Abstract
It is believed that an effective HCV vaccine must induce strong HCV-specific cytotoxic IFNγ+ CD8+ T cells able to migrate into and become fully activated within the liver, an organ known to suppress T-cell responses and induce tolerance. Given the importance of intrahepatic HCV-specific T cells in the clearance of acute infection, the goal of this present study was to determine if peripheral immunization was able to induce functional intrahepatic HCV-specific T cell-based immunity both in the presence and absence of HCV antigen expression within the liver. Using a novel HCV NS3/NS4A DNA vaccine, we show that peripheral immunization of C57BL/6 mice results in the formation of a large pool of fully functional HCV-specific cytotoxic IFNγ+ CD8+ T cells within the liver and that these cells were highly enriched within the liver as compared with the spleen. Following hepatic expression of cognate HCV antigen using a previously described liver transfection method, we show that this pool of vaccine-induced HCV-specific CD8+ T cells retained its ability to become highly activated as shown by the upregulation of IFNγ and CCR5 expression, as well as by the clearance of HCV NS3 expressing hepatocytes. Taken together, these findings suggest that T-cell effector function is preserved within the liver and that selective recruitment of antigen-specific T cells to the liver may play a previously unappreciated role in the process of immune surveillance, which may be exploited for future T cell-based HCV vaccines.
Key words: HCV DNA vaccine, NS3, NS4A, liver transfection, consensus
Numerous studies of both acutely infected humans and chimpanzees have shown that a strong HCV-specific T-cell response within the liver is critical for both reduction of viral load and clearance of acute infection.1,2 The best characterized of the intrahepatic responses to HCV infection is that of the CD8+ T cell, with increasing evidence pointing to the need for intrahepatic cytotoxic and IFNγ producing CD8+ T cells to effectively control and eliminate infection. Studies of convalescent chimpanzees have shown that localization of CD8+ T cells within the liver is required for termination of HCV replication and that control of viremia is dependent on a rapid and massive expansion of cytotoxic T cells.2 However, it is known that resolution of infection can occur in the absence of elevated serum transaminase levels, suggesting that production of pro-inflammatory cytokines, such as IFNγ, may be important factors in viral control as well.3 For example, IFNγ has been associated with large reductions in viral load1 and has been correlated with clearance of acute infection.4
While it is known that a strong intrahepatic T-cell response is critical for clearance of HCV infection, up to 70% of infected individuals fail to either mount or sustain these responses resulting in chronic infection.3 This failure may in part be due to the unique immunological properties of the liver and its ability to modulate T-cell responses. It is known that the liver harbors within it an abundance of antigen experienced T cells, many of which are preferentially found within the liver as compared with the peripheral blood, suggesting that the liver is able to specifically recruit and sequester antigen experienced T cells. Although the exact role of liver localized T cells has yet to be elucidated, they are thought to play an important role both in the continual process of immune surveillance and in tolerizing the body to food antigens.5,6 Receiving approximately 75 percent of its blood from the portal vein, the liver is constantly inundated with both food and bacterial antigens from the gut, as well as, exposed to potential pathogens.7 Under resting conditions it is believed that the liver maintains a default state of T-cell tolerance and that active recruitment of antigen experienced T cells to the liver is a mechanism for suppressing immune responses to gut antigens as evidenced by abundant IL-10 production,8 low expression of co-stimulatory molecules and selective deletion of activated T cells within the liver.9,10 In fact, antigenic priming of T cells in the liver has been shown to result in both the dysfunction and apoptosis of CD8+ T cells resulting in tolerance to food, transplant and hepatotropic viral antigens.11–14
Given the importance of intrahepatic HCV-specific T cells in the clearance of acute infection, the goal of this present study was to determine if peripheral immunization was able to induce intrahepatic HCV-specific T cell-based immunity and if so, determine how the liver may negatively modulate vaccine-specific responses both in the presence and absence of HCV antigen expression within the liver. In order to test this, we utilized both a novel HCV NS3/NS4A DNA vaccine and a previously described liver transfection method to induce HCV NS3 expression within the liver. We show that peripheral immunization results in a large pool of functional HCV-specific cytotoxic IFNγ+ CD8+ T cells that are selectively sequestered and enriched within the liver despite the absence of antigen expression. Additionally, following expression of cognate antigen within the liver, this pool of vaccine-induced HCV-specific T cells was able to become highly activated resulting in the upregulation of IFNγ and the clearance of HCV NS3 expressing hepatocytes. Taken together, these findings suggest that T-cell effector function is preserved within the liver and that selective recruitment of antigen-specific T cells to the liver may play a previously unappreciated role in the process of immune surveillance, which may be exploited for future T cell-based HCV vaccines.
Results
pConNS3/NS4A induces HCV-specific IFNγ+ and CD107A+ cytotoxic T cells in the periphery.
In order to follow and evaluate HCV-specific T-cell responses in the liver, we first confirmed that intramuscular immunization could induce a large pool of HCV-specific T cells in the periphery. HCV-specific T cells were generated by intramuscular immunization of C57BL/6 mice with pConNS3/NS4A. The same construct will be used to induce HCV NS3 expression within the liver. Each animal (n = 5) received a total of two intramuscular immunizations of 12.5 µg of pConNS3/NS4A followed by electroporation. The animals were sacrificed one week following the last immunization and the HCV NS3-specific T-cell responses of each animal were assessed by IFNγ ELISpot and flow cytometry. The immunization scheme is depicted in (Fig. 1A). Given that both cytotoxic and IFNγ+ HCV-specific T cells have been correlated with clearance of acute infection, we focused primarily on these responses when evaluating the immunogenicity of pConNS3/NS4A.
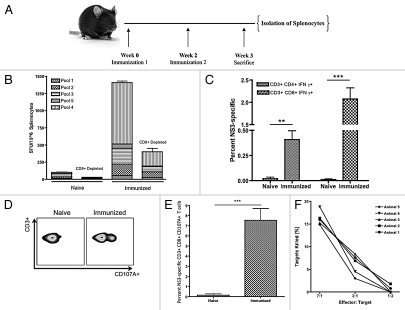
Figure 1.
Immunization with pConNS3/NS4A induces HCV NS3-specific IFNγ+ and cytotoxic T-cell responses in the periphery. (A) Immunization scheme, n = 5. (B) IFNγ ELISpot. HCV NS3-specific responses were reported as the average number of spot forming units (SFU) per million splenocytes. (C) Average percent HCV NS3-specific CD4+ and CD8+ IFNγ+ T cells. (D) Representative CD107A staining. (E) Average percent HCV NS3-specific CD107A expression for each group. (F) Percent HCV NS3-specific granzyme B mediated killing as normalized to the number of NS3-specific effector CD8+ T cells. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005; NS, not significant).
HCV NS3-specific IFNγ T-cell responses averaged approximately 1,400 SFU per million splenocytes with CD8+ T cells accounting for approximately 70 percent of the response (Fig. 1B). These results were confirmed with intracellular cytokine staining (ICS) for IFNγ and flow cytometry. The percentage of CD4+ IFNγ+ and CD8+ IFNγ+ T cells was 0.4% ± 0.09% and 2.1% ± 0.25%, respectively (Fig. 1C).
CD107A expression was used as a phenotypic marker for HCV NS3-specific CD8+ cytotoxic T cells. Immunized mice expressed a large percentage of HCV NS3-specific CD107A with an average of 7.5% ± 1.25% of CD8+ T cells staining positive (Fig. 1D and E). To confirm NS3-specific cytoxicity, a functional flow cytometric-based assay was employed to measure the amount of active granzyme B delivered to target cells. Target cells were pulsed with HCV NS3 antigens and co-incubated with effector cells that had been stimulated for 12 h with HCV NS3 peptides. A dose dependent decrease in the percentage of targets killed was observed with an average of 16% HCV NS3-specific killing seen at the maximum effector to target ratio of 7:1 (Fig. 1F). The number of antigen-specific effectors for each animal was determined by the activation markers CD44 and CD11a (Fig. S1).
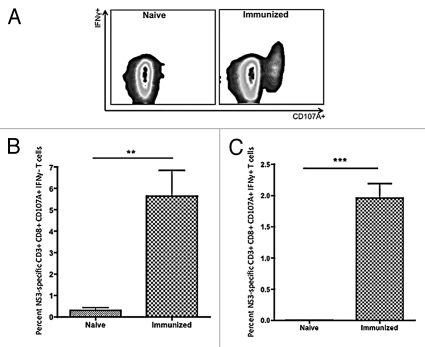
Further analysis revealed that all HCV NS3-specific CD8+ T cells stained positive for CD107A. However, when analyzed for IFNγ expression, HCV NS3-specific CD8+ T cells fell into one of two major populations, either CD8+ CD107A+ IFNγ− or CD8+ CD107A+ IFNγ+ (Fig. 2A). The percentage of HCV NS3-specific CD8+ CD107A+ IFNγ− and CD8+ CD107A+ IFNγ+ T cells averaged 5.6% ± 1.2% and 2.0% ± 0.23%, respectively (Fig. 2B and C).
Figure 2.
Flow cytometric analysis of HCV NS3-specific CD8+ T cells for CD107A and IFNγ expression. (A) Representative HCV NS3-specific CD8+ T cell staining for CD107A and IFNγ. (B) Average percentage of HCV NS3-specific CD8+ CD107A+ IFNγ− T cells. (C) Average percentage of HCV NS3-specific CD8+ CD107A+ IFNγ+ T cells. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005; NS, not significant). (n = 5).
HCV NS3-specific CD8+ T cells were enriched in the liver as compared with the periphery.
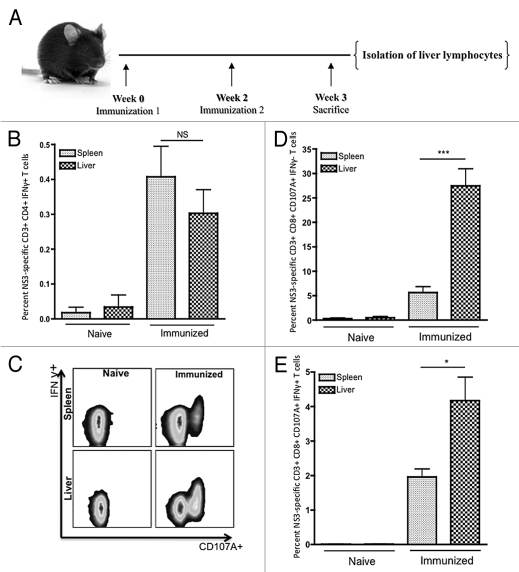
We next assessed whether peripheral immunization could also induce HCV-specific T cell-based intrahepatic immunity. Specifically, we were interested in determining whether HCV-specific T cells induced through peripheral immunization could migrate into and remain functional within the immunotolerant liver. Liver lymphocytes were analyzed from both groups (Fig. 3A). Results from flow cytometric analysis showed that both HCV NS3-specific CD4+ and CD8+ IFNγ+ T cells could be detected within the liver. Out of the CD4+ T cells isolated from the liver an average of 0.26% ± 0.09% expressed IFNγ (Fig. 3B). Likewise, both populations of HCV NS3-specific CD8+ T cells identified previously in the spleen were readily detected within the liver (Fig. 3C). The percentage of HCV NS3-specific CD8+ CD107A+ IFNγ− and CD8+ CD107A+ IFNγ+ T cells within the resting liver were 27.5% ± 3.5% and 4.2% ± 0.68%, respectively (Fig. 3D and E). Both populations of HCV NS3-specific CD8+ T cells were significantly enriched within the liver. Percentages of HCV NS3-specific CD8+ CD107A+ IFNγ− and CD8+ CD107A+ IFNγ+ T cells were approximately 5- and 2-fold higher in the liver as compared with the spleen, respectively.
Figure 3.
Flow cytometric analysis of HCV NS3-specific T-cell responses within the liver as compared with the spleen. (A) Immunization scheme, n = 5. (B) Average percent HCV NS3-specific CD4+ IFNγ+ T cells. (C) Representative CD107A and IFNγ staining for HCV NS3-specific CD8+ T cells. (D) Average percentage of HCV NS3-specific CD8+ CD107A+ IFNγ− T cells. (E) Average percentage HCV NS3-specific CD8+ CD107A+ IFNγ+ T cells. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005; NS, not significant).
To attempt to explain this enrichment, HCV-specific CD8+ T cells were stained for CCR5 and CXCR3, receptors that are believed to be important for the recruitment of antigen-specific T cells to the liver.15,16 However, neither population showed a significant increase in the percentage of CCR5 or CXCR3 positive T cells in the liver as compared with the spleen, suggesting that the enrichment of HCV NS3-specific CD8+ T cells was not chemokine receptor dependent (Fig. S2A and B).
Liver expression of HCV NS3 resulted in the activation of intrahepatic HCV NS3-specific CD8+ T cells.
While it had been shown that peripheral immunization could induce functional HCV-specific T cell based immunity within the resting liver, an effective vaccine strategy must also be able to induce HCV-specific T cells with the ability to become activated and remain functional following HCV antigen expression within the liver. However, observations of the apoptotic fate of selectively recruited T cells to the liver has led to theories that the liver may function as either a ‘graveyard’ or ‘killing field’ for activated CD8+ T cells, which is thought to be one possible mechanism by which the liver may be able to induce tolerance.7,8,10,17 Given these observations, we were curious as to the fate of our NS3-specific CD8+ T cells following activation by expression of their cognate antigen within the liver. In order to test this, we took advantage of a previously reported liver transfection method where tail vein injection of naked DNA was used to selectively transfect hepatocytes resulting in foreign protein expression in the liver.18–20 In order to induce liver expression of HCV NS3, C57BL/6 mice were tail vein injected with pConNS3/NS4A using a previously described method of liver transfection.19 Both naive and immunized animals received tail vein injections of 100 µg of pConNS3/NS4A and were sacrificed 48 h following injection (Fig. 4A). When the percentages of HCV NS3-specific CD8+ CD107A+ T cells were compared before and after liver transfection, no significant difference was seen. This finding suggested that the population of HCV NS3-specific CD8+ T cells observed prior to injection was the same observed following transfection (Fig. 4B).
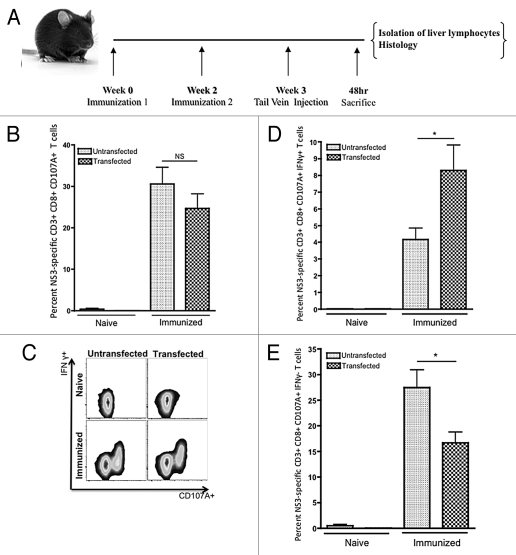
Figure 4.
Flow cytometric analysis of intrahepatic HCV NS3-specific T-cell responses following HCV NS3 transfection within the liver. (A) Immunization and injection scheme, n = 5. Animals were given a tail vein injection of 100 µg of pConNS3/NS4A. After 48 h, animals were sacrificed and liver lymphocytes were individually analyzed. (B) Average percentage of HCV NS3-specific CD8+ CD107A+ T cells detected within the untransfected vs. the transfected liver. (C) Representative CD107A and IFNγ staining of HCV NS3-specific CD8+ T cells before and after liver transfection. (D) Average percentage of HCV NS3-specific CD8+ CD107A+ IFNγ+ T cells detected within the untransfected vs. the transfected liver. (E) Average percentage of HCV NS3-specific CD8+ CD107A+ IFNγ− T cells detected within the untransfected vs. the transfected liver. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005; NS, not significant).
Intrahepatic lymphocytes were then analyzed for expression of both IFNγ and CD107A. A large shift toward a gain of function was observed in the population of HCV NS3-specific CD8+ T cells isolated from immunized animals following liver transfection (Fig. 4C). An approximately 2-fold increase in the percentage of HCV NS3-specific CD8+ CD107A+ IFNγ+ T cells was observed which coincided with an approximately 2-fold decrease of HCV NS3-specific CD8+ CD107A+ IFNγ− T cells (Fig. 4D and E). The percentages of HCV NS3-specific CD8+ CD107A+ IFNγ+ and CD8+ CD107A+ IFNγ− were 7.8% ± 1.5% and 16.7% ± 2.1%, respectively.
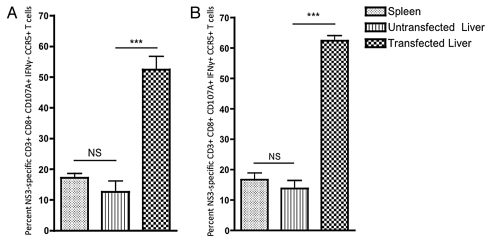
Given the observed increase in activation of HCV-specific CD8+ T cells as evidenced by the enhanced expression of IFNγ+, we wished to further confirm this finding by again comparing the percentage of CXCR3+ and CCR5+ HCV NS3-specific CD8+ T cells in the liver vs. the spleen. No significant change in the percentage of intrahepatic HCV NS3-specific CD8+ T cells expressing CXCR3 was observed following liver transfection (Fig. S3A and B). However, a large and highly significant increase in the percentage of HCV NS3-specific CCR5+ CD8+ CD107A+ IFNγ− and CCR5+ CD8+ CD107A+ IFNγ+ T cells was observed (Fig. 5A and B).
Figure 5.
CCR5+ expression on intrahepatic HCV NS3-specific CD8+ T cells following HCV NS3 transfection within the liver. Percentage of (A) CC R5+ CD8+ CD107A+ IFNγ− (B) CCR5+ CD8+ CD107A+ IFNγ+. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005; NS, not significant). (n = 5).
HCV NS3-specific cytotoxic CD8+ T cells rapidly clear HCV NS3 expressing hepatocytes.
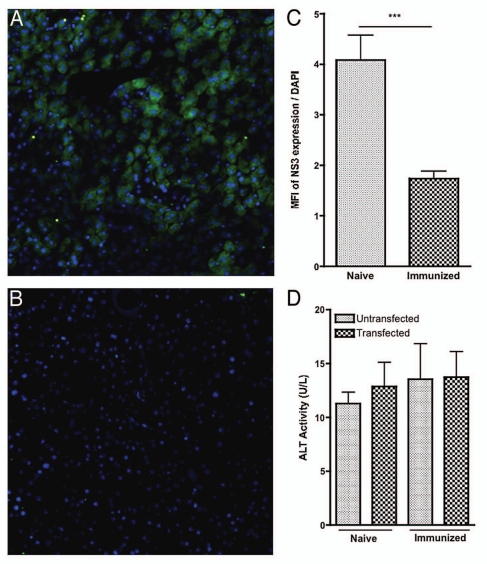
Cytotoxicity of HCV NS3-specific CD8+ T cells within the liver was assessed by the ability of immunized animals to clear HCV NS3 expressing hepatocytes compared with naive controls. Representative confocal images of this staining for each group are shown (Fig. 6A and B). Clearance of HCV NS3 transfected hepatocytes for each group was quantified by the mean florescent intensity (MFI) of HCV NS3 expression normalized to the number of cells present within each field as measured by the MFI of nuclear DAPI staining (Fig. 6C). Compared with the naïve control, dramatic reductions in the number of NS3 expressing hepatocytes were seen in the immunized group (Fig. 6C). Additionally, this response was found to be highly specific and did not result in generalized liver damage as measured by serum alanine aminotransferase (ALT) activity (Fig. 6D).
Figure 6.
Intrahepatic HCV NS3-specific cytotoxic T-cell responses within the liver following HCV NS3 transfection. Intrahepatic HCV NS3-specific cytotoxic T-cell responses within the liver following HCV NS3 transfection. (A and B) Confocal microscopy. Expression of HCV NS3 was detected with an anti-HA antibody (green). Nuclei were stained with DAPI (blue). (A) Representative liver confocal images of hepatocyte expression of HCV NS3 in the (A) naïve group (B) immunized group. (C) Average MFI ratio of expression of HCV NS3 as normalized to DAPI. For each group, four images were captured for each animal (n = 4, one animal from each group was dropped due to insufficient sample quality). MFI values for HCV NS3 were calculated and normalized to the MFI value for DAP I for each image. (D) Average ALT activity before and after liver transfection of each group. Significance was determined by Student's t-test (*p < 0.05, **p < 0.005 and ***p < 0.0005).
Discussion
While other studies have shown that liver transfection with HCV antigens can be used to assess the cytoxicity of the DNA vaccine induced CD8+ T cell responses in mice19 and HCV DNA immunization studies in chimpanzees have demonstrated the presence of HCV-specific IFNγ+ T cells within the liver,21 our study is the first to suggest that the liver actively recruits certain subsets of vaccine-specific T cells following peripheral immunization. Additionally, we have shown that the liver recruits vaccine-specific T cells despite the absence of cognate antigen and that this recruitment leads to the formation of a fully functional pool of vaccine-specific T cells able to both clear HCV protein expressing hepatocytes and to upregulate expression of CCR5 and IFNγ within the liver. While the liver is known to actively recruit and sequester certain subsets of antigen-specific CD8+ T cells, observations of the subsequent dysfunction and deletion of intrahepatic CD8+ T cells has led to the belief that this recruitment is an important mechanism by which the liver induces T cell tolerance to food and gut antigens.9,10 Consistent with this observation, we found the resting liver to be highly enriched for HCV NS3-specific CD8+ T cells. In total over 30% of the CD8+ T cells isolated from the liver were specific for HCV NS3. However, surprisingly, these cells were also found to be fully functional. Using our model, we were able to show that these cells retained their ability to become fully activated following mock HCV infection as evidenced by the large increase in CD107A and IFNγ expression, as well as, the nearly complete clearance of HCV NS3 positive hepatocytes.
This activation was further confirmed by the large increase in CCR5 expression. Numerous studies have shown a clear correlation between the expression of CCR5 on the surface of HCV-specific T cells and clearance of acute infection.22,23 While not initially responsible for their recruitment, CCR5 expression on intrahepatic HCV NS3-specific CD8+ T cells was highly upregulated following liver transfection. Since expression of CCR5 is believed to be important for retention and chemotaxis of T cells within the liver, expression of CCR5 on HCV NS3-specific T cells may have been an important factor in the retention and chemotaxis of these cells to sites of inflammation within the liver, providing an additional explanation for the rapid clearance of transfected hepatocytes.
Our results suggest that active recruitment of antigen-specific T cells and preservation of their functionality within the liver may serve as an important part of immune surveillance and may be one mechanism by which the liver guards itself against infection. While preservation of T-cell responses within the liver may appear contrary to what has classically been reported in the literature, our findings lend support to the emerging idea that liver-induced T-cell tolerance may not be universal. In fact, recent studies have suggested that induction of T-cell tolerance may be dependent on whether or not antigen is first encountered within the liver. For example, it has been reported that T cell priming in the liver results in dysfunctional T cells unable to induce hepatitis, however, studies have shown that T cell priming within the lymph nodes preserves T-cell function within the liver allowing these cells to retain the ability to cause hepatitis.9
Previously published studies, as well as the work reported here, lend increasing support for the idea that priming in the liver is responsible for the liver-induced T-cell tolerance and that active recruitment of antigen-specific T cells may be a mechanism by which the liver protects itself against infection given the fact it is unable to induce functional T-cell responses on its own. However, given that the liver maintains an environment with abundant amounts of IL-10,8 low expression of co-stimulatory molecules and is known to selectively delete activated T cells,9,10 it is unlikely that this environment would provide the necessary stimulation and survival factors in order to be able to activate antigen-specific T cells primed in the lymph nodes and therefore does not explain the large amounts of activation observed in our study following expression of HCV NS3 within the liver.
What may explain our findings is a newly emerging model that suggests that the liver maintains a delicate balance between both tolerizing and activating T cells, and that the switch to T-cell activation within the liver is dependent on the presence of inflammatory signals.7 During infection, inflammation within the liver is thought to bias the liver away from T-cell suppression and toward induction of T-cell responses. During inflammation, upregulation of type 1 IFNs is thought to promote the maturation of liver resident DCs8 and induce the synthesis of IL-15 which have been shown to result in enhanced CD8+ T-cell survival.24 Therefore, while priming T cells within the tolerizing environment of the liver may play an important role in inducing tolerance to food and gut antigens, the liver may also compensate for its inability to induce functional T cell responses by actively recruiting antigen-specific T cells from the periphery and allowing for their activation during times of infection within the liver.
While encouraging, these findings are limited to one inbred strain of mouse and were conducted at the peak of the immune response. Therefore, further investigation is needed to assess the ability of this response to persist within the liver and to form lasting, functional memory. Additionally, further experiments in humanized mice or rhesus macaques are also needed to ascertain whether these effects can be seen in animals with an immune system that more closely resembles that of humans.
However, in summary, our data suggests that peripheral immunization can subvert liver-induced T-cell tolerance resulting in the induction of HCV-specific T cell-based immunity within the liver and that the unique ability of the liver to both recruit and sequester antigen-specific T cells could be exploited by future HCV vaccines to induce the formation of a highly functional pool of intrahepatic HCV-specific T cells able to rapidly respond to infection.
Materials and Methods
Generation of pConNS3/4A.
The generation and expression in vivo of pConNS3/NS4A have been previously described in reference 25. The consensus sequence for NS3 was generated from 15 different genotype 1a sequences and 26 different genotype 1b sequences and the consensus sequence for NS4A was generated from 15 different genotype 1a sequences and 19 different genotype 1b sequences. All sequences were obtained from GenBank and aligned using Clustal X (version 1.8) software to generate the final NS3/NS4A consensus sequence. To the consensus NS3/NS4A sequence, an IgE leader sequence, endoproteolytic cleavage site and a C-terminal HA tag were also added. The consensus sequence was codon and RNA optiminized using GeneOptimizer™ (GENEART, Germany). The final consensus NS3/NS4A fusion gene was synthesized and sequence verified by GENEART (Germany).
Immunization/Electroporation.
Six to eight week old female C57BL/6 mice were purchased from Jackson Laboratories. Animals were maintained in accordance with the National Institutes of Health and the University of Pennsylvania Institutional Care and Use Committee (IACUC) guidelines.
Five animals were used per group.
Each animal received a total of two intramuscular immunizations of 12.5 µg of pConNS3/NS4A followed by in vivo electroporation with the CELLECTRA® adaptive constant current electroporation device (Inovio Pharmaceuticals, Blue Bell, PA). Two 0.1 Amp constant current square-wave pulses were delivered through a triangular 3-electrode array consisting of 26-gauge solid stainless steel electrodes. Each pulse was 52 ms in length with a 1 sec delay between pulses. Each immunization was given two weeks apart and animals were sacrificed one week following the last immunization.
Splenocyte isolation.
Spleens were crushed individually using a Stomacher machine (Seward, Bohemia NY), strained with a 40 µM cell strainer and treated 5 min with ACK lysis buffer (Biosource) to lyse the RBCs. The splenocytes were resuspended in complete media (RPMI 1640 with 2 mM/L L-glutamine supplemented with 10% heat inactivated FBS, 1x antibiotic/antimycotic and 55 µM/L β-mercaptoethanol).
Liver lymphocyte isolation.
Each liver was perfused with PBS and individually pulverized using a Stomacher machine (Seward). The resulting mixture was pelleted and resuspended in 10 ml ACK lysis buffer (Bioscience) for 5 min in order to clear the RBCs. The reaction was stopped with 10 ml R10 and pelleted. The pellet was resuspended in 10 ml of room temperature 40% isotonic percoll and centrifuged 1,200 rpm for 10 min in order to pellet the lymphocytes. The pelleted lymphocytes were resuspended in complete R10 media.
IFNγ ELISpot.
The mouse IFNγ ELISpot assays were conducted as previously described in reference 26. Splenocytes were stimulated with five pools of 15 mer peptides over lapping by 8 amino acids and spanning the length of pConNS3/NS4A. A total of 101 HCV NS3/NS4A peptides were synthesized by Invitrogen, resuspended in DMSO and pooled into five different pools at a concentration of 2 µg/ml/peptide. The splenocytes from each animal were plated at a concentration of 2,000,000 cells per well. Results were adjusted and graphed as the average number of spot forming units (SFU) per 1 × 106 splenocytes.
Flow cytometry.
Flow cytometry reagents. The following directly conjugated anti-mouse antibodies were used: CD3− allophycocyanin cyanine dye 7 (APC-Cy7) [clone 145-2C11], CD4− peridinin chlorophyll protein 5.5 (PerCP5.5) [clone RM4–5], CD8- allophycocyanin (APC) [clone 53-6.7], CD107A− fluorescein isothiocyanate (FITC) [clone 1D4B], IFNγ-Alexa Fluor 700 [clone XMG1.2] and CCR5− phycoerythryin cyanine (PE) [clone C34–3448] (all from BD Biosciences) and CXCR3− phycoerythryin cyanine dye 7 (PE-Cy7) [clone CXCR3-173], (Biolegend). Aqua Live/Dead fixable dead cell Stain Kit (Molecular Probes) was used according to manufacturer's protocol to identify live cells.
Intracellular cytokine staining. Splenocytes were resuspended in complete media at a concentration of 1.5 × 106 cells/100 µl and plated in a round bottom 96-well plate. Cells were stimulated with 100 µl of either: (1) 2 µg/ml pConNS3/NS4A pooled peptides (described in Methods section under “IFNγ ELISpot”), (2) 1 µg/ml Staphylococcus enterotoxin B (positive control; Sigma-Aldrich) or (3) 0.1% dimethyl sulfoxide (negative control) all diluted in complete media supplemented with GolgiStop, GolgiPlug (BD Bioscience) and anti-CD107A. After 5 h of stimulation at 37°C, splenocytes were washed three times with PBS and stained for viability, then stained extracellularly for the surface markers; anti- CD4, CD8, CCR5 and CXCR3 for 30 min at 4°C. Cells were then permeabilized and washed using BD Cytofix/Cytoperm Solution Kit (BD Bioscience) and then stained intracellularly with anti-IFNγ and CD3 for 45 min at 4°C. Specific function was reported as the percent function of the peptide stimulated group minus the percent function of the 0.1% dimethyl sulfoxide stimulated group (negative control) for each animal.
Granzyme B killing assay.
Splenocytes were resuspended to 1 × 106 cells/100 µl in complete RPMI and plated in 96-well plates. Splenocytes were stimulated with 100 µl of either a 1:200 dilution of HCV NS3/NS4A peptides (Genscript) or 1:200 dilution of DMSO (negative control) and incubated for 5.5 h at 37°C. Following incubation, cells were washed three times in PBS and stained with fluorophore-conjugated antibodies to cell surface antigens, CD3, CD4, CD8, CD11a and CD44. Following staining, the cells were again washed, and immediately analyzed on a flow cytometer.
The granzyme B cell-killing assay was performed using GranToxiLux PLUS (OncoImmunin) per manufacturer's instructions. Briefly, splenocytes from vaccinated animals were used as effectors and combined with autologous targets that had either been pulsed with HCV NS3/NS4A peptides for 1 h at 37°C or HIV gag peptides (negative control). Effector-to-target ratio was normalized based on expression of CD44 and CD11a on effector cells and NS3-specific killing was reported as percent killing in the HCV pulsed targets minus percent killing of HIV pulsed targets.
Confocal microscopy.
Liver biopsies were fixed with 2% paraformaldehyde and incubated 4°C overnight in 30% sucrose. Biopsies were quick frozen in Tissue-Tek °CT (Bayer Corporation). Staining was performed on tissue sections (6 µm) mounted on Superfrost Plus glass slides (Fisher Scientific), and kept at 80°C until use. For staining, slides were brought to room temperature and washed (three times with PBS), and blocked in PBS containing 10% normal serum and 0.1% Triton. Sections were incubated for 1 h at room temperature or overnight at 4°C in primary reagents. The secondary reagents were applied 30 min at room temperature. The sections were counterstained with DAPI (Invitrogen). Coverslips were mounted with Prolong Gold mounting media (Invitrogen). The following antibody was used, mouse anti-HA.11 (clone 16B12) from Covance. All images were obtained using a Zeiss Axiovert 100 inverted confocal microscope and analysis and quantification of florescence intensities was conducted using Image J software (NIH).
Liver transfection.
Animals each received a tail vein injection of 100 µg of pConNS3/NS4A diluted in 300 µl PBS, a method previously reported in reference 19. Animals were sacrificed 48 h following injection.
Measurement of ALT activity.
Whole blood was collected from mice via cardiac puncture, then centrifuged at 5,000 rpm for 5 min at 4°C to obtain serum. Serum alanine aminotransferase (ALT) activity was measured using an absorbance-based assay (Stanbio Laboratory, catalog # 0930) on a BioTek Synergy 2 microplate reader in accordance with the manufacturer's instructions. Results are reported as units per liter (U/L) and represent the amount of enzyme that oxidizes one µmol/L of NADH per minute. Activity of 0–38 U/L is considered normal.
Abbreviations
- ALT
alanine aminotransferase
- IP-10
interferon gamma-induced protein 10 kDa
- MIG
monokine induced by IFNγ
- MIP-1α
macrophage inflammatory protein
- NS3
non-structural protein 3
- NS4A
non-structural protein 4A
- SFU
spot forming units
Disclosures
This manuscript was supported in part by funding from the NIH to DBW and from Inovio pharmaceuticals, as well as, a grant from the state of Pennsylvania. The authors note possible commercial conflicts associated with this work, which may include; Wyeth, Inovio, BMS, Virxsys, Ichor, Merck, VGXi and Aldeveron.
Grant Support
Cure Grant—PA Department of Health—08-07-07.
Supplementary Material
References
- 1.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 4.Lechner F, Cuero AL, Kantzanou M, Klenerman P. Studies of human antiviral CD8+ lymphocytes using class I peptide tetramers. Rev Med Virol. 2001;11:11–22. doi: 10.1002/rmv.295. [DOI] [PubMed] [Google Scholar]
- 5.Thomas HC, Singer CR, Tilney NL, Folch H, MacSween RN. The immune response in cirrhotic rats. Antigen distribution, humoral immunity, cell-mediated immunity and splenic suppressor cell activity. Clin Exp Immunol. 1976;26:574–582. [PMC free article] [PubMed] [Google Scholar]
- 6.Gorczynski RM, Chan Z, Chung S, Cohen Z, Levy G, Sullivan B, et al. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation. 1994;58:816–820. [PubMed] [Google Scholar]
- 7.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 9.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- 11.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 12.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-8.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 14.Qian S, Lu L, Li Y, Fu F, Li W, Starzl TE, et al. Apoptosis of graft-infiltrating cytotoxic T cells: a mechanism underlying “split tolerance” in mouse liver transplantation. Transplant Proc. 1997;29:1168–1169. doi: 10.1016/S0041-1345(96)00521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalor PF, Adams DH. Adhesion of lymphocytes to hepatic endothelium. Mol Pathol. 1999;52:214–219. doi: 10.1136/mp.52.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, et al. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol. 1998;186:82–89. doi: 10.1002/(SICI)1096-9896(199809)186:1<82::AID-PATH151>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. doi: 10.1016/S1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Pimenov AA, Nayak JV, Plowey J, Falo LD, Jr, Huang L. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum Gene Ther. 2000;11:547–554. doi: 10.1089/10430340050015734. [DOI] [PubMed] [Google Scholar]
- 19.Ahlen G, Nystrom J, Pult I, Frelin L, Hultgren C, Sallberg M. In vivo clearance of hepatitis C virus nonstructural 3/4A-expressing hepatocytes by DNA vaccine-primed cytotoxic T lymphocytes. J Infect Dis. 2005;192:2112–2116. doi: 10.1086/498218. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 21.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 22.Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 24.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 25.Lang KA, Yan J, Draghia-Akli R, Khan A, Weiner DB. Strong HCV NS3- and NS4A-specific cellular immune responses induced in mice and Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Vaccine. 2008;26:6225–6231. doi: 10.1016/j.vaccine.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M, et al. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol Ther. 2007;15:411–421. doi: 10.1038/sj.mt.6300036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.