Abstract
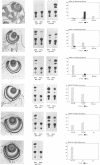
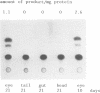
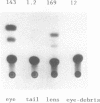
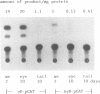
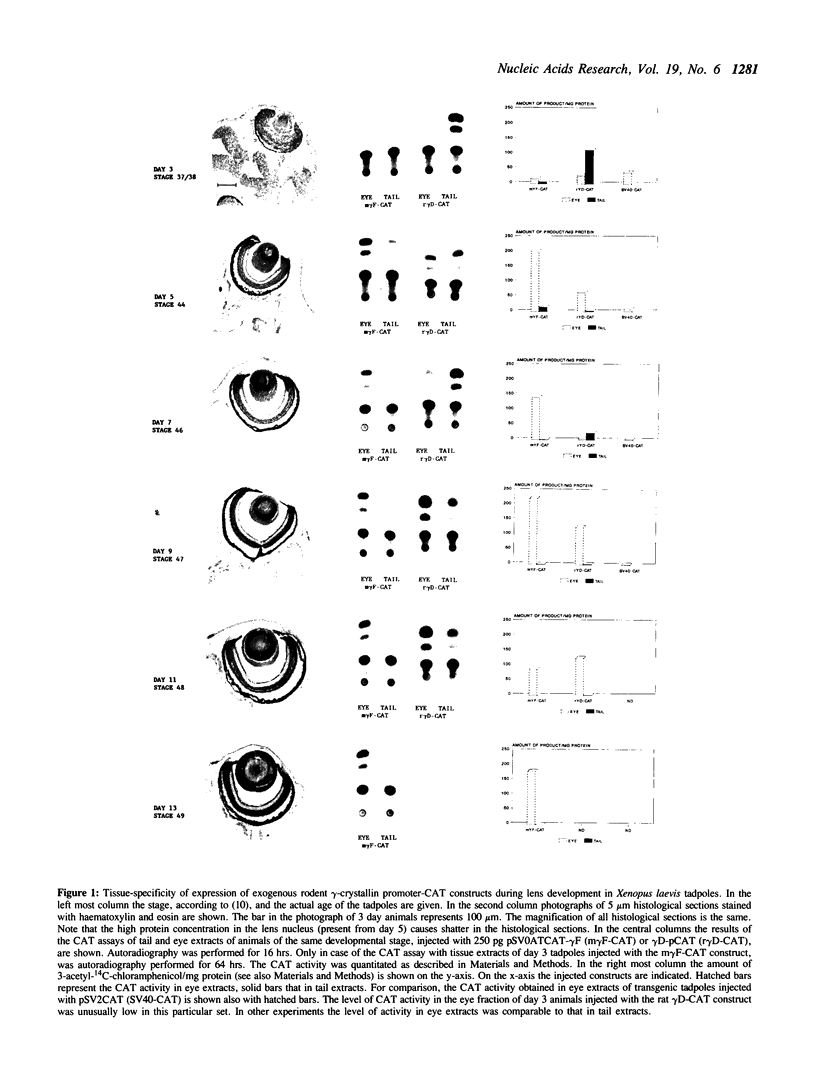
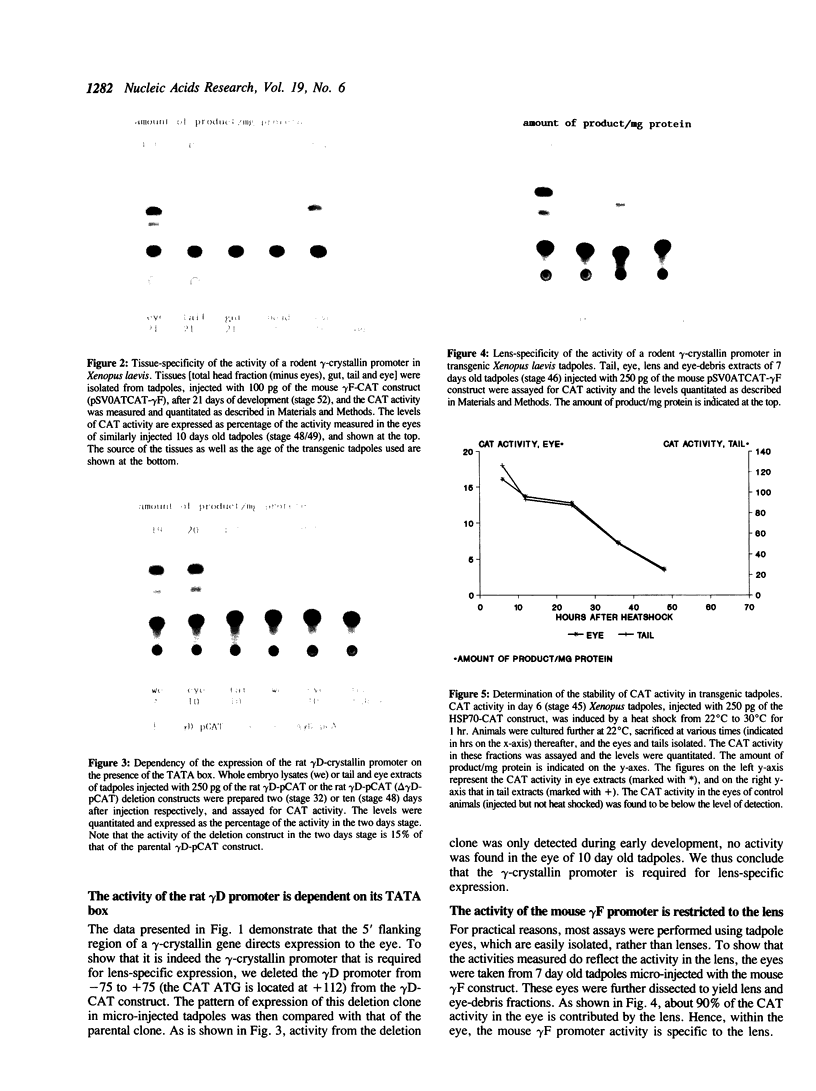
Rodent gamma-crystallin promoters were recognized as lens-specific promoters in micro-injected Xenopus laevis tadpoles and targeted the expression of the chloramphenicol acetyl transferase (CAT) reporter gene to the tadpole lens. The onset of expression coincided with lens cell formation. The level of expression continued to increase up to 9 days of development (stage 47), stayed at that level till at least day 13 and dropped by only 57% at day 21. In contrast, the level of expression of a non-tissue-specific promoter, the SV40 early promoter, decreased rapidly in the eye during development and was only detectable up to stage 44 (day 5). The stability of the CAT activity in the lens was assessed by delivering a pulse of activity from a heat shock promoter-CAT fusion gene. The half-life of the CAT activity in the eye was the same as that in the tail. The increase in CAT activity in the lens thus depends upon continued activity of the injected gamma-crystallin promoters. Our data demonstrate that mammalian promoters can be used to target gene expression to specific tissues during Xenopus laevis development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Muellener D. B., Ryffel G. U. Persistence, methylation and expression of vitellogenin gene derivatives after injection into fertilized eggs of Xenopus laevis. Nucleic Acids Res. 1984 Mar 12;12(5):2283–2302. doi: 10.1093/nar/12.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Etkin L. D., Pearman B. Distribution, expression and germ line transmission of exogenous DNA sequences following microinjection into Xenopus laevis eggs. Development. 1987 Jan;99(1):15–23. doi: 10.1242/dev.99.1.15. [DOI] [PubMed] [Google Scholar]
- Etkin L. d., Pearman B., Roberts M., Bektesh S. L. Replication, integration and expression of exogenous DNA injected into fertilized eggs of Xenopus laevis. Differentiation. 1984;26(3):194–202. doi: 10.1111/j.1432-0436.1984.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Giebelhaus D. H., Eib D. W., Moon R. T. Antisense RNA inhibits expression of membrane skeleton protein 4.1 during embryonic development of Xenopus. Cell. 1988 May 20;53(4):601–615. doi: 10.1016/0092-8674(88)90576-4. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Montag M., Steinbeisser H., Trendelenburg M. F. Plasmid and bacteriophage lambda-DNA show differential replication characteristics following injection into fertilized eggs of Xenopus laevis: dependence on period and site of injection. Cell Differ Dev. 1990 Apr;30(1):77–85. doi: 10.1016/0922-3371(90)90075-8. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Gurdon J. B. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990 Sep 13;347(6289):197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- Krone P. H., Heikkila J. J. Expression of microinjected hsp 70/CAT and hsp 30/CAT chimeric genes in developing Xenopus laevis embryos. Development. 1989 Jun;106(2):271–281. doi: 10.1242/dev.106.2.271. [DOI] [PubMed] [Google Scholar]
- Landel C. P., Chen S. Z., Evans G. A. Reverse genetics using transgenic mice. Annu Rev Physiol. 1990;52:841–851. doi: 10.1146/annurev.ph.52.030190.004205. [DOI] [PubMed] [Google Scholar]
- Lok S., Breitman M. L., Chepelinsky A. B., Piatigorsky J., Gold R. J., Tsui L. C. Lens-specific promoter activity of a mouse gamma-crystallin gene. Mol Cell Biol. 1985 Sep;5(9):2221–2230. doi: 10.1128/mcb.5.9.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Reddan J. R., Tsui L. C., Breitman M. L. A rabbit lens epithelial cell line supports expression of an exogenous crystallin gene characteristic of lens fiber cell differentiation. Exp Eye Res. 1989 Jan;48(1):131–137. doi: 10.1016/0014-4835(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R., van der Logt P., Lubsen N. H., Schoenmakers J. G. Tissue- and species-specific promoter elements of rat gamma-crystallin genes. Nucleic Acids Res. 1990 Mar 11;18(5):1189–1197. doi: 10.1093/nar/18.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S., Schaffner W. Transformation of frog embryos with a rabbit beta-globin gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5051–5055. doi: 10.1073/pnas.78.8.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török I., Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5' and 3' ends of the hsp 70 gene in the hybrid plasmid 56H8. Nucleic Acids Res. 1980 Jul 25;8(14):3105–3123. doi: 10.1093/nar/8.14.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leen R. W., Breuer M. L., Lubsen N. H., Schoenmakers J. G. Developmental expression of crystallin genes: in situ hybridization reveals a differential localization of specific mRNAs. Dev Biol. 1987 Oct;123(2):338–345. doi: 10.1016/0012-1606(87)90392-7. [DOI] [PubMed] [Google Scholar]
- Wilson C., Cross G. S., Woodland H. R. Tissue-specific expression of actin genes injected into Xenopus embryos. Cell. 1986 Nov 21;47(4):589–599. doi: 10.1016/0092-8674(86)90623-9. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]