Abstract
Background
It has been found that gap junction-associated intracellular Ca2+ [Ca2+]i disturbance contributes to the arrhythmogenesis and hyperconstriction in diseased heart. However, whether functional gaps are also involved in the regulation of normal Ca2+ signaling, in particular the basal [Ca2+]i activities, is unclear.
Methods and Results
Global and local Ca2+ signaling and gap permeability were monitored in cultured neonatal rat ventricular myocytes (NRVMs) and freshly isolated mouse ventricular myocytes by Fluo4/AM and Lucifer yellow (LY), respectively. The results showed that inhibition of gap communication by heptanol, Gap 27 and flufenamic acid or interference of connexin 43 (Cx43) with siRNA led to a significant suppression of LY uptake and, importantly, attenuations of global Ca2+ transients and local Ca2+ sparks in monolayer NRVMs and Ca2+ sparks in adult ventricular myocytes. In contrast, overexpression of rat-Cx43 in NRVMs induced enhancements in the above measurements, and so did in HEK293 cells expressing rat Cx43. Additionally, membrane-permeable inositol 1,4,5-trisphosphate (IP3 butyryloxymethyl ester) and phenylephrine, an agonist of adrenergic receptor, could relieve the inhibited Ca2+ signal and LY uptake by gap uncouplers, whereas blockade of IP3 receptor with xestospongin C or 2-aminoethoxydiphenylborate mimicked the effects of gap inhibitors. More importantly, all these gap-associated effects on Ca2+ signaling were also found in single NRVMs that only have hemichannels instead of gap junctions. Further immunostaining/immunoblotting single myocytes with antibody against Cx43 demonstrated apparent increases in membrane labeling of Cx43 and non-junctional Cx43 in overexpressed cells, suggesting functional hemichannels exist and also contribute to the Ca2+ signaling regulation in cardiomyocytes.
Conclusions
These data demonstrate that Cx43-associated gap coupling plays a role in the regulation of resting Ca2+ signaling in normal ventricular myocytes, in which IP3/IP3 receptor coupling is involved. This finding may provide a novel regulatory pathway for mediation of spontaneous global and local Ca2+ activities in cardiomyocytes.
Introduction
In myocardium gap junctions provide both electrical and metabolic exchange among connected myocytes, enabling a synchronized excitation and muscle contraction. Hemichannels are precursors of gap junctions, assembled by six connexin subunits that span the lipid bilayer. Like conventional ion channels, hemichannels do not remain continuously open, instead, they flip between open and closed states regulated by multiple stimuli. For instances, reduction in extracellular Ca2+, membrane depolarization, mechanical stress, metabolic inhibition, low intracellular redox potential, activation of purinergic receptors and intracellular kinase activity have all been implicated in the activation of hemichannel [1]–[6].
It has been demonstrated that functional connexin hemichannels also exist in isolated ventricular myocytes [6]. Open hemichannels are nonselective conduits for small molecules and cations, allowing the release of ATP [1], [2], [7] and NAD+ [8], and the influx of Ca2+ and Na+ [9]. Upon pathological insults such as ischemia and oxidative stress, hemichannels and gap coupling have been found to allow the passage of small molecules that contribute to cell injury [10], [11].
Intracellular Ca2+ ([Ca2+]i) transient represents the global intracellular Ca2+ signaling, while Ca2+ sparks are the building blocks of intracellular Ca2+ activity that derive from local, rapid and transient Ca2+ release from a cluster of ryanodine receptor (RyR) activation in the sarcoplasmic reticulum [12]. Both of the signal modes are important in regulation of normal heart function. Previous studies have shown that under pathological condition gap coupling is disordered and involved in the abnormal Ca2+ activities that potentially generate lethal arrhythmias and hyperconstriction in ventricles [11], [13]–[16], suggesting a functional role of the gap junction/intercellular communication in the regulation of Ca2+ signaling in diseased heart. Yet whether gap junction and hemichannels are also involved in the modulation of Ca2+ signaling, particularly, in the basal Ca2+ activities in normal heart, is presently unknown.
In this study, we used single cardiac myocytes to determine the effects of hemichannel on the [Ca2+]i activities and compared them with those found in monolayer myocytes that already form typical gap junctions. We found that both confluent and single myocytes exhibited downregulated Ca2+ signaling in response to gap uncouplers and interference of connexin43 (Cx43) expression the predominant connexin in the ventricles, while overexpression of Cx43 displayed enhanced Ca2+ activities in both densities of the cells. Therefore, this study demonstrates that Cx43-associated coupling plays a fundamental role in the mediation of local and global Ca2+ signaling in ventricular myocytes.
Materials and Methods
Materials and animals
Fluo-4/AM and Lucifer yellow (LY) were obtained from Molecular Probes (Invitrogen Inc, Carlsbad, California, USA). Myo-inositol 1,4,5-trisphosphate hexakis (butyryloxymethyl) ester (IP3/BM) was synthesized as instructed [17] (purity>95%). Xestospongin C was purchased from Calbiochem (Merck Inc, Darmstadt, Germany). All the antibodies and the reagents used, unless otherwise indicated, were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA. USA) and Sigma-Aldrich (St Louis, MO, USA), respectively.
C57BL mice (25–30 g) were obtained from the Experimental Animal Center of Capital Medical University (Beijing, China). The animals were housed at the animal care facility at 25°C with 12/12 h light/dark cycles and have free access to food and water ad libitum. All animal study protocols were approved by the Institutional Animal Research and Ethics Committee of Capital Medical University (Beijing, China, SCXK2009-0008).
Isolation and culture of neonatal rat ventricular myocytes
NRVMs were isolated from 1 to 2-day-old Sprague-Dawley rats by enzymatic digestion with 0.1% trypsin and 0.03% collegenase, as described [18]. After removing cardiac fibroblasts, NRVMs were plated onto 60 mm or 35 mm dishes at a density of 1×106 cells/ml for monolayer or dilute 10-fold for single cell study in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/ml penicillin/streptomycin, and 0.1 mM 5-bromo-2-deoxyuridine to inhibit fibroblast proliferation.
Isolation of ventricular myocytes from adult mice
C57BL mice were treated with heparin (2.5 units/g body weight) by intraperitoneal injection for 15 min before obtaining their hearts for perfusion. After proper anesthesia (10% chloral hydrate was intraperitoneally injected at 0.1 ml/20 g), the heart was rapidly excised and dropped into a beaker of cold modified HEPES buffered Tyrode solution (in mM NaCl 120, KCl 5.4, NaH2PO4 1.2, MgSO4 1.2 and glucose 5, HEPES 5, tuarine 5, 2,3-butanedione monoxime 10). After perfusion with Ca2+-free Tyrode solution and equilibration with 95%O2∶5%CO2 in Langendorff preparation, the heart was incubated in the enzyme solution (0.5 mg/ml collegenase and 0.1 mg/ml trypsin) gassed at 37°C for 15 min. Then the ventricle was broken into pieces by forceps and titrated gently with aspiration pipette, and the dispersed cells were harvested and filtered by 200 micron-pore sized cell sieve. Cells were incubated with Medium 199 containing 10% calf serum before usage for 2 h.
Confocal Ca2+ transient and spark imaging
Measurement of [Ca2+]i and the preparation of HEPES buffered physiological saline solution (HBSS) were performed as previously described [18]. All the NRVMs were used after 48 h culture, while the adult mouse ventricular myocytes were immediately used after isolation and equilibration with M199. Experiments were performed at room temperature (22–24°C).
LY uptake assay
NRVMs were incubated in a Ca2+-containing HBSS with the presence of vehicle or the drug of interest e.g. heptanol, Gap 27 and flufenamic acid (FFA) for 1–2 min, 30 min and 5 min, respectively. After washing with Ca2+-free HBSS twice, the cells were incubated with 2.5% LY (containing 1 mM EGTA) for 5 min at room temperature, and then the dye uptake was observed by Leica SP5 fluorescence laser scanning confocal microscopy (excitation at 405 nm and emission detection at 530 nm). Ten pictures were taken randomly from each dish for the statistical analysis.
Cell infection with adenovirus
All the construction of plasmids and adenovirus were performed by Invitrogen Inc. (Shanghai, China). In brief, Cx43 coding region was amplified by PCR reaction. The sequences for the pair of primers are 5′- CCGCTCGAGGCCACCATGGGTGACTGGAGTGCCTTGGG-3′ and 5′-CCGGAATTCTTAAATCTCCAGGTCATCAGGCCGA-3′. Then the PCR products were digested by XhoI and EcoRI and cloned into pIRES2-EGFP vector. The Cx43 overexpressing plasmids pGJA1-IRES-EGFP (wt-Cx43) was confirmed by sequencing and subcloned into pAD/CMV/V5-DEST vector by gateway reconstitution technique to make pAd-JX-GJA1-IRES2-EGFP adenovirus construct. To make the Cx43 knockdown plasmids, the complementary sequences 5′-TGCTGGATTCGCGTCTTCTTGTTGTCGTTTTGGCCACTGACTGACGACAACAAAGACGCGAATC-3′ and 5′-CCTGGATTCGCGTCTTTGTTGTCGTCAGTCAGTGGCCAAAACGACAACAAGAAGACGCGAATCC-3′ were verified to avoid the off-target silencing and inserted into pcDNA™6.2-GW/EmGFPmiRNA vector using BLOCK-iT™ Pol II miR RNAi Expression Vector Kit. After evaluation of the knockdown effects, it was also subcloned into pAD/CMV/V5-DEST vector. NRVMs were infected with adenovirus constructs (m.o.i. = 15) for 48 h to examine the overexpression or knockdown of Cx43 protein. Additionally, HEK293 cells were also expressed with wt-Cx43 by plasmid transfection (2 µg/ml) as previously described [19].
Immunocytochemistry
Cardiac myocytes were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 10 min and then permeabilized with 1% Triton X-100 PBS for 8 min at room temperature. After blocking in PBS containing 5% bovine serum albumin for 1 h, anti-Cx43 antibody was used overnight at 4°C at a dilution of 1∶100. The secondary antibody Alexa Fluor 488-labeled donkey anti-rabbit (Molecular Probes, Carlsbad, California, USA) were applied at a dilution of 1∶500 for 1 h at room temperature. The nucleus was labeled with Hoechst 33258 (1 µg/ml) for 5 min. Chemifluorescent detection was performed directly on a laser-scanning confocal microscopy (Leica SP 5) with a ×63 oil-immersion objective (NA 1.4). All negative controls were performed by taking host serum as the primary antibody.
Western blot
The extraction of non-junctional and junctional protein lysates was performed as previously described with some modification [20], [21]. In brief, NRVMs were incubated in lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton X-100) supplemented with 1 mM polymethylsulfonyl fluoride and 1× complete protease inhibitor cocktail on ice for 30 min. These samples were separated into Triton-soluble and -insoluble fractions by centrifugation at 14,000 rpm at 4°C for 30 min. Triton-insoluble fractions (pellets) were resuspended in the above lysis buffer supplemented with 2% Triton X-100 and 0.4% SDS and followed by brief sonication. After incubation on ice for 30 min, the lysates were centrifuged at 14,000 rpm at 4°C for 30 min to get the junctional protein lysates.
For the whole cell lysate, NRVMs were lysed in RIPA buffer containing 1 mM polymethylsulfonyl fluoride and 1× complete protease inhibitor cocktail. Lysates were boiled for 5 min, resolved on a 10% SDS-PAGE gel and transferred to PVDF membrane. Membranes were blocked with 5% nonfat milk powder in Tris-buffered saline containing 0.1% (v/v) Tween 20 for 60 min at room temperature. Anti- Cx43, -GAPDH and -α-actin antibodies were used overnight at 4°C at dilution of 1∶1500, 1∶3000 and 1∶1500, respectively. The immunoblotted membrane was then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h and immunoreactive bands were detected by using enhanced chemiluminescence.
Statistics
Data were analyzed and presented as means ± (S.E.) of n measurements. When appropriate, statistical comparisons between groups were carried out with 2-way paired or unpaired Student's t test. The accepted level of significance was P<0.05.
Results
Impairment of dye uptake by gap junction inhibitors in NRVMs
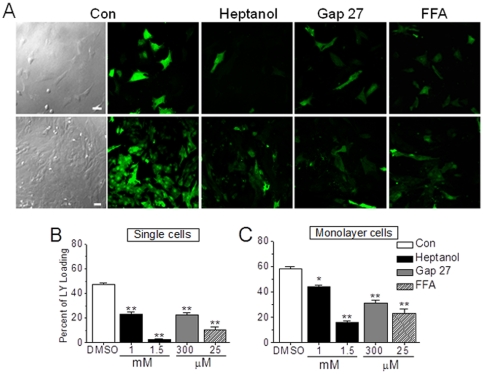
To determine whether hemichannels in cardiac myocytes are functional, first we examined and compared the cellular uptake of LY in Ca2+-free extracellular medium between single and monolayer NRVMs. LY (molecular weight 457 Dalton) can only enter cells through gaps, thus providing a rapid and noninvasive approach to determine the activity of hemichannels by evaluation of dye spreading [22]. A spectrum of drugs has been shown to inhibit gap junction communication with variable degrees of efficacy and specificity. These drugs include heptanol (nonspecific) [23], Gap 27 (specific), a peptide that mimics short sequences in the extracellular loop 2 of Cx43 and inhibits gap junction by direct interaction with exposed hemichannels in plasma membranes [24], and FFA as a hemichannel blocker [25], [26]. As shown in Figure 1, inhibition of gap junction by 1 mM and 1.5 mM heptanol significantly reduced the dye uptake by 52.3±2.33% and 95.1±4.54% in single cells and 23.6±1.25% and 74.4±3.31% in monolayer cells. Also, Gap 27 (300 µM) and FFA (25 µM) attenuated the LY uptake by 52.4±2.32% and 46.5±1.19%, and 78.3±3.63% and 60.3±2.32% in single and monolayer cells, respectively. Thus, these data suggest that functional gaps present not only in confluent cardiomyocytes, but also in single cells, in this case as hemichannels.
Figure 1. Impairment of dye uptake by gap junction inhibitors in NRVMs.
(A) Typical confocal images of Lucifer yellow (LY) uptake in different groups of single and confluent NRVMs treated with DMSO or heptanol (1 or 1.5 mM) for 2 min, Gap 27 (300 µM) for 30 min and FFA (25 µM) for 5 min as indicated. (B, C) Statistical data were obtained from 6–8 independent determinations for each bar. * and ** represent P<0.05 and P<0.01, vs. DMSO, respectively.
Inhibition of spontaneous Ca2+ signals by gap junction inhibitors in NRVMs
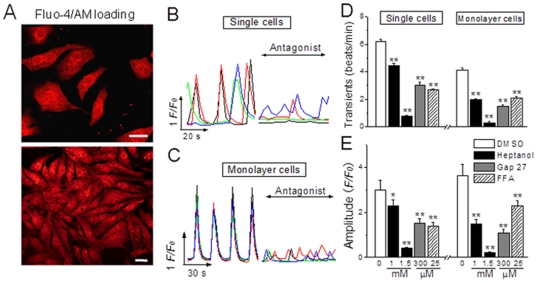
To determine if gap junctions regulate the resting Ca2+ signaling in ventricular myocytes, we monitored the spontaneous Ca2+ signals in unstimulated confluent NRVMs or non-contacting single NRVMs with or without the presence of gap uncouplers. As shown in Figure 2, both single and confluent NRVMs oscillated spontaneously at different rhythm, and it is obvious that monolayer cells displayed coordinate Ca2+ oscillations because of the formation of gap junctions (Figure 2A and C), whereas single cells generated uncoupled Ca2+ transients among the sighted cells due to lack of gap junctions (Figure 2A and B). Heptanol at the similar concentrations used for LY measurement could significantly inhibit the frequency and amplitude of the spontaneous Ca2+ transients in both single and monolayer cells, whereas the duration of the transient remained unaltered (Figure 2D and E). Similarly, Gap 27 and FFA also remarkably hindered the Ca2+ transients by reducing transient frequency and amplitude in either density of NRVMs, respectively. It appeared that all the gap uncouplers attenuated the Ca2+ transients with more potency in confluent cells than in single cells, likely implying that the hemichannels in single cells were not so sensitive to uncoupler as the hemichannels/gap channels in confluent cells. Nevertheless, these observations do demonstrate an obvious effect of hemichannel/gap channels on the resting global Ca2+ signaling in ventricular myocytes.
Figure 2. Inhibition of spontaneous Ca2+ signals by gap junction inhibitors in NRVMs.
(A) The intracellular Ca2+ alterations in NRVMs, loaded with Fluo4/AM, were monitored by confocal microscopy. (B, C) Typical traces represent the spontaneous Ca2+ transients in single and monolayer NRVMs prior to and after heptanol (1 mM or 1.5 mM) for 2 min, Gap 27 (300 µM) for 30 min or FFA (25 µM) for 5 min. (D, E) The statistical data of the Ca2+ transient frequency and amplitude in single and confluent NRVMs as indicated were obtained from 10–12 independent determinations for each bar. * and ** represents P<0.05 and P<0.01 vs. DMSO, respectively.
Role of Cx43 in global Ca2+ signal in NRVMs and non-muscle cells
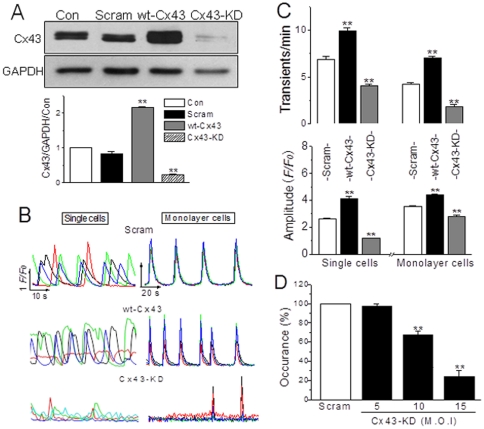
The above results demonstrated that gap junction inhibitors impaired LY uptake as well as spontaneous [Ca2+]i activity among monolayer NRVMs and in single NRVM. To assure if the gap permeability affected the Ca2+ signaling specifically, we constructed adenovirus carrying rat Cx43 gene with full sequences (wt-Cx43) and specific knockdown sequences encoding rat Cx43 (Cx43-KD, see Methods), the major connexin in ventricular myocytes. Transfection of the NRVMs for 48 h with wt-Cx43 or Cx43-KD virus displayed a significant overexpression or knockdown of Cx43 in these cells (Figure 3A).
Figure 3. Role of Cx43 in gap-mediated Ca2+ transients in NRVMs.
(A) NRVMs were infected with adenovirus vector or adenovirus carrying Cx43 full sequences (wt-Cx43) or Cx43 siRNA (m.o.i. = 15) for 48 h. The expression of Cx43 in NRVMs was determined by Western blot, which was normalized by the level of GAPDH. ** denotes P<0.01 vs. control. (B) Typical traces represent spontaneous Ca2+ transients in single and monolayer NRVMs with Cx43 overexpression or knockdown as indicated, and (C) Their statistical data of the transient frequency and amplitude were obtained from 5–6 independent experiments. (D) The statistical data of the dose-dependent effect of Cx43 deficiency on Ca2+ transients in single cells were obtained from 7–9 independent experiments. ** represents P<0.01 vs. scramble (Scram) for each panel.
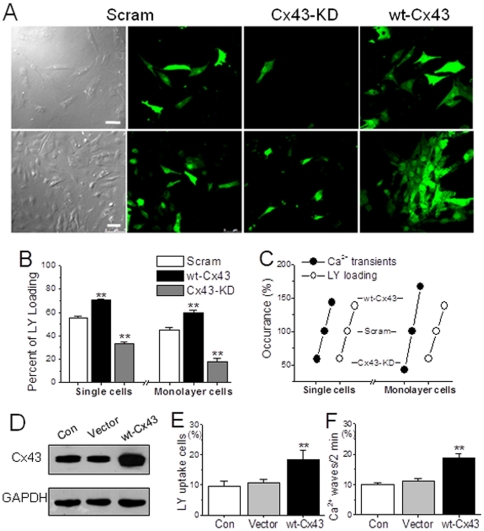
Then the effects of interfering Cx43 on spontaneous Ca2+ signals were examined in transfected NRVMs by the same protocol used above. In accordance with the findings in the uncoupler treatment, knockdown of Cx43 reduced the frequency and amplitude of Ca2+ transients, whereas overexpression of Cx43 greatly enhanced them in both single and monolayer NRVMs (Figure 3B and C). Furthermore, a Cx43 deficiency-dependent inhibitory effect on the Ca2+ oscillations was obtained by knocking down Cx43 with different concentrations of virus in single myocytes (Figure 3D). These data suggest a contribution of the endogenous Cx43-originated gaps/hemichannels for the spontaneous Ca2+ transient activity. At same time, corresponding results were also found in the evaluation of Cx43-associated permeability to LY, which was significantly blocked in Cx43-KD NRVMs, but dramatically potentiated in wt-Cx43 cells (Figure 4A and B). More importantly, taken the measurements in native NRVMs as control (100%), the values of LY transfer and Ca2+ transient rates were correlated well with the levels of Cx43 expression in both single and confluent transfected cells (Figure 4C).
Figure 4. Role of Cx43 in LY uptake in NRVMs.
(A, B) Typical confocal images of LY uptake in single and confluent NRVMs with Cx43 overexpression or silence by adenovirus infection (m.o.i. = 15). The LY uptake cells in each group as indicated were counted and expressed with percentage of the total recorded cells determined by 5–6 independent experiments for each bar. ** represents P<0.01 vs. scramble (Scram) cells. (C) Corresponding alterations of global Ca2+ transients and LY uptake in response to manipulations of Cx43 in both single and confluent monolayer NRVMs. (D) HEK293 cells were transfected with vector or plasmids carrying Cx43 for 48 h and determined their levels of Cx43 expression by Western blot. (E, F) Statistical data of the LY uptake percentage and Ca2+ wave frequency from different groups of HEK293 cells as indicated were obtained from 5–6 independent experiments. ** denotes P<0.01 vs. vector in all panels.
To further confirm the Cx43 mediation of spontaneous Ca2+ signaling, HEK293 cells were adopted because these cells also possess endogenous Cx43 and can overexpress rat Cx43 by plasmid transfection. Additionally, this type of cells generate no-spreading spontaneous Ca2+ waves among connected cells that can be observed clearly under laser confocal microscopy, thus LY uptake and spontaneous Ca2+ waves were evaluated and compared between wt-Cx43 and vector-control cells. As shown in Figure 4D–F, rat Cx43 expression in HEK293 cells caused a similar effect as that found in muscle cells, i.e. significant increases in the evaluations of LY uptake and occurrence of Ca2+ waves.
Role of Cx43 in local Ca2+ signal in cardiac myocytes
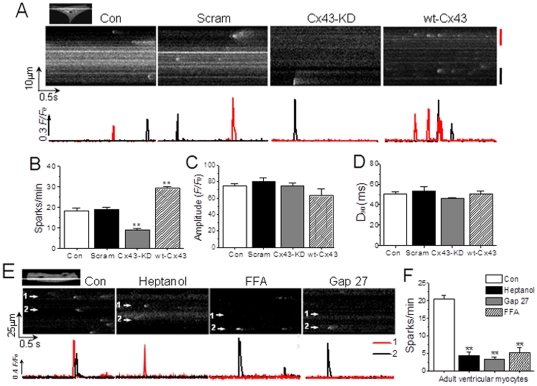
Furthermore, the local Ca2+ signal like the sparks was examined too in resting NRVMs and freshly isolated adult mouse ventricular myocytes. Similar to the findings in global Ca2+ signal measurement, both gap junction inhibitors, heptanol and Gap 27 (data not shown), and interfering Cx43 expression in NRVMs significantly affected the frequency but not the amplitude and duration of spontaneous Ca2+ sparks in single and confluent NRVMs (Figure 5A–D). Similarly, the isolated adult ventricular myocytes also demonstrated decreased Ca2+ sparks due to gap inhibitors at the similar concentration used in neonatal myocytes (Figure 5E and F).
Figure 5. Effect of gap inhibition on Ca2+ sparks in NRVMs and adult mouse cardiomyocytes.
(A) Typical linescan images of Ca2+ sparks (upper panel) and their F/F0 changes over the time (lower panel) in single NRVM from different groups as indicated. (B, C, D) Statistical data of the spark frequency, amplitude and duration in NRVMs in different groups as indicated were obtained from 4–5 independent determinations, n = 20–36 cells for each panel. ** represents P<0.01 vs. scramble. (E) Typical linescan images of Ca2+ sparks (upper panel) and their F/F0 changes over the time (lower panel) in adult mouse ventricular myocytes. The cells were respectively treated with vehicle (Con), 1.5 mM heptanol for 2 min, 300 µM Gap 27 for 30 min or 25 µM FFA for 5 min. (F) Statistical data from 3–5 independent determinations (n = 15–20 cells for each bar) show the effect of the drugs on the Ca2+ spark rate. ** represents P<0.01 vs. control.
Therefore, both silencing and overexpressing Cx43 influenced the resting [Ca2+]i activities and LY uptake in muscle and non-muscle cells, demonstrating a specific role of Cx43-associated coupling in the regulation of the fundamental Ca2+ signaling in these cells.
Corresponding Cx43 alterations in transfected single and monolayer NRVMs
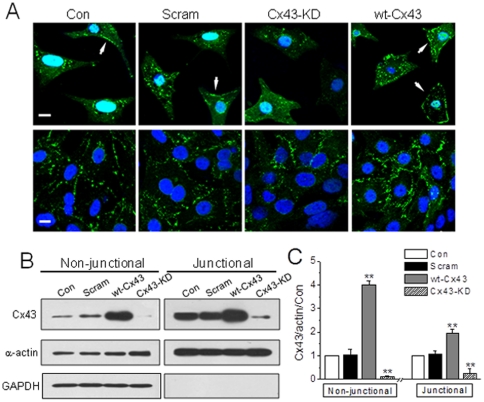
The interference of Cx43 by either gap inhibitors or gene manipulation demonstrated a significant disturbance in the basal Ca2+ signals in single NRVMs and adult cardiomyocytes, suggesting functional hemichannels possibly existed in these myocytes. Therefore, we examined the subcellular distribution of Cx43 in single cells and compared with that of monolayer cells by immunostaining and immunoblotting approaches. As shown in Figure 6A, typical punctuate Cx43 labeling was found in the interfaces between confluent NRVMs as reported, whereas the most Cx43 fluorescence was accumulated inside the cytosol in single NRVMs. However, membrane docked Cx43 indicated with white arrows in Figure 6A could be detected in some of the control and vector single cells (27.4±1.86% and 25.5±1.22%, n = 40 and 37 cells, respectively), and appeared to be increased in wt-Cx43 cells (34.2±1.43%, n = 35, P<0.05 vs. vector cells). The corresponding changes due to Cx43 overexpression and knockdown were further confirmed in western blotting Triton- insoluble and soluble fractions (see Methods), which represent gap junctions and precursors of gaps including hemichannels, respectively [20], [21]. While Cx43 was accordingly increased or reduced to Cx43-overexpression or Cx43-KD treatment in both soluble and insoluble fractions of monolayer cells (Figure 6B and C), Cx43 labeling was only detected in the soluble fraction of single cells that also responded to Cx43 manipulations (data not shown).
Figure 6. Distribution and expression level of Cx43 in single and monolayer NRVMs transfected with different virus.
(A) Single and confluent NRVMs with Cx43 overexpression or knockdown by adenovirus infection (m.o.i. = 15) for 48 h. The subcellular distribution of Cx43 in different groups as indicated was determined by immunostaining the cells with antibody specific for Cx43. Cell nucleus are indicated by Hoechest 33258 (1 µg/ml), and the arrows indicate the membrane-associated Cx43. (B, C) NRVMs were infected with adenovirus vector or adenovirus carrying Cx43 full sequences (wt-Cx43) or Cx43 siRNA (Cx43-KD, both m.o.i. = 15) for 48 h. The expression of Cx43 in NRVMs was determined in Triton X-100 soluble and insoluble fractions by Western blot (B) and normalized by the level of α-actin and then the level of Cx43 in control cells (C). The detection of GAPDH in non-junctional but not in junctional fraction represents a successful separation of non-junctional and junctional Cx43. ** denotes P<0.01 vs. vector or scramble.
Therefore, the corresponding alterations in [Ca2+]i activity to the level of non-junctional Cx43 observed in single cells suggest a role of functional hemichannel in the regulation of Ca2+ signaling in ventricular myocytes.
Signaling pathways contributing to Cx43-mediated Ca2+ activities in NRVMs
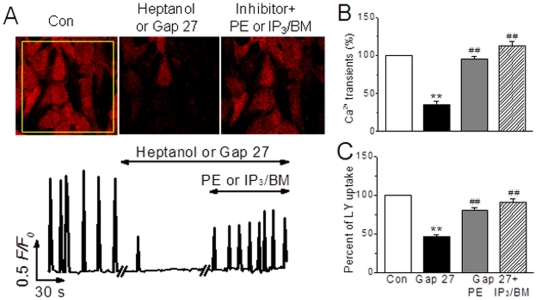
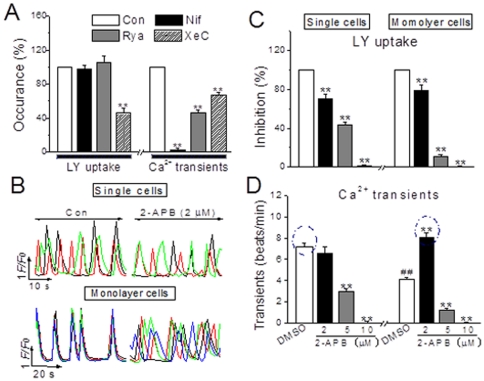
To further investigate the mechanism(s) underlying the role of Cx43 in this regard, several important Ca2+ signaling pathways in the heart such as L-type Ca2+ channel/RyR and G-protein coupled receptors/inositol 1,4,5-trisphosphte receptor (IP3R) [12], [18], [27] and hemichannel-associated regulators like ATP and IP3 [7], [28]–[31] were examined. In monolayer NRVMs, phenylephrine (PE), an agonist of α-adrenergic receptor, and IP3/BM, a membrane permeable IP3, rescued the depressed occurrence of Ca2+ transients and LY loading induced by heptanol (1 mM) or Gap 27 (300 µM) (Figure 7A–C), but isoprenaline, an agonist of β-adrenergic receptor to activate L-type Ca2+ channels, and phorbol myristate acetate, an activator of PKC, showed no any effect (data not shown). Furthermore, like gap uncouplers, inhibition of IP3R with xestospongin C (XeC, 10 µM for 20 min), a selective IP3R inhibitor [18], caused significant inhibitions of LY loading and Ca2+ transients (Figure 8A). Due to an increase in basal [Ca2+]i by XeC at higher concentration, another IP3R antagonist 2-aminoethoxydiphenyl borate (2-APB, 10 min) was used and it induced concentration-dependent blockades of LY uptake and Ca2+ transients in single and monolayer cells, except that 2-APB at concentration of 2 µM made the coordinate oscillations to desynchronized Ca2+ spikings in monolayer cells, mimicking the pattern of Ca2+ transients in single cells (Figure 8B–D). Additionally, the measurement of gap communication by fluorescence recovery after bleaching (FRAP) confirmed the above observation in LY loading (unpublished data). And, inhibitions of L-type channel with nifedipine (1 µM, 10 min) and RyR with ryanodine (100 µM, 10 min) induced tremendous suppression of Ca2+ oscillating, but the levels in LY uptake remained unchanged, a sign of normal gap communication, in these cells (Figure 8A), neither suramin, an antagonist of purinergic 2z/2x receptor, affected the gap inhibitor-regulated [Ca2+]i activity and LY loading (data not shown). Thus, these results demonstrate IP3-IP3R pathway involves in the Cx43-mediated Ca2+ signaling, and further confirmations and study on signaling transduction are presented and submitted separately.
Figure 7. Recovery effect of inositol 1,4,5-trisphosphate on gap inhibitor-induced Ca2+ transient inhibition in monolayer NRVMs.
(A) Images and traces represent spontaneous global Ca2+ oscillations in monolayer cells prior to and 1 or 30 min after heptanol (1 mM) or Gap 27 (300 µM), and then phenylephrine (PE 20 µM, 5 min) or IP3/BM (20 µM, 6 min) treatments. (B, C) The summarized data indicate the recovery effect of IP3 analogues on Gap 27-inhibited Ca2+ transients and LY uptake. **P<0.01 vs. control; ## P<0.01 vs. Gap 27 alone, from 10–18 independent determinations for each bar.
Figure 8. Pathways involved in the Cx43-associated mediation of Ca2+ activities in single and monolayer NRVMs.
(A) The summarized data of the effects of nifedipine (Nif 1 µM, 10 min), ryanodine (Rya 100 µM, 10 min) and xestospongin C (XeC 10 µM, 20 min) on the LY uptake and intracellular Ca2+ frequency in NRVMs. (B) Typical traces represent the spontaneous Ca2+ transients in single and monolayer NRVMs prior to (Con) and after 2-APB (2 µM) for 10 min treatment. (C, D) The statistical data of the concentration-dependent effects of 2-APB on LY loading and Ca2+ transient frequency in single and confluent NRVMs as indicated were obtained from 8–12 determinations for each bar. **P<0.01 vs. DMSO in each panel; ## P<0.01 vs. DMSO treated single cells.
Discussion
The present study demonstrated that functional Cx43-relevant gap junction/hemichannels play an important role in the regulation of spontaneous Ca2+ signaling in unstimulated NRVMs and adult myocytes. This proposal is mainly based on the following observations (i) in corresponding to the LY uptake inhibition, spontaneous Ca2+ transients and sparks were significantly affected by gap junction inhibitors and specific Cx43 interference in single and monolayer NRVMs; (ii) spontaneous Ca2+ sparks in adult cardiomyoctes displayed similar response to gap uncoupler treatments; (iii) overexpression of Cx43 in NRVMs or HEK293 cells exhibited enhancement in spontaneous Ca2+ activities and LY uptake; (iv) specific hemichannel inhibitor FFA showed a similar effect on [Ca2+]i activities as gap uncouplers in neonatal and adult myocytes; and (v) like in monolayer cells, the changes in Ca2+ signals and LY uptake were well correlated with the level of cell surface Cx43 labeling and non-junctional Cx43 in single NRVMs.
In agreement with the chemical inhibitors of gap junction, specific overexpression or knockdown of Cx43 in NRVMs by adenovirus infection, the predominant connexin in ventricular myocytes, could upregulate or downregulate the gap permeability as well as the spontaneous Ca2+ signaling accordingly. In fact, a direct impact of Ca2+ spreading among adjacent cells has already been demonstrated in Cx43-expression manipulated cells in other studies. For instances, it has been shown that Cx43-expression in other cell types is correlated with both intercellular dye transfer [32], [33] and propagated intercellular Ca2+ waves through gaps [2], [34]. Similarly, mutation of Cx43 in NRVMs can induce desynchronization of Ca2+ transients that further hampers the synchronous beating [35]. All these studies demonstrate a critical role of gap junctions in the spreading of physiological Ca2+ signals among connected cells. The present study further depicts a role of Cx43-associated gap coupling in the regulation of intrinsic Ca2+ signaling inside the ventricular myocytes. Such effect of Cx43-coupling should be unrelated with the instant electrical exchange between myocytes, because similar observations found in monolayer myocytes were also spotted in single unconnected cells, suggesting a potential role of Cx43-relevant hemichannels in the regulation of spontaneous Ca2+ signaling in normal cardiac myocytes.
Interestingly, a release of ATP or IP3 has been found to be responsible for the hemichannel-mediated intercellular Ca2+ wave initiation and spreading in non-muscle cells [1], [2], [28], [29]. In this regard, ATP facilitates the Ca2+ activities of adjacent unconnected cells by ATP release to the buffer and entrance into these unconnected cells via hemichannels [1], [2], [30], [31]. However, ATP release may not be the main contributor for the hemichannel-related regulation of Ca2+ signaling in this study, for suramin, an antagonist of purinergic 2z/2x receptor, did not affect the spontaneous Ca2+ transients in the wild type NRVMs, nor did on the potentiated spontaneous Ca2+ oscillations in the wt-Cx43 NRVMs (data not shown). In contrast, inhibition of IP3R with XeC or 2-APB inhibited the global spontaneous Ca2+ signals as well as the gap exchange in NRVMs, whereas blockade of L-type Ca2+ channel or RyR, two important effectors of Ca2+ signaling, did not show such parallel effects on Ca2+ activity and intercellular communication. Importantly, both membrane-permeable IP3 and PE, to produce endogenous IP3, could completely recover Ca2+ oscillations inhibited by heptanol or Gap 27. Moreover, knockdown of the endogenous IP3R mimicked the 2-APB effects on NRVMs and, interesting, affected the phosphorylation level of Cx43 (unpublished data in a separate manuscript). Therefore, these data indicate that IP3/IP3R-associated pathway contributes significantly to Cx43-regulated Ca2+ signaling, a proposal is also supported by other studies that demonstrate a direct IP3 release mediated by Cx43 [29] and remained inside the cells when gap junction is not formed [36].
In pathological situations, gap junction remodeling characterized by Cx43 lateralization and down-regulation are typical features in failing myocardium [37], [38], in which Ca2+ signaling is severely compromised, e.g. lower amplitude and longer duration of Ca2+ transient compared with those in normal myocardium [12], [13], [39], [40]. These observations further imply a tight connection between the state/function of connexin and Ca2+ signaling in cardiomyocytes. Therefore, the present study demonstrates that in addition to contributing for Ca2+ spreading between adjacent cells in normal heart and abnormal Ca2+ signal formation in diseased heart, Cx43-associated coupling may also play a role in the regulation of basal intracellular Ca2+ activities in normal ventricular myocytes.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the National Natural Science Foundation (30973537), and the Beijing Natural Science Foundation (7082018 and 5122006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 2.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Saez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci U S A. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 5.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 6.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 7.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Sugishita K, Su Z, Ueda I, Barry WH. Activation of connexin-43 hemichannels can elevate [Ca2+]i and [Na+]i in rabbit ventricular myocytes during metabolic inhibition. J Mol Cell Cardiol. 2001;33:2145–2155. doi: 10.1006/jmcc.2001.1477. [DOI] [PubMed] [Google Scholar]
- 10.Shintani-Ishida K, Uemura K, Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714–1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Dorado D, Inserte J, Ruiz-Meana M, Gonzalez MA, Solares J, et al. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circ. 1997;96:3579–3586. doi: 10.1161/01.cir.96.10.3579. [DOI] [PubMed] [Google Scholar]
- 12.Wier WG, Balke CW. Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation-contraction coupling in normal heart muscle. Circ Res. 1999;85:770–776. doi: 10.1161/01.res.85.9.770. [DOI] [PubMed] [Google Scholar]
- 13.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H. Does remodeling of gap junctions and connexin expression contribute to arrhythmogenesis? Study in an immobilization rat model. Circ J. 2010;74:2558–2559. doi: 10.1253/circj.cj-10-0983. [DOI] [PubMed] [Google Scholar]
- 15.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Meana M, Garcia-Dorado D, Hofstaetter B, Piper HM, Soler-Soler J. Propagation of cardiomyocyte hypercontracture by passage of Na+ through gap junctions. Circ Res. 1999;85:280–287. doi: 10.1161/01.res.85.3.280. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Schultz C, Llopis J, Tsien RY. Membrane-permeant esters of inositol polyphosphates, chemical syntheses and biological applications. Tetrahedron; 1997;53:12017–12040. [Google Scholar]
- 18.Luo D, Yang D, Lan X, Li K, Li X, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–174. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Meng Q, Jing X, Xu P, Luo D. A role for protein kinase C in the regulation of membrane fluidity and Ca2+ flux at the endoplasmic reticulum and plasma membranes of HEK293 and Jurkat cells. Cell Signal. 2010;23:497–505. doi: 10.1016/j.cellsig.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 20.VanSlyke JK, Musil LS. Analysis of connexin intracellular transport and assembly. Methods. 2000;20:156–164. doi: 10.1006/meth.1999.0933. [DOI] [PubMed] [Google Scholar]
- 21.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opsahl H, Rivedal E. Quantitative determination of gap junction intercellular communication by scrape loading and image analysis. Cell Adhes Commun. 2000;7:367–375. doi: 10.3109/15419060009109019. [DOI] [PubMed] [Google Scholar]
- 23.Watts SW, Tsai ML, Loch-Caruso R, Webb RC. Gap junctional communication and vascular smooth muscle reactivity: use of tetraethylammonium chloride. J Vasc Res. 1994;31:307–313. doi: 10.1159/000159057. [DOI] [PubMed] [Google Scholar]
- 24.Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes. 2007;14:265–273. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- 25.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 26.Tong D, Gittens JE, Kidder GM, Bai D. Patch-clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain specific in the mouse. Am J Physiol Cell Physiol. 2006;290:C290–297. doi: 10.1152/ajpcell.00297.2005. [DOI] [PubMed] [Google Scholar]
- 27.Tilley DG. G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function. Circ Res. 2011;109:217–230. doi: 10.1161/CIRCRESAHA.110.231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gossman DG, Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes. 2008;15:305–315. doi: 10.1080/15419060802357217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 32.Naus CC, Zhu D, Todd SD, Kidder GM. Characteristics of C6 glioma cells overexpressing a gap junction protein. Cell Mol Neurobiol. 1992;12:163–175. doi: 10.1007/BF00713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu D, Caveney S, Kidder GM, Naus CC. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci U S A. 1991;88:1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry T, Evans JH, Sanderson MJ. Propagation of intercellular calcium waves in C6 glioma cells transfected with connexins 43 or 32. Microsc Res Tech. 2001;52:289–300. doi: 10.1002/1097-0029(20010201)52:3<289::AID-JEMT1014>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Oyamada Y, Zhou W, Oyamada H, Takamatsu T, Oyamada M. Dominant-negative connexin43-EGFP inhibits calcium-transient synchronization of primary neonatal rat cardiomyocytes. Exp Cell Res. 2002;273:85–94. doi: 10.1006/excr.2001.5411. [DOI] [PubMed] [Google Scholar]
- 36.Leite MF, Hirata K, Pusl T, Burgstahler AD, Okazaki K, Ortega JM, Goes AM, Prado MAM, Spray DC, Nathanson MH. Molecular basis for pacemaker cells in epithelia. J Biol Chem. 2002;227:16313–16323. doi: 10.1074/jbc.M109207200. [DOI] [PubMed] [Google Scholar]
- 37.Kostin S, Rieger M, Dammer S, Hein S, Richter M, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 38.Ai X, Zhao W, Pogwizd SM. Connexin43 knockdown or overexpression modulates cell coupling in control and failing rabbit left ventricular myocytes. Cardiovasc Res. 2010;85:751–762. doi: 10.1093/cvr/cvp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297:H1235–1242. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circ. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]