Abstract
Lactococcus lactis is a well-studied bacterium widely used in dairy fermentation and capable of producing metabolites with organoleptic and nutritional characteristics. For fine tuning of the distribution of glycolytic flux at the pyruvate branch from lactate to diacetyl and balancing the production of the two metabolites under aerobic conditions, a constitutive promoter library was constructed by randomizing the promoter sequence of the H2O-forming NADH oxidase gene in L. lactis. The library consisted of 30 promoters covering a wide range of activities from 7,000 to 380,000 relative fluorescence units using a green fluorescent protein as reporter. Eleven typical promoters of the library were selected for the constitutive expression of the H2O-forming NADH oxidase gene in L. lactis, and the NADH oxidase activity increased from 9.43 to 58.17-fold of the wild-type strain in small steps of activity change under aerobic conditions. Meanwhile, the lactate yield decreased from 21.15±0.08 mM to 9.94±0.07 mM, and the corresponding diacetyl production increased from 1.07±0.03 mM to 4.16±0.06 mM with the intracellular NADH/NAD+ ratios varying from 0.711±0.005 to 0.383±0.003. The results indicated that the reduced pyruvate to lactate flux was rerouted to the diacetyl with an almost linear flux variation via altered NADH/NAD+ ratios. Therefore, we provided a novel strategy to precisely control the pyruvate distribution for fine tuning of the lactate and diacetyl production through promoter engineering in L. lactis. Interestingly, the increased H2O-forming NADH oxidase activity led to 76.95% lower H2O2 concentration in the recombinant strain than that of the wild-type strain after 24 h of aerated cultivation. The viable cells were significantly elevated by four orders of magnitude within 28 days of storage at 4°C, suggesting that the increased enzyme activity could eliminate H2O2 accumulation and prolong cell survival.
Introduction
Lactococcus lactis has long been used in dairy fermentation processes and considered as one of the most important starter cultures. It produces multifarious end metabolites during dairy fermentation, such as lactate, diacetyl, acetoin, vitamins and extracellular exopolysaccharides which contribute to the organoleptic and health-promoting properties of the fermented products [1]–[4]. L. lactis has become a model for rational industrial strain improvement because of its relatively small genome, simple metabolism and industrial relevance [5].
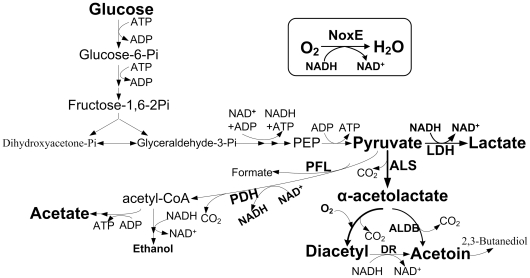
Diacetyl is an important aroma compound and essential for the flavor of dairy products. Normally, L. lactis undergoes homolactic fermentation, and most of the central intermediate pyruvate is converted to lactate, a reaction catalyzed by lactate dehydrogenase (LDH) with the oxidation of NADH to NAD+ for maintaining a redox balance [6]. Under aerobic conditions, the activities of α-acetolactate synthase (ALS) and NADH oxidase (NOX) are strongly increased [7]. ALS catalyzes the pyruvate to α-acetolactate. After decarboxylation, α-acetolactate is further converted to acetoin and diacetyl. The reoxidation of NADH by NOX would replace the role of the LDH in the regeneration of NAD+, allowing the accumulation of these two aroma compounds (Figure 1) [8]. However, in the presence of O2, L. lactis displays the metabolic shift from homolactic to mixed-acid product formation, including lactate, acetate and CO2. Hence, diacetyl accumulation is rather limited [9]. Therefore, several approaches to improve diacetyl production in L. lactis have been developed, such as the overexpression of the als and nox-2 genes and the inactivation of the ldh and α-acetolactae decarboxylase (aldB) genes. The herein excessive pyruvate was channeled to acetoin or diacetyl via ALS, whereas the flux from pyruvate to lactate was almost abolished [8], [10]–[12]. Considering that lactate is an important metabolite that prevents fermented products from spoilage and contributes to the texture of dairy products, the strong overexpression or complete deletion of target genes limits the application of genetically modified lactic acid bacteria as starter cultures for industrial production of dairy products. Thus, strategies for the fine-tuning of gene expression are required to control the aimed metabolic fluxes.
Figure 1. Metabolic pathway of glycolysis and diacetyl biosynthesis in L. lactis.
PEP, phosphoenolpyruvate; LDH, lactate dehydrogenase; ALS, α-acetolactate synthase; ALDB, acetolactate decarboxylase; PDH, pyruvate dehydrogenase; DR, diacetyl reductase; PFL, pyruvate formate lyase; NoxE, H2O-forming NADH oxidase.
Promoter engineering is interpreted as the creation of a functional promoter library for precisely controlling gene expression to perform metabolic optimization or control analysis. It has promising perspectives with respect to the research of functional genomics, cell network analysis and synthetic biology [13]–[15]. For example, a series of mutant L. lactis strains have been constructed based on synthetic promoters to demonstrate that LDH exerted virtually no control on the glycolytic flux at the wild-type enzyme level and the lactate production [16]. In addition, a synthetic promoter library has been used to evaluate the influence of different production levels of glucose-6-phosphate dehydrogenase on xylose fermentation and ethanol yield in Saccharomyces cerevisiae [17]. These previous studies have illustrated the valuable applications of the promoter engineering in the precise control and the quantitative assessment of gene expression level.
L. lactis is a facultatively anaerobic bacterium and O2 has negative effects on both cell growth and survival, because in vivo O2 is converted into reactive oxygen species (ROS) which cause protein, lipid and nucleic acid damage [18]. To cope with oxygen toxicity, L. lactis could grow via respiratory metabolism when heme is available [19]. Some lactic acid bacteria have antioxidant enzymes to detoxify O2-derived compounds [20]. Aside from the toxic effects of O2, aeration could induce the metabolic shift from homolactic to mixed-acid fermentation, making pyruvate metabolism more flexible. Therefore, lowering cytoplasmic O2 is economically significant in improving cell growth and survival under aerobic conditions.
Here we constructed a constitutive promoter library by randomizing the space sequence between the two conserved motifs of the promoter of the H2O-forming NADH oxidase (noxE) gene in L. lactis. Under the control of individual random promoters, the fine tuning of lactate and diacetyl production was achieved by precisely regulating the intracellular NADH/NAD+ ratios in L. lactis. Furthermore, the beneficial effects of the increased NoxE activity on cell survival were also investigated in this study.
Materials and Methods
Plasmids, bacterial strains and growth conditions
The plasmids used in this study are listed in Table 1. E. coli DH5α was grown aerobically in Luria Bertani broth at 37°C. The plasmid-free strain L. lactis MG1363 was used as host for the construction of the constitutive promoter library and considered as a wild-type strain in this study [21]. The L. lactis DA strain was a derivative of L. lactis MG1363 with the aldB gene deletion. L. lactis was grown in M17 broth (Oxoid, Basingstoke, United Kingdom) containing 0.25% (wt/vol) glucose (GM17) at 30°C. For milk fermentation, L. lactis was incubated in 12.5% (wt/vol) sterile, reconstituted skim milk (RSM) supplemented with 1% glucose (wt/vol). The following antibiotics were added at the indicated concentrations: chloramphenicol, 5 µg/mL for L. lactis or 10 µg/mL for E. coli; erythromycin, 10 µg/mL for L. lactis; and ampicillin, 100 µg/mL for E. coli.
Table 1. Plasmids used in this study.
| Plasmid | Relevant characteristics | Reference or source |
| pMD18-T | Cloning vector, Amp R | Takara |
| pT-GFP | BglII-PstI-gfp-EcoRI fragment cloned in pMD18-T, Amp R | This study |
| pSec∶Leiss∶Nuc | pWV01 replicon, expresses Nuc under PnisA control, Cm R | [55] |
| pGFP | Promoter probing vector used in the construction of promoter library; pSec∶leiss∶Nuc derivative with PnisA and Nuc fragment replaced by gfp gene fragment, Cm R | This study |
| pOgfp | pGFP derivative, carrying the native promoter O159, Cm R | This study |
| pMgfp | pGFP derivatives, carrying individual random promoters of the promoter library, Cm R | This study |
| pG+ host4 | Derivative of pGK12 used for homologous recombination, Erm R | [28] |
| pEnox | pMgfp derivative with gfp gene fragment replaced by noxE gene fragment, carrying eleven selected promoters, respectively, Cm R | This study |
Construction of the constitutive promoter library
The primers used in this study are listed in Table 2. All molecular manipulations were performed as described previously [22]. Taq polymerase, restriction enzymes and T4 DNA ligase were used as stated by standard procedures (TaKaRa, Tokyo, Japan).
Table 2. Oligonucleotide primers used in this study.
| Primer | Sequences (5′-3′) | Restriction sites |
| NOXEp-for | GGTAGATCTTTTGATTCAGAAACTATGTGG | BglII |
| NOXEp-rev | GATCTGCAGACTAATAGGTCTCCTTTA | PstI |
| NOXEp-mut | CGGAGATCTNNNNNNNNTTGACANNNNNNNNNNNNNNNNNNNTANAATNNNNNTTTCACAATGTTCACAAGCGCTTAC | BglII |
| GFP-for | TTCTGTCAGTGGAGAGGGT | |
| GFP-rev | GGATAACGGGAAAAGCATT | |
| GAP-for | GCGACAGGTTTCTTTGCGA | |
| GAP-rev | CGTCTGCCATTGGTGCTAA | |
| NOXE-for | CCTCTGCAGGTATGAAAATCGTAGTTATC | PstI |
| NOXE-rev | TTCGTCGACTTATTTGGCATTCAAAGCT | SalI |
| TU-for | ACTCTCGAGCACTAAAATGCGTCAGTCAAT | XhoI |
| TU-rev | GGCGAATTCATTTCTCTTTTCTATCTCAT | EcoRI |
| TD-for | GCGGAATTCGATATTGATGTAGCTGA | EcoRI |
| TD-rev | TTGCGGCCGCTCCACTATCTATAAAATG | NotI |
| DC-for | ATAATGAATCAGTCGAATGCAAGA | |
| DC-rev | TTTGGGCAATCCAGCAACTCCTA |
The restriction sites in the primer sequences are underlined.
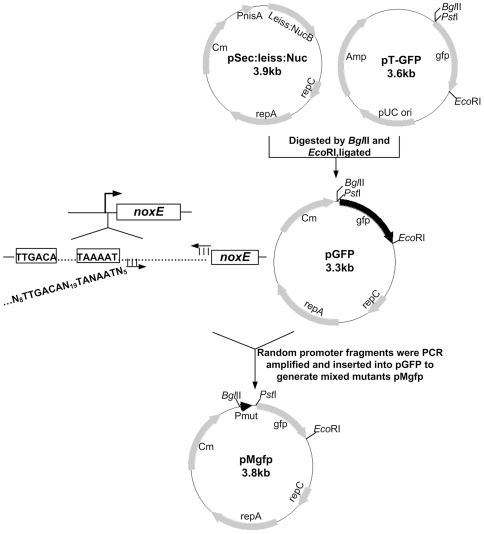
The scheme for generating the promoter library is shown in Figure 2. The nisin-inducible promoter PnisA and nuc gene fragment of the E. coli/L. lactis shuttle vector pSec∶Leiss∶Nuc was replaced by the promoterless green fluorescent protein (gfp) gene fragment, resulting in the promoter probing vector pGFP.
Figure 2. Scheme for generating the constitutive promoter library in L. lactis.
N = A, G, T or C.
The noxE promoter fragment was PCR amplified from the genomic DNA of L. lactis MG1363 with primers NOXEp-for and NOXEp-rev. The PCR product was digested with BglII and PstI and ligated to compatible ends of the digested pGFP to generate pOgfp. To develop the constitutive promoter library for L. lactis, the fragments containing randomized promoters were amplified using primers NOXEp-mut and NOXEp-rev by degenerate PCR with the plasmid pOgfp DNA as template. After digested with BglII and PstI, the random promoter fragments were inserted into the corresponding sites of the probing vector pGFP to generate the mixed plasmids pMgfp. The mixed plasmids pMgfp were then electrotransformed into the competent cells of L. lactis MG1363, and the cells were plated onto the GM17 agar containing chloramphenicol [23]. The colonies ranging in color from white to dark green were picked from the GM17 agar plates. After overnight static incubation at 30°C, each culture was diluted 100-fold in 5 mL of fresh GM17 medium and further incubated for 10 h at 30°C. The culture turbidity was monitored by OD600. Cells were collected from 1 mL cultures by centrifugation at 7,000 g for 5 min, washed twice and resuspended in 500 µL of phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4). The fluorescence intensity of the suspension was determined by an LS-50B spectrofluorometer (PerkinElmer) with excitation at 488 nm and emission at 511 nm. To obtain a relative fluorescence unit (RFU), the fluorescence intensity of the cells carrying the promoter probing vector pGFP was used as a background and subtracted from that of cells containing plasmids pMgfp.
Characterization of the constitutive promoter library
Eleven colonies with various promoter activities were selected and their promoters were sequenced. The promoter strength was evaluated as described by Alper et al. [13]. Growth was monitored by OD600 every thirty minutes over five hours. At the same intervals, the cultures were sampled and the fluorescence intensities were measured as above. The slope of the fluorescence versus culture turbidity was considered as the exponential growth phase steady-state concentration of GFP.
The cultures in GM17 medium were collected at the exponential phase and the total RNA was extracted with a RNA simple Total RNA Kit (TIANGEN, Beijing, China) according to the manufacturer's protocols. The quantity and purity of RNA were determined spectrophotometrically at 260 nm and 280 nm. Reverse transcription was performed with Random 6 mers and Oligo dT primer using the PrimeScript RT reagent Kit (TaKaRa, Tokyo, Japan) according to the manufacturer's instructions. Real-time PCR was performed with the SYBR Premix Ex TaqII (TaKaRa, Tokyo, Japan) applying the protocol in the Real-Time PCR Detection Systems (Bio-Rad, Hercules, CA, USA). The gfp transcript was PCR amplified with the primers GFP-for and GFP-rev. The gapA (D-glyceraldehyde-3-phosphate dehydrogenase) transcript used as internal standard was amplified with the primers GAP-for and GAP-rev.
Expression of the noxE gene with selected promoters
The noxE gene was PCR amplified from the genomic DNA of L. lactis MG1363 using primers NOXE-for and NOXE-rev. The PCR product was digested by PstI and SalI and inserted into the same sites of vector pMgfp to replace the gfp gene, yielding pEnox (E: eleven individual promoters selected). Subsequently, the plasmids were introduced into L. lactis DA to obtain recombinant L. lactis DA/pEnox, respectively. The expression of the noxE gene under the control of eleven promoters was analyzed via the intracellular NoxE activity assay. See below for details.
Fermentation conditions and analytical methods
The recombinant strains were pre-cultured in 5 mL of GM17 medium, and 2 mL of the overnight cultures were incubated in 500-mL Erlenmeyer flasks with 100 mL of GM17 medium with 200 rpm orbital shaking at 30°C.
Cell growth was monitored by OD600. Glucose, acetate, lactate, formate and ethanol were analyzed by high-performance liquid chromatography (HPLC; Shimazu, Japan) using a column of Aminex HPX-87H Ion Exclusion particles (300 mm×7.8 mm, Bio-Rad, Hercules, CA, USA), at a column temperature of 65°C with 5 mM sulfuric acid as the mobile phase at a flow rate of 0.6 mL/min. Acetoin, α-acetolactate and diacetyl were determined according to Benson et al. [24]. H2O2 was measured using the H2O2 quantified analysis Kit (Sangon Biotech, Shanghai, China). NADH and NAD+ were extracted as described previously [25], and their concentrations were measured by enzyme cycling assay [26], [27].
Enzymatic measurements
Cells from 5 mL cultures were collected by centrifugation and washed twice with potassium phosphate buffer (pH 7.0). The cells were resuspended in 2.5 mL of potassium phosphate buffer and disrupted by sonication on ice (400 w, sonication for 3 s, intermission for 8 s). The supernatant was recovered by centrifugation at 12,000 g for 10 min to determine the in vivo enzyme activities. The protein concentration was determined using the Bradford protein assay with bovine serum albumin as standard.
The NoxE activity was determined spectrophotometrically by measuring the initial rate of NADH oxidation at 25°C. The total 200 µL assay mixture contained 0.3 mM NADH and 0.3 mM EDTA in 50 mM potassium phosphate buffer. The reaction was initiated by adding 10 µL of cell extract and monitored by the decrease at OD340nm using Spectra MAX 190 (Molecular Devices Corporation, U.S.A.). A unit enzyme was defined as the amount which catalyzed the oxidation of 1 µmol of NADH to NAD+ per minute at 25°C. LDH activity was determined according to the method of Andersen et al. [16].
Deletion of the aldB gene
The upstream and downstream sequences of the aldB gene were PCR amplified from the genomic DNA of L. lactis MG1363 with primers TU-for/TU-rev and TD-for/TD-rev, and then inserted into the vector pG+host4, respectively. Subsequently, the resulting plasmid was used to perform homologous double-cross in the L. lactis MG1363 chromosome as the modified method described previously [28]. Gene deletion was verified by PCR amplification with the primer DC-for and DC-rev.
Results
Construction of the constitutive promoter library
The sequence alignment of the noxE promoter located at L. lactis MG1363 genome with the consensus promoter sequences of L. lactis revealed that the −35 region had high identity with the TTGACA sequence, whereas the −10 region was less conserved with the sequence TAAAAT deviating from the canonical TATAAT sequence in the 3rd position. In our strategy, the −35 region nucleotides were kept constant and the −10 region was generally maintained except the 3rd base A was randomized, yielding TANAAT (N = A, T, G or C). The spacing between the −10 and −35 region had 19 completely random nucleotides. Furthermore, the randomization was also introduced into 8 bases upstream of the −35 region and 5 bases downstream of the −10 region for higher diversity of random promoters.
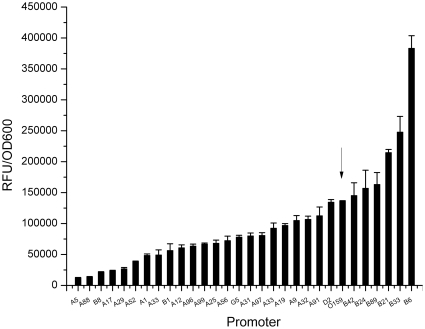
To screen the random promoters easily, a promoter probing vector pGFP was constructed using GFP as reporter based on the E. coli/L. lactis shuttle vector pSec∶leiss∶Nuc. The randomized promoter fragments were inserted into pGFP, and the resulting plasmids pMgfp were transformed into L. lactis MG1363. Five hundred colonies of L. lactis MG1363/pMgfp carrying individual random promoters were picked by observation of the green color on the GM17 plates. To assess the promoter activities, the fluorescence intensities of the colonies were determined and 30 representative random promoters were selected to form a constitutive promoter library (Figure 3). The promoter activities of the library spanned from 7,000 to 380,000 RFU, covering 3 to 4 logs of expression levels in small increments. Six random promoters showing higher activity than the native promoter O159 were obtained, and the most potent promoter B6 exhibited a 2.8-fold activity increment.
Figure 3. Promoter library for constitutive gene expression in L. lactis.
The activity of the promoter is measured as RFU per OD600. The data are the means and standard deviations of results from five independent experiments. The arrowhead indicates the native promoter O159.
Characterization of the promoter library
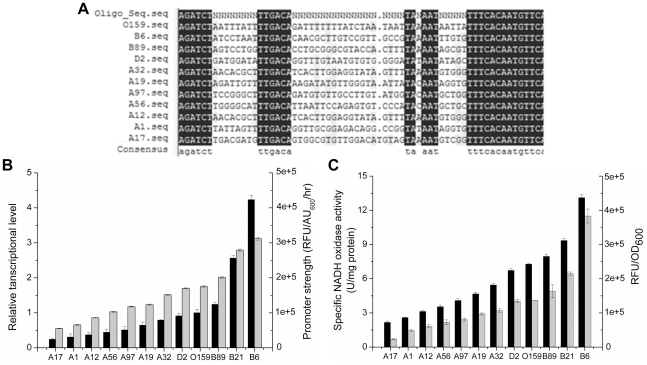
Eleven promoters (B6, B21, B89, D2, A32, A19, A97, A56, A12, A1 and A17) with activities covering from 23,000 to 380,000 RFU were selected for the sequence analysis. As shown in Figure 4A, all selected promoters had a DNA sequence identical to specific sequence of the designed oligonucleotide NOXEp-mut. No base change was observed in the −35 region, while base changes were found in the −10 region of five promoters which exhibited lower activity than that of the native promoter O159. The base T insertion was observed in the spacer of promoter A17, resulting in a drastic reduction in promoter activity. The alignment showed that the higher similarity of sequences outside the conserved regions between the random promoter and the native led to stronger promoter activity.
Figure 4. Characterization of the promoter library.
(A) Sequence analysis of the selected promoters. N = A, G, T or C. (B) Determination of promoter strength from the transcript quantification of the gfp gene (black) and dynamics of GFP production based on fluorescence intensity (gray). (C) Specific NoxE activity (black) under the control of eleven selected promoters (promoter activity is shown as gray). In panels (B) and (C) the values are means ± standard deviations of three independent experiments.
To eliminate the effects of protein maturation, degradation and cell growth rate on the GFP fluorescence intensity, the eleven promoters strength were determined by a dynamic model in which the influencing factors were comprehensively considered [13]. Based on this model, the promoter strength of the library varied from 55,006±79 to 311,982±410 and increased by 17.51% on average between the adjacent promoters (Figure 4B).
To characterize the promoter library at transcriptional level, relative mRNA levels of the gfp transcripts under the control of eleven promoters were analyzed by Real-Time PCR. The relative level of the gfp transcript spanned a 17.49-fold variation and correlated well with the promoter strength (Figure 4B).
Tuning of the lactate and diacetyl production in L. lactis
To investigate the regulation capacity of different promoters on the distribution of the pyruvate flux to lactate and diacetyl, the sequenced promoters were fused with the noxE gene from L. lactis MG1363. Then, the resulting plasmids (pB6nox, pB21nox, pB89nox, pD2nox, pA32nox, pA19nox, pA97nox, pA56nox, pA12nox, pA1nox and pA17nox) were introduced into L. lactis DA which was unable to convert α-aectolactate to acetoin by acetolactate decarboxylase. The NoxE activities of the recombinant strains were determined at the late exponential phase. As expected, the NoxE activity of L. lactis DA was very low (0.23±0.05 U/mg protein). In the eleven recombinants, it showed linear increase with the promoter strength, from 2.17±0.12 U/mg protein to 13.11±0.28 U/mg protein (Figure 4C). However, the differences in the enzyme activity between the adjacent promoters did not exceed 1.5 units except promoter B6 which was 3.8 units higher than the next promoter B21. Moreover, the LDH activity of the recombinant strains was very similar to that of wild-type (7.21±0.14 U/mg protein).
After 12 h of aerobic culture in GM17 medium, the cell growth and glucose consumption rate of L. lactis DA and eleven recombinant strains were similar to those of the wild-type L. lactis MG1363, indicating that genetic modification had little influence on L. lactis growth. As shown in Table 3, the intracellular NADH/NAD+ ratios varied from 0.711±0.005 to 0.383±0.003 by controlling the expression of the NoxE. The lactate accumulation exhibited a nearly linear decrease from 21.15±0.08 mM to 9.94±0.07 mM, whereas the diacetyl yield showed a gradual increase from 1.07±0.03 mM to 4.16±0.06 mM. These results indicated that the decreased pyruvate flux to the LDH pathway was rerouted to the ALS pathway accompanied by the enhancement of NoxE activity. Meanwhile, acetate yield in the recombinant strains was on average 2.48 mM higher than that of the strain L. lactis DA and acetoin accumulation was still detectable in the recombinant strains. In the aldB-deficient strains, the unstable intermediate α-acetolactate was directly converted to diacetyl, which was subsequently reduced to acetoin by diacetyl reductase in the presence of O2. The acetoin and diacetyl yields were undetectable in L. lactis DA, whereas the lactate and acetate production were 23.41±0.11 mM and 8.47±0.47 mM, respectively.
Table 3. pH, product and NADH/NAD+ ratios of different recombinant strains at initial growth pH of 7.3 after 12 h aerobic culture.
| Concentrations of pyruvate metabolites (mM) | ||||||
| Strains | Final pH | Lactate | Acetate | Diacetyl | Acetoin | NADH/NAD+ ratio |
| DA | 5.93±0.03 | 23.41±0.11 | 8.47±0.14 | ND | ND | 0.741±0.009 |
| DA/pA17nox | 6.02±0.04 | 21.15±0.08 | 8.40±0.15 | 1.07±0.03 | 0.69±0.02 | 0.711±0.005 |
| DA/pA1nox | 6.05±0.02 | 20.28±0.13 | 8.43±0.09 | 1.19±0.01 | 0.87±0.05 | 0.689±0.006 |
| DA/pA12nox | 6.05±0.05 | 19.86±0.29 | 9.26±0.1 | 1.51±0.03 | 0.92±0.03 | 0.681±0.002 |
| DA/pA56nox | 6.07±0.02 | 19.09±0.12 | 9.12±0.14 | 1.8±0.01 | 1.01±0.04 | 0.556±0.008 |
| DA/pA97nox | 6.10±0.01 | 18.15±0.21 | 10.29±0.13 | 1.8±0.04 | 1.05±0.01 | 0.55±0.005 |
| DA/pA19nox | 6.10±0.01 | 17.46±0.17 | 11.08±0.07 | 1.84±0.05 | 1.05±0.03 | 0.541±0.005 |
| DA/pA32nox | 6.12±0.02 | 16.24±0.1 | 11.96±0.08 | 2.02±0.04 | 1.38±0.01 | 0.462±0.002 |
| DA/pD2nox | 6.14±0.04 | 14.21±0.13 | 13.11±0.12 | 2.26±0.02 | 1.67±0.05 | 0.457±0.002 |
| DA/pB89nox | 6.18±0.01 | 13.00±0.09 | 13.42±0.21 | 2.39±0.06 | 1.96±0.04 | 0.413±0.004 |
| DA/pB21nox | 6.22±0.02 | 11.76±0.16 | 13.09±0.01 | 3.38±0.12 | 2.14±0.04 | 0.406±0.003 |
| DA/pB6nox | 6.32±0.03 | 9.94±0.07 | 12.34±0.14 | 4.16±0.06 | 2.83±0.06 | 0.383±0.003 |
ND, not detected. The values are means ± standard deviations for three independent experiments.
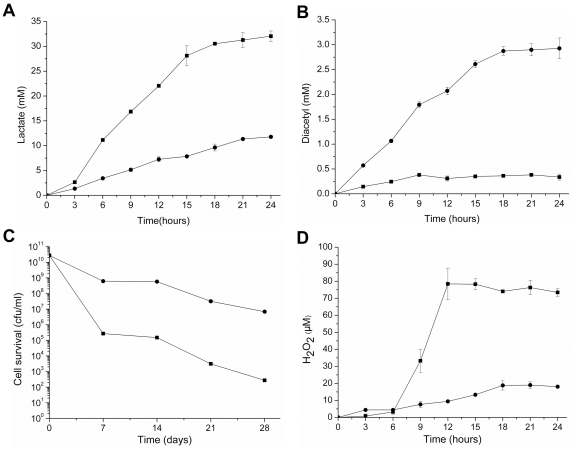
Among the eleven recombinant strains, L. lactis DA/pB6nox consumed 33.96% and 23.88% of the carbon flux towards lactate and diacetyl under aerobic conditions, respectively. After L. lactis DA/pB6nox incubation in RSM supplemented with 1% glucose for 24 h, lactate production was 11.78±0.32 mM and diacetyl production was 2.93±0.21 mM with the pH decreasing from 6.63 to 5.02±0.01. The lactate yield in L. lactis DA was 32.05±1.05 mM with the pH decreasing from 6.61 to 4.67±0.03, whereas little diacetyl was accumulated (Figure 5A and 5B).
Figure 5. Metabolite accumulation and cell survival of L. lactis DA and the recombinant strain.
Lactate production (A) and diacetyl production (B) by strains L. lactis DA (▪) and L. lactis DA/pB6nox (•) in RSM added 1% (wt/vol) glucose. Cell survival (C) and H2O2 accumulation (D) in L. lactis DA (▪) and L. lactis DA/pB6nox (•) after aerobic cultivation in GM 17 medium. In panels (A), (B), (C) and (D) the values are means ± standard deviations of three independent experiments.
Cell survival and H2O2 accumulation
To evaluate the effects of increased NoxE activity on the viability of L. lactis under aerobic conditions, L. lactis DA and L. lactis DA/pB6nox were cultured aerobically for 24 h, after which the cultures were transferred to 4°C and cell viability was examined at intervals over one month. As shown in Figure 5C, initially, the cell populations of the two cultures reached approximately 2.0×1010 per mL. Viable counts of L. lactis DA dynamically dropped as time progressed, and a 108-fold decrease was observed after 28 days of storage. Comparatively, the viability of the L. lacits DA/pB6nox culture remained approximately 6.8×106 cells per mL, four orders of magnitude higher than that of L. lactis DA.
The H2O2 concentration of the L. lactis DA and L. lactis DA/pB6nox cultures were tested to elucidate the cause of cell death under aerobic conditions. As shown in Figure 5D, in L. lactis DA, H2O2 accumulated rapidly within 6 h of incubation and reached a concentration of 78.44±9.29 µM after 24 h. In contrast, H2O2 accumulation was below 10 µM during 12 h of cultivation in L. lactis DA/pB6nox. After 24 h, the final H2O2 concentration of L. lactis DA/pB6nox was 18.08±0.33 µM, which was 76.95% lower than that of L. lactis DA. The reduction in the H2O2 concentration of L. lactis DA/pB6nox might have resulted from the elevated NoxE activity which catalyzed the oxidation of NADH by simultaneously reducing O2 to H2O.
Discussion
With the advent of metabolic engineering, L. lactis has received increasing attention with the aim to promote the flavor and health advantages of fermented products, through the production of, for example, homoalanine, diacetyl, mannitol and folate [10], [29]–[33]. This study provided a platform for precisely regulating the metabolic flux via promoter engineering instead of the gene inactivation or overexpression in L. lactis. The partial redistribution of the pyruvate flux from lactate to diacetyl was achieved by controlling the noxE gene expression through a constitutive promoter library in L. lactis. Furthermore, we newly demonstrated that the elevated NoxE activity had a positive role in eliminating H2O2 and prolonging the cell-survival of L. lactis.
Sequence alignment showed that the promoter of the noxE gene from L. lactis MG1363 possessed the typical promoter properties [34]. Therefore, the mutant strategy was performed to randomize the space sequence of the noxE promoter based on the previous method [35]. A total of 30 random promoters from 500 mutants were selected to form the constitutive promoter library, which displayed broad variability with small steps of activity change between 0.1 and 2.8-fold of the native promoter. Sequence analysis verified that any alteration of the bases in the conserved motifs and changes in the spacer length could lead to a drastic decrease of promoter strength, which supported the postulates in the previous report [36]. Moreover, the sequences outside the −10 and −35 region may influence promoter strength. Eleven typical promoters were used to confirm the effective and stable characteristics of the library through promoter strength measurements and the mRNA transcript levels of the gfp gene. In addition, the noxE gene was used to prove the broad application range of the promoter library. NoxE activity showed a nearly linear correlation with promoter activity. Consequently, we developed a constitutive promoter library with a wide promoter activity range for fine-tuning of gene expression in L. lactis, regardless of the target gene context.
Although the nisin controlled gene expression (NICE) system has been utilized extensively in L. lactis [37], some shortcomings confined it to laboratory experimental conditions, including inducer usage, expression delay and heterogeneity of transcription levels in cell population [35]. Therefore, practical and stable properties of constitutive gene expression systems are desired in large-scale processes. The stable expression of GFP and NoxE driven by random promoters confirmed that the constitutive promoter library could meet the demands of the industrial fermentation process.
Cofactors are essential in completing a large number of biochemical reactions, and their manipulation has been proved to have great influences on metabolic networks [38]. The H2O-forming NADH oxidase specifically utilizes NADH and provides an extra route for the regeneration of NAD+ when O2 is available [39], [40]. In this study, eleven typical promoters from the promoter library were chosen to precisely control the noxE expression and the intracellular NADH/NAD+ ratios were pinpointly regulated. The direct oxidation of NADH necessary for pyruvate reduction by the increased NoxE activity resulted in a diminished pyruvate flux towards lactate via LDH, and the pyruvate flux was redistributed to the ALS pathway. Subsequently, α-acetolactate was decarboxylated into diacetyl in the presence of O2. The Metabolic Control Analysis (MCA) prediction and experimental observation showed that the glycolytic flux to the α-acetolactate branch was less than 0.1% in wild-type L. lactis [41]. However, the increasing NoxE activity driven by the eleven promoters led to the increase of 5.98% to 23.88% flux towards α-acetolactate and retained 67.29% to 33.96% flux to lactate. Acetate production exhibited a slight increase, probably due to the specific PDH activity which catalyzed the conversion of pyruvate to acetyl-CoA with the regeneration of NADH under aerobic conditions [42]. Moreover, neither formate nor ethanol was detected, indicating that no flux was distributed to the pyruvate formate lyase pathway and the alcohol dehydrogenase pathway, which was in agreement with the previous report [43]. In the milk fermentation process, the carbon flux was apportioned to lactate and diacetyl in 4∶1 proportion in L. lactis DA/pB6nox, in which the diacetyl yield was significantly improved as compared to the wild-type strain. Accordingly, through the precise control of the noxE gene expression levels by the constitutive promoter library, the tight constraint on the end-product fluxes in the wild type was alleviated by the gradual lowering of NADH/NAD+ ratios, yielding a series of recombinant strains with small differences in the proportion of lactate and diacetyl production among the end metabolites, which provide potential strains to optimize metabolite distribution.
Generally, the tolerance of lactic acid bacteria to O2 requires the presence of either catalase, NADH oxidases (dehydrogenase), superoxidase dismutase (SOD) or thiol-active enzyme system [44]. There are more than seven NADH oxidase and dehydrogenase genes in the L. lactis genome, including noxA, noxB, noxC, noxD, noxE, yphA and aphF [45], [46]. NoxA and NoxB are two membrane-integrated NADH-dehydrogenases and have been demonstrated to be components of the electron transfer chain (ETC) [47]. NoxC and NoxD are described as H2O2-forming NADH oxidases, however there is no experimental evidence to support this [48]. NoxE is a well characterized H2O-forming NADH oxidase [39]. The yphA gene encodes a NADH dehydrogenase similar to that of Aquifex aeolicus, which participates in aerobic energy metabolism [45]. AhpF is an H2O2-forming NADH oxidase, constituting the alkyl hydroperoxide reductase (AhpR) system [49]. Whole-genome transcriptome analysis has revealed that the H2O2-forming NADH oxidase and SOD were induced to alleviate oxidative stress under aerobic conditions, resulting in H2O2 accumulation [50]. In addition, a small amount of H2O2 in aerated cultures of L. lactis may also result from pyruvate oxidase activity (POX, encoded by the poxL gene) [51]. However, L. lactis is catalase-negative, so the considerable H2O2 accumulation leads to oxidative damage [52]. Figure 5C and 5D showed that the H2O2 concentration of the recombinant strain L. lactisDA/pB6nox was markedly reduced and the cell viability was significantly elevated compared with the wild-type strain. This variance could be the result of the elevated NoxE activity in the recombinant strain. Firstly, more dissolved O2 was consumed in the H2O-forming reaction by the elevated NoxE activity, which reduced the substrate O2 involved in the H2O2-forming reaction. Secondly, in L. lactis, NADH can be consumed by NoxE, LDH and H2O2-forming NADH oxidase. Because NoxE possesses much higher affinity for NADH (Km = 4.1 µM) than the other H2O2-forming NADH oxidase (for example, the Km value of NADH for AhpF was 76 µM), it succeeded in competing with the H2O2-forming NADH oxidase for the substrate NADH, leading to lower H2O2 production [39], [53]. Moreover, although the Km value of the LDH (10 µM) for NADH could compare with that of the NoxE, there is no H2O2 generation in the reaction catalyzed by LDH. Subsequently, decreased H2O2 accumulation could effectively prevent the formation of HO· via the Fenton reaction and reduce ROS [54], therefore enabling L. lactis to be resistant to oxidative stress for achieving long-term cell survival.
In conclusion, here promoter engineering was successfully used to avoid the disadvantages brought by the massive expression of controlling enzyme and elimination of the branching flux. This study proved that promoter engineering was a useful genetic toolbox for metabolic pathway analysis and optimal metabolite distribution. As rapid acidification and flavor compound generation are crucial criteria for starter cultures of lactic acid bacteria, the recombinant strains constructed in this study showed both reasonable ratios of end products and long-term cell survival, which opens perspectives for rational improvement of starter cultures in dairy fermentation industry.
Acknowledgments
We would like to thank N. Galleron for kindly providing the plasmid pSec∶leiss∶Nuc. We also thank I. Biswas and E. Maguin for their generous gift of the plasmid pG+ host4.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was financially supported by a grant from the National Natural Science Foundation of China (NSFC, grant no. 31070091, http://www.nsfc.gov.cn/Portal0/default106.htm); a grant from the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, grant no. 20100131110035, http://www.cutech.edu.cn/cn/index.htm); and Hi-tech Research and Development Program of China (grant no. 2011AA100902, http://www.863.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kleerebezem M, Hugenholtz J. Metabolic pathway engineering in lactic acid bacteria. Curr Opin Biotechnol. 2003;14:232–237. doi: 10.1016/s0958-1669(03)00033-8. [DOI] [PubMed] [Google Scholar]
- 2.Leroy F, De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science & Technology. 2004;15:67–78. [Google Scholar]
- 3.Hugenholtz J. The lactic acid bacterium as a cell factory for food ingredient production. International Dairy Journal. 2008;18:466–475. [Google Scholar]
- 4.Kleerebezem M, Boels IC, Groot MN, Mierau I, Sybesma W, et al. Metabolic engineering of Lactococcus lactis: the impact of genomics and metabolic modelling. Journal of biotechnology. 2002;98:199–213. doi: 10.1016/s0168-1656(02)00132-3. [DOI] [PubMed] [Google Scholar]
- 5.de Vos WM, Hugenholtz J. Engineering metabolic highways in Lactococci and other lactic acid bacteria. Trends Biotechnol. 2004;22:72–79. doi: 10.1016/j.tibtech.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Neves AR, Pool WA, Kok J, Kuipers OP, Santos H. Overview on sugar metabolism and its control in Lactococcus lactis-the input from in vivo NMR. FEMS microbiology reviews. 2005;29:531–554. doi: 10.1016/j.femsre.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Bassit N, Boquien CY, Picque D, Corrieu G. Effect of Initial Oxygen Concentration on Diacetyl and Acetoin Production by Lactococcus lactis subsp. lactis biovar diacetylactis. Appl Environ Microbiol. 1993;59:1893–1897. doi: 10.1128/aem.59.6.1893-1897.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez de Felipe F, Kleerebezem M, de Vos WM, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koebmann BJ, Andersen HW, Solem C, Jensen PR. Experimental determination of control of glycolysis in Lactococcus lactis. Antonie Van Leeuwenhoek. 2002;82:237–248. [PubMed] [Google Scholar]
- 10.Kleerebezem M, Hols P, Hugenholtz J. Lactic acid bacteria as a cell factory: rerouting of carbon metabolism in Lactococcus lactis by metabolic engineering. Enzyme and microbial technology. 2000;26:840–848. doi: 10.1016/s0141-0229(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 11.Platteeuw C, Hugenholtz J, Starrenburg M, van Alen-Boerrigter I, de Vos WM. Metabolic engineering of Lactococcus lactis: influence of the overproduction of alpha-acetolactate synthase in strains deficient in lactate dehydrogenase as a function of culture conditions. Appl Environ Microbiol. 1995;61:3967–3971. doi: 10.1128/aem.61.11.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SQ. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int J Food Microbiol. 2003;83:115–131. doi: 10.1016/s0168-1605(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 13.Alper H, Fischer C, Nevoigt E, Stephanopoulos G. Tuning genetic control through promoter engineering. Proc Natl Acad Sci U S A. 2005;102:12678–12683. doi: 10.1073/pnas.0504604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijakovic I, Petranovic D, Jensen PR. Tunable promoters in systems biology. Curr Opin Biotechnol. 2005;16:329–335. doi: 10.1016/j.copbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Hammer K, Mijakovic I, Jensen PR. Synthetic promoter libraries–tuning of gene expression. Trends Biotechnol. 2006;24:53–55. doi: 10.1016/j.tibtech.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Andersen HW, Pedersen MB, Hammer K, Jensen PR. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcus lactis. European Journal of Biochemistry. 2001;268:6379–6389. doi: 10.1046/j.0014-2956.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeppsson M, Johansson B, Jensen PR, Hahn-Hägerdal B, Gorwa-Grauslund MF. The level of glucose-6-phosphate dehydrogenase activity strongly influences xylose fermentation and inhibitor sensitivity in recombinant Saccharomyces cerevisiae strains. Yeast. 2003;20:1263–1272. doi: 10.1002/yea.1043. [DOI] [PubMed] [Google Scholar]
- 18.Duwat P, Sourice S, Cesselin B, Lamberet G, Vido K, et al. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J Bacteriol. 2001;183:4509–4516. doi: 10.1128/JB.183.15.4509-4516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudu P, Vido K, Cesselin B, Kulakauskas S, Tremblay J, et al. Respiration capacity and consequences in Lactococcus lactis. Antonie Van Leeuwenhoek. 2002;82:263–269. [PubMed] [Google Scholar]
- 20.Marty-Teysset C, de la Torre F, Garel J. Increased production of hydrogen peroxide by Lactobacillus delbrueckii subsp. bulgaricus upon aeration: involvement of an NADH oxidase in oxidative stress. Appl Environ Microbiol. 2000;66:262–267. doi: 10.1128/aem.66.1.262-267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Holo H, Nes IF. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson K, Godon JJ, Renault P, Griffin H, Gasson M. Effect of ilvBN-encoded α-acetolactate synthase expression on diacetyl production in Lactococcus lactis. Applied microbiology and biotechnology. 1996;45:107–111. [Google Scholar]
- 25.San KY, Bennett GN, Berrios-Rivera SJ, Vadali RV, Yang YT, et al. Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metabolic engineering. 2002;4:182–192. doi: 10.1006/mben.2001.0220. [DOI] [PubMed] [Google Scholar]
- 26.Bernofsky C, Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973;53:452–458. doi: 10.1016/0003-2697(73)90094-8. [DOI] [PubMed] [Google Scholar]
- 27.Leonardo MR, Dailly Y, Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- 30.Hols P, Kleerebezem M, Schanck AN, Ferain T, Hugenholtz J, et al. Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol. 1999;17:588–592. doi: 10.1038/9902. [DOI] [PubMed] [Google Scholar]
- 31.Hugenholtz J, Kleerebezem M, Starrenburg M, Delcour J, De Vos W, et al. Lactococcus lactis as a cell factory for high-level diacetyl production. Appl Environ Microbiol. 2000;66:4112–4114. doi: 10.1128/aem.66.9.4112-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisselink HW, Mars AE, Van Der Meer P, Eggink G, Hugenholtz J. Metabolic engineering of mannitol production in Lactococcus lactis: influence of overexpression of mannitol 1-phosphate dehydrogenase in different genetic backgrounds. Appl Environ Microbiol. 2004;70:4286–4292. doi: 10.1128/AEM.70.7.4286-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, De Vos WM, et al. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl Environ Microbiol. 2003;69:3069–3076. doi: 10.1128/AEM.69.6.3069-3076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vos WM, Simons G, Gasson M. Gene cloning and expression systems in lactococci. Genetics and biotechnology of lactic acid bacteria. 1994:52–105. [Google Scholar]
- 35.Jensen PR, Hammer K. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Environ Microbiol. 1998;64:82–87. doi: 10.1128/aem.64.1.82-87.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solem C, Jensen PR. Modulation of gene expression made easy. Appl Environ Microbiol. 2002;68:2397–2403. doi: 10.1128/AEM.68.5.2397-2403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 38.Heux S, Cachon R, Dequin S. Cofactor engineering in Saccharomyces cerevisiae: Expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab Eng. 2006;8:303–314. doi: 10.1016/j.ymben.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Lopez de Felipe F, Hugenholtz J. Purification and characterisation of the water forming NADH-oxidase from Lactococcus lactis. International Dairy Journal. 2001;11:37–44. [Google Scholar]
- 40.Neves AR, Ventura R, Mansour N, Shearman C, Gasson MJ, et al. Is the Glycolytic Flux in Lactococcus lactis Primarily Controlled by the Redox Charge? Journal of Biological Chemistry. 2002;277:28088–28098. doi: 10.1074/jbc.M202573200. [DOI] [PubMed] [Google Scholar]
- 41.Hoefnagel MH, Starrenburg MJ, Martens DE, Hugenholtz J, Kleerebezem M, et al. Metabolic engineering of lactic acid bacteria, the combined approach: kinetic modelling, metabolic control and experimental analysis. Microbiology. 2002;148:1003–1013. doi: 10.1099/00221287-148-4-1003. [DOI] [PubMed] [Google Scholar]
- 42.Papagianni M, Avramidis N, Filiousis G. Glycolysis and the regulation of glucose transport in Lactococcus lactis spp. lactis in batch and fed-batch culture. Microb Cell Fact. 2007;6:16. doi: 10.1186/1475-2859-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen NB, Melchiorsen CR, Jokumsen KV, Villadsen J. Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl Environ Microbiol. 2001;67:2677–2682. doi: 10.1128/AEM.67.6.2677-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel RF, Pavlovic M, Ehrmann MA, Wiezer A, Liesegang H, et al. Genomic analysis reveals Lactobacillus sanfranciscensis as stable element in traditional sourdoughs. Microb Cell Fact. 2011;10(Suppl 1):S6. doi: 10.1186/1475-2859-10-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11:731–753. doi: 10.1101/gr.169701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189:3256–3270. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tachon S, Michelon D, Chambellon E, Cantonnet M, Mezange C, et al. Experimental conditions affect the site of tetrazolium violet reduction in the electron transport chain of Lactococcus lactis. Microbiology. 2009;155:2941–2948. doi: 10.1099/mic.0.029678-0. [DOI] [PubMed] [Google Scholar]
- 48.Odamaki T, Xiao J, Yonezawa S, Yaeshima T, Iwatsuki K. Improved viability of bifidobacteria in fermented milk by cocultivation with Lactococcus lactis subspecies lactis. Journal of dairy science. 2011;94:1112–1121. doi: 10.3168/jds.2010-3286. [DOI] [PubMed] [Google Scholar]
- 49.Tachon S, Brandsma JB, Yvon M. NoxE NADH oxidase and the electron transport chain are responsible for the ability of Lactococcus lactis to decrease the redox potential of milk. Appl Environ Microbiol. 2010;76:1311–1319. doi: 10.1128/AEM.02120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen MB, Garrigues C, Tuphile K, Brun C, Vido K, et al. Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: identification of a heme-responsive operon. J Bacteriol. 2008;190:4903–4911. doi: 10.1128/JB.00447-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pudlik AM, Lolkema JS. Citrate uptake in exchange with intermediates in the citrate metabolic pathway in Lactococcus lactis IL1403. J Bacteriol. 2011;193:706–714. doi: 10.1128/JB.01171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rezaïki L, Cesselin B, Yamamoto Y, Vido K, van West E, et al. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol Microbiol. 2004;53:1331–1342. doi: 10.1111/j.1365-2958.2004.04217.x. [DOI] [PubMed] [Google Scholar]
- 53.Jiang R, Bommarius AS. Hydrogen peroxide-producing NADH oxidase (nox-1) from Lactococcus lactis. Tetrahedron: Asymmetry. 2004;15:2939–2944. [Google Scholar]
- 54.Bruno-Barcena JM, Andrus JM, Libby SL, Klaenhammer TR, Hassan HM. Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl Environ Microbiol. 2004;70:4702–4710. doi: 10.1128/AEM.70.8.4702-4710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Loir Y, Gruss A, Ehrlich SD, Langella P. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol. 1998;180:1895–1903. doi: 10.1128/jb.180.7.1895-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]