Abstract
Two new compounds, peptide-polyketide glycoside totopotensamide A (1) and its aglycone totopotensamide B (2), were isolated from Streptomyces sp. cultivated from the gastropod mollusk, Lienardia totopotens collected in the Philippines. The compounds contain a previously undescribed polyketide component, a novel 2,3-diaminobutyric acid-containing macrolactam, and a new amino acid, 4-chloro-5,7-dihydroxy-6-methylphenylglycine. The application of Marfey’s method to phenylglycine derivatives was explored using quantum mechanical calculations and NMR.
Microbial associates of marine animals provide an excellent and still comparatively untapped source of new molecules.1–3 One hypothesis holds that examination of new animal groups will lead to discovery of new animal-symbiont interactions, and therefore to new chemistry. Shelled gastropod mollusks are relatively little explored for their symbiotic bacterial content, especially in terms of natural products discovery. We have been examining the natural products of bacteria cultivated from shelled gastropods as the source of new compounds with neuroactivity and other biological effects.4,5 In particular, we have applied a collection methodology, lumun-lumun6 that has led to diverse and previously unappreciated mollusk taxa.6 The method involves laying a net for a one-to-six month period, during which time the net is colonized by many different small mollusk species, some of which are as yet undescribed. In the course of these studies, a new species of conoidean mollusk, Lienardia totopotens, was found in a lumun-lumun net in the Philippines.7 At the same time that the type specimen of this new species was collected, identical samples were obtained from the same net and used to cultivate bacterial associates, including Streptomyces sp. 1053U.I.1a.1b. Here, we report characterization of structurally novel peptides totopotensamides A (1) and B (2), isolated from Streptomyces sp. 1053U.I.1a.1b. Although the compounds did not exhibit biological activity in a wide range of assays, they contained relatively unusual structural features and so were further studied.
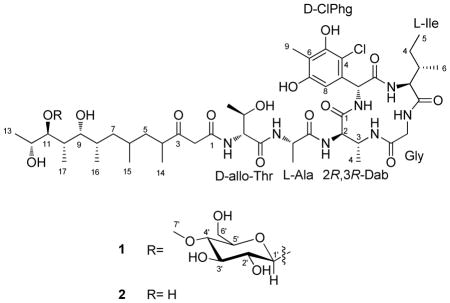
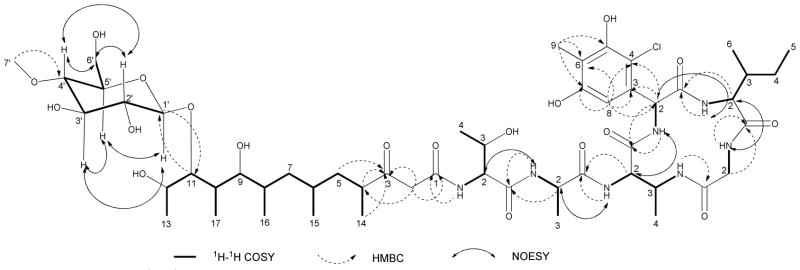
Totopotensamide A (1) was isolated as a pale yellow solid and assigned the molecular formula C52H84ClN7O19 on the basis of HRESIMS analysis. The isotopic distribution of the ions indicated the presence of chlorine. Analysis of the 2D NMR spectra, including COSY, HSQC, and HMBC in DMSO-d6 (Table 1 and Figure 1) allowed for the complete spectroscopic assignment of five common amino acids: one unit each of isoleucine, threonine, alanine, glycine, and 2,3-diaminobutyric acid (Dab). The 13C NMR spectrum (Table 1) showed six aromatic carbon signals representing five quaternary sp2 carbons (δC 131.3, C-3ClPhg; 111.8, C-4ClPhg; 153.2, C-5ClPhg; 114.5, C-6ClPhg; 155.8, C-7ClPhg) and one protonated sp2 carbon (δC 107.9, C-8ClPhg). The HMBC correlations (Figure 1) from the singlet methyl protons (δH 2.04, H-9ClPhg) to C-5ClPhg, C-6ClPhg and C-7ClPhg, from the singlet aromatic proton (δH 6.61, H-8ClPhg) to C-3ClPhg, C-4ClPhg and C-7ClPhg, and from an α proton (δH 5.71, H-2ClPhg) to C-3ClPhg, C-4ClPhg, C-8ClPhg and a carbonyl carbon (δC 172.3, C-1ClPhg) together with their chemical shifts revealed the presence of 4-chloro-5,7-dihydroxy-6-methylphenylglycine (ClPhg). The amino acid sequence and cyclic structure of 1 was deduced from HMBC and NOESY correlations (Figure 1). HMBC correlations between the H-α protons or amide NH signals of one residue and the carbonyl carbons of adjacent residues indicated the sequence was Gly-Ile-ClPhg-Dab-Ala-Thr. 1H – 1H NOESY correlations between the H-α protons of one residue and the amide NH signals of adjacent residues (Figure 1) confirmed the sequence. The HMBC correlation from 3-NHDab to the carbonyl and α carbons of Gly (δC 168.7; 43.7) established the Dab-Gly linkage and allowed assignment of the peptide macrocyclic portion of 1.
Table 1.
NMR Spectroscopic Data (500 MHz for 1H, 125 Hz for 13C, DMSO-d6) for Totopotensamides A and B (1 and 2).
| unit | No | 1 | 2 | ||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| L-Gly | 1 | 168.7, C | - | 169.3, C | - |
| 2 | 43.7, CH2 | 4.07, m; 3.44, m | 43.8, CH2 | 4.07, m; 3.42, m | |
| NH | 8.07, m | 8.05, m | |||
| L-Ile | 1 | 172.0, C | - | 171.6, C | - |
| JH2~H3 = 10.0 Hz in CD3OD | 2 | 61.5, CH | 3.77, m | 61.9, CH | 3.76, m |
| 3 | 35.6, CH | 1.77, m | 35.7, CH | 1.75, m | |
| 4 | 27.1, CH2 | 1.19, m; 1.05, m | 27.1, CH2 | 1.23, m; 1.04, m | |
| 5 | 11.5, CH3 | 0.78, t (6.3) | 11.6, CH3 | 0.79, t (6.3) | |
| 6 | 17.3, CH3 | 0.73, d (7.0) | 17.2, CH3 | 0.74, d (7.0) | |
| NH | 7.09, d (6.0) | 7.09, d (6.0) | |||
| L-ClPhg | 1 | 172.3, C | - | 172.1, C | - |
| 2 | 57.7, CH | 5.71, d (10.0) | 57.7, CH | 5.71 d (10.0) | |
| 3 | 131.3, C | - | 131.2, C | - | |
| 4 | 111.8, C | - | 112.0, C | - | |
| 5 | 153.2, C | - | 153.3, C | - | |
| 6 | 114.5, C | - | 114.6, C | - | |
| 7 | 155.8, C | - | 155.8, C | - | |
| 8 | 107.9, CH | 6.61, s | 107.1, CH | 6.61, s | |
| 9 | 11.3, CH3 | 2.04, s | 11.0, CH3 | 2.03, s | |
| NH | 8.93, d (10.0) | 8.90, d (10.1) | |||
| 2R,3S-Dab | 1 | 169.9, C | - | 169.8, C | - |
| JH2~H3 = 3.5 Hz in CD3OD | 2 | 56.4, CH | 4.46, m | 56.5, CH | 4.45, m |
| 3 | 46.7, CH | 4.16, m | 47.1, CH | 4.09, m | |
| 4 | 16.5, CH3 | 1.06, d (6.7) | 16.6, CH3 | 1.06, d (6.5) | |
| 2-NH | 8.02, d (8.2) | 8.01, d (8.1) | |||
| 3-NH | 6.24, d (9.7) | 6.22, d (9.8) | |||
| L-Ala | 1 | 172.9, C | - | 173.0, C | - |
| 2 | 49.6, CH | 4.35, m | 49.7, CH | 4.34, m | |
| 3 | 19.6, CH3 | 1.19, d (7.0) | 19.8, CH3 | 1.18, d (7.0) | |
| NH | 8.05, d (8.0) | 8.03, d (8.0) | |||
| D-allo-Thr | 1 | 170.9, C | - | 171.0, C | - |
| JH2~H3 = 12.0 Hz in CD3OD | 2 | 59.8, CH | 4.23, m | 60.1, CH | 4.21, m |
| 3 | 68.4, CH | 3.80, m | 68.6, CH | 3.80, m | |
| 4 | 21.1, CH3 | 1.07, d (6.7) | 21.1, CH3 | 1.07, d (6.6) | |
| NH | 8.24, d (8.1) | 8.24, d (8.1) | |||
| Polyketide | 1 | 167.5, C | - | 167.6, C | - |
| 2 | 50.5, CH2 | 3.52, d (15.2); 3.42, d (15.2) | 50.6, CH2 | 3.53, d (15.1); 3.41, d (15.1) | |
| 3 | 210.4, C | - | 210.4, C | - | |
| 4 | 44.8, CH | 2.74, m | 44.8, CH | 2.72, m | |
| 5 | 40.4, CH2 | 1.73, m; 0.77, m | 40.4, CH2 | 1.72, m; 0.77, m | |
| 6 | 29.2, CH | 1.38, m | 29.1, CH | 1.38, m | |
| 7 | 42.6, CH2 | 1.12, m; 0.78, m | 42.6, CH2 | 1.10, m; 0.76, m | |
| 8 | 34.9, CH | 1.50, m | 34.9, CH | 1.51, m | |
| 9 | 73.9, CH | 3.53, m | 75.5, CH | 3.44, m | |
| 10 | 37.6, CH | 1.75, m | 37.7, CH | 1.78, m | |
| 11 | 88.4, CH | 3.41, m | 79.8, CH | 3.19, m | |
| 12 | 67.6, CH | 3.61, m | 68.3, CH | 3.54, m | |
| 13 | 20.3, CH3 | 1.04, d (6.0) | 20.2, CH3 | 1.06, d (6.1) | |
| 14 | 19.1, CH3 | 0.99, d (6.8) | 19.1, CH3 | 0.99, d (6.8) | |
| 15 | 22.6, CH3 | 0.81, d (6.4) | 22.6, CH3 | 0.80, d (6.5) | |
| 16 | 17.9, CH3 | 0.84, d (6.4) | 17.9, CH3 | 0.82, d (6.4) | |
| 17 | 17.3, CH3 | 0.73, d (6.9) | 17.3, CH3 | 0.75, d (6.9) | |
| Methyl-glucose | 1′ | 104.8, CH | 4.21, d (8.2) | ||
| 2′ | 75.3, CH | 2.99, dd (9.0, 8.2) | |||
| 3′ | 77.9, CH | 3.29, dd (9.0, 8.6) | |||
| 4′ | 80.8, CH | 2.91, dd (9.0, 8.6) | |||
| 5′ | 76.9, CH | 3.14, m | |||
| 6′ | 62.1, CH2 | 3.60, m, 3.46, m | |||
| 7′-OMe | 61.3, CH3 | 3.41, s | |||
Figure 1.
Selected 1H-1H COSY, HMBC and NOESY correlations for 1.
By using the advanced Marfey’s method,8,9 it was possible to assign the absolute configuration of α carbons for the amino acid residues. Compound 1 was hydrolyzed, and the resulting amino acids were converted to both Nα-(2,4-dinitro-5-fluorophenyl)-L-leucinamide (L-FDLA) and Nα-(2,4-dinitro-5-fluorophenyl)-D-leucinamide (D-FDLA) derivatives, which were characterized by LC-MS in order of elution for D- and L-FDLA derivatives (Supporting Information). Thus, configurations were assigned as 2R-Thr, 2S-Ile and L-Ala. For 2R-Thr, a large coupling constant between H-2Thr and H-3Thr (JH2~H3 = 12.0 Hz) and by a NOESY correlation between H3-4Thr and NHThr revealed the absolute configuration of C-3Thr to be R.10 Similarly, a large coupling between H-2Ile and H-3Ile (JH2~H3 = 10.0 Hz) in Ile and a NOESY correlation observed between H-6Ile and NHIle revealed the absolute configuration of C-3Ile to be S. Using two authentic L-2,3-Dab (2S,3R-Dab and 2S,3S-Dab) standards, it was confirmed that the retention time for L-FDLA-L-2,3-Dab derivative was earlier than D-FDLA-L-2,3-Dab derivative (Supporting Information). The retention time for D-FDLA-D-2,3-Dab in 1 was identical to L-FDLA-2S,3S-Dab. So, D-2R,3R-Dab was identified in 1. The 3R absolute configuration of 2,3-Dab in 1 was also confirmed by the small coupling constant between H-2Dab and H-3Dab (JH2~H3 = 3.5 Hz) and by a NOESY correlation between H3-4Dab and NHDab.10 Furthermore, the acid hydrolysates of 1 were converted to Nα-(2,4-dinitro-5-fluorophenyl)-L-alaninamide derivatives (L-FDAA), which were characterized by LC-MS in comparison with authentic standards (D-allo-Thr, D-Thr and L-Ile). The results confirmed that 1 contained D-allo-Thr and L-Ile.
Because the use of Marfey’s method with phenylglycine derivatives has not been rigorously explored, we synthesized standards using both D-phenylglycine (Phg) and L-3,5-dihydroxyphenylglycine (DhPhg). While the FDLA derivatives of Phg exhibited the expected elution order by HPLC, the DhPhg elution order was the opposite of what is expected in comparison to proteinogenic amino acids. We further investigated this phenomenon using molecular modeling with quantum mechanical energy analysis. It was found that the lowest energy conformations of Phg-FDLA derivatives conform to the expected shape adopted by proteinogenic amino acids. By contrast, a strong H-bond between the DhPhg phenolic OH and the leucinyl residue of FDLA stabilized a different conformation. This conformational difference drastically changes the relative orientation of the Leu and Phg α protons in the low-energy conformations (Figure 2). The α protons of the Leu and Phg both oriented to the same side of the dinitrobenzene plane in the S,R isomer, but opposite in the S,S isomer. By contrast, with DhPhg, there was less of an apparent difference in the orientation of the α protons of the Leu and DhPhg. Gratifyingly, when 1H NMR spectra of both diastereomers of Phg-FDLA and DhPhg-FDLA were compared, this prediction was borne out: there was a smaller difference in the Δδ (0.24 ppm and 0.08 ppm, respectively) of α protons of the Leu and DhPhg in the DhPhg-FDLA derivatives, while in Phg derivatives this values were 0.35 ppm and 0.13 ppm, respectively (Supporting Information), supporting the reliability of the model. Quantum mechanical calculations indicated that ClPhg found in 1 should adopt a similar low-energy conformation to DhPhg (Figure 2). Because elution order is proposed to be based upon the shape adopted by derivatives,8 and ClPhg and DhPhg should adopt the same shape, the configuration of ClPhg in 1 was assigned as D (R). Thus, amino acid configurations in 1 were determined to be L-Ile, D-ClPhg, 2R,3R-Dab, L-Ala and D-allo-Thr.
Figure 2.
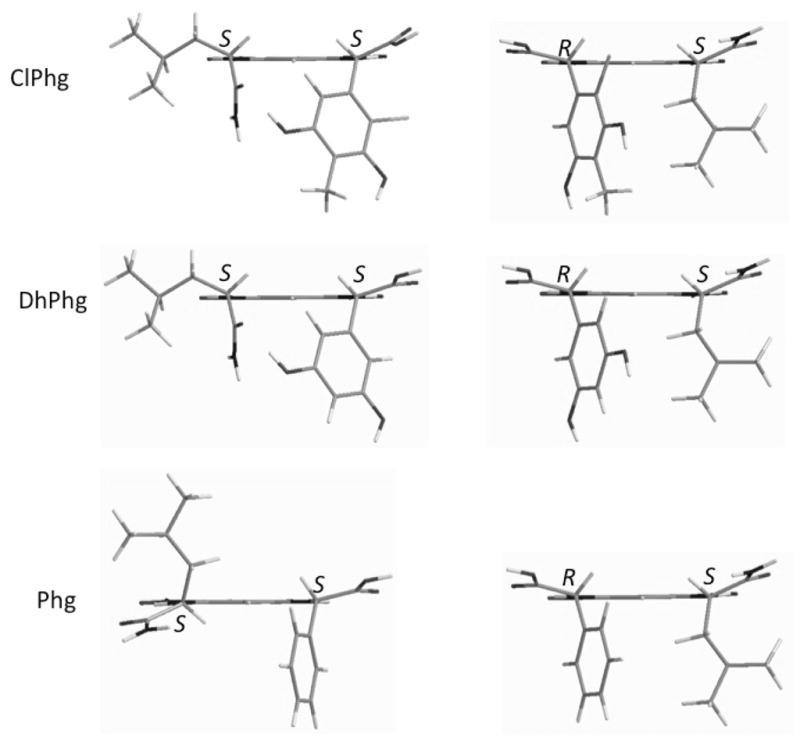
Molecular modeling of FDLA derivatives of Phg, DHPhg, and ClPhg with lowest quantum mechanical energy.
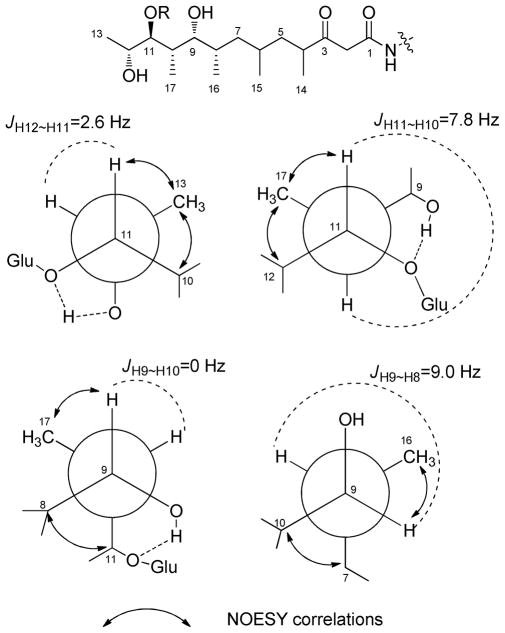
The polyketide component of 1 was deduced as follows. A proton network from H-4 through H3-13 and four doublet methyls (H3-14, H3-15, H3-16 and H3-17) indicated a methyl-branched polyketide chain. HMBC correlations between C-3 (δC 210.4) and H-2, H-4, and H-5 indicated the presence of a ketone. Further, an isolated methylene spin system (δH 3.52 d (15.2), 3.42 d (15.2); δC 50.5, C-2) correlated by HMBC to both a carbonyl at C-1 (δC 167.5) and C-3. This placement predicted that the H-2 protons should be exchangeable. Indeed, incubation of 1 in CD3OD led to solvent exchange of the H-2 protons (Supporting Information). HMBC correlations from the NH (δH 6.24) of D-allo-Thr to polyketide C-1 established the connection between the polyketide and peptide portions of the molecule. The relative configuration of the C-8 to C-13 region was established by analysis of 1H-1H coupling constants and NOESY data (Figure 3). C-11/C-12 was assigned as anti based on the small JH-11~H-12 value (2.6 Hz) and NOESY correlations for H-11/H3-13 and H-10/H3-13. C-10/C-11 was assigned as anti because of the large JH-10~H-11 value (7.8 Hz) and NOESY correlations for H-11/H3-17 and H-12/H3-17. The C-9/C-10 portion was syn based on the small JH-9~H-10 value (0 Hz) and NOESY correlations for H-9/H3-17 and H-8/H3-11. The C-8/C-9 portion was assigned as syn from the large JH-8~H-9 value (9.0 Hz) and NOESY correlations for H-9/H3-16 and H-7/H-10. The configurations of C-4 and C-6 could not be unambiguously determined.
Figure 3.
Key NOESY correlations and rotational model for the polyketide unit in totopotensamide A (1).
Finally, the remaining NMR signals were assigned to a methyl-hexose moiety using 1H-1H COSY and HMBC experiments (Figure 1). An HMBC correlation between H3-7′ (δH 3.41) and C-4′ (δC 80.8) determined placement of the methyl group, while an HMBC correlation from H-1′ (δH 4.21) to polyketide C-11 (δC 88.4) determined the point of attachment to the polyketide chain. The relative configuration of the 4-methyl-hexose moiety was elucidated on the basis of NOESY and 1H-1H coupling constant data (Figure 1 and Table 1). NOESY correlations between H-1′/H-3′/H-5′ suggested that the hexose moiety took a chair form with axial orientations for these protons. The anti orientations of these to H2′ and H-4′ were deduced from the large vicinal coupling constants (JH-1′~H-2′ = 8.2 Hz, JH-2′~H-3′ = 9.0 Hz, JH-3′~H-4′ = 8.6 Hz, JH-4′~H-5′ = 9.0 Hz). Thus, the 4-O-methyl-hexose moiety was assigned as 4-O-methyl-1-α-glucoside.
Totopotensamide B (2) was obtained as a pale yellow solid. Its molecular formula was determined as C45H72ClN7O14 on the basis of HRESIMS data, which differed from 1 by the loss of C7H12O5. Because of this mass difference and the absence of NMR signals for the methyl glucose unit (Table 1), 2 was assigned as the aglycone of 1. Indeed, the NMR spectra were very similar, with the notable exception of an 8.6 ppm upfield shift of C-11 in 2 compared to 1, supporting the absence of the glycosidic bond at C-11 in 2. COSY, HSQC, and HMBC experiments further supported these assignments (Supporting Information). The absolute configuration of the components of 2 were identical to those of 1. The 1H and 13C NMR data, including coupling constants, (Table 1) were similar in both compounds. The hydrolysate of 2 was analyzed using the advanced Marfey’s method,8,9 confirming that the absolute configuration of the amino acids in 2 were identical to those of 1.
Totopotensamides are not closely related to known compounds and exhibit several unusual features not found in other natural products, to the best of our knowledge. They contain a new amino acid, ClPhg. Modified phenylglycine (Phg) is found occasionally in bacterial natural products, most notably in vancomycin-like compounds of the actaplanin11/ristomycin group.12 The 2-position of the aromatic ring in Phg is relatively easily halogenated. For example, in risocetin this position was synthetically iodinated.12 Although Dab is relatively common in nonribosomal peptides, the presence of Dab in such a small, constrained macrocycle has not been previously observed in natural products. Third, the polyketide sidechain has no precedent, in that the organization of substituents such as methyl, hydroxyl, and keto groups has not been previously observed. The functionality is somewhat related to macrolides, such as erythromycin and relatives,13 but overall there are no reported compounds that are close to this organization. In addition, the β-ketoamide-amino acid motif is reminiscent of many tetramic acids.14 In the case of tetramic acid synthesis, the amino acid undergoes a Dieckmann condensation with the side chain, leading to termination of chain extension,15 but here the compounds are further extended by amino acids. Finally, 4-O-methyl-glucose is usually associated with eukaryotic metabolism, but it has been found occasionally in actinomycete products such as glycosylated nucleosides (tubericidin, dapiramicin).16
Totopotensamides were examined in a range of broad-net phenotypic assays, at concentrations up to 100 μM. The compounds did not exhibit activity against panels of bacterial, fungal, or mammalian cell lines. They exhibited no effects on receptors or ion channels, as gauged using mouse dorsal root ganglion neurons and human receptor screens at the NIMH Psychoactive Drug Screening Program. The compounds caused no observable phenotypic effects on zebrafish larvae, nor did they affect the producing bacteria as hormones or in any other observable way. Thus, the biological activity of these unusual products if any has yet to be determined.
The discovery of new molecules from a bacterium associated with a new species of mollusk is perhaps only coincidence, but we feel that linking exploration for biodiversity and natural products can accelerate discovery in both areas. Complications in making this assessment include the difficulty in determining whether bacteria are truly symbiotic, or whether they are casual associates of the animals in question. For example, although we have shown that actinobacteria are likely specific symbionts of cone snails,17 in this particular case we do not know whether the cultivated bacteria are symbionts or casual associates. The lumun-lumun method, in providing access to animal diversity, has already been shown to yield new animal species and new animal venoms,6 and could also provide a rich source of new molecules from associated symbiotic bacteria. This report represents an early stage exploration of that potential.
EXPERIMENTAL SECTION
General Experimental Procedures
Optical rotations were measured on a Jasco DIP-370 polarimeter. UV spectra were obtained using a Perkin-Elmer Lambda2 UV/vis spectrometer. NMR data were collected using either a Varian INOVA 500 (1H 500 MHz, 13C 125 MHz) NMR spectrometer with a 3 mm Nalorac MDBG probe or a Varian INOVA 600 (1H 600 MHz, 13C 150 MHz) NMR spectrometer equipped with a 5 mm 1H[13C,15N] triple resonance cold probe with a z-axis gradient, utilizing residual solvent signals for referencing. High-resolution mass spectra (HRMS) were obtained using a Bruker (Billerica, MA) APEXII FTICR mass spectrometer equipped with an actively shielded 9.4 T superconducting magnet (Magnex Scientific Ltd.), an external Bruker APOLLO ESI source, and a Synrad 50W CO2 CW laser. Supelco Discover HS (4.6 × 150 mm) and semipreparative (10 × 150 mm) C18 (5 μm) columns were used for analytical and semipreparative HPLC, respectively, as conducted on a Hitachi Elite Lachrom System equipped with a Diode Array L-2455 detector.
Bacterial Material
Streptomyces sp. 1053U.I.1a.1b was cultivated from Lienardia totopotens obtained by professional collectors near Mactan Island, Cebu, Philippines as previously described.5 Relevant permission from local and national authorities in the Philippines was obtained prior to beginning this study. The strain was cultured from dissected hepatopancreas tissue, purified, and later the strain was recovered from a glycerol stock and used for further chemical analysis.5 The 16S gene was cloned using primers 8-27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGA CTT-3′) and submitted to GenBank, accession number JN982561. The taxonomically described relative with the closest sequence match was Streptomyces carnosus.
Fermentation and Extraction
During the neurological activity screening by DRG assay5 and chemistry screening by LS-DAD-MS, the extract of Streptomyces sp. 1053U.I.1a.1b was not active, but shown to contain compounds with unusual MS ions feature. In order to characterize the structures of those compounds, the strain was cultured at 30 °C with bioflo110 fermentor containing 10 L of the medium ISP2 (0.4% yeast extract, 1% malt extract, 0.4% glucose, 2% NaCl). After 8 days, the broth was centrifuged and the supernatant was extracted with HP-20 resin for 4 h. The resin was filtered through cheesecloth and washed with H2O to remove salts. The filtered resin was eluted with MeOH to yield an extract.
Purification
The extract (500 mg) was separated into 4 fractions (Fr1-Fr4) on a C18 column using step-gradient elution of MeOH in H2O (40%, 60%, 70%, 80%). Fr 2 eluting in 60% MeOH was passed through a LH-20 column (to remove diketopiperazines). The early elution fractions from the LH-20 column were combined according to the HPLC analysis and further purified by C18 HPLC using 35% CH3CN in H2O with 0.1% TFA to obtain compound 1 (3.5 mg, tR = 6.4 min) and 2 (1.0 mg, tR=7.7 min).
Totopotensamide A (1)
pale yellow solid; [α]25D +20 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 235 (2.11), 282 (1.45) nm; 1H and 13C NMR (see Table 1); HRESIMS m/z 1146.5598 [M+H]+ (calcd for C52H84ClN7O19, 1146.5589).
Totopotensamide B (2)
pale yellow solid; [α]25D +43 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 235 (2.10), 282 (1.35) nm; 1H and 13C NMR (see Table 1); HRESIMS m/z 992.4793 [M+Na]+ (calcd for C45H72ClN7O14, 992.4723).
Acid Hydrolysis of Peptides
Compounds 1 and 2 (100 μg each) were separately dissolved in 6 N HCl (500 μL) and heated in sealed ampule vials at 110 °C for 16 h. The solvent was removed in vacuo.
LC-MS Analysis of D/L-FDLA Derivatives
The acid hydrolysates of 1 and 2 were dissolved in H2O separately. To a 50 μL aliquot of each were added 1 N NaHCO3 (20 μL) and 1-fluoro-2,4-dinitrophenyl-5-L-leucinamide (1% solution in acetone, 100 μL), and the mixture was heated to 40 °C for 50 min. The solutions were cooled to room temperature, neutralized with 1 N HCl (20 μL), and then dried in vacuo. The residues were dissolved in 1:1 CH3CN-H2O and then analyzed by LC-MS. The analysis of the L- and D-FDLA derivatives was performed on a supelcosil LC-18 column (150 × 4.6 mm, 5 μm) employing a linear gradient of from 25% to 70% CH3CN in 0.01 M formic acid at 0.5 mL/min over 45 min. The retention times of the D- and L-FDLA derivatives, respectively, were as follows: L-Ala: 29.00, 25.30 min, m/z 382 [M − H]−; D-allo-Thr: 21.29, 23.02 min, m/z 412 [M − H]−; D-Dab: 38.95, 44.35 min, m/z 705 [M − H]−; L-ClPhg: 39.22, 31.18 min, m/z 524 [M − H]−; L-Ile: 39.24, 31.16 min, m/z 424 [M − H]−.
LC-MS Analysis of L-FDAA Derivatives
L-FDAA was used to derivatize the acid hydrolysates of 1 and 2 and three standard amino acids (D-allo-Thr, D-Thr and L-Ile). The reaction with L-FDAA was performed with the same procedure as that used for FDLA. The retention times of the L-FDAA derivatives were as follows: D-allo-Thr: 13.50 min, m/z 370 [M − H]−; D-Thr: 14.80 min, m/z 370 [M − H]−; L-Ile: 24.32 min, m/z 382 [M − H]−.
Quantum Mechanical Energy Analysis of FDLA derivertives of Phg, DhPhg and ClPhg
The conformational energetics of each isomers (L-Phg-L-FDLA, D-Phg-L-FDLA, L-DhPhg-L-FDLA, D-DhPhg-L-FDLA, L-ClPhg-L-FDLA, and D-ClPhg-L-FDLA) was studied using quantum mechanical energy analysis. To understand which conformation was energetically preferred, L-Phg-L-FDLA, D-Phg-L-FDLA, L-DhPhg-L-FDLA, and D-DhPhg-L-FDLA were each built in five likely starting conformations. An additional hydrogen bonding conformation was also built for L-DhPhg-L-FDLA and D-DhPhg-L-FDLA. These starting structures were optimized with Gaussian09 using density functional theory.18 Specifically, the PBEPBE exchange and correlation functional19,20 was employed with the 6-311g++(3df,3pd) basis set using automatic density fitting. To mimic the presence of water, the IEFPCM implicit solvent was used during optimization.21 Four conformations each of L-ClPhg-L-FDLA and D-ClPhg-L-FDLA were also built and optimized, starting with the two lowest energy structures from L-DhPhg-L-FDLA and D-DhPhg-L-FDLA and adding the chlorine atom oriented either inward or outward.
Supplementary Material
Acknowledgments
This work was funded by ICBG grant U01TW008163 from Fogarty (NIH). We thank the government of the Philippines and the community of Mactan Island for permission to conduct this study. We also acknowledge computer time from the Center for High Performance Computing at the University of Utah and on Blacklight at the Pittsburgh Supercomputing Center through NSF XSEDE allocations MCA01S027. We thank a reviewer of this manuscript for suggesting further examination of Phg absolute configuration.
Footnotes
Supporting Information Available:
NMR data and HRESIMS data for totopotensamides A and B (1 and 2). Ion chromatograms of the D/L-FDLA derivatives and the L-FDLA derivatives of the hydrolysis product of totopotensamides. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gil-Turnes MS, Hay ME, Fenical W. Science. 1989;4926:116–118. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- 2.Jensen PR, Fenical W. Ann Rev Microbiol. 1994;48:559–584. doi: 10.1146/annurev.mi.48.100194.003015. [DOI] [PubMed] [Google Scholar]
- 3.Piel J. Nat Prod Rep. 2009;26:338–362. doi: 10.1039/b703499g. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Reilly CA, Antemano R, Hughen RW, Marett L, Concepcion GP, Haygood MG, Olivera BM, Light A, Schmidt EW. J Med Chem. 2011;54:3746–3755. doi: 10.1021/jm101621u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, Antemano RR, Hughen RW, Tianero MDB, Peraud O, Haygood MG, Concepcion GP, Olivera BM, Light A, Schmidt EW. J Nat Prod. 2010;73:1922–1926. doi: 10.1021/np100588c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seronay RA, Fedosov AE, Astilla MAQ, Watkins M, Saguil N, Heralde FM, III, Tagaro S, Poppe GT, Alino PM, Oliverio M, Kantorc YI, Concepciona GP, Olivera BM. Toxicon. 2010;56:1257–1266. doi: 10.1016/j.toxicon.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg G, Stahlschmidt P. Proc Acad Nat Sci Philad. 2011;161:105–115. doi: 10.1635/053.161.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii K, Ikai Y, Mayumi T, Oka H, Suzuki M, Harada K. Anal Chem. 1997;69:3346–3352. [Google Scholar]
- 9.Fujii K, Ikai Y, Mayumi T, Oka H, Suzuki M, Harada K. Anal Chem. 1997;69:5146–5151. [Google Scholar]
- 10.Lu Z, Van Wagoner RM, Harper MK, Baker HL, Hooper JNA, Bewley CA, Ireland CM. J Nat Prod. 2011;74:185–193. doi: 10.1021/np100613p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber FM, Michel KH, Hunt AH, Martin JW, Molloy RM. J Antibiot. 1988;41:798–801. doi: 10.7164/antibiotics.41.798. [DOI] [PubMed] [Google Scholar]
- 12.Harris CM, Fesik SW, Thomas AM, Kanna R, Harris TM. J Org Chem. 1986;51:1509–1513. [Google Scholar]
- 13.Ma X, Ma S. Curr Med Chem. 2011;18:1993–2015. doi: 10.2174/092986711795590075. [DOI] [PubMed] [Google Scholar]
- 14.Royles BJL. Chem Rev. 1995;95:1981–2001. [Google Scholar]
- 15.Sims JW, Schmidt EW. J Am Chem Soc. 2008;130:11149–11155. doi: 10.1021/ja803078z. [DOI] [PubMed] [Google Scholar]
- 16.Shomura T, Nishizawa N, Iwata M, Yoshida J, Ito M, Amano S, Koyama M, Kojima M, Inouye S. J Antibiot. 1983;36:1300–1304. doi: 10.7164/antibiotics.36.1300. [DOI] [PubMed] [Google Scholar]
- 17.Peraud O, Biggs JS, Hughen RW, Light AR, Concepcion GP, Olivera BM, Schmidt EW. Appl Environ Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.1. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- 19.Perdew JP, Burke K, Ernzerhof M. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 20.Wadnerkar N, Kalamse V, Chaudhari A. Int J Hydrogen Energy. 2011;36:664–670. [Google Scholar]
- 21.Tomasi J, Mennucci B, Cancès E. J Mol Struct. 1999;464:211–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.