Abstract
L-type bovine spongiform encephalopathy (BSE) is an atypical form of BSE. To characterize the Japanese L-type BSE prion, we conducted a comparative study of the Japanese and foreign L-type BSE isolates. The L-type BSE isolates of Japan, Germany, France and Canada were intracerebrally inoculated into bovinized prion protein-overexpressing transgenic mice (TgBoPrP). All the examined L-type BSE isolates were transmitted to TgBoPrP mice, and no clear differences were observed in their biological and biochemical properties. Here, we present evidence that the Japanese and Canadian L-type BSE prions are identical to those from the European cases.
Key words: prion, atypical BSE, L-type BSE
Bovine spongiform encephalopathy (BSE) is one of the transmissible spongiform encephalopathies (TSEs), or prion diseases, in cattle. TSE is characterized by spongiform changes in the central nervous system (CNS) and the accumulation of an abnormal prion protein (PrPSc) in the CNS.1 PrPSc has been regarded as the major component of TSE pathogens.2
BSE was detected in the UK in 1986,3 and subsequently spread to the other European countries, Japan and North America.4–6 BSE is thought to be caused by a single prion strain, based on the analyses of its biological and biochemical characteristics.7 From 2003, however, several atypical neuropathological and molecular phenotypes of BSE (atypical BSE) have been detected in Japan, several European countries and North America.6,8–17 Currently, based on the molecular size of the proteinase-digested non-glycosylated form of PrPSc, atypical BSE is classified into two groups (L-type and H-type).14
L-type BSE cases have been identified in the European countries, including Italy, France, Germany, Netherland, Poland and in Canada and Japan.8–15 Two L-type BSE cases have been identified in Japan. One case was detected in a healthy 23-mo-old Holstein steer (BSE/JP8),8 and the other was detected in a 14-y-old black Japanese beef cattle (BSE/JP24).9 The latter case was successfully transmitted to bovinized transgenic mice and cattle, and the biological and biochemical properties differed from that of classical BSE (C-BSE).18,19 However, it is unclear whether Japanese L-type BSE prion is identical to that of L-type BSE isolates from other countries. To characterize the Japanese L-type BSE isolate, we performed a comparative study of the Japanese and foreign L-type BSE isolates.
A transmission study using experimental animals is a useful approach for prion characterization. Therefore, we performed a transmission study of the L-type BSE isolates in bovinized prion protein (PrP)-overexpressing transgenic mice (TgBoPrP).20 Brain samples of L-type BSE-affected cattle from Japan (BSE/JP24),9 France,10 Germany11 and Canada12 were used in this study. The brain homogenates were intracerebrally inoculated into TgBoPrP using previously described methods in reference 18. All animal experiments were reviewed by the Committee of the Ethics on Animal Experiment of the National Institute of Animal Health.
All the examined L-type BSE isolates were transmitted to TgBoPrP, and the affected mice developed progressive neurological diseases. Japanese L-type BSE isolate-affected TgBoPrP exhibited a unique clinical sign, the circling behavior. The same phenotype was observed when TgBoPrP were inoculated with German, French and Canadian L-type BSE isolates. On the other hand, in the first passage the incubation period for the Japanese L-type BSE isolate was significantly different from that of the other L-type BSE isolates (Table 1). We then performed serial passages of these isolates for further characterization. The incubation periods in the second passage were shorter than those in the first passage. In the third passage, the incubation periods for all the L-type BSE isolates converged at about 145 d. These results suggest that the L-type BSE isolates in the primary passage were not fully adapted to the TgBoPrP mice. Furthermore, the different incubation periods in the first passage may be caused by the lower titer of the Japanese L-type BSE prion.
Table 1.
Transmission of L-type BSE isolates in TgBoPrP mice
| Incubation period (days) | ||||
| JPN | CAN | GER | FRA | |
| First passage | 197.7 (3.4)† | 172.8 (4.0)* | 173.3 (3.3)* | 175.7 (5.6)* |
| (10/10·) | (12/12) | (12/12) | (10/10) | |
| Second passage | 152.0 (1.7) | 145.7 (1.8) | 143.1 (5.7) | 143.1 (3.9) |
| (24/24) | (23/23) | (18/18) | (18/18) | |
| Third passage | 145.1 (3.6) | 143.7 (4.6) | 145.3 (8.6) | 141.6 (4.7) |
| (21/21) | (25/25) | (12/12) | (20/20) | |
Mean (standard deviation)
Number of affected mice/number of inoculated mice
p < 0.05 for Japanese L-type BSE isolate vs. other L-type BSE isolates in the first passage (Student's t-test)
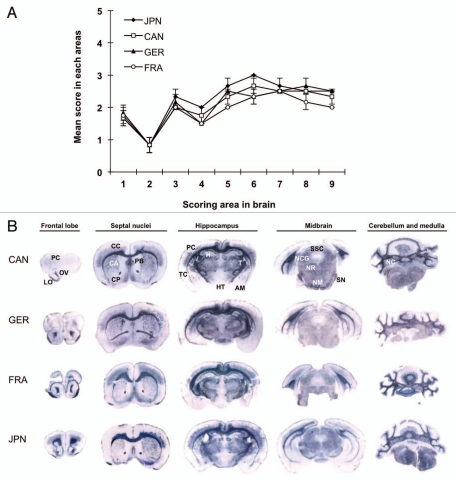
Neuropathological examination of the L-type BSE isolate-affected TgBoPrP were performed using previously described methods.18 Lesion profile analysis revealed that the degree of brain vacuolation due to the Japanese L-type BSE isolate was similar to that caused by the other L-type BSE isolates (Fig. 1A). All the L-type BSE isolates caused severe spongiform changes in the hippocampus, septal nuclei of the paraterminal body and cerebral cortex. We next examined the PrPSc deposition pattern in the brain using paraffin-embedded tissue (PET) blot, as described previously in reference 18. The distributions of PrPSc deposits in Japanese L-type BSE isolate-inoculated mice were similar to that of mice inoculated with the other L-type BSE isolates; fine punctate and fine granular PrPSc were predominantly and uniformly distributed in the pons, cerebellar medulla, midbrain, thalamus and corpus callosum (Fig. 1B). Furthermore, similar PrPSc deposits and distribution patterns were observed in the brain in the first and subsequent passages of all the L-type BSE isolates (data not shown).
Figure 1.
Neuropathological analysis of L-type BSE isolate-affected TgBoPrP. (A) Lesion profile in the first passage. The vacuolation in the following brain regions was scored on a scale of 0–5 (mean values): 1, dorsal medulla; 2, cerebellar cortex; 3, superior cortex; 4, hypothalamus; 5, thalamus; 6, hippocampus; 7, septal nuclei of the paraterminal body; 8, cerebral cortex at the levels of the hypothalamus and thalamus; and 9, cerebral cortex at the level of the septal nuclei of the paraterminal body. The data are presented as mean ± standard deviation (n = 5). ◆, Japanese L-type BSE (JPN); □, Canadian L-type BSE (CAN); ▲, German L-type BSE (GER); ○, French L-type BSE (FRA). (B) The neuroanatomical distribution of PrPSc in the brain of TgBoPrP mice infected with Canadian (CAN), German (GER), French (FRA) and Japanese (JPN) L-type BSE isolate by PET-blot analysis. The PET-blot analysis reveals preferential and intense PrPSc immunolabeling along with periventricular areas, corpus callosum and cerebellar gray matter. Widespread PrPSc immunolabeling is also detected in the thalamic and brainstem nuclei, while PrPSc immunostaining in the cerebral and cerebellar cortices and basal ganglia is less conspicuous. Dewaxed membranes were treated with PK (80 µg/mL), followed by denaturation with 3 M guanidine thiocyanate. The monoclonal antibody (mAb) SAF84 was used. Blots corresponding to the brain areas at the level of frontal lob, septal nuclei, hippocampus, midbrain and medulla and cerebellum. FC, frontal cortex; OV, olfactory ventricle; LO, lateral orbital cortex; CC, cingulated cortex; CP, caudate putamen; PB, paraterminal body; CP, caudate putamen; PC, parietal cortex; TC, temporal cortex; H, hippocampus; T, thalamus; HT, hypothalamus; AM, amygdala; SSC, stratum moleculare of the cerebellum; NCG, nucleus corporis geniculati; NR, nucleus rubber; SN, substantia nigra; NM, nucleus mammillaris; NC, deep nuclei of the cerebellum.
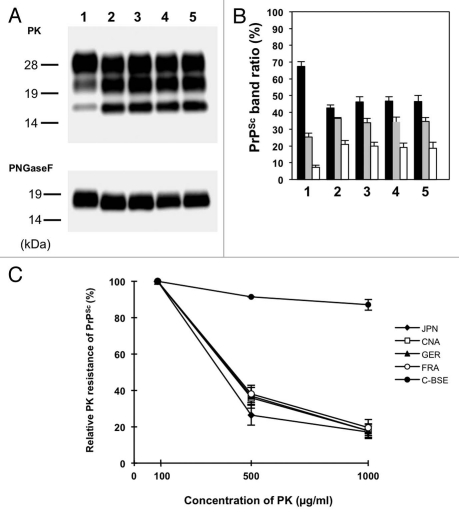
We further examined the biochemical properties of PrPSc, such as the glycoform ratio and molecular mass of proteinase K (PK)-digested PrPSc (PrPcore). PrPSc were extracted from the brain of L-type BSE isolate-affected TgBoPrP using previously described methods in reference 18. Western blotting analysis revealed that the glycoform patterns and molecular mass of the PrPcore of the Japanese L-type BSE isolate resembled that of the other L-type BSE isolates. In contrast, clear differences were observed between C-BSE and L-type BSE isolates (Fig. 2A and B). Next, we examined the relative PK resistance of PrPSc from L-type BSE isolate-affected TgBoPrP, as described previously in reference 18. The PrP concentration of the sample was adjusted using the signal intensity of western blot. The PK resistance of PrPSc from the Japanese L-type BSE was similar to that of the foreign L-type BSE isolates. The PrPSc of C-BSE-affected TgBoPrP was resistant to digestion with 1,000 µg/ml of PK. In contrast to C-BSE, the PrPSc signal from the L-type BSE isolates decreased when digested with 500 µg/ml of PK (Fig. 2C).
Figure 2.
Western blot analysis of proteinase K (PK)-digested prion protein (PrPcore) from the brain of L-type BSE isolate-affected TgBoPrP. (A) Lane 1, Classical-BSE; Lane 2, Japanese L-type BSE; Lane 3, Canadian L-type BSE; Lane 4, French L-type BSE; Lane 5, German L-type BSE. All the samples were digested with 50 µg/ml PK at 37°C for 1 h (upper part), and digested aliquots were treated with N-glycosidase F (PNGaseF), according to the manufacturer's instructions (bottom part). PrPcore was detected with mAb 6H4. Molecular markers are shown on the left (kDa). (B) The relative amounts of the diglycosylated (solid black bar), monoglycosylated (gray bar), and unglycosylated (clear bar) forms in the PrPcore from the brain of L-type BSE isolate-affected TgBoPrP. The lane numbers are as listed in (A). The results are presented as mean ± standard deviation from 5 experiments. (C) Relative PK resistance of PrPSc from L-type BSE isolate-affected TgBoPrP. The PrPSc concentration of the sample was adjusted using the western blot signal intensity. The samples were treated with various concentrations of PK (100–1,000 µg/mL). The results are presented as mean ± standard deviation from 3 experiments. PrPSc was detected with mAb 6H4. ◆, Japanese L-type BSE (JPN); □, Canadian L-type BSE (CAN); ▲, German L-type BSE (GER); ○, French L-type BSE (FRA); ●, Classical-BSE (C-BSE).
The analyses of L-type BSE cases have been performed using different bovinized PrP-overexpressing transgenic mice, such as TgBoPrP,18 Tgbov XV11,21 and Tg540.22 Thus, it has been impossible to compare the properties of L-type BSE isolates in detail. In this study, therefore, we performed a transmission study of the L-type BSE isolates using identical bovinized PrP-overexpressing transgenic mice to further characterize the Japanese L-type BSE prion. All the L-type BSE isolates transmitted to TgBoPrP, and their incubation periods converged at approximately 145 d following serial passages (Table 1). Similar degrees of vacuolation and PrPSc deposition patterns in the brain were observed among the L-type BSE isolates (Fig. 1A and B). Besides the biological characteristics, no differences were observed in the biochemical characteristics of PrPSc from the L-type BSE isolates (Fig. 2A–C). These findings suggest that the examined L-type BSE cases were caused by prions with identical characteristics.
Italian L-type BSE cases are called bovine amyloid spongiform encephalopathy (BASE). We could not compare the characteristics of the Japanese L-type BSE with those of the Italian isolates. In a transmission study using transgenic mice, the French L-type BSE isolate and BASE exhibit similar biological characteristics.22 Our data indicated that the properties of the Japanese L-type BSE prion are identical to those of the French L-type BSE isolate. It has also been reported that the characteristics of Japanese L-type BSE isolate closely resemble those of BASE in an experimental transmission study in cattle.19
The origin of L-type BSE prion is unknown. The present study showed that the Japanese and Canadian L-type BSE prions are identical to those from the European cases. The fact that identical L-type BSE prions exhibit a worldwide distribution is important insight for devising atypical BSE control measures.
Acknowledgments
We would like to thank Dr. Stefanie Czub for providing the Canadian L-type BSE isolate, Dr. Martin H. Groschup for providing the German L-type BSE isolate, and Dr. Thierry Baron for providing the French L-type BSE isolate. We would also like to thank Ms. Naoko Tabeta, Atsuko Ojima and Naomi Furuya for their technical assistance, and Dr. Morikazu Shinagawa for his encouragement. We are also grateful to Mr. Manabu Aida, Ms. Sei Chouei, Ms. Chizuru Kuramochi, Ms. Che Jing Zh and animal laboratory staff at the National Institute of Animal Health for maintaining the mouse colony. This study was supported by a Grant-in-Aid from the BSE and Other Prion Disease Control Project of the Ministry of Agriculture, Forestry and Fisheries, Japan, and in part by a grant for BSE research from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- BSE
bovine spongiform encephalopathy
- BSE/JP8
the 8th BSE case in Japan
- BSE/JP24
the 24th BSE case in Japan
- BASE
bovine amyloid spongiform encephalopathy
- CNS
central nervous system
- C-BSE
classical BSE
- H-type BSE
high-type BSE
- L-type BSE
low-type BSE
- mAb
monoclonal antibody
- PET blot
paraffin-embedded tissue blot
- PK
proteinase K
- PNGaseF
N-glycosidase F
- PrP
prion protein
- PrPcore
proteinase K-digested PrPSc
- PrPSc
abnormal prion protein
- TSEs
transmissible spongiform encephalopathies
References
- 1.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 2.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 3.Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, et al. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987;121:419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- 4.Ducrot C, Arnold M, de Koeijer A, Heim D, Calavas D. Review on the epidemiology and dynamics of BSE epidemics. Vet Res. 2008;39:15. doi: 10.1051/vetres:2007053. [DOI] [PubMed] [Google Scholar]
- 5.Giles J. Mad cow disease comes to Japan. Nature. 2001;413:240. doi: 10.1038/35095180. [DOI] [PubMed] [Google Scholar]
- 6.Richt JA, Kunkle RA, Alt D, Nicholson EM, Hamir AN, Czub S, et al. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J Vet Diagn Invest. 2007;19:142–154. doi: 10.1177/104063870701900202. [DOI] [PubMed] [Google Scholar]
- 7.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 8.Yamakawa Y, Hagiwara K, Nohtomi K, Nakamura Y, Nishijima M, Higuchi Y, et al. Expert Committee for BSE Diagnosis, Ministry of Health, Labour and Welfare of Japan. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn J Infect Dis. 2003;56:221–222. [PubMed] [Google Scholar]
- 9.Hagiwara K, Yamakawa Y, Sato Y, Nakamura Y, Tobiume M, Shinagawa M, et al. Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine spongiform encephalopathy case in Japan. Jpn J Infect Dis. 2007;60:305–308. [PubMed] [Google Scholar]
- 10.Biacabe AG, Laplanche JL, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 2004;5:110–115. doi: 10.1038/sj.embor.7400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, Hoffmann C, et al. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet Microbiol. 2006;117:103–116. doi: 10.1016/j.vetmic.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Dudas S, Yang J, Graham C, Czub M, McAllister TA, Coulthart MB, et al. Molecular, biochemical and genetic characteristics of BSE in Canada. PLoS One. 2010;5:10638. doi: 10.1371/journal.pone.0010638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, et al. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA. 2004;101:3065–3070. doi: 10.1073/pnas.0305777101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs JG, Langeveld JP, Biacabe AG, Acutis PL, Polak MP, Gavier-Widen D, et al. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J Clin Microbiol. 2007;45:1821–1829. doi: 10.1128/JCM.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polak MP, Zmudzinski JF, Jacobs JG, Langeveld JP. Atypical status of bovine spongiform encephalopathy in Poland: a molecular typing study. Arch Virol. 2008;153:69–79. doi: 10.1007/s00705-007-1062-6. [DOI] [PubMed] [Google Scholar]
- 16.Tester S, Juillerat V, Doherr MG, Haase B, Polak M, Ehrensperger F, et al. Biochemical typing of pathological prion protein in aging cattle with BSE. Virol J. 2009;6:64. doi: 10.1186/1743-422X-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry LA, Jenkins R, Thorne L, Everest SJ, Chaplin MJ, Davis LA, et al. First case of H-type bovine spongiform encephalopathy identified in Great Britain. Vet Rec. 2007;160:873–874. doi: 10.1136/vr.160.25.873. [DOI] [PubMed] [Google Scholar]
- 18.Masujin K, Shu Y, Yamakawa Y, Hagiwara K, Sata T, Matsuura Y, et al. Biological and biochemical characterization of L-type-like bovine spongiform encephalopathy (BSE) detected in Japanese black beef cattle. Prion. 2008;2:123–128. doi: 10.4161/pri.2.3.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda S, Iwamaru Y, Imamura M, Masujin K, Shimizu Y, Matsuura Y, et al. Intraspecies transmission of L-type-like Bovine Spongiform Encephalopathy detected in Japan. Microbiol Immunol. 2009;53:704–707. doi: 10.1111/j.1348-0421.2009.00169.x. [DOI] [PubMed] [Google Scholar]
- 20.Scott MR, Safar J, Telling G, Nguyen O, Groth D, Torchia M, et al. Identification of a prion protein epitope modulating transmission of bovine spongiform encephalopathy prions to transgenic mice. Proc Natl Acad Sci USA. 1997;94:14279–14284. doi: 10.1073/pnas.94.26.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, et al. Conversion of the BASE prion strain into the BSE strain: the origin of BSE? PLoS Pathog. 2007;3:31. doi: 10.1371/journal.ppat.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Béringue V, Andréoletti O, Le Dur A, Essalmani R, Vilotte JL, Lacroux C, et al. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci. 2007;27:6965–6971. doi: 10.1523/JNEUROSCI.0693-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]