Frontal, cell body, and rear regions perform distinct functions in the complex process of cell migration. A low-capacity, directional mechanism in the front coupled to a high-capacity, nondirectional mechanism in the middle represents a highly appealing model for driving cell migration under high mechanical load.
Abstract
To understand the mechanism of cell migration, we cultured fibroblasts on micropatterned tracks to induce persistent migration with a highly elongated morphology and well-defined polarity, which allows microfluidic pharmacological manipulations of regional functions. The function of myosin II was probed by applying inhibitors either globally or locally. Of interest, although global inhibition of myosin II inhibited tail retraction and caused dramatic elongation of the posterior region, localized inhibition of the cell body inhibited nuclear translocation and caused elongation of the anterior region. In addition, local application of cytochalasin D at the tip inhibited frontal extension without inhibiting forward movement of the cell nucleus, whereas local treatment posterior to the nucleus caused reversal of nuclear movement. Imaging of cortical dynamics indicated that the region around the nucleus is a distinct compression zone where activities of anterior and posterior regions converge. These observations suggest a three-component model of cell migration in which a contractile middle section is responsible for the movement of a bulky cell body and the detachment/retraction of a resistive tail, thereby allowing these regions to undergo coordinated movement with a moving anterior region that carries little load.
INTRODUCTION
Cell migration is an essential, tightly regulated process in development and homeostasis. Abnormal cell migration during embryogenesis may lead to birth defects, whereas failure of cell migration in the adult body affects wound-healing and inflammation responses. Deregulated cell motility may also play a critical role in cancer metastasis. Directional cell migration relies on establishing a polarity with distinct anterior and posterior regions (Petrie et al., 2009). However, how these regions coordinate to generate forward movement of the cell body has not been clearly elucidated.
Likely as a result of short- and long-range positive/negative feedback mechanisms (Satulovsky et al., 2008), anterior and posterior regions of migrating cells exhibit distinct activities—extension and adhesion at the front, and retraction and de-adhesion at the rear (Sheetz et al., 1999). The anterior region also carries such distinct structures as the leading lamellipodium, with actin flux and nascent focal adhesions (Wang, 1985; Ponti et al., 2004; Choi et al., 2008); the lamella, where retrograde cortical transport takes place (Heath 1983; Cramer et al., 1997); and a convergence zone, where lamellum retrograde flow meets with slow anterograde flow (Salmon et al., 2002; Vallotton et al., 2004). Compared to the anterior region, the posterior region shows much more pronounced cycles of stretching and shortening during cell migration. The cell body, which carries structures such as nucleus, centrosomes, and Golgi apparatus, is located between these two distinct regions.
Most studies of cell migration have focused on the frontal lamellipodia (Vallotton and Small, 2009) and the associated traction forces (Munevar et al., 2001a). Although few mechanical details are known, cell migration clearly involves force interactions between structures in different regions undergoing different mechanical activities. Furthermore, traction forces reflect only mechanical output to the environment, whereas forces involved in cell migration may engage in much more complex interactions that take place intracellularly and be undetectable on the underlying substrate.
Among the force-generating mechanisms, myosin II–dependent contractility is believed to provide the major forces of the actin–myosin system that drive cell migration (Jay et al., 1995; Beningo et al., 2006; Meili et al., 2010). Myosin II isoforms show different distributions between the anterior and posterior regions (Even-Ram et al., 2007; Vicente-Manzanares et al., 2008), consistent with its region-specific mechanical functions. However, the function of myosin II contractility in cell migration is ambiguous. Inhibition of myosin II in fish keratocytes halts cell body translocation (Oliver et al., 1999; Verkhovsky et al., 1999), whereas knockout of myosin II heavy chains in Dictyostelium suppresses cell migration under overlaid agar but causes only subtle effects on two-dimensional (2D) surfaces (Delozanne and Spudich, 1987; Wessels et al., 1988; Uchida et al., 2003). Furthermore, whereas myosin II–dependent traction forces are concentrated at the front, it is believed that myosin II is involved in tail retraction (Munevar et al., 2001a; Vicente-Manzanares et al., 2009). These results suggest different functions of myosin II in different regions of the cell, which may play distinct roles in cell migration under different conditions.
A careful examination of the interplay among different regions of the cell during directional migration may shed important light on the mechanism of cell translocation. However, whereas global manipulations of specific proteins have become routine using pharmacological, gene manipulation, or small interfering RNA–based approaches, few techniques allow localized disruption of protein functions for the dissection of their site-specific functions. In this study, we combined micropatterning and microfluidic approaches to focus on the biophysical and mechanical mechanisms of cell body translocation. Micropatterning was used for generating well-polarized cells with distinct anterior and posterior regions, which were then probed with localized pharmacological treatments using micropipette-based microfluidic approaches.
RESULTS
Distinct dynamics of anterior and posterior regions during cell migration
To facilitate unambiguous dissection of functions in different regions of a migrating cell, we developed a simple, economical substrate micropatterning method using linear polyacrylamide as the blocking agent, which provides strong and stable resistance against cell adhesion in blocked regions and allows long-term maintenance of cell patterning (Supplemental Figure S1; Guo and Wang, 2011).
When cultured on strips 2–12 μm in width, most NIH3T3 fibroblasts showed highly persistent migration with well-defined anterior and posterior regions (89.2% of 157 cells; Supplemental Movies S1 and S2; Doyle et al., 2009). In contrast to retraction-induced protrusion for cells spread on 2D surfaces (Chen, 1979), the anterior region maintained a relatively constant morphology and migration rate despite cycles of stretching and retractions in the posterior region (Figure 1, A and B; and Supplemental Movies S1 and S2), suggesting that anterior and posterior regions are at best loosely coupled mechanically and that the anterior region is more rigid than the rest of the cell. In addition, posterior retraction was followed by rearward extension in which retracted tail appeared to reextend (Supplemental Movies S1 and S2), suggesting active forces driving the cytoplasm backward upon tail retraction.
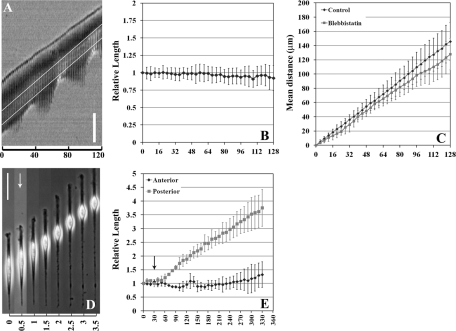
FIGURE 1:
Cell migration on micropatterned strips with distinct behaviors in anterior and posterior regions. Typical cell behavior of NIH3T3 cells migrating along a micropatterned strip is shown as a kymograph (A). While the tail undergoes striking cyclical retractions and reextension, the frontal region translocates steadily without discernible correlation with tail retraction cycles. Note that the nucleus, located between the lines, migrates in a similar way to the frontal region. Strong correlation between the movement of the leading edge and the nucleus is indicated as a nearly constant distance between them (B; n = 8). Treatment with 10 μM blebbistatin causes failure of tail retraction; however, frontal protrusion and migration of the nucleus were not affected (D). Steady migration was observed for at least 3 h after blebbistatin treatment (D). As a result, the anterior segment maintains a relatively constant length (measured as the distance from the leading edge to the nucleus; E, lower curve) similar to control cells (B), whereas the posterior region shows a striking elongation (measured as the distance from the nucleus to the tip of the tail; E, upper curve; n = 11; all 11 cells showed a similar behavior). Arrows indicate the time of blebbistatin addition (D, E). The speed of nuclear translocation also appeared unaffected by the strong inhibition of tail retraction for at least 2 h (C; p = 0.388). Numbers on the x-axis are time in minutes (A–C, and E) or hours (D). Error bar, SD. Bar, 100 μm for A and 50 μm for D.
Functional roles of myosin II and actin in anterior and posterior regions
To dissect the function of different regions of the cell, we applied pharmacological agents both globally to determine any differential effects in different regions and locally to determine any effects on global and local migration behavior. We first inhibited myosin II–mediated contractility with blebbistatin, an inhibitor of myosin II ATPase and traction forces (Straight et al., 2003; Beningo et al., 2006). Global application of blebbistatin completely prevented the tail from shortening and detaching, without noticeably affecting the anterior region. The cell body became more compact (Figure 1D). However, quantification of the effects, as permitted by the present approach, indicated that the rate of protrusion, the length of the anterior segment (measured as the distance from the leading edge to the center of nucleus), or the phase morphology of the anterior region appeared different from those of control cells (Figure 1, D and E, and Supplemental Movie S3). The translocation of nucleus was also unaffected by the strong inhibition of tail retraction (Figure 1, C and D, and Supplemental Movie S3), as confirmed by Student's t test of the speed before and after treatment (p = 0.388). Similar results were obtained with Y-27632, an inhibitor of small GTPase Rho–mediated myosin II activation (Maekawa et al., 1999). Although tail elongation following blebbistatin treatment was reported previously with unpatterned cells (Kolega, 2006), the lack of effects in the anterior region was much more difficult to assess without a well-defined polarity and persistent migration as in the present case.
The foregoing results suggest distinct functions of myosin II–dependent contractility in anterior and posterior regions. We then applied myosin II inhibitors locally using double-microneedle microfluidic drug release (Bradke and Dotti, 1999; O'Connell et al., 2001), which uses a release needle paired with an aspiration needle nearby to constrain the distribution of compounds and maintain a focused drug distribution, such that the concentration drops by 50% from the tip of the release needle over a short distance of 10–15 μm. Application of blebbistatin or Y-27632 in the anterior region had no significant effect on frontal protrusion, nuclear translocation, or tail activities (Figure 2, A, B, and F, Supplemental Movie S4, and Supplemental Figure S3), suggesting that traction forces generated by anterior myosin II are not transmitted across the cell body to drive nuclear movement or tail retraction. Similarly, localized inhibition of myosin II at the tail did not affect tail retraction (Figure 2E, Supplemental Movie S5, and Supplemental Figure S4). The most striking effects were observed when myosin II was inhibited in the cell body, which caused nuclear movement to halt. It is perhaps not surprising that tail retraction was also inhibited due to the lack of cell body movement. However, the frontal region continued to move forward, resulting in a highly elongated anterior region connected through a thin thread with the cell body (Figure 2, C, D, and F, Supplemental Movie S6, and Supplemental Figure S5). This result suggests that contractility is required for maintaining the position of the cell body relative to the frontal region, thereby providing a dynamic link that releases the front end upon localized inhibition of myosin II.
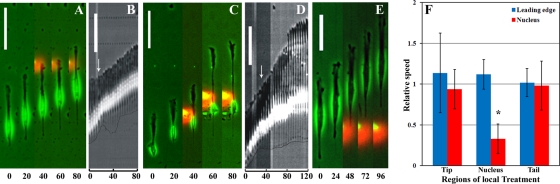
FIGURE 2:
Myosin II–dependent forces near the nucleus are indispensable for nuclear translocation and tail retraction. Local application of Y-27632 at the leading edge, as indicated by the red fluorescence, has no significant effect on protrusion, tail release, or nuclear translocation (A and B; experiment represents 13 of 18 cells observed, 72%). Similarly, inhibition of myosin II with either Y-27632 or blebbistatin at the tail did not affect tail retraction (E; 11 of 11 cells observed, 100%). However, treatment of the region near the nucleus causes strong inhibition of nuclear translocation without significantly affecting frontal movement, which results in striking elongation of the anterior region (C, D; 12 of 18 cells observed, 67%). Note that tail elongation and retraction are inhibited due to the inhibition of nuclear movement. Bar graph indicates the relative average speed of the leading edge and nucleus over a period of 60 min before and after local treatment of Y-27632 in different regions (F; average speed after treatment divided by average speed before treatment). Note that treatment at the leading edge, nucleus, or tail does not change the average speed of the leading edge (p = 0.91, 0.6, and 0.97, respectively). On the other hand, although application at the tip and tail does not affect nucleus movement (p = 0.81 and 0.66 respectively), treatment near the nucleus significantly reduced the average speed of nuclear translocation (p < 0.001; indicated by asterisk in F). Arrows indicate the starting point of local drug application. Numbers indicate time in minutes. Outlines in kymographs (B, D) mark the tracks of tail movement. Error bars in the bar chart represent SD. Bar, 100 μm for A–D and 40 μm for E.
We then applied cytochalasin D to probe the requirement of actin structures in cell migration. As expected, global application of cytochalasin D inhibited cell migration and abolished cell polarity. Locally applying cytochalasin D to the frontal region halted frontal protrusion (Figure 3, A–C, and Supplemental Movie S7). However, the nucleus continued to move forward, whereas tail retraction appeared unaffected (Figure 3, A and B), suggesting that the cell body does not simply passively follow lamellipodial extension (Anderson et al., 1996). The cell eventually switched its polarity by forming a new leading edge at the opposite end. Application of cytochalasin D at the tip of the tail had no effect on either nuclear movement or cell polarity. Of interest, cytochalasin D applied to the cell body just posterior to the nucleus caused reversal of nuclear movement and subsequent reversal of cell polarity (Figure 3, D–F, and Supplemental Movie S8). The rear end of the cell protrudes despite the presence of cytochalasin D, likely as a result of shifting force balance within the cell body that pushes cytoplasmic materials backward (Figure 3E). In addition, this result implies that the cell body may contribute to cell directionality, despite a general consensus that the sensing and steering mechanism resides at the front.
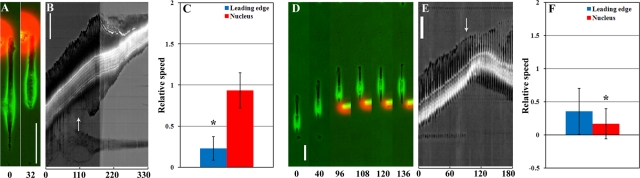
FIGURE 3:
Distinct responses of anterior and posterior regions to local application of cytochalasin D. Local application of cytochalasin D at the leading edge of the cell does not affect nuclear translocation or tail retraction, although it does suppress frontal protrusion, as shown in A. Kymograph from a separate cell shows a similar response over a prolonged period of time (B). In contrast, local application of cytochalasin D in a region just posterior to the nucleus causes reversal of nuclear movement (D, E; for kymograph of the same cell; 11 of 11 cells observed, 100%). Bar graphs indicate the relative average speed of the leading edge and nucleus over a period of 60 min before and after local treatment of cytochalasin D at the leading edge (C; average speed after treatment divided by average speed before treatment; n = 10) or near the nucleus (F; n = 11). Note that although treatment at the leading edge significantly reduced leading edge protrusion (p < 0.005; indicates by asterisk in E) without affecting nuclear translocation (p = 0.61), application of cytochalasin D just posterior to the nucleus causes significantly reduced net nuclear translocation within 60 min and eventual reversal of nuclear movement (p < 0.05; indicates as by asterisk in F). The treatment is stopped after the formation of a new leading edge at the opposite end. Arrows indicate the starting point of local drug application. Numbers indicate time in minutes. Error bars in bar charts represent SD. Bar, 50 μm.
The distinct pharmacological responses as described here suggest distinct functions of different regions. To determine whether the actin cortex also showed different activities, we tracked features on the ventral actin cortex in cells expressing green fluorescent protein (GFP)–α-actinin as a marker, which is localized both at focal adhesions and stress fibers and at cortical punctate structures (Hotulainen and Lappalainen, 2006; Supplemental Figure S2). We observed distinct patterns of dynamics between anterior and posterior cortices using spinning disk confocal optics, with particular attention on the cell body and posterior region. Structures in the anterior region were largely stationary relative to the substrate (Figure 4A; Cramer et al., 1997; Anderson et al., 2008), whereas those in nonretracting posterior regions showed a gradient of forward movement, with the most pronounced movement occurring just posterior to the nucleus (Figure 4B). During retraction, structures near the tip of the tail surged forward (Figure 4C). Thus, while the anterior cortex moves backward relative to the nucleus, the frontal end of the posterior cortex is relatively stationary except during retraction, such that anterior and posterior cortices converge near the nucleus to create a contraction zone in the cell body. The only effect of blebbistatin on cortical dynamics was to inhibit tail retraction and the associated surge of posterior structures, which then became increasingly stretched as the cell migrated forward (Figure 4, D and E).
FIGURE 4:
Distinct patterns of cortical actin dynamics in anterior and posterior regions during cell migration. Using GFP–α-actinin as a marker for the ventral actin cytoskeleton, we observed distinct patterns of cortical dynamics in different regions (all movements analyzed as kymographs): stationary or slow forward transport in the anterior region (A; line indicates the extension of leading edge), compression in the region near the nucleus (B; N marks the nuclear region), and stretching and retraction at the tail (B, C). Structures at tip of the tail are stationary before tail retraction (B, bottom) but undergo rapid forward movement during tail retraction (C; the line indicates tail retraction). Inhibiting myosin II by either blebbistatin or Y-27632 does not significantly affect cortical dynamics in the anterior region or the region just behind the nucleus (D; near the top edge). The only effect of blebbistatin on cortical dynamics is to inhibit the surge of posterior structures due to tail retraction. Posterior structures become increasingly stretched as the cell migrates forward (E). Numbers indicate time in minutes. Bar, 10 μm.
DISCUSSION
Prevalent models of fibroblast migration emphasize a frontal pulling mechanism based on actin-driven protrusion coupled with myosin II–dependent traction forces (Munevar et al., 2001b). Tail region plays a secondary and possibly coordinating role, stimulating frontal protrusion upon each episode of retraction for cells spread on 2D surfaces (Chen, 1979). The present results point to a different picture for cells migrating in one dimension—that a myosin II–independent mechanism, driven possibly by cytoskeletal dynamics (Miao et al., 2003), actin polymerization (Abraham et al., 1999), actin cross-linking/bundling coupled to depolymerization (Sun et al., 2010), or motors other than myosin II, is responsible for the movement of the anterior region. In addition, the anterior region appears to maintain a relatively constant length and morphology, showing little response to either the retraction of the tail or the inhibition of myosin II. Forces generated by anterior myosin II may instead be involved in sensing the mechanical environment and steering the migration (Rape et al., 2011).
Judging from the fixed position of its cortex relative to the substrate (Figure 4; Hay 1989; Cramer et al., 1997), movement of the anterior region likely involves assembling new structures at the distal end (Wang, 1985) and disassembling old structures at the proximal end while maintaining a firm adhesion with the underlying substrate. Previous observations further support the hypothesis that actin cytoskeleton at the leading edge contributes to the contractile bundles elsewhere (Svitkina et al., 1997; Hotulainen and Lappalainen, 2006; Anderson et al., 2008; Nemethova et al., 2008), with a compression zone of actin and myosin II at the lamellipodial/cell body transition zone (Svitkina et al., 1997; Schaub et al., 2007).
In contrast to the anterior region, the posterior region appears to be much more elastic, showing cycles of shortening and elongation as cells migrate forward. Myosin II is required for the retraction of the tail and appears essential for its apparent elasticity, as the tail stretches to great length in the presence of myosin II inhibitors until it breaks. Furthermore, tail reextension following each episode of retraction suggests compression activities somewhere near the cell body, which would push the cytoplasm back toward a weakened tail.
An intriguing observation is that global inhibition of myosin II has no effect on nuclear translocation, whereas local inhibition of myosin II at the cell body inhibits its forward movement. In neither case was frontal extension affected, which then resulted in the dramatic elongation of the posterior region upon global inhibition and elongation of the anterior region upon inhibition of the cell body. Combined with the compression activities of the cortex around the cell body, these observations may be explained by a three-component model for fibroblast migration: an active, myosin II–dependent contractile central cell body, flanked by a rigid anterior region whose migration and mechanical properties depend on structural assembly/disassembly but are independent of myosin II, and a posterior region whose elasticity and retraction are highly dependent on myosin II (Figure 5A). During cell migration, the anterior region moves actively forward, maintaining a constant length, while the posterior region remains attached to the central cell body and the substrate. Myosin II–dependent contraction of the cell body then causes the cell body to move forward and the posterior region to detach or elongate, as dictated by the differential rigidity/resistance of the anterior region and posterior region. In addition, following tail release, contraction of the central cell body would cause the tail to reextend as a result of its minimal resistance.
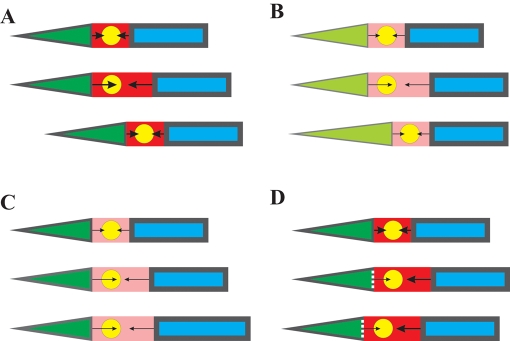
FIGURE 5:
A three-component model for the migration of adherent cells. During normal migration (A), the anterior region moves forward while maintaining a fixed length and a rigid cortex (represented as a thick line), whereas posterior region is more elastic. Contraction of the cell body coupled to asymmetric resistance causes the nucleus to move forward and the posterior region to stretch or detach. On global inhibition of myosin II (B), anterior movement is independent of myosin II and is unaffected. However, tail elasticity is myosin II dependent, and the resistance to cell migration decreases dramatically. This allows the cell body to migrate forward despite the loss of most contractile forces (reduced arrows). The tail becomes highly stretched as a result. Localized inhibition of myosin II in the cell body does not affect anterior protrusion or posterior resistance (C). However, contractile forces in the cell body are weakened, causing the inhibition of cell body movement and elongation of the region in front of the nucleus. Disrupting the posterior cytoskeleton with cytochalasin D reduces posterior resistance and/or contractility in the posterior portion of the cell body (D), causing the cytoplasm and nucleus to be pushed backward.
Together these observations indicate that contractility of the cell body plays an important role in maintaining cell integrity during migration. The cell body in essence functions as a myosin II–dependent dynamic linkage between the anterior and posterior regions, which releases upon local inhibition of myosin II. As the weakened contractile forces in the cell body are no longer able to overcome the tail resistance in order to follow the forward movement of the anterior region, the frontal region walks away from the cell body as a result (Figure 5C). Note that a similar mechanism may be responsible for the movement of the nucleus in migratory neurons during development (Schaar and McConnell, 2005), whereas the down-regulation of myosin II may be coupled to the outgrowth of axons away from a stationary cell body during later phases of brain development.
It is equally important to note that, upon global inhibition of myosin II, the tail region loses its ability to retract and appears to generate little resistance to the forward movement of the cell body, while the anterior region maintains its shape. The decrease in tail resistance, together with the decrease in focal adhesion–mediated anchorage (Chrzanowska-Wodnicka and Burridge, 1996), allows the cell body to maintain its forward movement despite the decrease in traction forces (Figure 5B). In contrast, local application of blebbistatin to the tail affected neither tail retraction nor cell migration, suggesting that forces for tail retraction are generated not locally in the tail region but in the cell body or at least distal to the tail region (Kaverina et al., 2000).
Equally interesting are the responses to local applications of cytochalasin D. The response to tip application is similar to that reported for fish keratocytes (Fournier et al., 2010) and may be explained by the inhibition of leading-edge advancement upon the inhibition of actin assembly without affecting the contractility and the resulting forward translocation of the cell body. The response to cytochalasin D treatment in the cell body posterior to the nucleus may be explained by the local disintegration of structures and reduction of posterior mechanical resistance, such that forces from the contraction of the cell body would cause the cytoplasm and nucleus to be pushed toward the rear (Figure 5D). The effect may be similar to tail reextension as seen in control cells, except that it occurs without the release of the tail. In addition, an actin-independent (thus cytochalasin-resistant) cell protrusion process could also play a role in the reversal of cell polarity (Peckham et al., 2001).
The present results suggest that fibroblast migration involves mechanical interactions among three discrete regions of the cell: the anterior region, which moves largely independently of myosin II; a central cell body, which undergoes concomitant myosin II–dependent contractions; and a posterior region, which generates strong resistance to the forward migration and retracts periodically. Assuming that the anterior region moves without direct involvement of motor molecules, it is expected to have a limited load-bearing capacity despite its ability to move rapidly and directionally, similar to an automobile in high gear. The ability to carry the cargo of cell body and to overcome tail resistance is then conferred by the contraction of the cell body. The combination of a low load-bearing anterior that detects environmental cues and a high load-bearing cell body that follows the directionality guidance of the front creates a highly efficient and responsive mechanism for the migration of adhesive cells. The present model is different from a recently proposed “all-wheel drive” model for the migration of fish epidermal keratocytes (Fournier et al., 2010), in which the lack of an adhesive tail and a compact, laterally oriented cell body may make the detection of distinct mechanical parts difficult.
In summary, we showed that anterior, cell body, and posterior regions perform distinct functions in the complex process of cell migration. In addition, we propose that a low-capacity, directional mechanism in the front coupled to a high-capacity, nondirectional mechanism in the middle creates a highly appealing model for driving cell migration under high mechanical load.
MATERIALS AND METHODS
Cell culture, plasmids, and drug administration
NIH3T3 mouse fibroblasts (American Type Culture Collection, Rockville, MD) were maintained in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% donor calf serum (HyClone, Logan, UT), 50 μg/ml streptomycin, 50 U/ml penicillin, and 2 mM l-glutamine. All cells were maintained in an incubator with 5% CO2 at 37°C.
GFP–α-actinin was kindly provided by Juergen Wehland (Helmholtz Centre for Infection Research, Braunschweig, Germany). NIH3T3 was nucleofected using Amaxa Nucleofector I Kit R (Amaxa, Gaithersburg, MD). Y-27632 (Calbiochem, San Diego, CA) was prepared as 10 mM stock solution in phosphate-buffered saline (PBS). Stock solution of blebbistatin (100 mM; Calbiochem) was prepared in dimethyl sulfoxide (DMSO) and stored at –20°C. All reagents were diluted from the stock solution into medium immediately before use. The final concentration was 100 μM for Y-27632 and 10 μM for blebbistatin unless specifically indicated.
Double-microneedle focal drug release was performed as previously reported (O'Connell et al., 2001). Briefly, cytochalasin D or Y-27632 was diluted into 0.5 mg/ml fluorescein dextran (Molecular Probes, Invitrogen, Carlsbad, CA) in DMEM to obtain a working concentration of 2 μM for cytochalasin D in 0.008% DMSO, 50 μM for blebbistatin in 0.025% DMSO, or 100 μM for Y-27632 in PBS. The solution was ultracentrifuged to remove particulates and loaded into a release microneedle connected to a source of compressed air. A suction microneedle with a large tip was prepared by breaking the tip and heat polishing using a microforge. The release and suction needles were mounted on a custom double-needle micromanipulator to allow simultaneous positioning. Positive and negative pressures were regulated with custom-made regulators. Highly localized distribution of the drug, as monitored with fluorescence optics, was obtained at minimal pressures. Localized release of drug was maintained throughout the period of observation.
Micropatterning using linear polyacrylamide
The strategy of micropatterning cell–matrix adhesion using linear polyacrylamide as the blocking agent is shown in Supplemental Figure S1. The detailed protocol was published elsewhere (Guo and Wang, 2011). Briefly, coverslips were activated with Bind-Silane (GE Healthcare, Piscataway, NJ), and areas for cell adhesion were covered selectively with positive photoresist SPR-220 (MicroChem, Newton, MA) through UV exposure, baking, and development. The remaining area is made nonadhesive by grafting linear polyacrylamide to the Bind-Silane–activated glass surface. The photoresist-protected adhesive region is then exposed by stripping off the photoresist.
Live-cell imaging, immunofluorescence staining, and image analysis
Live-cell images were recorded every 2 or 4 min with a cooled slow-scan charge-coupled device (CCD) camera (NTE/CCD-512-EBFT; Princeton Instruments, Trenton, NJ) or an ultra-low-light electron multiplication CCD camera (iXon DV887DCS-BV; Andor Technology, South Windsor, CT) attached to a Zeiss Axiovert 200M microscope equipped with a 10×, CP-Achromat phase objective lens phase-contrast or a 100×, Plan-Neofluar phase objective lens for GFP-actinin on a stage incubator for time-lapse recording (Carl Zeiss, Thornwood, NY).
For immunofluorescence staining, cells were fixed with 4% formaldehyde (EMS, Hatfield, PA) and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Samples were blocked with 1% bovine serum albumin/PBS for 15 min at room temperature and then incubated for 60 min at 37°C with 1:200 dilution of primary antibodies against paxillin (Santa Cruz Biotechnology, Santa Cruz, CA) and Alexa dye–conjugated anti-rabbit or anti-mouse secondary antibodies (obtained from Molecular Probes, Life Technologies, Eugene, OR) and applied at a dilution of 1:400 for 30 min at 37°C. Images were collected with an inverted microscope (Axiovert 200M) equipped with a 100× Plan-Neofluar numerical aperture 1.3 oil lens. Fluorescence intensities were measured and kymographs produced with custom software. Statistical significance for most experiments was based on the high frequency of a given outcome when an experiment was repeated 8–18 times; each repetition typically involved hours of time-lapse recording while maintaining a consistent drug exposure.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grant GM-32476 to Y.L.W. We thank Samantha Cavolo for technical support.
Abbreviations used:
- CCD
charge-coupled device
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- 2D
two-dimensional
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-06-0556) on March 7, 2012.
REFERENCES
- Abraham VC, Krishnamurthi V, Taylor DL, Lanni F. The actin-based nanomachine at the leading edge of migrating cells. Biophys J. 1999;77:1721–1732. doi: 10.1016/S0006-3495(99)77018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KI, Wang YL, Small JV. Coordination of protrusion and translocation of the keratocyte involves rolling of the cell body. J Cell Biol. 1996;134:1209–1218. doi: 10.1083/jcb.134.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver f-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol Biol Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Chen WT. Induction of spreading during fibroblast movement. J Cell Biol. 1979;81:684–691. doi: 10.1083/jcb.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:U1039–U1036. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J Cell Biol. 1997;136:1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy-chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin microtubule crosstalk. Nat Cell Biol. 2007;9:299-U104. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Fournier MF, Sauser R, Ambrosi D, Meister JJ, Verkhovsky AB. Force transmission in migrating cells. J Cell Biol. 2010;188:287–297. doi: 10.1083/jcb.200906139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W-H, Wang Y-L. Micropatterning cell-substrate adhesions using linear poly-acrylamide as the blocking agent. Cold Spring Harb Protoc. 2011;2011((1)):prot5582. doi: 10.1101/pdb.prot5582. [DOI] [PubMed] [Google Scholar]
- Hay ED. Theory for epithelial-mesenchymal transformation based on the fixed cortex cell motility model. Cell Motil Cytoskeleton. 1989;14:455–457. doi: 10.1002/cm.970140403. [DOI] [PubMed] [Google Scholar]
- Heath JP. Behavior and structure of the leading lamella in moving fibroblasts. 1. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell-surface. J Cell Sci. 1983;60:331–354. doi: 10.1242/jcs.60.1.331. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay PY, Pham PA, Wong SA, Elson EL. A mechanical function of myosin-II in cell motility. J Cell Sci. 1995;108:387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Gimona M, Beningo KA, Wang YL, Small JV. Enforced polarisation and locomotion of fibroblasts lacking microtubules. Curr Biol. 2000;10:739–742. doi: 10.1016/s0960-9822(00)00544-3. [DOI] [PubMed] [Google Scholar]
- Kolega J. The role of myosin II motor activity in distributing myosin asymmetrically and coupling protrusive activity to cell translocation. Mol Biol Cell. 2006;17:4435–4445. doi: 10.1091/mbc.E06-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Meili R, Alonso-Latorre B, del Alamo JC, Firtel RA, Lasheras JC. Myosin II is essential for the spatiotemporal organization of traction forces during cell motility. Mol Biol Cell. 2010;21:405–417. doi: 10.1091/mbc.E09-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L, Vanderlinde O, Stewart M, Roberts TM. Retraction in amoeboid cell motility powered by cytoskeletal dynamics. Science. 2003;302:1405–1407. doi: 10.1126/science.1089129. [DOI] [PubMed] [Google Scholar]
- Munevar S, Wang YL, Dembo M. Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol Biol Cell. 2001a;12:3947–3954. doi: 10.1091/mbc.12.12.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munevar S, Wang YL, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J. 2001b;80:1744–1757. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemethova M, Auinger S, Small JV. Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella. J Cell Biol. 2008;180:1233–1244. doi: 10.1083/jcb.200709134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell CB, Warner AK, Wang YL. Distinct roles of the equatorial and polar cortices in the cleavage of adherent cells. Curr Biol. 2001;11:702–707. doi: 10.1016/s0960-9822(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M, Miller G, Wells C, Zicha D, Dunn GA. Specific changes to the mechanism of cell locomotion induced by overexpression of beta-actin. J Cell Sci. 2001;114:1367–77. doi: 10.1242/jcs.114.7.1367. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Rape A, Guo W-H, Wang Y-L. Responses of cells to adhesion-mediated signals: a universal mechanism. In: Johnson A Wagoner, Harley BAC., editors. Mechanobiology of Cell-Cell and Cell-Matrix Interactions. New York: Springer; 2011. pp. 1–10. [Google Scholar]
- Salmon WC, Adams MC, Waterman-Storer CM. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J Cell Biol. 2002;158:31–37. doi: 10.1083/jcb.200203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satulovsky J, Lui R, Wang YL. Exploring the control circuit of cell migration by mathematical modeling. Biophys J. 2008;94:3671–3683. doi: 10.1529/biophysj.107.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S, Bohnet S, Laurent VM, Meister JJ, Verkhovsky AB. Comparative maps of motion and assembly of filamentous actin and myosin II in migrating cells. Mol Biol Cell. 2007;18:3723–3732. doi: 10.1091/mbc.E06-09-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP, Felsenfeld D, Galbraith CG, Choquet D. Cell migration as a five-step cycle. Biochem Soc Symp. 1999;65:233–243. [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Sun SX, Walcott S, Wolgemuth CW. Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr Biol. 2010;20:R649–R654. doi: 10.1016/j.cub.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida KSK, Kitanishi-Yumura T, Yumura S. Myosin II contributes to the posterior contraction and the anterior extension during the retraction phase in migrating Dictyostelium cells. J Cell Sci. 2003;116:51–60. doi: 10.1242/jcs.00195. [DOI] [PubMed] [Google Scholar]
- Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc Natl Acad Sci USA. 2004;101:9660–5. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallotton P, Small JV. Shifting views on the leading role of the lamellipodium in cell migration: speckle tracking revisited. J Cell Sci. 2009;122:1955–1958. doi: 10.1242/jcs.042036. [DOI] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma XF, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL. Exchange of actin subunits at the leading-edge of living fibroblasts—possible role of treadmilling. J Cell Biol. 1985;101:597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D, Soll DR, Knecht D, Loomis WF, Delozanne A, Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy-chain. Dev Biol. 1988;128:164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.