrocytes, but only after a lag of several minutes (Bittner et al., 2011). On the other hand, a prolonged application of glutamate results in a switch of the astrocytic metabolism from glycolytic to oxidative, which is manifested as a stimulation of mitochondrial activity, decreased glucose uptake and decreased glycolytic lactate production (Dienel, 2004; Liao and Chen, 2003). This may be related to the fact that glutamate is an energy substrate in astrocytes (Hertz et al., 2007). It was recently shown that glutamate added to the extracellular solution containing 3 mM of glucose results in a significant decrease in cytosolic glucose concentration in astrocytes (Prebil et al., 2011a). The high glutamate concentration may interfere with the intermediates of the TCA cycle (Yu et al., 1982). This suggests that elevated glutamate may be used by astrocytes as an energy source and that glucose may be directed towards glycogen synthesis, hence a decrease in cytosolic glucose concentration. The time-course of the glucose concentration decrease has a time-constant of approximately 50 s with a delay to onset of the change of 24 s after stimulation, comparable with the delay of glutamate-stimulated hexose uptake (Loaiza et al., 2003). The glutamate together with K+ triggers an increase in the rate of glycolysis in astrocytes (Bittner et al., 2010). On the other hand, when glutamate is added to a glucose-free extracellular medium, a slow increase in the cytosolic glucose concentration was detected (Prebil et al., 2011a). In this case the glutamate is likely to be used as an energy source (see Hertz et al., 2007) and it enabled glucose to be spared from immediate use. This mechanism was confirmed by the inhibition of glycogen mobilization using a GPa inhibitor DAB (diaminobenzidine), where such an increase in intracellular glucose after glutamate addition was attenuated (Prebil et al., 2011a). This confirms that glutamate may be used as an alternative source of energy (Swanson et al., 1990) and that the glycogen-derived glucose may be preserved in hypoglycaemic conditions. The increase in intracellular glucose in glucose-free extracellular medium is in agreement with the view that astrocytes may provide an endogenous source of brain glucose, since they express glucose-6-phosphatase-β (Forsyth et al., 1993; Ghosh et al., 2005); however, the role of astrocytes as glucose-releasing cells by this mechanism is controversial and has to be further investigated (Dringen and Hamprecht, 1993; Forsyth, 1996).
Modulation of glucose metabolism in astrocytes by noradrenaline, adrenaline and ATP
Receptors for multiple neurotransmitters co-exist on astrocytes and can regulate energy metabolism (Magistretti, 1988). In astrocytes, noradrenaline activates both α- and β-adrenergic receptors (Northam et al., 1989; Hertz et al., 2010), which induces glycogen breakdown (Subbarao and Hertz, 1990a, 1991; Pellerin and Magistretti, 1994; Fray et al., 1996; Gibbs et al., 2008; Walls et al., 2009; Obel et al., 2012). In addition to rapid glycogen breakdown, noradrenaline stimulates long-term glycogen re-synthesis (Pellerin et al., 1997). The increase in glycogen turnover was found to be dependent on the activation of α2 adrenergic receptors involving the Gi/o-PI3K (phosphoinositide 3-kinase) pathway in chick astrocytes (Hutchinson et al., 2011). Although β1-adrenergic receptors are the predominant β-adrenergic receptors in mouse astrocytes, the activation of β2- and β3-adrenergic receptors was found to increase glucose uptake in mouse astrocytes (Catus et al., 2011). Noradrenaline-induced pyruvate decarboxylation was found to result from an increase in intra-mitochondrial concentration of Ca2+ in astrocytes (Chen and Hertz, 1999). This significantly stimulates the TCA cycle in astrocytes (Subbarao and Hertz, 1990b, 1991). The inhibition of β2-adrenergic stimulation of glycogen synthesis is associated with cognitive impairment (Hertz and Gibbs, 2009).
An application of adrenaline or noradrenaline results in increased cytosolic glucose concentration from 0.3 to 0.5 mM, with the initial rates of [glc]i rising at 1.6 μM/s (Prebil et al., 2011a). This is similar to the total glycolytic rate (1.8 μM/s) measured in astrocytes bathed in the GLUT inhibitor cytochalasin B (Bittner et al., 2010). The β-adrenergic stimulation of astrocytes modulates cytosolic glucose via changes in cytosolic cAMP levels (Pellerin et al., 1997). In cells stimulated with noradrenaline and preincubated with DAB, a GPa inhibitor, cells displayed only one-third of the [glc]i increase compared with noradrenaline-stimulated cells without DAB preincubation (Prebil et al., 2011a). α- and β-adrenergic receptors induce glycogen breakdown in astrocytes (Subbarao and Hertz, 1990a, 1991; Pellerin and Magistretti, 1994; Fray et al., 1996; Gibbs et al., 2008) and the increased availability of cytosolic glucose after adrenaline or noradrenaline stimulation suggests that the stimulated glycogen breakdown exceeds the cytosolic glucose utilization in astrocytes (Prebil et al., 2011a). On the other hand, noradrenaline-stimulated glucose uptake was also demonstrated (Yu et al., 1993).
Using the FRET-based glucose nanosensor protein FLIPglu-600 μ in 3T3-L1 fibroblasts and adipocytes revealed that the changes in cytosolic glucose concentration were only detected in 56% of 3T3-L1 fibroblasts and in 14% of 3T3-L1 adipocytes, where insulin increased cytosolic glucose concentration by a factor of 4. On the other hand, adrenaline increased cytosolic glucose concentration in fibroblasts but not in adipocytes (Kovacic et al., 2011). Similarly, adrenaline inhibits glycogen synthase and activates GP in muscle (Villa-Moruzzi et al., 1979). In astrocytes, glycogen is continuously synthesized and degraded (Shulman et al., 2001), and lactate originating from glycogen is compartmentalized from that derived from glucose (Sickmann et al., 2005).
ATP is a major factor mediating intercellular communication and triggers a variety of different biological effects (Brake and Julius, 1996) and is considered to be the dominant extracellular messenger for astrocyte-to-astrocyte communication (Cotrina et al., 1998; Guthrie et al., 1999; Wang et al., 2000; Pangrsic et al., 2007; Parpura and Zorec, 2010). It is released from astrocytes upon mechanical stimulation (Guthrie et al., 1999) or glutamatergic receptor activation (Queiroz et al., 1997; Pangrsic et al., 2007). Astrocytes respond to ATP with a propagating wave of intracellular calcium increases (Guthrie et al., 1999), a process that is thought to serve as a long-range signalling system in the CNS (central nervous system; Cornell-Bell et al., 1990; Koizumi et al., 2005). ATP stimulation promotes exocytosis in astrocytes (Pangrsic et al., 2006) and ATP released from astrocytes as a result of neuronal activity modulates synaptic transmission (Zhang et al., 2003). Furthermore, astrocytes are capable of ATP-induced ATP release (Anderson et al., 2004). It should be noted that there is some controversy regarding release of ATP during hypoxic conditions (Martín et al., 2007; Fujita et al., 2012).
ATP is an agonist for P2Y and P2X receptors (Ralevic and Burnstock, 1998). Primary rat cortical astrocytes express ligand-gated P2X ion channels (i.e. P2X1–5 and P2X7) and G-protein-coupled P2Y receptors (i.e. P2Y1, P2Y2, P2Y4, P2Y6 and P2Y12) (Fumagalli et al., 2003). Up-regulation of receptors in astrocytes after injury has been found (Franke et al., 2001, 2004). The P2X7 subtype acts also as a permeabilization pore that can induce cell death under prolonged activation by ATP (Innocenti et al., 2004). Astrocyte-released ATP mediates a paracrine activation of microglial P2X7 receptors that triggers a perturbation of calcium homoeostasis and finally leads to microglial cell death (Verderio and Matteoli, 2001).
Stimulation of isolated astrocytes with ATP decreases cytosolic glucose concentration with a time constant of approximately 150 s (Prebil et al., 2011b). The mechanism of ATP-dependent glucose concentration decrease is not yet fully understood, and may potentially affect glucose transport or metabolism. In astrocytes, purinergic receptors, particularly the P2X7 subtype, are coupled to the PI3K/Akt [also known as PKB (protein kinase B)] pathway (Jacques-Silva et al., 2004). On the other hand, in renal mesangial cells ATP was reported to activate Akt via the P2Y receptor (Huwiler et al., 2002). In astrocytes, ATP stimulates Akt phosphorylation, where calcium, PI3K and a Src family kinase are involved (Jacques-Silva et al., 2004), which may result in several cellular downstream effects related to the Akt pathway, such as glycogen synthesis (Brazil and Hemmings, 2001). Activated (phosphorylated) Akt phosphorylates and inactivates GSK3 (GS kinase 3), which is a major protein kinase involved in the regulation of glucose metabolism in muscle (Medina and Castro, 2008). Inactivated GSK3 results in less phosphorylated and thus more active glycogen synthase (Cross et al., 1995; Lawrence and Roach, 1997; McManus et al., 2005), which may lead to a decline in cytosolic glucose levels as observed in ATP-stimulated astrocytes (Prebil et al., 2011b). On the other hand, stimulation of P2X7 receptors is associated with the activation of PKC (protein kinase C) and phospholipase D in astrocytes (Sun et al., 1999), which may represent an alternative pathway of glycogen synthase activation. ATP stimulation of P2 receptors of rat cortical astrocytes was shown to result in inhibition of GSK3 activity by a PKC-dependent pathway that is independent of Akt (Neary and Kang, 2006).
AMINO ACID METABOLISM
Astrocytes are obviously involved in metabolism of all amino acids but this review will be focused on the role of astrocytes in the metabolic homoeostasis of the two major neuroactive amino acids, glutamate and GABA (γ-aminobutyric acid) mediating the vast majority of excitatory and inhibitory neurotransmitter signalling respectively. The key enzymes involved in metabolic reactions pertinent to the turnover of the neurotransmitters glutamate and GABA as well as their prevailing cellular localization has recently been reviewed by Waagepetersen et al. (2009). It should be noted that GS is exclusively expressed in astrocytes (Norenberg and Martinez-Hernandez, 1979) and glutamate decarboxylase is only present in GABAergic neurons and not in astrocytes (Hertz et al., 1992). In addition, it is of functional importance that the activity of PAG (phosphate-activated glutaminase) is higher in neurons than in astrocytes (Schousboe et al., 1979; Drejer et al., 1985; Larsson et al., 1985; Lovatt et al. 2007). This difference in the expression level of GS and PAG in astrocytes forms the basis for the glutamate-glutamine cycle which was originally proposed on the basis of studies of glutamate and glutamine metabolism in brain tissue preparations which indicated different cellular compartments of these amino acids with different turnover rates (Berl et al., 1961, 1962; Van den Berg and Garfinkel, 1971). The glutamate–glutamine cycle is in short the clearance of glutamate from the synaptic cleft by uptake into astrocytes and the subsequent amidation of glutamate into glutamine which is transferred into neurons for re-synthesis of glutamate (Figure 2). Hence the cycle leads to a net transfer of nitrogen from the astrocytic to the neuronal compartment. In order to maintain nitrogen homoeostasis in the ‘tripartite’ microenvironment, i.e. the pre- and post-synaptic neuron and the surrounding astrocyte, this nitrogen must be transferred back to the astrocyte (Figure 2). This may be accomplished by transfer of an amino acid, e.g. alanine, which is thought to be transaminated forming glutamate from which the amino group may be liberated by the action of GDH (glutamate dehydrogenase; Waagepetersen et al. 2000; Schousboe et al., 2003; Bak et al., 2006). The amino group may subsequently take part in the GS reaction. For the shuttle to operate stoichiometrically, the GDH reaction has to operate in both directions, i.e. reductive amination in neurons and oxidative deamination in astrocytes. The high content of ammonia in the glutamatergic neurons may overcome the problem that deamination seems to be favoured (Plaitakis and Zaganas, 2001). Additionally, the branched chain amino acids have been proposed to provide the amino nitrogen for de novo synthesis of glutamate via pyruvate carboxylation in astrocytes followed by amidation by GS and transfer of glutamine to the neurons (Lieth et al., 2001). In order for the glutamate–glutamine cycle to operate stoichiometrically, all glutamate taken up by astrocytes via high affinity glutamate transporters (Danbolt, 2001) must be converted to glutamine in the GS-catalysed reaction (Cotman et al., 1981). However, numerous metabolic studies have demonstrated considerable oxidative metabolism of glutamate via the TCA cycle (Yu et al., 1982; McKenna et al, 1996; Sonnewald et al., 1997). The conversion of the carbon skeleton of glutamate to α-KG can take place by two different enzymatic pathways, i.e. via the GDH-catalysed oxidative deamination or by transamination (Figure 3). The latter process may be catalysed by any aminotransferase, but since AAT (aspartate aminotransferase) is the member of this family of enzymes having by far the highest activity in the brain (Erecinska and Silver, 1990), this is the most likely enzyme to catalyse this reaction. It is probable that oxidative deamination catalysed by GDH plays a prominent role, since the aminotransferase inhibitor AOAA (aminooxyacetic acid) in several studies has been shown to inhibit oxidation of glutamate in the TCA cycle (e.g. Yu et al., 1982; Westergaard et al., 1996). The conclusion from the above-mentioned considerations is that a substantial fraction of the glutamate taken up into astrocytes during glutamatergic activity is oxidatively metabolized in the TCA cycle (see Westergaard et al., 1995) and hence, the glutamate–glutamine cycle is not operating stoichiometrically (McKenna et al., 2012). This imposes a need for de novo synthesis of the glutamate carbon skeleton which is dependent on the pyruvate carboxylase reaction that, like GS, is confined to astrocytes (Yu et al., 1983). It should also be pointed out that oxidation of the carbon skeleton of glutamate, i.e. α-KG, requires pyruvate recycling (Figure 3), a process that has been shown to occur in astrocytes (Sonnewald et al., 1996; Waagepetersen et al., 2002). In this pathway, malate originating from the TCA cycle is converted to pyruvate by malic enzyme and subsequently decarboxylated by pyruvate dehydrogenase and oxidized in the TCA cycle (Bak et al., 2007; Obel et al., 2012).
The demonstration of a significant albeit low activity of PAG in cultured astrocytes (Schousboe et al., 1979) is compatible with the observation that glutamine can be oxidatively metabolized in astrocytes after conversion to first glutamate and then α-KG (Yu and Hertz., 1983; Hertz et al., 1988). In line with this, the use of 13C-labelled glutamine and MR spectroscopy has demonstrated substantial metabolism of glutamine in astrocytes, a process coupled to pyruvate recycling (Sonnewald et al., 1996). Metabolism of glutamine via PAG leads to production of not only glutamate but also ammonia and in the case of glutamate is oxidatively metabolized by GDH and an additional molecule of ammonia is produced. This ammonia must eventually be disposed of which likely occurs predominantly by conversion of glutamate to glutamine by GS. The fact that the PAG- and GS-catalysed reactions are intracellularly separated taking place in the mitochondrial (PAG) and the cytoplasmic (GS) compartments respectively, allows regulatory control. Nevertheless, exposure of astrocytes to elevated glutamine concentrations leads to adverse effects on mitochondria caused by ammonia liberated in the PAG reaction as demonstrated by Jayakumar et al. (2004).
Astrocytic uptake and metabolism of GABA appears to be of importance for the functional capacity of GABAergic neurotransmission, since inhibitors of astrocytic GABA transporters as well as GABA-T (GABA-aminotransferase) act as anticonvulsants (Sarup et al., 2003; Schousboe et al. 2010). GABA will be metabolized into succinic semi-aldehyde in the astrocytic mitochondria that contain appreciable activity of GABA-T (Waagepetersen et al., 2009). Succinic semi-aldehyde dehydrogenase catalyses the subsequent oxidation of succinic semi-aldehyde to succinate which may be used for glutamate and glutamine synthesis via conversion to α-KG using acetyl-CoA from glucose metabolism (Waagepetersen and Schousboe, 2007) or oxidized to CO2 via pyruvate recycling.
METABOLIC COMPARTMENTATION OF ENERGY METABOLISM
Metabolic compartmentation at the level of the single cell is defined as the presence of multiple, distinct intracellular pools of identical metabolites that are not in equilibrium. The energy metabolism of astrocytes in culture has been shown numerous times to be compartmentalized (Schousboe et al., 1993; Sonnewald et al., 1993; Bouzier et al., 1998; Qu et al., 1999; Cruz et al., 2001; Waagepetersen et al., 2001, 2006; Zwingmann et al., 2001). As mentioned above, the pyruvate pool seems to be highly compartmentalized (Figure 4) since lactate released from cultured astrocytes is derived from the metabolism of extracellular glucose but not breakdown of glycogen (Sickmann et al., 2005). This may be difficult to understand since metabolites are seemingly diffusing freely inside the aqueous environment of the cell. There are several possible answers to this; importantly, the intracellular compartment of an astrocyte, or indeed any cell, cannot be regarded as being analogous to a glass of water with all metabolites existing in a thermodynamically ideal mixture. Cytoplasm is a very heterogeneous environment containing high concentrations of small metabolites, macromolecules, ions and multiple membrane-bound boundaries between organelles. These aspects are discussed in an almost 30-year-old review by Clegg (1984). Interestingly, recent mathematical modelling of the diffusion of metabolites inside cells shows that cytosolic compartmentation of high-concentration metabolites (e.g. glucose, lactate, pyruvate and ATP) due to molecular sinks such as enzymes and transporters should not be possible and that such compartmentation is restricted to signalling molecules such as cytosolic Ca2+ (Martinez et al., 2010; Barros and Martinez, 2007). Thus, metabolic compartmentation may not arise due to localized consumption of e.g. ATP or glucose. However, a number of issues complicate matters somewhat. First, the astrocyte (as well as the neuron) has a complex morphology and may be divided into functional domains (Kimelberg and Nedergaard, 2010). Secondly, as implied above, the astrocyte may be regarded as a crowded place; metabolites and organelles are moving around in a morphologically and functionally complex manner and diverse cell in a semi-aqueous cytosolic environment with internal physical barriers (i.e. organellar membranes) to isotropic diffusion. Previous work in cardiomyocytes has shown that diffusion of ATP is anisotropic and 2–3 times slower than in dilute solution (Vendelin and Birkedal, 2008); these authors suggested that the anisotropy was caused by intracellular membranes hindering diffusion. Whether a 2–3 times slower rate of diffusion is sufficient to contribute to intracellular compartmentation of metabolism remains to be established. Thirdly, the existence of functionally and metabolically heterogeneous pools of mitochondria has been suggested and indeed mitochondria are very dynamic and heterogeneous organelles (Hollenbeck and Saxton, 2005; Waagepetersen et al., 2001). Lastly, some (astrocytic) biochemical processes, such as ATP-consuming membrane pumps, depend on ATP produced by substrate-level phosphorylation in the glycolytic pathway rather than the bulk pool of ATP; this might be explained by the formation of supramolecular complexes of enzymes and transporters such that at least some glycolytic ATP never enters the bulk cytosol (Schousboe et al., 2011). Solid evidence at the molecular level that mitochondria are indeed metabolically heterogeneous came from an immunogold-labelling experiment in cultured astrocytes conducted by Waagepetersen et al. (2006). Electron microscopic investigation of immunogold-labelled α-KG dehydrogenase, a key TCA cycle enzyme, showed heterogeneous distribution among mitochondria within the same cell, indicating differential capacity for mitochondria to perform oxidative metabolism. This implies that some mitochondria may be tuned to produce energy in the form of ATP, whereas others may be tuned to perform a different task, e.g. anaplerotic reactions for synthesis of glutamine for export to neurons as a precursor for neurotransmitter glutamate and GABA synthesis (Figure 4 and Waagepetersen et al., 2001). Indeed, metabolic compartmentation of astrocytic (energy) metabolism is complex and much is still left to be learned about this subject.
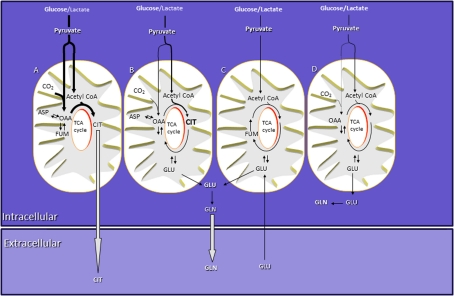
Figure 4. Schematic presentation of multiple compartments in astrocytes.
Synthesis of a large amount of releasable citrate via pyruvate carboxylase occurs in compartment A, a compartment for preferential glucose metabolism. Releasable glutamine is synthesized from glutamate originating from compartments B and C. Glucose is the main oxidative substrate for compartment B, whereas lactate and glucose are metabolized to the same extent in compartments C and D. The main intracellular pool of glutamine is synthesized from glutamate originating from compartment D. The size of the arrows provide an estimate of the relative magnitudes of the respective fluxes. GLN, glutamine; GLU, glutamate; CIT, citrate; OAA, oxaloacetate; ASP, aspartate; FUM, fumarate.
CONCLUDING REMARKS
Brown and Prior (2006) noted that over the last few decades the methods to study brain-energy metabolism have evolved from inflating a pneumatic cuff in order to occlude the carotid arteries in ‘volunteer’ prisoners, to MRI (magnetic resonance imaging). They also note that there is a “desire to measure energy metabolism in a single cell (or even different regions of a single cell) in the brain in real-time” (Brown and Prior, 2006). However, some years ago this was not possible due to a limited spatial and temporal resolution of monitoring techniques. New implications of FRET-based nanosensors may exemplify such a high spatial and high temporal resolution technique to address brain energy metabolism at the subcellular level.
Pertinent research questions to be asked are many when it comes to subcellular compartmentation of (energy) metabolism, such as why are seemingly identical metabolic pathways such as glycolysis starting from extracellular glucose segregated from glycolysis starting from glucose-6-phosphate derived from breakdown of glycogen? And, are metabolically heterogeneous mitochondria localized to different cellular compartments? Heterogeneous populations of mitochondria between organs have been shown and might be therapeutically targeted (Jayakumar et al., 2007); in the same way, it might in the future be both desirable and possible to target specific cellular populations of mitochondria, the latter e.g. based on differences in their membrane potential.
REFERENCES
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Mercer AJ, Haorah J. Ethanol impairs glucose uptake by human astrocytes and neurons: protective effects of acetyl-l-carnitine. Int J Physiol Pathophysiol Pharmacol. 2011;3:48–56. [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl) 2011;216:75–84. doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- Allen N, Barres B. Neuroscience: glia–more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Anderson C, Bergher J, Swanson R. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Arluison M, Quignon M, Nguyen P, Thorens B, Leloup C, Penicaud L. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain – an immunohistochemical study. J Chem Neuroanat. 2004;28:117–136. doi: 10.1016/j.jchemneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Bak LK, Waagepetersen HS, Melø TM, Schousboe A, Sonnewald U. Complex glutamate labeling from [U-13C]glucose or [U-13C]lactate in co-cultures of cerebellar neurons and astrocytes. Neurochem Res. 2007;32:671–680. doi: 10.1007/s11064-006-9161-4. [DOI] [PubMed] [Google Scholar]
- Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem. 2009;109(Suppl 1):87–93. doi: 10.1111/j.1471-4159.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2010;63:149–159. doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Barros LF, Martinez C. An enquiry into metabolite domains. Biophysical Journal. 2007;92:3878–3884. doi: 10.1529/biophysj.106.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer P, Ross B. Oxidative stress in the central nervous system: monitoring the metabolic response using the pentose phosphate pathway. Dev Neurosci. 1994;16:328–336. doi: 10.1159/000112127. [DOI] [PubMed] [Google Scholar]
- Bergles D, Dzubay J, Jahr C. Glutamate transporter currents in Bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci. 1997;USA.94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S, Lajtha A, Waeelsch H. Amino acid and protein metabolism–IV. Cerebral compartments of glutamic acid metabolism. J Neurochem. 1961;7:186–197. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Berl S, Takagaki G, Clarke DD, Waelsch H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962;237:2562–2569. [PubMed] [Google Scholar]
- Bittner C, Loaiza A, Ruminot I, Larenas V, Sotelo-Hitschfeld T, Gutiérrez R, Córdova A, Valdebenito R, Frommer W, Barros L. High resolution measurement of the glycolytic rate. Front Neuroenergetics. 2010;15:26. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martín A, Gutiérrez R, Zambrano M, Barros LF. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier AK, Goodwin R, de Gannes FM, Valeins H, Voisin P, Canioni P, Merle M. Compartmentation of lactate and glucose metabolism in C6 glioma cells. A 13C and 1H NMR study. J Biol Chem. 1998;273:27162–27169. doi: 10.1074/jbc.273.42.27162. [DOI] [PubMed] [Google Scholar]
- Brake A, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brown AM, Prior MJW. Neuronal lactate metabolism–the first piece of the jigsaw falls in to place. Physiol News. 2006;64:23–25. [Google Scholar]
- Brown A, Ransom B. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Brown A, Wender R, Ransom B. Metabolic substrates other than glucose support axon function in central white matter. J Neurosci Res. 2001;66:839–843. doi: 10.1002/jnr.10081. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo A, Broadwell R. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. Br J Pharmacol. 2011;162:1700–1715. doi: 10.1111/j.1476-5381.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Lehre K, van Lookeren Campagne M, Ottersen O, Danbolt N, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hertz L. Noradrenaline effects on pyruvate decarboxylation: correlation with calcium signaling. J Neurosci Res. 1999;58:599–606. doi: 10.1002/(sici)1097-4547(19991115)58:4<599::aid-jnr13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Cheng C, Glover G, Banker G, Amara S. A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin–Darby canine kidney cells and hippocampal neurons. J Neurosci. 2002;22:10643–10652. doi: 10.1523/JNEUROSCI.22-24-10643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsaki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci. 2010;30:15298–15303. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JS. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984;246:R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Contreras L, Satrustegui J. Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem. 2009;284:7091–7099. doi: 10.1074/jbc.M808066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell A, Finkbeiner S, Cooper M, Smith S. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Foster A, Lanthorn T. An overview of glutamate as a neurotransmitter. Adv Biochem Psychopharmacol. 1981;27:1–27. [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cruz F, Villalba M, Garcia-Espinosa MA, Ballesteros P, Bogonez E, Satrustegui J, Cerdan S. Intracellular compartmentation of pyruvate in primary cultures of cortical neurons as detected by (13)C NMR spectroscopy with multiple (13)C labels. J Neurosci Res. 2001;66:771–781. doi: 10.1002/jnr.10048. [DOI] [PubMed] [Google Scholar]
- Danbolt N. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Derouiche A. The perisynaptic astrocyte process as a glial compartment-immunolabelling for gluthamine synthetase and other glial markers. In: Hertz L, editor. Nonneuronal Cells of the Nervous System. Amsterdam, The Netherlands: Elsevier; 2004. pp. 147–163. [Google Scholar]
- Dienel G. Lactate muscles its way into consciousness: fueling brain activation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R519–R521. doi: 10.1152/ajpregu.00377.2004. [DOI] [PubMed] [Google Scholar]
- Dienel G, Cruz N. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood–borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Maraviglia B, Giove F. Why does the brain (not) have glycogen? Bioessays. 2011;33:319–326. doi: 10.1002/bies.201000151. [DOI] [PubMed] [Google Scholar]
- Drejer J, Larsson OM, Kvamme E, Svenneby G, Hertz L, Schousboe A. Ontogenetic development of glutamate metabolizing enzymes in cultured cerebellar granule cells and in cerebellum in vivo. Neurochem Res. 1985;10:49–62. doi: 10.1007/BF00964771. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hamprecht B. Differences in glycogen metabolism in astroglia-rich primary cultures and sorbitol-selected astroglial cultures derived from mouse brain. Glia. 1993;8:143–149. doi: 10.1002/glia.440080302. [DOI] [PubMed] [Google Scholar]
- Dzubay J, Jahr C. The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci. 1999;19:5265–5274. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Fehr M, Lalonde S, Lager I, Wolff M, Frommer W. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J Biol Chem. 2003;278:19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- Fellows L, Boutelle M, Fillenz M. Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J Neurochem. 1992;59:2141–2147. doi: 10.1111/j.1471-4159.1992.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fillenz M, Lowry J, Boutelle M, Fray A. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol Scand. 1999;167:275–284. doi: 10.1046/j.1365-201x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Forsyth R. Astrocytes and the delivery of glucose from plasma to neurons. Neurochem Int. 1996;28:231–241. doi: 10.1016/0197-0186(95)00094-1. [DOI] [PubMed] [Google Scholar]
- Forsyth R, Bartlett K, Burchell A, Scott H, Eyre J. Astrocytic glucose-6-phosphatase and the permeability of brain microsomes to glucose 6-phosphate. Biochem J. 1993;294:145–151. doi: 10.1042/bj2940145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier K, González M, Robinson M. Rapid trafficking of the neuronal glutamate transporter, EAAC1: evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem. 2004;279:34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
- Fox P, Raichle M, Mintun M, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Franke H, Grosche J, Schädlich H, Krügel U, Allgaier C, Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001;108:421–429. doi: 10.1016/s0306-4522(01)00416-x. [DOI] [PubMed] [Google Scholar]
- Franke H, Krügel U, Grosche J, Heine C, Härtig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Fray A, Forsyth R, Boutelle M, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol. 1996;496:49–57. doi: 10.1113/jphysiol.1996.sp021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Williams EK, Jensen TK, Smith NA, Takano T, Tieu K, Nedergaard M. Cultured astrocytes do not release adenosine during hypoxic conditions. J Cereb Blood Flow Metab. 2012;32:e1–e7. doi: 10.1038/jcbfm.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D'Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–203. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Gandhi G, Cruz N, Ball K, Dienel G. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Cheung Y, Mansfield B, Chou J. Brain contains a functional glucose-6-phosphatase complex capable of endogenous glucose production. J Biol Chem. 2005;280:11114–11119. doi: 10.1074/jbc.M410894200. [DOI] [PubMed] [Google Scholar]
- Gibbs M, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32:927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gordon G, Choi H, Rungta R, Ellis-Davies G, MacVicar B. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L, Gelb B, Adams V, McCabe E. Developmental expression of hexokinase 1 in the rat. Biochim Biophys Acta. 1992;1129:309–317. doi: 10.1016/0167-4781(92)90508-w. [DOI] [PubMed] [Google Scholar]
- Guthrie P, Knappenberger J, Segal M, Bennett M, Charles A, Kater S. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. The blood–brain barrier and glutamate. Am J Clin Nutr. 2009;90:867S–874S. doi: 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heni M, Hennige AM, Peter A, Siegel-Axel D, Ordelheide AM, TCA N, Machicao F, Fritsche A, Häring HU, Staiger H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE. 2011;6:e21594. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Glutamate, a neurotransmitter-and so much more. A synopsis of Wierzba III. Neurochem Int. 2006;48:416–425. doi: 10.1016/j.neuint.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel G. Energy metabolism in the brain. Int Rev Neurobiol. 2002;51:1–102. doi: 10.1016/s0074-7742(02)51003-5. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke H. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs M. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Drejer J, Schousboe A. Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochem Res. 1988;13:605–610. doi: 10.1007/BF00973275. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Westergaard N, Yudkoff M, Schousboe A. Neuronal-astrocytic interactions in metabolism of transmitter amino acids of the glutamate family. In: Schousboe A, Diemer NH, Kofod H, editors. Drug Research Related to Neuroactive Amino Acids, Alfred Benzon Symposium 32. Munksgaard, Copenhagen: 1992. pp. 30–48. [Google Scholar]
- Hertz L, Swanson RA, Newman GC, Marrif H, Juurlink BH, Peng L. Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis? Dev Neurosci. 1998;20:339–347. doi: 10.1159/000017329. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel G. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hertz L, Lovatt D, Goldman S, Nedergaard M. Adrenoceptors in brain: Cellular gene expression and effects on astrocytic metabolism and [Ca(2+)](i). Neurochem Int. 2010;57:411–20. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wilson G. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Catus SL, Merlin J, Summers RJ, Gibbs ME. α2-Adrenoceptors activate noradrenaline-mediated glycogen turnover in chick astrocytes. J Neurochem. 2011;117:915–926. doi: 10.1111/j.1471-4159.2011.07261.x. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Rölz W, Dorsch S, Ren S, Pfeilschifter J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y(2) purinoceptor in renal mesangial cells. Br J Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. Glycogen and its related enzymes of metabolism in the central nervous system. Adv Anat Embryol Cell Biol. 1975;52:3–89. doi: 10.1007/978-3-642-86875-7. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E. ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci. 2004;24:8577–8583. doi: 10.1523/JNEUROSCI.2812-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi S, Kawahara K. Inducible astrocytic glucose transporter-3 contributes to the enhanced storage of intracellular glycogen during reperfusion after ischemia. Neurochem Int. 2011;59:319–325. doi: 10.1016/j.neuint.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Jacques-Silva M, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez F, Weisman G, Neary J. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol. 2004;141:1106–1117. doi: 10.1038/sj.bjp.0705685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Rama Rao KV, Schousboe A, Norenberg MD. Glutamine-induced free radical production in cultured astrocytes. Glia. 2004;46:296–301. doi: 10.1002/glia.20003. [DOI] [PubMed] [Google Scholar]
- Jayakumar DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS. Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol. 2007;292:C689–C697. doi: 10.1152/ajpcell.00108.2006. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kasischke K. Lactate fuels the neonatal brain. Front Neuroenergetics. 2011;3:4. doi: 10.3389/fnene.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K. Regulation of cell-to-cell communication mediated by astrocytic ATP in the CNS. Purinergic Signal. 2005;1:211–217. doi: 10.1007/s11302-005-6321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic PB, Chowdhury HH, Velebit J, Kreft M, Jensen J, Zorec R. New insights into cytosolic glucose levels during differentiation of 3T3-L1 fibroblasts into adipocytes. J Biol Chem. 2011;286:13370–13381. doi: 10.1074/jbc.M110.200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon P, Zorec R. Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- Lai J, Behar K, Liang B, Hertz L. Hexokinase in astrocytes: kinetic and regulatory properties. Metab Brain Dis. 1999;14:125–133. doi: 10.1023/a:1020761831295. [DOI] [PubMed] [Google Scholar]
- Larsson OM, Drejer J, Kvamme E, Svenneby G, Hertz L, Schousboe A. Ontogenetic development of glutamate and GABA metabolizing enzymes in cultured cerebral cortex interneurons and in cerebral cortex in vivo. Int J Dev Neurosci. 1985;3:177–185. doi: 10.1016/0736-5748(85)90008-5. [DOI] [PubMed] [Google Scholar]
- Lawrence JC, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- Leino R, Gerhart D, van Bueren A, McCall A, Drewes L. Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res. 1997;49:617–626. doi: 10.1002/(SICI)1097-4547(19970901)49:5<617::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat P, Ferré P, Pénicaud L. Glucose transporter 2 (GLUT 2): expression in specific brain nuclei. Brain Res. 1994;638:221–226. doi: 10.1016/0006-8993(94)90653-x. [DOI] [PubMed] [Google Scholar]
- Leo G, Driscoll B, Shank R, Kaufman E. Analysis of [1-13C]D-glucose metabolism in cultured astrocytes and neurons using nuclear magnetic resonance spectroscopy. Dev Neurosci. 1993;15:282–288. doi: 10.1159/000111346. [DOI] [PubMed] [Google Scholar]
- Liao S, Chen C. L-glutamate decreases glucose utilization by rat cortical astrocytes. Neurosci Lett. 2003;348:81–84. doi: 10.1016/s0304-3940(03)00721-3. [DOI] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712–1723. doi: 10.1046/j.1471-4159.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- Loaiza A, Porras O, Barros L. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JHC, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;47:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R, Fogarty K, Fay F. Modulation of hexokinase association with mitochondria analysed with quantitative three-dimensional confocal microscopy. J Cell Biol. 1991;112:385–395. doi: 10.1083/jcb.112.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Ceña V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- McKenna M, Stevenson J, Huang X, Hopkins I. Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int. 2000;37:229–241. doi: 10.1016/s0197-0186(00)00042-5. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A. Energy metabolism of the brain. In: Brady ST, Siegel GJ, Albers RW, Price DI, editors. Basic Neurochemistry: Principles of Molecular, Cellular, and Medical Neurobiology, 8th ed. Waltham, MA, USA: Academic Press, Elsevier; 2012. pp. 200–231. [Google Scholar]
- Magistretti P. Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabetes Metab. 1988;14:237–246. [PubMed] [Google Scholar]
- Magistretti P. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci S, Simpson I. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- Martinez C, Kalise D, Barros LF. General requirement for harvesting antennae at ca and h channels and transporters. Front Neuroenergetics. 2010;10:27. doi: 10.3389/fnene.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, Castro A. Glycogen synthase kinase-3 (GSK-3) inhibitors reach the clinic. Curr Opin Drug Discov Devel. 2008;11:533–543. [PubMed] [Google Scholar]
- Miller A, Hawkins R, Veech R. Decreased rate of glucose utilization by rat brain in vivo after exposure to atmospheres containing high concentrations of CO2. J Neurochem. 1975;25:553–558. doi: 10.1111/j.1471-4159.1975.tb04367.x. [DOI] [PubMed] [Google Scholar]
- Morgello S, Uson R, Schwartz E, Haber R. The human blood–brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Nakamichi Y, Inoue N, Inoue M, Nishino H, Sawa H. Rat C6 glioma cell growth is related to glucose transport and metabolism. Biochem J. 1996;319:477–482. doi: 10.1042/bj3190477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J, Kang Y. P2 purinergic receptors signal to glycogen synthase kinase-3beta in astrocytes. J Neurosci Res. 2006;84:515–524. doi: 10.1002/jnr.20969. [DOI] [PubMed] [Google Scholar]
- Needels D, Wilson J. The identity of hexokinase activities from mitochondrial and cytoplasmic fractions of rat brain homogenates. J Neurochem. 1983;40:1134–1143. doi: 10.1111/j.1471-4159.1983.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Nie X, Olsson Y. Endothelin peptides in brain diseases. Rev Neurosci. 1996;7:177–186. doi: 10.1515/revneuro.1996.7.3.177. [DOI] [PubMed] [Google Scholar]
- Niitsu Y, Hori O, Yamaguchi A, Bando Y, Ozawa K, Tamatani M, Ogawa S, Tohyama M. Exposure of cultured primary rat astrocytes to hypoxia results in intracellular glucose depletion and induction of glycolytic enzymes. Brain Res Mol Brain Res. 1999;74:26–34. doi: 10.1016/s0169-328x(99)00245-4. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Northam W, Bedoy C, Mobley P. Pharmacological identification of the alpha-adrenergic receptor type which inhibits the beta-adrenergic activated adenylate cyclase system in cultured astrocytes. Glia. 1989;2:129–133. doi: 10.1002/glia.440020209. [DOI] [PubMed] [Google Scholar]
- Obel LF, Andersen KM, Bak LK, Scousboe A, Waagepetersen HS. Effects of adrenergic agents on intracellular Ca2+ homeostasis and metabolism of glucose in astrocytes with an emphasis on pyruvate carboxylation, oxidative decarboxylation and recycling: implications for glutamate neurotransmission and excitotoxicity. Neurotox Res. 2012;21:405–417. doi: 10.1007/s12640-011-9296-1. [DOI] [PubMed] [Google Scholar]
- O'Kane R, Martínez-López I, DeJoseph M, Viña J, Hawkins R. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood–brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–31895. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Haydon P, Zorec R, Kreft M. Astrocyte swelling leads to membrane unfolding, not membrane insertion. J Neurochem. 2006;99:514–523. doi: 10.1111/j.1471-4159.2006.04042.x. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Baker B, Jeras M, Zorec R. Regulated exocytosis in astrocytic signal integration. Neurochem Int. 2010;57:451–459. doi: 10.1016/j.neuint.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti P. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti P. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti P. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Stolz M, Sorg O, Martin J, Deschepper C, Magistretti P. Regulation of energy metabolism by neurotransmitters in astrocytes in primary culture and in an immortalized cell line. Glia. 1997;21:74–83. doi: 10.1002/(sici)1098-1136(199709)21:1<74::aid-glia8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore A, Aubert A, Serres S, Merle M, Costalat R, Magistretti P. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Zaganas I. Regulation of human glutamate dehydrogenases: implications for glutamate, ammonia and energy metabolism in brain. J Neurosci Res. 2001;66:899–908. doi: 10.1002/jnr.10054. [DOI] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF. Na (+)-Ca (2+) cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia. 2008;56:59–68. doi: 10.1002/glia.20589. [DOI] [PubMed] [Google Scholar]
- Prebil M, Vardjan N, Jensen J, Zorec R, Kreft M. Dynamic monitoring of cytosolic glucose in single astrocytes. Glia. 2011a;59:903–913. doi: 10.1002/glia.21161. [DOI] [PubMed] [Google Scholar]
- Prebil M, Chowdhury HH, Zorec R, Kreft M. Changes in cytosolic glucose level in ATP stimulated live astrocytes. Biochem Biophys Res Commun. 2011b;405:308–313. doi: 10.1016/j.bbrc.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Qu H, Faero E, Jorgensen P, Dale O, Gisvold SE, Unsgard G, Sonnewald U. Decreased glutamate metabolism in cultured astrocytes in the presence of thiopental. Biochem Pharmacol. 1999;58:1075–1080. doi: 10.1016/s0006-2952(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Gebicke-Haerter P, Schobert A, Starke K, von Kügelgen I. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience. 1997;78:1203–1208. doi: 10.1016/s0306-4522(96)00637-9. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Robinson M. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Rothstein J, Martin L, Levey A, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl R. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein J, Dykes-Hoberg M, Pardo C, Bristol L, Jin L, Kuncl R, Kanai Y, Hediger M, Wang Y, Schielke J, Welty D. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sarup A, Larsson OM, Schousboe A. GABA transporters and GABA-transaminase as drug targets. Curr Drug Targ CNS Neurol Dis. 2003;2:269–277. doi: 10.2174/1568007033482788. [DOI] [PubMed] [Google Scholar]
- Scheiber IF, Dringen R. Copper accelerates glycolytic flux in cultured astrocytes. Neurochem Res. 2011;36:894–903. doi: 10.1007/s11064-011-0419-0. [DOI] [PubMed] [Google Scholar]
- Schmidt MM, Rohwedder A, Dringen R. Effects of chlorinated acetates on the glutathione metabolism and on glycolysis of cultured astrocytes. Neurotox Res. 2011;19:628–637. doi: 10.1007/s12640-010-9209-8. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Hertz L, Svenneby G, Kvamme E. Phosphate activated glutaminase activity and glutamine uptake in primary cultures of astrocytes. J Neurochem. 1979;32:943–950. doi: 10.1111/j.1471-4159.1979.tb04579.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sonnewald U, Waagepetersen HS. Differential roles of alanine in GABAergic and glutamatergic neurons. Neurochem Int. 2003;43:311–315. doi: 10.1016/s0197-0186(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Madsen KK, White HS. GABA transport inhibitors and seizure protection: the past and future. Future Med Chem. 2010;3:183–187. doi: 10.4155/fmc.10.288. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sickmann HM, Bak LK, Schousboe I, Jajo FS, Faek SAA, Waagepetersen HS. Neuron–glia interactions in glutamatergic neurotransmission: roles of oxidative and glycolytic adenosine triphosphate as energy source. J Neurosci Res. 2011;89:1926–1934. doi: 10.1002/jnr.22746. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L. Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci. 1993;15:359–366. doi: 10.1159/000111356. [DOI] [PubMed] [Google Scholar]
- Shulman R, Hyder F, Rothman D. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann H, Schousboe A, Fosgerau K, Waagepetersen H. Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem Res. 2005;30:1295–1304. doi: 10.1007/s11064-005-8801-4. [DOI] [PubMed] [Google Scholar]
- Silver I, Erecińska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Hassel B, Muller TB, Unsgard G, Fonnum F, Hertz L, Schousboe A, Petersen SB. NMR spectroscopic studies of 13C acetate and 13C glucose metabolism in neocortical astrocytes: evidence for mitochondrial heterogeneity. Dev Neurosci. 1993;15:351–358. doi: 10.1159/000111355. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Jones P, Taylor A, Bachelard HS, Schousboe A. Metabolism of [U-13C5]glutamine in cultured astrocytes studied by NMR spectroscopy: First evidence of astrocytic pyruvate recycling. J Neurochem. 1996;67:2566–2572. doi: 10.1046/j.1471-4159.1996.67062566.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21:99–105. doi: 10.1002/(sici)1098-1136(199709)21:1<56::aid-glia6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Grilc S, Pangrsic T, Zorec R. EAAT2 density at the astrocyte plasma membrane and Ca(2+)-regulated exocytosis. Mol Membr Biol. 2008;25:203–215. doi: 10.1080/09687680701790925. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990a;536:220–226. doi: 10.1016/0006-8993(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Hertz L. Noradrenaline induced stimulation of oxidative metabolism in astrocytes but not in neurons in primary cultures. Brain Res. 1990b;527:346–349. doi: 10.1016/0006-8993(90)91157-c. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Hertz L. Stimulation of energy metabolism by alpha-adrenergic agonists in primary cultures of astrocytes. J Neurosci Res. 1991;28:399–405. doi: 10.1002/jnr.490280312. [DOI] [PubMed] [Google Scholar]
- Sun SH, Lin LB, Hung AC, Kuo JS. ATP-stimulated Ca2+ influx and phospholipase D activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J Neurochem. 1999;73:334–343. doi: 10.1046/j.1471-4159.1999.0730334.x. [DOI] [PubMed] [Google Scholar]
- Swanson R. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol. 1992;70 Suppl:S138–144. doi: 10.1139/y92-255. [DOI] [PubMed] [Google Scholar]
- Swanson R, Choi D. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- Swanson R, Yu A, Chan P, Sharp F. Glutamate increases glycogen content and reduces glucose utilization in primary astrocyte culture. J Neurochem. 1990;54:490–496. doi: 10.1111/j.1471-4159.1990.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Alvarez R, Tabernero A, Medina J. Endothelin-1 stimulates the translocation and upregulation of both glucose transporter and hexokinase in astrocytes: relationship with gap junctional communication. J Neurochem. 2004;89:703–714. doi: 10.1046/j.1471-4159.2004.02398.x. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Chaudhuri B, Frommer W. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim Biophys Acta. 2008;1778:1091–1099. doi: 10.1016/j.bbamem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti P. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CJ, Garfinkel D. A simulation study of brain compartments: metabolism of glutamate and related substances in mouse brain. Biochem J. 1971;23:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelin M, Birkedal R. Anisotropic diffusion of fluorescently labeled ATP in rat cardiomyocytes determined by raster image correlation spectroscopy. Am J Physiol Cell Physiol. 2008;295:C1302–C1315. doi: 10.1152/ajpcell.00313.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol. 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- Vesce S, Bezzi P, Volterra A. The active role of astrocytes in synaptic transmission. Cell Mol Life Sci. 1999;56:991–1000. doi: 10.1007/s000180050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Moruzzi E, Locci-Cubeddu T, Bergamini E. Effects of adrenaline administration on the activity of glycogen metabolizing enzymes in different muscle types. Pflugers Arch. 1979;379:301–302. doi: 10.1007/BF00581437. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Hansen GH, Fenger K, Lindsay JG, Gibson G, Schousboe A. Cellular mitochondrial heterogeneity in cultured astrocytes as demonstrated by immunogold labeling of alpha-ketoglutarate dehydrogenase. Glia. 2006;53:225–231. doi: 10.1002/glia.20276. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Schousboe A. Glial GABA and glutamate metabolism. In: Squire L, Albright T, Bloom F, Gage F, Spitzer N, editors. New Enclyclopedia in Neuroscience. Oxford, UK: Elsevier Ltd; 2007. [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. Energy and amino acid neurotransmitter metabolism in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Berlin, Germany: Springer; 2009. pp. 177–199. [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem. 2000;75:471–479. doi: 10.1046/j.1471-4159.2000.0750471.x. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. Multiple compartments with different metabolic characteristics are involved in biosynthesis of intracellular and released glutamine and citrate in astrocytes. Glia. 2001;35:246–252. doi: 10.1002/glia.1089. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Hertz L, Sonnewald U, Schousboe A. Demonstration of pyruvate recycling in primary cultures of neocortical astrocytes but not in neurons. Neurochem Res. 2002;27:1431–1437. doi: 10.1023/a:1021636102735. [DOI] [PubMed] [Google Scholar]
- Walls A, Heimbürger C, Bouman S, Schousboe A, Waagepetersen H. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Wang Z, Haydon P, Yeung E. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem. 2000;72:2001–2007. doi: 10.1021/ac9912146. [DOI] [PubMed] [Google Scholar]
- Wender R, Brown A, Fern R, Swanson R, Farrell K, Ransom B. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Drejer J, Schousboe A, Sonnewald U. Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia. 1996;17:160–168. doi: 10.1002/(SICI)1098-1136(199606)17:2<160::AID-GLIA7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wiesinger H, Hamprecht B, Dringen R. Metabolic pathways for glucose in astrocytes. Glia. 1997;21:22–34. doi: 10.1002/(sici)1098-1136(199709)21:1<22::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yu A, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Yu A, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982;39:954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yu ACH, Hertz L. Metabolic sources of energy in astrocytes. Neurol Neurobiol. 1983;7:431–438. [Google Scholar]
- Yu N, Martin J, Stella N, Magistretti P. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc Natl Acad Sci USA. 1993;90:4042–4046. doi: 10.1073/pnas.90.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang H, Ye C, Ge W, Chen Y, Jiang Z, Wu C, Poo M, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- Zielke H, Collins RJ, Baab P, Huang Y, Zielke C, Tildon J. Compartmentation of [14C]glutamate and [14C]glutamine oxidative metabolism in the rat hippocampus as determined by microdialysis. J Neurochem. 1998;71:1315–1320. doi: 10.1046/j.1471-4159.1998.71031315.x. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Richter-Landsberg C, Leibfritz D. 13C isotopomer analysis of glucose and alanine metabolism reveals cytosolic pyruvate compartmentation as part of energy metabolism in astrocytes. Glia. 2001;34:200–212. doi: 10.1002/glia.1054. [DOI] [PubMed] [Google Scholar]