Abstract
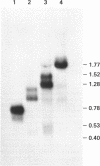
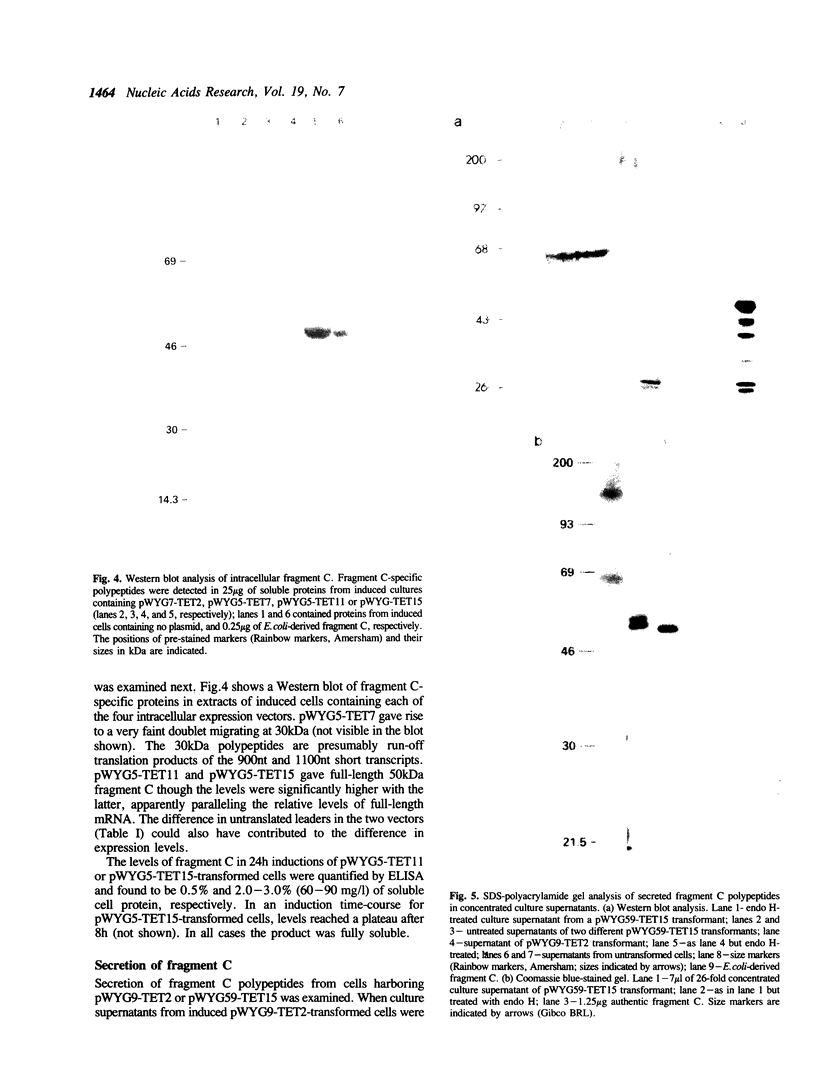
Fragment C is a non-toxic 50 kDa fragment of tetanus toxin which is a candidate subunit vaccine against tetanus. The AT-rich Clostridium tetani DNA encoding fragment C could not be expressed in Saccharomyces cerevisiae due to the presence of several fortuitous polyadenylation sites which gave rise to truncated mRNAs. The polyadenylation sites were eliminated by chemically synthesising the DNA with increased GC-content (from 29% to 47%). Synthesis of the entire gene (1400 base pairs) was necessary to generate full-length transcripts and for protein production in yeast. Using a GAL1 promoter vector, fragment C was expressed to 2-3% of soluble cell protein. Fragment C could also be secreted using the alpha-factor leader peptide as a secretion signal. The protein was present at 5-10 mg/l in the culture medium in two forms: a high molecular mass hyper-glycosylated protein (75-200 kDa) and a core-glycosylated protein (65 kDa). Intracellular fragment C was as effective in vaccinating mice against tetanus authentic fragment C. The glycosylated material was inactive, though it was rendered fully active by de-glycosylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus P. W., Mihaly G. W., Morgan D. J., Smallwood R. A. Oxygen dependence of omeprazole clearance and sulfone and sulfide metabolite formation in the isolated perfused rat liver. J Pharmacol Exp Ther. 1989 Sep;250(3):1043–1047. [PubMed] [Google Scholar]
- Baker S. M., Johnston S. A., Hopper J. E., Jaehning J. A. Transcription of multiple copies of the yeast GAL7 gene is limited by specific factors in addition to GAL4. Mol Gen Genet. 1987 Jun;208(1-2):127–134. doi: 10.1007/BF00330433. [DOI] [PubMed] [Google Scholar]
- Beesley K. M., Francis M. J., Clarke B. E., Beesley J. E., Dopping-Hepenstal P. J., Clare J. J., Brown F., Romanos M. A. Expression in yeast of amino-terminal peptide fusions to hepatitis B core antigen and their immunological properties. Biotechnology (N Y) 1990 Jul;8(7):644–649. doi: 10.1038/nbt0790-644. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. S., Platt T. RNA processing generates the mature 3' end of yeast CYC1 messenger RNA in vitro. Science. 1988 Dec 2;242(4883):1270–1274. doi: 10.1126/science.2848317. [DOI] [PubMed] [Google Scholar]
- Butler J. S., Sadhale P. P., Platt T. RNA processing in vitro produces mature 3' ends of a variety of Saccharomyces cerevisiae mRNAs. Mol Cell Biol. 1990 Jun;10(6):2599–2605. doi: 10.1128/mcb.10.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Brust P. F., Koutz P. J., Waters A. F., Harpold M. M., Gingeras T. R. Isolation of alcohol oxidase and two other methanol regulatable genes from the yeast Pichia pastoris. Mol Cell Biol. 1985 May;5(5):1111–1121. doi: 10.1128/mcb.5.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Nau H. H. Analysis of the immune response to papain digestion products of tetanus toxin. Acta Pathol Microbiol Immunol Scand C. 1984 Feb;92(1):59–63. doi: 10.1111/j.1699-0463.1984.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Cohen E. H. Sequences responsible for transcription termination on a gene segment in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1515–1520. doi: 10.1128/mcb.4.8.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Kelly J. D., Cohen E. H. Transcription terminates in yeast distal to a control sequence. Cell. 1983 Jun;33(2):607–614. doi: 10.1016/0092-8674(83)90441-5. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Oxer M. D., Romanos M. A., Fairweather N. F., Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989 Dec 25;17(24):10191–10202. doi: 10.1093/nar/17.24.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Volkert F., Broach J. Components of the site-specific recombination system encoded by the yeast plasmid 2-micron circle. Cold Spring Harb Symp Quant Biol. 1984;49:779–787. doi: 10.1101/sqb.1984.049.01.088. [DOI] [PubMed] [Google Scholar]
- Murray J. A., Cesareni G. Functional analysis of the yeast plasmid partition locus STB. EMBO J. 1986 Dec 1;5(12):3391–3399. doi: 10.1002/j.1460-2075.1986.tb04655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne B. I., Guarente L. Mutational analysis of a yeast transcriptional terminator. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4097–4101. doi: 10.1073/pnas.86.11.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Romanos M. A., Boyd A. A transcriptional barrier to expression of cloned toxin genes of the linear plasmid k1 of Kluyveromyces lactis: evidence that native k1 has novel promoters. Nucleic Acids Res. 1988 Aug 11;16(15):7333–7350. doi: 10.1093/nar/16.15.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Russo P., Sherman F. Transcription terminates near the poly(A) site in the CYC1 gene of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8348–8352. doi: 10.1073/pnas.86.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A. J., Cussell D., Hughes M. Production and characterization of monoclonal antibodies to tetanus toxin. Infect Immun. 1984 Feb;43(2):710–714. doi: 10.1128/iai.43.2.710-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Boyd A., Mileham A. J., Romanos M. A. The plasmid-encoded killer system of Kluyveromyces lactis: a review. Yeast. 1990 Jan-Feb;6(1):1–29. doi: 10.1002/yea.320060102. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. Mutationally altered 3' ends of yeast CYC1 mRNA affect transcript stability and translational efficiency. J Mol Biol. 1984 Jul 25;177(1):107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Lu H. S., Fieschko J. C., Goldstein L., Davis J., Duker K., Suggs S. V., Lai P. H., Bitter G. A. Protein secretion from Saccharomyces cerevisiae directed by the prepro-alpha-factor leader region. J Biol Chem. 1986 May 5;261(13):5858–5865. [PubMed] [Google Scholar]