Abstract
RNA expression profiles are increasingly used to diagnose and classify disease, based on expression patterns of as many as several thousand RNAs. To ensure quality of expression profiling services in clinical settings, a standard operating procedure incorporates multiple quality indicators and controls, beginning with preanalytic specimen preparation and proceeding thorough analysis, interpretation, and reporting. Before testing, histopathological examination of each cellular specimen, along with optional cell enrichment procedures, ensures adequacy of the input tissue. Other tactics include endogenous controls to evaluate adequacy of RNA and exogenous or spiked controls to evaluate run- and patient-specific performance of the test system, respectively. Unique aspects of quality assurance for array-based tests include controls for the pertinent outcome signatures that often supersede controls for each individual analyte, built-in redundancy for critical analytes or biochemical pathways, and software-supported scrutiny of abundant data by a laboratory physician who interprets the findings in a manner facilitating appropriate medical intervention. Access to high-quality reagents, instruments, and software from commercial sources promotes standardization and adoption in clinical settings, once an assay is vetted in validation studies as being analytically sound and clinically useful. Careful attention to the well-honed principles of laboratory medicine, along with guidance from government and professional groups on strategies to preserve RNA and manage large data sets, promotes clinical-grade assay performance.
Microarray technology and RNA signatures have a long and somewhat rocky track record in basic science laboratories. In the past few years, advances in technology and quality assurance suggest that genomic profiling is reliable enough for medical decision making in clinical trials and ultimately in routine patient care. Factors contributing to adoption in clinical settings include the following: i) good manufacturing practices for reagents, supplies, control instruments, and software; ii) progress in biospecimen science that promotes RNA integrity; iii) advances in bioinformatics, facilitating interpretation of complex data; and iv) novel strategies for quality control, ensuring that each patient test performs as expected.
Microarrays permit measurement of hundreds or even tens of thousands of RNAs simultaneously, including coding and noncoding RNAs of human and microbial sources.1 RNA profiles that are unique to clinical status can assist with diagnosis, prognosis, monitoring, and predicting efficacy of therapy. To warrant adoption in clinical settings, the assay must provide added value beyond what is already available to patients or their health care system.2
RNA profiling is guided by high standards and well-honed principles of laboratory medicine that ensure quality while allowing innovation and progress. The quality systems that are being adapted to expression profiling are emblematic of the novel strategies being developed to manage a wide range of multianalyte technologies, such as full-genome sequencing, proteomics, and single-nucleotide polymorphism chips. The two most important contributors to good outcomes are competent personnel to perform each assay and controls and quality indicators that help these personnel identify and correct problems.3 Meticulous attention to detail, expert technical knowledge, and medical judgment are required to validate and maintain each assay. During validation work, a standard operating procedure is refined and tested, and evidence is collected to substantiate choices regarding specimen requirements, indications for testing, recommended clinical use of test results, step-by-step analysis, and quality control processes.4–6 Each new assay or modification to an assay must be vetted by the laboratory director as being analytically sound and clinically useful enough to justify implementation as a medical service. This article reviews the quality assurance measures that are established during assay validation and that are used on a daily basis to ensure good outcomes.
Governmental Oversight of Laboratory Services in the United States
Scrupulous attention to detail and high standards ensure the quality of laboratory work performed in clinical settings. Governmental oversight of testing laboratories, and of the manufacturers providing reagents or test systems to such laboratories, is codified in federal regulations of the Clinical Laboratory Improvement Amendments and Title 21, directing the Food and Drug Administration (FDA). Many RNA-based assays have been FDA cleared or approved, such as tests to detect HIV, hepatitis C virus, mycobacterium tuberculosis, and influenza virus. Quantitative RT-PCR (RT-qPCR) is the technology most frequently used in clinical molecular laboratories, and panels of RT-qPCRs can reasonably be performed on dozens to thousands of different analytes at once.7,8 For human gene discovery research, even denser arrays provide expression data on virtually all (approximately 22,000) human protein-coding genes and >1000 microRNAs.1,9
To date, 10 multianalyte RNA assays have been FDA cleared on Agilent (Santa Clara, CA), Affymetrix (Santa Clara, CA), RT-qPCR, and bead-type platforms. These include Mammaprint (Agendia, Irvine, CA) to help manage selected breast cancer patients,10,11 Tissue of Origin Test (Pathwork Diagnostics, Redwood City, CA) to help pathologists refine the type of malignancy,12 AlloMap blood test for acute cellular rejection of a transplanted heart (XDx, Brisbane, CA), BLN assay for breast tumor metastasis to lymph nodes (Veridex, Raritan, NJ; this assay was voluntarily withdrawn from the US market), and six respiratory virus panels from Idaho Technologies (Salt Lake City, UT), Nanosphere (Northbrook, IL), Applied Biosystems (Carlsbad, CA), Focus Diagnostics (Cypress, CA), Gen-Probe (San Diego, CA), and Luminex (Toronto, ON, Canada). After approval of a predicate in vitro diagnostic device, the FDA often issues guidance for manufacturers validating similar assays. Many laboratory-developed tests have been implemented as medical services under Clinical Laboratory Improvement Amendment regulations, and examples of such laboratory-developed tests characterizing RNA are BCR-ABL1 in leukemia13 and Genomic Health's (Redwood City, CA) Oncotype Dx assay in breast cancer.14
The MicroArray Quality Control project began as an initiative to assess the quality of RNA-based microarray expression profiles and to recommend improvements. The project was launched by the FDA and involves nearly 200 academic institutions, commercial manufacturers, and governmental divisions. In work to date, sample exchanges and data set analyses showed that expression profiling is technically robust, is biologically informative, and generates similar profiles across multiple platforms when viewed by functional means.15–18 Several approaches to prediction modeling were deemed effective.15,19 The European Union's EMERALD project (Empowering the Microarray-Based European Research Area to Take a Lead in Development and Exploitation) developed software tools for quality metrics of array data.20
Guidance from Professional Groups
Several laboratory professional groups offer guidance on clinical-grade procedures and standards. In particular, checklists marketed by the College of American Pathologists (CAP) as part of their worldwide laboratory accreditation program are a blueprint for achieving good outcomes (http://www.cap.org, see Reference Resources and Publications section of website; last accessed June 30, 2011). The Clinical and Laboratory Standards Institute produces documents thoroughly describing principles and best practices, such as Diagnostic Nucleic Acid Microarrays,21 Verification and Validation of Multiplex Nucleic Acid Assays,22 and Use of External RNA Controls in Gene Expression Assays.23 Three European organizations developed joint guidelines for microarray profiling of leukemia,24 and most of the recommended principles and procedures extend to other diseases.
Some multiplexed tests target thousands of RNAs at once, although the minimum number of analytes composing an array is only 11, according to the American Medical Association Current Procedural Terminology Editorial Panel. In the United States, reimbursement by payers for microarray services is accomplished using the physician fee schedule, emphasizing that a pathologist or other laboratory physician is responsible for technical work and uses medical judgment in expert interpretation of the findings. Among the many requirements of Clinical Laboratory Improvement Amendment certification is that a physician consultant be available to discuss indications for testing and patient-specific result interpretation for all tests offered by high-complexity clinical laboratories.
Preanalytic Specimen Preparation
Preanalytic collection, stabilization, transport, and storage conditions are critical for obtaining accurate analytic test results.25–29 Interestingly, procedures that may seem irrelevant can affect outcome, such as first versus second pass of a needle-core biopsy30 or freezing of blood or tissue.31,32 Guidance from professional groups addresses these preanalytic concerns.33,34 The testing laboratory takes a trust-and-verify approach by educating health care workers in proper collection and handling procedures, while using quality checks, such as housekeeping transcript levels, to evaluate specimen quality.
RNA is notorious for rapidly degrading if special precautions are not taken to preserve it.33,35 In stored blood and marrow, Ma et al36 showed similar RNA profiles for frozen versus TRIzol-preserved cells. Stabilization of RNA at the bedside is feasible using commercial blood collection systems that must be validated for their intended use.37–42 The advantages of RNA stability must be weighed against the cost of stocking special collection vials at every pertinent blood collection site and the incapacity to interpret smears or apply cell enrichment procedures once the preservative is added.
For solid tissue, 10% neutral-buffered formalin is the fixative favored by nearly all histopathology laboratories. Formalin functions by aldehyde cross-linking to generate a scaffold preventing tissue degradation and diminishing unwanted enzymatic activity (eg, RNases). Diffusion of formalin into tissue is a function of distance and density; thus, slicing tissue into thin pieces promotes rapid fixation. The duration of fixation is critical, with sufficient time required to ensure adequate preservation of analytes, while avoiding overfixation that hardens tissue and increases cross-links with macromolecules, rendering intact RNA difficult to recover.43–45 A study by Chung et al46 showed that fixation between 4 and 48 hours was reasonable, although 12 to 24 hours was ideal with respect to downstream RNA quality. Incubating RNA in warm Tris buffer is predicted to reverse, at least in part, the adverse effects of formalin.45 In validation work, consider splitting fresh specimens and testing pertinent preanalytic variables to gather the evidence required to set limits on acceptable specimen preparation for the particular assay.47,48
Fresh or frozen tissue tends to have much better-quality RNA than does formalin-fixed tissue, but formalin-fixed tissue is readily available in clinical settings and, thus, is the specimen of choice. Acid decalcification is not recommended because depurination fragments nucleic acid. Alternative fixatives, particularly alcohol-based solutions, often yield high-quality RNA,49–51 but these fixatives may suffer when rated by pathologists for histological detail, not to mention the adverse impact on immunostains and other histochemical procedures that are frequently applied in clinical settings.52 It appears that a molecular test is most likely to be adopted if the test is robust enough to work on customary specimens, recognizing that the term customary encompasses a wide range of nonstandardized formalin fixation protocols.47 Fortunately, multiple groups53–55 have succeeded in profiling RNA from archival formalin-fixed tissues. Because some, but not all, target RNA levels are equivalent in paraffin blocks versus matched frozen tissue,56–61 it is clear that each RNA-based test must be validated for its relevant specimen preparation.
Enrichment of Lesional Cells
To generate an accurate RNA signature, the input tissue must be appropriately representative of the organ or lesion being evaluated.62 To accomplish this, a pathologist examines a stained slide to confirm histopathological findings and to assess tissue adequacy for the particular test.63 If indicated, lesional cell enrichment is done by macrodissection or microdissection.64–66 For blood, marrow, or other cytologic fluids, cell enrichment is achieved using flow cytometry or magnetic bead separation.67 For plasma or serum, variations in the protocol for cell separation can affect downstream RNA measurement.68,69
Criteria for tissue selection are defined during validation work. In tumor profiling, the amount and nature of the malignant and stromal cell components can influence the profile, depending on which RNAs compose the test panel, the anatomical site (eg, primary versus metastatic), and whether the stroma is normal, fibrotic, desmoplastic, or inflammatory.62,70 In preanalytic work, a pathologist typically selects a portion of tissue in which malignant cell nuclei exceed a given proportion of all nuclei present, which is different from circling malignant areas because those areas might contain only scattered malignant cells in a sea of reactive stromal cells. Expert interpretation of downstream molecular results is done in the context of the input tissue and other correlative data.
RNA Extraction and Quantification
In busy clinical laboratories, automated RNA extraction instruments help control costs and improve reproducibility. Staal et al24 reported that the extraction method (TRIzol versus RNeasy) affects RNA expression patterns in blood, providing cautionary evidence that substituting extraction protocols can adversely affect results. Skipping extraction altogether is feasible for some robust technologies.71,72
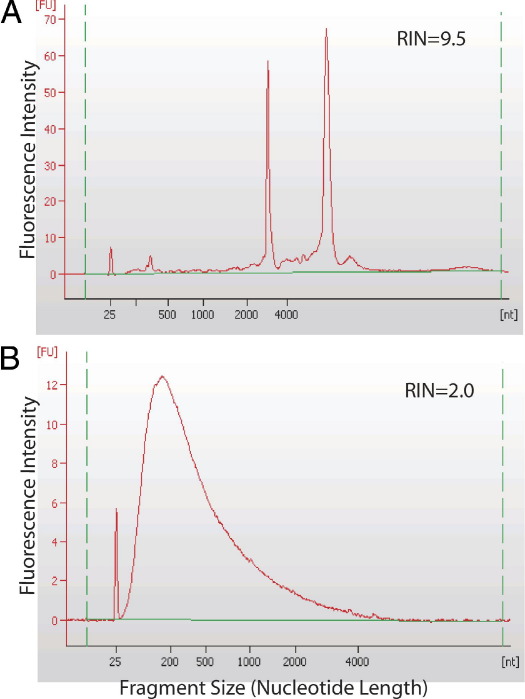
It is often worth evaluating RNA quantity and quality before subjecting a specimen to expensive microarray analysis. RNA concentration is measurable by UV spectrophotometry or fluorimetry, and RNA size distribution may be visualized on an electropherogram and scored using a software algorithm (eg, RNA integrity score).73 The spectrum of RNA size is dramatically larger in fresh or frozen tissue compared with formalin-fixed, paraffin-embedded tissue (Figure 1). A 1-hour delay in fresh specimen processing does not adversely affect the RNA integrity score, although it may disturb individual analytes.52,74,75 By RT-qPCR, amplicons >500 bp are infrequently achieved from formalin-fixed tissues compared with frozen tissue, and RNA integrity scores do not predict amplifiability from paraffin tissue blocks; instead, levels of housekeeping transcripts, and separate hybridizations of 5′ and 3′ ends of such transcripts, are helpful for assessing specimen quality.58 The usual precautions to protect RNA are in order, such as use of RNase-free reagents and plasticware, wearing and frequently changing gloves, and keeping a clean work environment by wiping surfaces with 10% bleach or RNase ZAP (Ambion) and using disposable bench covers.
Figure 1.
Agilent Bioanalyzer electropherograms reflect RNA size spectrum. A: Intact RNA from frozen tissue has prominent 18S and 28S rRNA peaks surrounded by other relatively large RNA molecules. B: Degraded RNA from matched paraffin-embedded tissue has much smaller RNA fragments and a lower RNA integrity (RIN) score.
Controls and Limits on Their Acceptability
Quality control is among the most important of quality assurance measures.3 Although traditional single-analyte assays require inclusion of a positive and a negative control in every run, it is clear that microarray runs cannot possibly include controls for each of the dozens to thousands of target analytes, not to mention the impossibility of representing each of the permutations and combinations of expressed genes that might be found in a given specimen. Thus, a new paradigm of quality control has emerged to accommodate multianalyte arrays.76 In short, the approach engenders confidence that the pattern of expression is accurate and reproducible.
Controls are used to check assay performance, with special focus on the least robust components. Multiple types of controls are used in RNA profiling. A “no template” control can evaluate background signal and contamination by stray nucleic acid. An exogenous control is run alongside patient specimens to evaluate assay performance in a general manner. A separate exogenous control, representing each of the main outcome groups, could be included for every X patients who are run, with X chosen based on the medical impact of an erroneous classification and the timeliness required to correct any error, recognizing that failure of a control will launch an investigation that questions the results for those patients tested since the last time that the control performed as expected.
An endogenous control checks an inherent feature of patient sample, such as a housekeeping transcript, which is particularly valuable for assessing preanalytic factors (eg, viable cellularity, collection, preservative, shipping, and storage). Because of inherent biological variability in levels of any given gene product, it is recommended to identify several housekeepers that are consistently expressed at low to high levels in the relevant tissue or fluid. Adequate expression of these housekeepers reflects suitable hybridizable RNA, thus permitting rejection of poor-quality specimens or those with faulty technical analysis. In addition, housekeepers can serve as a normalizer by which to quantify target RNAs.73,77–79 Caution is warranted because ex vivo degradation proceeds at different rates for different analytes, reinforcing the need to validate the control and normalization strategy to promote comparability of results across specimens.
Spiked controls are yet another way of checking assay performance. Thanks to the work of the External RNA Controls Consortium, commercial RNA standards are newly available to assess technical accuracy.80,81 These nonhuman, nonpathogen, spiked RNA products are marketed [by Invitrogen (Carlsbad, CA) and VWR (Radnor, PA)] as mixes of multiple synthetic RNAs at a known concentration and a known sequence. They are spiked into each patient specimen at the earliest informative time point (eg, with lysis buffer) to permit downstream evaluation of assay performance within the patient specimen. Results can detect technical failure and iatrogenic inhibition (eg, residual phenol or heparin anticoagulant) or endogenous interfering substances (eg, hemoglobin or background autofluorescence). Finally, combinations of spiked molecules are proposed as a way of tracking specimen identity through the many steps of specimen preparation and analysis.82
Limits on acceptable performance of controls are empirically set by replicate analysis. Consider running a control many times (across different days, technologists, instruments, and lot numbers), and then calculate the mean ± 2 SDs as the limit on its performance. When multiple controls are used, the expected failure rate increases accordingly. For example, a failure rate of 5% for any one control implies that a combination of four controls will fail 18% of the time. This high failure rate emphasizes the benefits of a quality control strategy that includes multiple controls for the many critical aspects of the assay and synthesizes multiple data points to interpret overall success or failure of an assay.
Control results falling outside acceptable limits are documented and investigated for root cause and, when feasible, to take corrective action. When combined with quality indicators (eg, spectrophotometry or histopathological characteristics), results of controls can help pinpoint the source of the problem. Affected patient tests are usually repeated, when sufficient specimen remains. Records of problems, investigations, and potential solutions are fodder for the laboratory's quality improvement program.
Sources of Control Materials
Exogenous controls should resemble patient specimens. Human tissue remaining after clinical analysis can be fractionated and stored for use as a control, although some heterogeneity is expected across aliquots. Xenografts are an alternative source of abundant human cells, although contamination with cells of the host species merits study to show that the xenograft is sufficiently representative of the RNA profile of interest. Fresh blood controls are particularly hard to find, so it is reasonable to use stored white cell pellets or residual RNA or cDNA from previously tested specimens.
A mock specimen may be prepared by mixing a cell line or RNA derived from that cell line with appropriate matrix, and serial dilutions can be used to challenge analytic sensitivity or linearity. Some lot-to-lot variation is expected even when precise criteria are defined for cell culture and harvest. Some scientists favor a mixture of several cell lines to fill in gaps that an individual cell line might have (eg, nonexpressed genes). This approach is exemplified by the Agilent/Stratagene reference RNA manufactured from a mixture of 10 cell lines and well characterized by virtue of its analysis in multiple quality-assurance studies.16,83,84
Single-use aliquots prevent multiple freeze-thaw cycles. When the same control material is used in multiple runs, selected numeric results can be tracked over time using Levey-Jennings charts to visualize drift or shift.85
Linear Amplification, Labeling, and Two-Color Arrays
When RNA quantity is limited, linear preamplification can boost the signal,58,64,86–89 and a label can be incorporated to permit downstream detection after hybridization.84 Labeling efficiency can be checked before hybridization.
In some hybridization strategies, a competitor RNA is cohybridized with patient RNA in a two-color approach.90 Compared with one-color assays, two-color assays are more expensive and arguably avoidably complex given that MicroArray Quality Control project II studies91,92 confirm that both one- and two-color strategies perform well. Although the merits and risks of various assay designs can be debated, it is imperative to use the same assay protocol in patient care that was vetted in validation studies.
cDNA Preparation
Before cDNA preparation, DNA is usually removed using DNase. The cDNA production is driven off random primers, oligo dT primers targeting poly A tails of mRNA, or specific primers targeting each RNA. The preparation of cDNA is among the most inconsistent features of RT-qPCRs. Whether replicates are required for this or any other step of analysis depends on the variability observed during reproducibility experiments performed in the validation study. In general, an assay that is so finicky as to require replicate testing may not be reliable enough for clinical use.93 As a rule, significant differences in expression pattern between two patients must largely represent true biological differences rather than technical error.
Equipment, Reagents, and Test Systems
Lessons learned in the early days of expression profiling led to many improvements in the manufacturing of hardware, reagents, supplies, and software-supporting expression profiling. Manufacturers are vital for supplying testing laboratories with reliable products.94 Purchasing decisions favor suppliers who comply with the FDA's medical device current good manufacturing practices (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/QualitySystemsRegulations, last accessed June 30, 2011) or comparable International Standardization Organization systems (http://www.iso.org/iso/iso_9000_essentials, last accessed June 30, 2011), promoting quality and consistency.95,96 Ideally, users and manufacturers communicate so that substantial changes to the product are conveyed to users, and performance of the product in users' hands drives improvement by the manufacturer. Even after extensive vetting by a manufacturer, it remains incumbent on the testing laboratory to ensure that each product performs adequately for its intended use.
Hybridization
Although assay design-related error should have been addressed during assay validation (eg, cross-reactivity, secondary structure diminishing intended base pairing, and competition), other systematic errors may crop up after implementation (eg, defective lot number or instrument). In addition to the error detection strategies previously addressed, consider designing redundancy into the test system by targeting critical analytes numerous times (eg, in different physical quadrants of the array or by probing both ends of a given transcript). Likewise, one could test multiple components of a critical biochemical pathway, multiple markers of a critical phenotype, or multiple conserved segments of an RNA viral genome. These scenarios capitalize on the array's strength in multiplexed testing.
Analytic Interpretation of Results
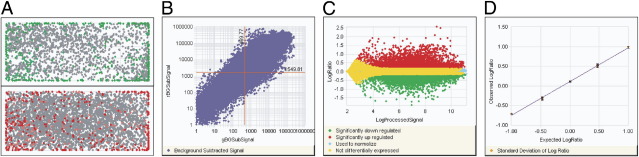
Analytic interpretation involves generating a reportable result after first evaluating raw data or data generated using decision-support tools, for each control and for the patient at hand. As an example, acceptable input RNA for Affymetrix profiling of paraffin-embedded tissue was defined by Roberts et al56 as RNA that, by RT-qPCR, had an endogenous ACTB 3′/5′ ratio of <20, a CT <7 between ACTB and the exogenous Agilent/Stratagene Universal Reference RNA, and an endogenous 28s ribosomal RNA CT <15. In our laboratory, quality indicators before Agilent profiling are listed in Table 1. After hybridization, an example of quality metrics used to vet Agilent array data on frozen tissue is shown in Figure 2. For Affymetrix arrays, Staal et al24 checked the 3′/5′ ratio for selected housekeeping genes (ideal, 1; good, <3); noise; scaling factor (should be lower than three-fold); visual check of image for bubbles, scratches, and grid alignment; homogeneity of hybridization and uniformity of background; and percentage of genes detectable (>25%).
Table 1.
Example Quality Indicators on RNA Processed from Frozen Tissue in Preparation for Expression Profiling of Cancer
| Quality indicator | Description |
|---|---|
| 1 | The tissue specimen is rejected if there are not at least 30% malignant cells, as determined by a pathologist using microscopy |
| 2 | The RNA specimen is rejected if either of the following is true: A260/A280 is <1.8, as determined using Nanodrop (Wilmington, DE) spectrophotometry; or the RNA integrity no. is <6.5, as determined using an Agilent Bioanalyzer 2100 |
| 3 | The labeled cRNA is rejected if either of the following is true based on results of Nanodrop fluorometry: there is <10 μg of amplified labeled cRNA or specific activity is <8 pmol Cy5/μg labeled cRNA |
Quality indicators and limits of acceptability must be established using evidence gathered during validation studies for the particular test being developed.
Figure 2.
Quality metrics are displayed by Agilent feature extraction software (version 10.5.1.1) from a two-color gene expression experiment on an Agilent microarray system. Acceptability limits might include uniform spatial distribution, with local background of red and green signal <2% and numbers of features nonuniform <5% (A); the dynamic range of expression exceeding five orders of magnitude (B); even distribution of significantly up- or down-regulated genes across signal intensities (C); and spike-in RNA measurements being linear, with a slope >0.9, R2 > 0.85, and replicate reproducibility signified by BGSubSignal <13 and processed signal <6 (D).
Individual markers of critical importance may be chosen as a quality check, such as ESR1 (estrogen receptor) RNA expression in breast cancer compared with ESR1 protein that has been tested by immunohistochemistry (IHC) on the same surgical specimen.97 Although it is recognized that RNA and protein-based tests may be discrepant for biological rather than technical reasons, the frequency of such discrepancies can be determined during validation studies to identify quality indicators that raise a red flag to be weighed in concert with results of other controls and quality checks.
When redundant assays are present on the array, replicates are examined for consistency or to find problematic variations.98 As an example, a highly proliferative tumor (identified by visualizing mitotic figures during the tissue selection phase) is expected to express proliferation markers on the array, thus confirming coordinated expression of those phenotypic markers that instill confidence in the array-based results.99
Data analysis is done in the context of a thorough understanding of the technical strengths and weaknesses of the test system, based on prior experience gathered during validation work and subsequent clinical practice. Software is relied on to present data in a manner that facilitates its interpretation.100 A typical standard operating procedure for data analysis involves normalization for background or housekeeping transcript abundance, log transformation to aid in comparing numeric results with those in the validation set, and graphic display of pertinent findings.77,78,101–104 Overmanipulation of the data should be avoided.105
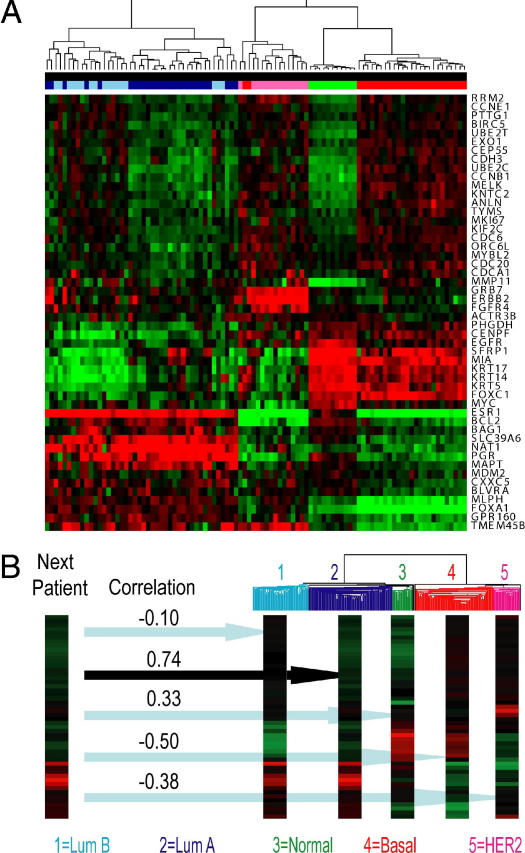
To categorize results in a given patient, a predictive model may be applied that finds patterns across many analytes, as facilitated by a single-sample predictor algorithm.21,59,106–110 Grouping global patterns of gene expression is a unique strength of RNA expression profiling. A categorical assignment is typically accompanied by a statistic (eg, Spearman's correlation coefficient) representing degree of confidence12 (Figure 3).
Figure 3.
Raw data are interpreted after evaluation of virtually all approximately 22,000 human protein-coding genes using Agilent microarrays. A: A heat map shows gene expression profiles of 96 specimens tested for 50 listed genes, and unsupervised hierarchical clustering reveals distinct patterns of expression. B: To classify intrinsic subtypes of breast cancer, a single-sample predictor algorithm compares each patient's expression pattern with the consensus pattern for each of the five intrinsic subtypes, and Spearman's correlation coefficients help estimate the certainty of the classification. In the example shown, the patient's profile matches most closely with luminal (Lum) A, although Lum B subtype cannot be excluded, whereas three other subtypes (normal, basal, and HER2) are excluded based on low correlation coefficients.
Computer-generated scores or predictors are checked to ensure that they make sense after evaluating pertinent raw data. Unsupervised clustering with a heat map dendrogram visually displays results of a given patient alongside results of a panel of known specimens, to help confirm categorization of the patient into the pre-established group that most closely shares its expression profile.110 It is important to recognize the limitations of hierarchical clustering, especially because the patient may not belong to any of the pre-established groups. Exogenous controls should be chosen to check categorization at the most critical medical decision points, rather than using only canonical examples that minimally challenge the test system.
Clinical Interpretation and Reporting
Clinical interpretation is the process by which the medical significance of the result is judged in light of the clinical indication for which the test was ordered. Even if the end result of analytic interpretation is an objective numeric score or a discrete disease classification, it is useful for that result to be further interpreted in light of input tissue characteristics; pertinent limitations of the assay, as revealed by controls and quality checks (eg, specimen thawed during transport and marginal housekeeping RNAs recovered); and the level of confidence in the result. Furthermore, correlative analysis with other patient information (eg, age, sex, tumor stage, IHC results, or flow cytometric findings) paints a more complete picture beyond the snapshot of expressed genes. More important, the clinician deserves a clear message about implications for clinical decision making and recommended follow-up. A report describing the BCR-ABL1 profile in a newly diagnosed lymphoblastic leukemia could expound on the implications for response to tyrosine kinase inhibitor therapy.111,112 Furthermore, follow-up testing by RT-qPCR might be recommended to identify an amplifiable translocation of BCR-ABL1 p210 or p190 that could be serially measured as a biomarker of response during therapy.112
To maximize the benefit of array-based testing, interpretation by a physician who is expert in disease pathobiological features and molecular technology is required. This laboratory physician takes responsibility for the analysis and for the quality control processes that were used in generating their interpretation. An experienced pathologist is capable of recognizing unusual or outlier results, analogous to unforeseen results generated from histopathological characteristics. For example, careful analysis of RNA expression data may reveal erroneous software-generated calls at variance with clinicopathological findings. A melanoma tissue that was categorized into good versus bad prognosis groups by a software algorithm might, on closer inspection of raw data, lack the typical melanoma markers, thus triggering investigation of whether the patient has a look-alike tumor. Known melanocyte markers that could be used in this evaluation include MAGEA1, MITF, MART1 (melan-A), CMM (HMB45), S100, and TYR (tyrosinase), each of which is confirmable using IHC for the corresponding protein.
It is clear that medical judgment is required to identify misleading data and to interpret the significance of findings in light of evidence from patient records, published literature, validation work, databases, and other reliable sources.113 Pertinent results and interpretation are incorporated into a concise, yet informative, report that is entered into the patient's medical record. As described in CAP guidelines for molecular test reporting,114 the document should include a written summary of results and an interpretation facilitating medical decision making. Review of draft reports can help catch clerical errors. Related quality assurance tactics include periodic independent appraisals, checking report format after electronic transmission, and surveying client satisfaction.115
Data Set Retention
CAP suggests that patient test records be retained for at least 2 years. The US federal regulations protect the privacy of those records and require that the corresponding procedure manual be retained, which serves to annotate each patient data set with the methods that were used to generate it. When the full human transcriptome is profiled, but only selected results are required for data analysis and interpretation, software can then be programmed to mask irrelevant data. Later, one could mine the same data set for the patient's benefit, analogous to re-examination of histopathological slides and blocks in light of the following: i) refined criteria for microscopic diagnosis, ii) availability of novel immunostains, iii) concerns about accuracy, or iv) changes in clinical status. Although RNA profiling might initially be used to help make a diagnosis, later, the same data set could be mined sequentially for prognosis and prediction of response to first- and second-line therapies.
Design Strategies to Minimize Error
Clinical laboratorians tend to choose methods that are tried and true. It is, therefore, not surprising that clinical laboratories are just now adopting expression profiling after years of use and refinement in research settings.116–118 RNA profiling is a complex process, and each unique reagent, piece of equipment, or manual action could be faulty. A principle of good assay design is to restrict assay components to those that are safe (for patients and health care workers) and simple, while still meeting the clinical objective. Unlike in research settings, turnaround time is critical, so the reliability of each assay component is a key factor in the success of the medical service. Translational research teams should include clinicians who will order the test and act on test results, technology experts who advise on platforms and reagents, and clinical laboratorians who will vet, perform, and interpret test results.
The transfer of a specimen or its derivative to a new vessel requires meticulous care to maintain specimen identity and integrity.119,120 Barcodes facilitate labeling and tracking throughout the many stages of testing.120,121 Robotic instruments should be programmed to minimize risk of carryover or aerosolization, and each instrument undergoes regular maintenance and is checked regularly for its performance. Data transfer requires a similarly scrupulous process for maintaining its integrity.
Proficiency Testing
Formal proficiency surveys are offered for many individual RNA-based tests, such as hepatitis C viral load and BCR-ABL1 quantification. The CAP's Cytogenomic Microarray Survey is a model for interlaboratory comparison of array-based data, although this particular survey challenges DNA-based, rather than RNA-based, microarray services.122 Proficiency surveys are meant to do the following: i) periodically check assay performance, ii) serve an educational function for laboratory members, and iii) promote quality improvement by sharing information on current methods.123–125 The CAP and the Association for Molecular Pathology have many resources supporting quality assurance and proficiency, including help in identifying another provider with whom to exchange specimens. In addition to interlaboratory exchange programs to check for comparable signatures or individual analytic test results, an alternative assessment method is to reanalyze internal samples as if they were unknowns.126
On the Horizon
This article describes the many principles of laboratory medicine that guide us in validating, implementing, and maintaining RNA-based tests. Strategies are described to check patient-specific and generic assay performance at critical junctures during each multistep protocol. With proper attention to assay design and quality assurance, it is clear that RNA profiling provides robust, accurate, and reproducible results that are powerful by virtue of the number of analytes that are evaluated, the informative signatures reflecting disease status in meaningful new ways, and the redundancy that boosts confidence in the findings. On the horizon are full transcriptome sequencing technologies that are likely to bring greater precision for quantifying RNA and characterizing splice variants.118
Acknowledgments
We thank our colleagues at the University of North Carolina at Chapel Hill for helpful discussions, especially Drs. Charles M. Perou, Greg Ray, Karen Weck, Yan Li, Jessica Booker, and Myla Lai-Goldman.
Footnotes
Supported by the Department of Pathology and Laboratory Medicine, the University Cancer Research Fund, the Alliance for Clinical Trials in Oncology [National Cancer Institute (NCI) U10 CA031946], an NIH Clinical Translational Science Award (U54 RR024383), and an award for Innovative Technologies for Molecular Analysis of Cancer (NCI R21 CA155543), all to University of North Carolina at Chapel Hill.
CME Disclosure: M.L.G. is a consultant for McKesson, Roche Molecular Systems, and Abbott Laboratories, and serves on the clinical advisory board of Generation Health. None of the other authors disclosed any relevant financial relationships.
References
- 1.Auer H., Newsom D.L., Kornacker K. Expression profiling using Affymetrix GeneChip Microarrays. Methods Mol Biol. 2009;509:35–46. doi: 10.1007/978-1-59745-372-1_3. [DOI] [PubMed] [Google Scholar]
- 2.Lai-Goldman M., Faruki H. Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genet Med. 2008;10:874–878. doi: 10.1097/GIM.0b013e31818de71c. [DOI] [PubMed] [Google Scholar]
- 3.Noble M.A., Ahler M.E., Bennett B., Coffey J., Connolly K., Daly J.A., Fleming C., Giribaldi S.C., Hilborne L., Leibach E.K., Mahoney J.J., Parsons B., Reynolds C.D., Valenstein P.N. Clinical and Laboratory Standards Institute; Wayne, PA: 2010. Clinical Laboratory Standards Institute Document GP35-A: Development and Use of Quality Indicators for Process Improvement and Monitoring of Laboratory Quality; Approved Guideline. [Google Scholar]
- 4.Jennings L., Van Deerlin V.M., Gulley M.L. Recommended principles and practices for validating clinical molecular pathology tests. Arch Pathol Lab Med. 2009;133:743–755. doi: 10.5858/133.5.743. [DOI] [PubMed] [Google Scholar]
- 5.Burd E.M. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev. 2010;23:550–576. doi: 10.1128/CMR.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohl M. Development and validation of predictive molecular signatures. Curr Mol Med. 2010;10:173–179. doi: 10.2174/156652410790963376. [DOI] [PubMed] [Google Scholar]
- 7.Spurgeon S.L., Jones R.C., Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS One. 2008;3:e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derveaux S., Vandesompele J., Hellemans J. How to do successful gene expression analysis using real-time PCR. Methods. 2010;50:227–230. doi: 10.1016/j.ymeth.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Arikawa E., Sun Y., Wang J., Zhou Q., Ning B., Dial S.L., Guo L., Yang J. Cross-platform comparison of SYBR Green real-time PCR with TaqMan PCR, microarrays and other gene expression measurement technologies evaluated in the MicroArray Quality Control (MAQC) study. BMC Genomics. 2008;9:328. doi: 10.1186/1471-2164-9-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotiriou C., Piccart M.J. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer. 2007;7:545–553. doi: 10.1038/nrc2173. [DOI] [PubMed] [Google Scholar]
- 11.Mook S., Bonnefoi H., Pruneri G., Larsimont D., Jaskiewicz J., Sabadell M.D., Macgrogan G., Van't Veer L.J., Cardoso F., Rutgers E.J. Daily clinical practice of fresh tumour tissue freezing and gene expression profiling: logistics pilot study preceding the MINDACT trial. Eur J Cancer. 2009;45:1201–1208. doi: 10.1016/j.ejca.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Monzon F.A., Medeiros F., Lyons-Weiler M., Henner W.D. Identification of tissue of origin in carcinoma of unknown primary with a microarray-based gene expression test. Diagn Pathol. 2010;5:3. doi: 10.1186/1746-1596-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN Chronic Myelogenous Leukemia Clinical Practice Guidelines in Oncology (Version 2.2011) National Comprehensive Cancer Network, Inc.; Fort Washington, PA: 2011. [PubMed] [Google Scholar]
- 14.NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 2.2011) National Comprehensive Cancer Network, Inc.; Fort Washington, PA: 2011. [Google Scholar]
- 15.Fan X., Lobenhofer E.K., Chen M., Shi W., Huang J., Luo J., Zhang J., Walker S.J., Chu T.M., Li L., Wolfinger R., Bao W., Paules R.S., Bushel P.R., Li J., Shi T., Nikolskaya T., Nikolsky Y., Hong H., Deng Y., Cheng Y., Fang H., Shi L., Tong W. Consistency of predictive signature genes and classifiers generated using different microarray platforms. Pharmacogenomics J. 2010;10:247–257. doi: 10.1038/tpj.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L., Reid L.H., Jones W.D., Shippy R., Warrington J.A., Baker S.C. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nature Biotechnol. 2006;24:1151–1161. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi W., Bessarabova M., Dosymbekov D., Dezso Z., Nikolskaya T., Dudoladova M., Serebryiskaya T., Bugrim A., Guryanov A., Brennan R.J., Shah R., Dopazo J., Chen M., Deng Y., Shi T., Jurman G., Furlanello C., Thomas R.S., Corton J.C., Tong W., Shi L., Nikolsky Y. Functional analysis of multiple genomic signatures demonstrates that classification algorithms choose phenotype-related genes. Pharmacogenomics J. 2010;10:310–323. doi: 10.1038/tpj.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillinghast G.W. Microarrays in the clinic. Nature Biotechnol. 2010;28:810–812. doi: 10.1038/nbt0810-810. [DOI] [PubMed] [Google Scholar]
- 19.Bustin S.A., Beaulieu J.F., Huggett J., Jaggi R., Kibenge F.S., Olsvik P.A., Penning L.C., Toegel S. MIQE precis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beisvåg V., Kauffmann A., Malone J., Foy C., Salit M., Schimmel H., Bongcam-Rudloff E., Landegren U., Parkinson H., Huber W., Brazma A., Sandvik A.K. Contributions of the EMERALD project to assessing and improving microarray data quality. Biotechniques. 2011;50:27–31. doi: 10.2144/000113591. [DOI] [PubMed] [Google Scholar]
- 21.Hackett J.L., Archer K.J., Gaigalas A.K., Garrett C.T., Joseph L.J., Kock W.H., Kricka L.J., McGlennen R.C., Van Deerlin V., Vasquez G.B. Clinical and Laboratory Standards Institute; Wayne, PA: 2006. Clinical and Laboratory Standards Institute Document MM12-A: Diagnostic Nucleic Acid Microarrays; Approved Guideline. [Google Scholar]
- 22.Wilson J.A., Zoccoli M.A., Jacobson J.W., Kalman L., Krunic N., Matthijs G., Pratt V.M., Schoonmaker M.M., Tezak Z. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Clinical Laboratory Standards Institute Document MM17-A: Verification and Validation of Multiplex Nucleic Acid Assays; Approved Guideline. [Google Scholar]
- 23.Warrington J.A., Corbisier P., Feilotter H., Hackett J.L., Reid L.H., Salit M.L., Wagar E.A., Williams P.M., Wolber P. Clinical and Laboratory Standards Institute; Wayne, PA: 2006. Clinical Laboratory Standards Institute Document MM16-A: Use of External RNA Controls in Gene Expression Assays; Approved Guideline. [Google Scholar]
- 24.Staal F.J., Cario G., Cazzaniga G., Haferlach T., Heuser M., Hofmann W.K., Mills K., Schrappe M., Stanulla M., Wingen L.U., van Dongen J.J., Schlegelberger B. Consensus guidelines for microarray gene expression analyses in leukemia from three European leukemia networks. Leukemia. 2006;20:1385–1392. doi: 10.1038/sj.leu.2404274. [DOI] [PubMed] [Google Scholar]
- 25.De Cecco L., Musella V., Veneroni S., Cappelletti V., Bongarzone I., Callari M., Valeri B., Pierotti M.A., Daidone M.G. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009;9:409. doi: 10.1186/1471-2407-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G., Chance J.J., Church S., Dazzi P., Fontana R., Giavarina D., Grankvist K., Huisman W., Kouri T., Palicka V., Plebani M., Puro V., Salvagno G.L., Sandberg S., Sikaris K., Watson I., Stankovic A.K., Simundic A.M. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49:1113–1126. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt M., Mengele K., Schueren E., Sweep F.C., Foekens J.A., Brunner N., Laabs J., Malik A., Harbeck N. European Organisation for Research and Treatment of Cancer (EORTC) Pathobiology Group standard operating procedure for the preparation of human tumour tissue extracts suited for the quantitative analysis of tissue-associated biomarkers. Eur J Cancer. 2007;43:835–844. doi: 10.1016/j.ejca.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Lim M.D., Dickherber A., Compton C.C. Before you analyze a human specimen, think quality, variability, and bias. Anal Chem. 2011;83:8–13. doi: 10.1021/ac1018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald J.S., Milosevic D., Reddi H.V., Grebe S.K., Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57:833–840. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- 30.Drubin D., Smith J.S., Liu W., Zhao W., Chase G.A., Clawson G.A. Comparison of cryopreservation and standard needle biopsy for gene expression profiling of human breast cancer specimens. Breast Cancer Res Treat. 2005;90:93–96. doi: 10.1007/s10549-004-3269-5. [DOI] [PubMed] [Google Scholar]
- 31.Jochumsen K.M., Tan Q., Dahlgaard J., Kruse T.A., Mogensen O. RNA quality and gene expression analysis of ovarian tumor tissue undergoing repeated thaw-freezing. Exp Mol Pathol. 2007;82:95–102. doi: 10.1016/j.yexmp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Vartanian K., Slottke R., Johnstone T., Casale A., Planck S.R., Choi D., Smith J.R., Rosenbaum J.T., Harrington C.A. Gene expression profiling of whole blood: comparison of target preparation methods for accurate and reproducible microarray analysis. BMC Genomics. 2009;10:2. doi: 10.1186/1471-2164-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rainen L., Arbique J.C., Asthana D., Earley M.C., Geiszler R.L., Krieg-Schneider F., Mannhalter C., Ogino S., Parish G.T., Ballas C., Chandler L.J., Fernandes H., Ferrari M., Lechpammer M., WalkerPeach C.R., Williams L.O. Clinical and Laboratory Standards Institute; Wayne, PA: 2005. Clinical Laboratory Standards Institute Document MM13-A: Collection, Transport, Preparation, and Storage of Specimens for Molecular Methods; Approved Guideline. [Google Scholar]
- 34.Leyland-Jones B.R., Ambrosone C.B., Bartlett J., Ellis M.J., Enos R.A., Raji A., Pins M.R., Zujewski J.A., Hewitt S.M., Forbes J.F., Abramovitz M., Braga S., Cardoso F., Harbeck N., Denkert C., Jewell S.D. Recommendations for collection and handling of specimens from group breast cancer clinical trials. J Clin Oncol. 2008;26:5638–5644. doi: 10.1200/JCO.2007.15.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botling J., Edlund K., Segersten U., Tahmasebpoor S., Engstrom M., Sundstrom M., Malmstrom P.U., Micke P. Impact of thawing on RNA integrity and gene expression analysis in fresh frozen tissue. Diagn Mol Pathol. 2009;18:44–52. doi: 10.1097/PDM.0b013e3181857e92. [DOI] [PubMed] [Google Scholar]
- 36.Ma W., Wang M., Wang Z.Q., Sun L., Graber D., Matthews J., Champlin R., Yi Q., Orlowski R.Z., Kwak L.W., Weber D.M., Thomas S.K., Shah J., Kornblau S., Davis R.E. Effect of long-term storage in TRIzol on microarray-based gene expression profiling. Cancer Epidemiol Biomarkers Prev. 2010;19:2445–2452. doi: 10.1158/1055-9965.EPI-10-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams M.A. Stabilizing the code: methods to preserve RNA prove their worth. Biomark Insights. 2010;5:139–143. doi: 10.4137/BMI.S6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asare A.L., Kolchinsky S.A., Gao Z., Wang R., Raddassi K., Bourcier K., Seyfert-Margolis V. Differential gene expression profiles are dependent upon method of peripheral blood collection and RNA isolation. BMC Genomics. 2008;9:474. doi: 10.1186/1471-2164-9-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matheson L.A., Duong T.T., Rosenberg A.M., Yeung R.S. Assessment of sample collection and storage methods for multicenter immunologic research in children. J Immunol Methods. 2008;339:82–89. doi: 10.1016/j.jim.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Debey S., Zander T., Brors B., Popov A., Eils R., Schultze J.L. A highly standardized, robust, and cost-effective method for genome-wide transcriptome analysis of peripheral blood applicable to large-scale clinical trials. Genomics. 2006;87:653–664. doi: 10.1016/j.ygeno.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Shou J., Dotson C., Qian H.R., Tao W., Lin C., Lawrence F., N′Cho M., Kulkarni N.H., Bull C.M., Gelbert L.M., Onyia J.E. Optimized blood cell profiling method for genomic biomarker discovery using high-density microarray. Biomarkers. 2005;10:310–320. doi: 10.1080/13547500500218583. [DOI] [PubMed] [Google Scholar]
- 42.Weber D.G., Casjens S., Rozynek P., Lehnert M., Zilch-Schoneweis S., Bryk O., Taeger D., Gomolka M., Kreuzer M., Otten H., Pesch B., Johnen G., Bruning T. Assessment of mRNA and microRNA stabilization in peripheral human blood for multicenter studies and miobanks. Biomark Insights. 2010;5:95–102. doi: 10.4137/bmi.s5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung J.Y., Hewitt S.M. An optimized RNA extraction method from archival formalin-fixed paraffin-embedded tissue. Methods Mol Biol. 2010;611:19–27. doi: 10.1007/978-1-60327-345-9_2. [DOI] [PubMed] [Google Scholar]
- 44.Macabeo-Ong M., Ginzinger D.G., Dekker N., McMillan A., Regezi J.A., Wong D.T., Jordan R.C. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod Pathol. 2002;15:979–987. doi: 10.1097/01.MP.0000026054.62220.FC. [DOI] [PubMed] [Google Scholar]
- 45.Evers D.L., Fowler C.B., Cunningham B.R., Mason J.T., O'Leary T.J. The effect of formaldehyde fixation on RNA: optimization of formaldehyde adduct removal. J Mol Diagn. 2011;13:282–288. doi: 10.1016/j.jmoldx.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung J.Y., Braunschweig T., Williams R., Guerrero N., Hoffmann K.M., Kwon M., Song Y.K., Libutti S.K., Hewitt S.M. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:1033–1042. doi: 10.1369/jhc.2008.951863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hewitt S.M., Lewis F.A., Cao Y., Conrad R.C., Cronin M., Danenberg K.D., Goralski T.J., Langmore J.P., Raja R.G., Williams P.M., Palma J.F., Warrington J.A. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929–1935. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- 48.Ferruelo A., El-Assar M., Lorente J.A., Nin N., Penuelas O., Fernandez-Segoviano P., Gonzalez C., Esteban A. Transcriptional profiling and genotyping of degraded nucleic acids from autopsy tissue samples after prolonged formalin fixation times. Int J Clin Exp Pathol. 2011;4:156–161. [PMC free article] [PubMed] [Google Scholar]
- 49.Cox M.L., Schray C.L., Luster C.N., Stewart Z.S., Korytko P.J., Khan K.N.M., Paulauskis J.D., Dunstan R.W. Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity. Exp Mol Pathol. 2006;80:183–191. doi: 10.1016/j.yexmp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Cox M.L., Eddy S.M., Stewart Z.S., Kennel M.R., Man M.Z., Paulauskis J.D., Dunstan R.W. Investigating fixative-induced changes in RNA quality and utility by microarray analysis. Exp Mol Pathol. 2008;84:156–172. doi: 10.1016/j.yexmp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Lawson M.H., Rassl D.M., Cummings N.M., Russell R., Morjaria J.B., Brenton J.D., Murphy G., Rintoul R.C. Tissue banking of diagnostic lung cancer biopsies for extraction of high quality RNA. J Thorac Oncol. 2010;5:956–963. doi: 10.1097/JTO.0b013e3181ddbbe9. [DOI] [PubMed] [Google Scholar]
- 52.Medeiros F., Rigl C.T., Anderson G.G., Becker S.H., Halling K.C. Tissue handling for genome-wide expression analysis: a review of the issues, evidence, and opportunities. Arch Pathol Lab Med. 2007;131:1805–1816. doi: 10.5858/2007-131-1805-THFGEA. [DOI] [PubMed] [Google Scholar]
- 53.Budczies J., Weichert W., Noske A., Muller B.M., Weller C., Wittenberger T., Hofmann H.P., Dietel M., Denkert C., Gekeler V. Genome-wide gene expression profiling of formalin-fixed paraffin-embedded breast cancer core biopsies using microarrays. J Histochem Cytochem. 2011;59:146–157. doi: 10.1369/jhc.2010.956607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saleh A., Zain R.B., Hussaini H., Ng F., Tanavde V., Hamid S., Chow A.T., Lim G.S., Abraham M.T., Teo S.H., Cheong S.C. Transcriptional profiling of oral squamous cell carcinoma using formalin-fixed paraffin-embedded samples. Oral Oncol. 2010;46:379–386. doi: 10.1016/j.oraloncology.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Ton C.C., Vartanian N., Chai X., Lin M.G., Yuan X., Malone K.E., Li C.I., Dawson A., Sather C., Delrow J., Hsu L., Porter P.L. Gene expression array testing of FFPE archival breast tumor samples: an optimized protocol for WG-DASL sample preparation. Breast Cancer Res Treat. 2011;125:879–883. doi: 10.1007/s10549-010-1159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts L., Bowers J., Sensinger K., Lisowski A., Getts R., Anderson M.G. Identification of methods for use of formalin-fixed, paraffin-embedded tissue samples in RNA expression profiling. Genomics. 2009;94:341–348. doi: 10.1016/j.ygeno.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Parker J.S., Mullins M., Cheang M.C., Leung S., Voduc D., Vickery T., Davies S., Fauron C., He X., Hu Z., Quackenbush J.F., Stijleman I.J., Palazzo J., Marron J.S., Nobel A.B., Mardis E., Nielsen T.O., Ellis M.J., Perou C.M., Bernard P.S. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdueva D., Wing M., Schaub B., Triche T., Davicioni E. Quantitative expression profiling in formalin-fixed paraffin-embedded samples by Affymetrix microarrays. J Mol Diagn. 2010;12:409–417. doi: 10.2353/jmoldx.2010.090155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duenwald S., Zhou M., Wang Y., Lejnine S., Kulkarni A., Graves J., Smith R., Castle J., Tokiwa G., Fine B., Dai H., Fare T., Marton M. Development of a microarray platform for FFPET profiling: application to the classification of human tumors. J Transl Med. 2009;7:65. doi: 10.1186/1479-5876-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X., Chen J., Radcliffe T., Lebrun D.P., Tron V.A., Feilotter H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J Mol Diagn. 2008;10:513–519. doi: 10.2353/jmoldx.2008.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mittempergher L., de Ronde J.J., Nieuwland M., Kerkhoven R.M., Simon I., Rutgers E.J., Wessels L.F., Van't Veer L.J. Gene expression profiles from formalin fixed paraffin embedded breast cancer tissue are largely comparable to fresh frozen matched tissue. PLoS One. 2011;6:e17163. doi: 10.1371/journal.pone.0017163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roepman P., de Koning E., van Leenen D., de Weger R.A., Kummer J.A., Slootweg P.J., Holstege F.C. Dissection of a metastatic gene expression signature into distinct components. Genome Biol. 2006;7:R117. doi: 10.1186/gb-2006-7-12-r117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Botling J., Micke P. Biobanking of fresh frozen tissue from clinical surgical specimens: transport logistics, sample selection, and histologic characterization. Methods Mol Biol. 2011;675:299–306. doi: 10.1007/978-1-59745-423-0_16. [DOI] [PubMed] [Google Scholar]
- 64.Luttges J., Hahn S.A., Heidenblut A.M. Manual microdissection combined with antisense RNA-longSAGE for the analysis of limited cell numbers. Methods Mol Biol. 2010;576:135–154. doi: 10.1007/978-1-59745-545-9_8. [DOI] [PubMed] [Google Scholar]
- 65.Burgemeister R. Nucleic acids extraction from laser microdissected FFPE tissue sections. Methods Mol Biol. 2011;724:117–129. doi: 10.1007/978-1-61779-055-3_8. [DOI] [PubMed] [Google Scholar]
- 66.Wang S., Wang L., Zhu T., Gao X., Li J., Wu Y., Zhu H. Improvement of tissue preparation for laser capture microdissection: application for cell type-specific miRNA expression profiling in colorectal tumors. BMC Genomics. 2010;11:163. doi: 10.1186/1471-2164-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hess C., Denkers F., Ossenkoppele G., Waisfisz Q., McElgunn C., Eldering E., Schouten J., Schuurhuis G. Gene expression profiling of minimal residual disease in acute myeloid leukaemia by novel multiplex-PCR-based method. Leukemia. 2004;18:1981–1988. doi: 10.1038/sj.leu.2403520. [DOI] [PubMed] [Google Scholar]
- 68.Wan H., Seth A., Rainen L., Fernandes H. Coamplification of HIV-1 proviral DNA and viral RNA in assays used for quantification of HIV-1 RNA. J Clin Microbiol. 2010;48:2186–2190. doi: 10.1128/JCM.02034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anwar A., Wan G., Chua K.B., August J.T., Too H.P. Evaluation of pre-analytical variables in the quantification of dengue virus by real-time polymerase chain reaction. J Mol Diagn. 2009;11:537–542. doi: 10.2353/jmoldx.2009.080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elloumi F., Hu Z., Li Y., Parker J.S., Gulley M.L., Amos K.D., Troester M.A. Systematic bias in genomic classification due to contaminating non-neoplastic tissue in breast tumor samples. BMC Med Genomics. 2011;4:54. doi: 10.1186/1755-8794-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malkov V.A., Serikawa K.A., Balantac N., Watters J., Geiss G., Mashadi-Hossein A., Fare T. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Res Notes. 2009;2:80. doi: 10.1186/1756-0500-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaume M., Hernandez D., Docquier M., Delucinge-Vivier C., Descombes P., Francois P. Orientation and expression of methicillin-resistant Staphylococcus aureus small RNAs by direct multiplexed measurements using the nCounter of NanoString technology. J Microbiol Methods. 2011;84:327–334. doi: 10.1016/j.mimet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 73.Becker C., Hammerle-Fickinger A., Riedmaier I., Pfaffl M.W. mRNA and microRNA quality control for RT-qPCR analysis. Methods. 2010;50:237–243. doi: 10.1016/j.ymeth.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Rudloff U., Bhanot U., Gerald W., Klimstra D.S., Jarnagin W.R., Brennan M.F., Allen P.J. Biobanking of human pancreas cancer tissue: impact of ex-vivo procurement times on RNA quality. Ann Surg Oncol. 2010;17:2229–2236. doi: 10.1245/s10434-010-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Botling J., Micke P. Fresh frozen tissue: RNA extraction and quality control. Methods Mol Biol. 2011;675:405–413. doi: 10.1007/978-1-59745-423-0_25. [DOI] [PubMed] [Google Scholar]
- 76.Shi L., Perkins R.G., Fang H., Tong W. Reproducible and reliable microarray results through quality control: good laboratory proficiency and appropriate data analysis practices are essential. Curr Opin Biotechnol. 2008;19:10–18. doi: 10.1016/j.copbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vermeulen J., De Preter K., Lefever S., Nuytens J., De Vloed F., Derveaux S., Hellemans J., Speleman F., Vandesompele J. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011;39:e63. doi: 10.1093/nar/gkr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devonshire A.S., Elaswarapu R., Foy C.A. Evaluation of external RNA controls for the standardisation of gene expression biomarker measurements. BMC Genomics. 2010;11:662. doi: 10.1186/1471-2164-11-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan X., Fang H., Hong H., Perkins R., Shi L., Tong W. Correlation analysis of external RNA controls reveals its utility for assessment of microarray assay. Anal Biochem. 2009;385:203–207. doi: 10.1016/j.ab.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Walter M., Honegger A., Schweizer R., Poths S., Bonin M. Utilization of AFFX spike-in control probes to monitor sample identity throughout Affymetrix GeneChip Array processing. Biotechniques. 2010;48:371–378. doi: 10.2144/000113421. [DOI] [PubMed] [Google Scholar]
- 83.Novoradovskaya N., Whitfield M.L., Basehore L.S., Novoradovsky A., Pesich R., Usary J., Karaca M., Wong W.K., Aprelikova O., Fero M. Universal reference RNA as a standard for microarray experiments. BMC Genomics. 2004;5:20. doi: 10.1186/1471-2164-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma C., Lyons-Weiler M., Liang W., LaFramboise W., Gilbertson J.R., Becich M.J., Monzon F.A. In vitro transcription amplification and labeling methods contribute to the variability of gene expression profiling with DNA microarrays. J Mol Diagn. 2006;8:183–192. doi: 10.2353/jmoldx.2006.050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Westgard J.O. Internal quality control: planning and implementation strategies. Ann Clin Biochem. 2003;40:593–611. doi: 10.1258/000456303770367199. [DOI] [PubMed] [Google Scholar]
- 86.Vermeulen J., Derveaux S., Lefever S., De Smet E., De Preter K., Yigit N., De Paepe A., Pattyn F., Speleman F., Vandesompele J. RNA pre-amplification enables large-scale RT-qPCR gene-expression studies on limiting sample amounts. BMC Res Notes. 2009;2:235. doi: 10.1186/1756-0500-2-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Roca E., Garcia-Albeniz X., Rodriguez-Mulero S., Gomis R.R., Kornacker K., Auer H. Accurate expression profiling of very small cell populations. PLoS One. 2010;5:e14418. doi: 10.1371/journal.pone.0014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corbi F.C., Inaoka R.J., Felix R.S., Andrade V.C., Etto L.Y., Vettore A.L., Franco M.F., Colleoni G.W. Comparative expression of a set of genes to an internal housekeeping control in CDNA amplified and not amplified by PolyAPCR in non-Hodgkin's lymphoma samples obtained from fine-needle aspiration cytology. Diagn Mol Pathol. 2010;19:40–44. doi: 10.1097/PDM.0b013e3181b0b618. [DOI] [PubMed] [Google Scholar]
- 89.Ferreira E.N., Maschietto M., Silva S.D., Brentani H., Carraro D.M. Evaluation of quantitative rt-PCR using nonamplified and amplified RNA. Diagn Mol Pathol. 2010;19:45–53. doi: 10.1097/PDM.0b013e3181ae8186. [DOI] [PubMed] [Google Scholar]
- 90.Shack S. Gene expression profiling of tissues and cell lines: a dual-color microarray method. Methods Mol Biol. 2011;700:125–143. doi: 10.1007/978-1-61737-954-3_9. [DOI] [PubMed] [Google Scholar]
- 91.Oberthuer A., Juraeva D., Li L., Kahlert Y., Westermann F., Eils R., Berthold F., Shi L., Wolfinger R.D., Fischer M., Brors B. Comparison of performance of one-color and two-color gene-expression analyses in predicting clinical endpoints of neuroblastoma patients. Pharmacogenomics J. 2010;10:258–266. doi: 10.1038/tpj.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patterson T.A., Lobenhofer E.K., Fulmer-Smentek S.B., Collins P.J., Chu T.M., Bao W., Fang H., Kawasaki E.S., Hager J., Tikhonova I.R., Walker S.J., Zhang L., Hurban P., de Longueville F., Fuscoe J.C., Tong W., Shi L., Wolfinger R.D. Performance comparison of one-color and two-color platforms within the MicroArray Quality Control (MAQC) project. Nature Biotechnol. 2006;24:1140–1150. doi: 10.1038/nbt1242. [DOI] [PubMed] [Google Scholar]
- 93.Toll A.D., Liu J.M., Gulati G., Behling E.M., Kocher W.D. Does routine repeat testing of critical values offer any advantage over single testing? Arch Pathol Lab Med. 2011;135:440–444. doi: 10.5858/2010-0025-OA.1. [DOI] [PubMed] [Google Scholar]
- 94.Daly T.M., Dumaual C.M., Dotson C.A., Farmen M.W., Kadam S.K., Hockett R.D. Precision profiling and components of variability analysis for Affymetrix microarray assays run in a clinical context. J Mol Diagn. 2005;7:404–412. doi: 10.1016/S1525-1578(10)60570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuscoe J.C., Tong W., Shi L. QA/QC issues to aid regulatory acceptance of microarray gene expression data. Environ Mol Mutagen. 2007;48:349–353. doi: 10.1002/em.20293. [DOI] [PubMed] [Google Scholar]
- 96.Hackett J.L., Gutman S.I. Introduction to the Food and Drug Administration (FDA) regulatory process. J Proteome Res. 2005;4:1110–1113. doi: 10.1021/pr050059a. [DOI] [PubMed] [Google Scholar]
- 97.Muller B.M., Kronenwett R., Hennig G., Euting H., Weber K., Bohmann K., Weichert W., Altmann G., Roth C., Winzer K.J., Kristiansen G., Petry C., Dietel M., Denkert C. Quantitative determination of estrogen receptor, progesterone receptor, and HER2 mRNA in formalin-fixed paraffin-embedded tissue: a new option for predictive biomarker assessment in breast cancer. Diagn Mol Pathol. 2011;20:1–10. doi: 10.1097/PDM.0b013e3181e3630c. [DOI] [PubMed] [Google Scholar]
- 98.Smyth G.K., Michaud J., Scott H.S. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 99.Ho Y.Y., Cope L., Dettling M., Parmigiani G. Statistical methods for identifying differentially expressed gene combinations. Methods Mol Biol. 2007;408:171–191. doi: 10.1007/978-1-59745-547-3_10. [DOI] [PubMed] [Google Scholar]
- 100.Fox P., Hendler J. Changing the equation on scientific data visualization. Science. 2011;331:705–708. doi: 10.1126/science.1197654. [DOI] [PubMed] [Google Scholar]
- 101.Expression profiling: best practices for data generation and interpretation in clinical trials Tumor Analysis Best Practices Working Group. Nat Rev Genet. 2004;5:229–237. doi: 10.1038/nrg1297. [DOI] [PubMed] [Google Scholar]
- 102.Ritchie M.E., Silver J., Oshlack A., Holmes M., Diyagama D., Holloway A., Smyth G.K. A comparison of background correction methods for two-colour microarrays. Bioinformatics. 2007;23:2700–2707. doi: 10.1093/bioinformatics/btm412. [DOI] [PubMed] [Google Scholar]
- 103.Fan J., Niu Y. Selection and validation of normalization methods for c-DNA microarrays using within-array replications. Bioinformatics. 2007;23:2391–2398. doi: 10.1093/bioinformatics/btm361. [DOI] [PubMed] [Google Scholar]
- 104.Zahurak M., Parmigiani G., Yu W., Scharpf R.B., Berman D., Schaeffer E., Shabbeer S., Cope L. Pre-processing Agilent microarray data. BMC Bioinformatics. 2007;8:142. doi: 10.1186/1471-2105-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McMullen P.D., Morimoto R.I., Amaral L.A.N. Physically grounded approach for estimating gene expression from microarray data. Proc Natl Acad Sci. 2010;107:13690–13695. doi: 10.1073/pnas.1000938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi L., Campbell G., Jones W.D., Campagne F., Wen Z., Walker S.J. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nature Biotechnol. 2010;28:827–838. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glaves P.D., Tugwood J.D. Generation and analysis of transcriptomics data. Methods Mol Biol. 2011;691:167–185. doi: 10.1007/978-1-60761-849-2_10. [DOI] [PubMed] [Google Scholar]
- 108.Simon R. Analysis of DNA microarray expression data. Best Pract Res Clin Haematol. 2009;22:271–282. doi: 10.1016/j.beha.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parry R.M., Jones W., Stokes T.H., Phan J.H., Moffitt R.A., Fang H., Shi L., Oberthuer A., Fischer M., Tong W., Wang M.D. k-Nearest neighbor models for microarray gene expression analysis and clinical outcome prediction. Pharmacogenomics J. 2010;10:292–309. doi: 10.1038/tpj.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simon R. Interpretation of genomic data: questions and answers. Semin Hematol. 2008;45:196–204. doi: 10.1053/j.seminhematol.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schultz K.R., Bowman W.P., Aledo A., Slayton W.B., Sather H., Devidas M., Wang C., Davies S.M., Gaynon P.S., Trigg M. Improved early event-free survival with imatinib in Philadelphia chromosome–positive acute lymphoblastic leukemia: a Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Izraeli S. Application of genomics for risk stratification of childhood acute lymphoblastic leukaemia: from bench to bedside? Br J Haematol. 2010;151:119–131. doi: 10.1111/j.1365-2141.2010.08312.x. [DOI] [PubMed] [Google Scholar]
- 113.Jelier R., Goeman J.J., Hettne K.M., Schuemie M.J., den Dunnen J.T., ‘t Hoen P.A. Literature-aided interpretation of gene expression data with the weighted global test. Brief Bioinform. 2011;12:518–529. doi: 10.1093/bib/bbq082. [DOI] [PubMed] [Google Scholar]
- 114.Gulley M.L., Braziel R.M., Halling K.C., Hsi E.D., Kant J.A., Nikiforova M.N., Nowak J.A., Ogino S., Oliveira A., Polesky H.F., Silverman L., Tubbs R.R., Van Deerlin V.M., Vance G.H., Versalovic J. Clinical laboratory reports in molecular pathology. Arch Pathol Lab Med. 2007;131:852–863. doi: 10.5858/2007-131-852-CLRIMP. [DOI] [PubMed] [Google Scholar]
- 115.Schiff G.D., Hasan O., Kim S., Abrams R., Cosby K., Lambert B.L., Elstein A.S., Hasler S., Kabongo M.L., Krosnjar N., Odwazny R., Wisniewski M.F., McNutt R.A. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169:1881–1887. doi: 10.1001/archinternmed.2009.333. [DOI] [PubMed] [Google Scholar]
- 116.Coppée J.Y. Do DNA microarrays have their future behind them? Microbes Infect. 2008;10:1067–1071. doi: 10.1016/j.micinf.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Feng L., Liu H., Liu Y., Lu Z., Guo G., Guo S., Zheng H., Gao Y., Cheng S., Wang J., Zhang K., Zhang Y. Power of deep sequencing and agilent microarray for gene expression profiling study. Mol Biotechnol. 2010;45:101–110. doi: 10.1007/s12033-010-9249-6. [DOI] [PubMed] [Google Scholar]
- 118.Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szecsi P.B., Odum L. Error tracking in a clinical biochemistry laboratory. Clin Chem Lab Med. 2009;47:1253–1257. doi: 10.1515/CCLM.2009.272. [DOI] [PubMed] [Google Scholar]
- 120.Fabbretti G. Risk management: correct patient and specimen identification in a surgical pathology laboratory: the experience of Infermi Hospital, Rimini, Italy. Pathologica. 2010;102:96–101. [PubMed] [Google Scholar]
- 121.Valenstein P.N., Alpern G.A., Keren D.F. Responding to large-scale testing errors. Am J Clin Pathol. 2010;133:440–446. doi: 10.1309/AJCPXLZE0YYNID0X. [DOI] [PubMed] [Google Scholar]
- 122.Brothman A.R., Dolan M.M., Goodman B.K., Park J.P., Persons D.L., Saxe D.F., Tepperberg J.H., Tsuchiya K.D., Van Dyke D.L., Wilson K.S., Wolff D.J., Theil K.S. College of American Pathologists/American College of Medical Genetics proficiency testing for constitutional cytogenomic microarray analysis. Genet Med. 2011;13:765–769. doi: 10.1097/GIM.0b013e31821d3165. [DOI] [PubMed] [Google Scholar]
- 123.Staines H., Garcia-Fernandez L., Pogothata R., Wallace P., MacKay W., Van Loon A. Monitoring performance of nucleic acid-based diagnostic measurement system users by EQA: accreditation and quality assurance. J Qual Comparability Reliability Chem Measurement. 2009;14:243–252. [Google Scholar]
- 124.Tholen D.W., Berte L.M., Boone D.J., Cooper W.G., Gun-Munro J., Noble M.A., Sarewitz S.J., Williams M.L. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Clinical Laboratory Standards Institute Document GP27-A2: Using Proficiency Testing to Improve the Clinical Laboratory; Approved Guideline-Second Edition. [Google Scholar]
- 125.Madej R.M., Cao Z., Dolinger D.L., Hall L., Neuwald P., Williams L.O. Clinical and Laboratory Standards Institute; Wayne, PA: 2005. Clinical and Laboratory Standards Institute Document MM14-A: Proficiency Testing (External Quality Assessment) for Molecular Methods; Approved Guideline. [Google Scholar]
- 126.Sarewitz S.J., George H., Miller W.G., Tholen D.W., Valenstein P.N. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Clinical Laboratory Standards Institute Document GP29-A2: Assessment of Laboratory Tests When Proficiency Testing Is Not Available; Approved Guideline-ed 2. [Google Scholar]