Abstract
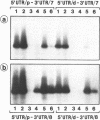
Recently we have found evidence that the human embryonic myosin alkali light chain (MLC1 emb) gene has two functional promoters and that its mRNAs exhibit heterogeneity in their 3'untranslated regions (UTR). To study this more in detail we have isolated and characterized the human MLC1emb gene. We focussed in particular on 2 kilobases of 5'flanking region and the alternative 3'UTRs. RNA primer extension and S1 mapping analyses revealed that the MLC1emb gene can indeed be driven either by a proximal or a distal promoter, both in fetal and adult cardiac tissue. These MLC1emb RNAs can contain either the proximal or distal 3'UTR. In contrast to this, in fetal as well as adult masseter muscle MLC1emb mRNA is predominantly transcribed from the proximal promoter and contains mainly the distal 3'UTR. These results explain the known heterogeneity of MLC1emb mRNAs. Finally, we present evidence that the murine MLC1emb gene also contains a functional distal promoter element which has hitherto been undetected.
Full text
PDF


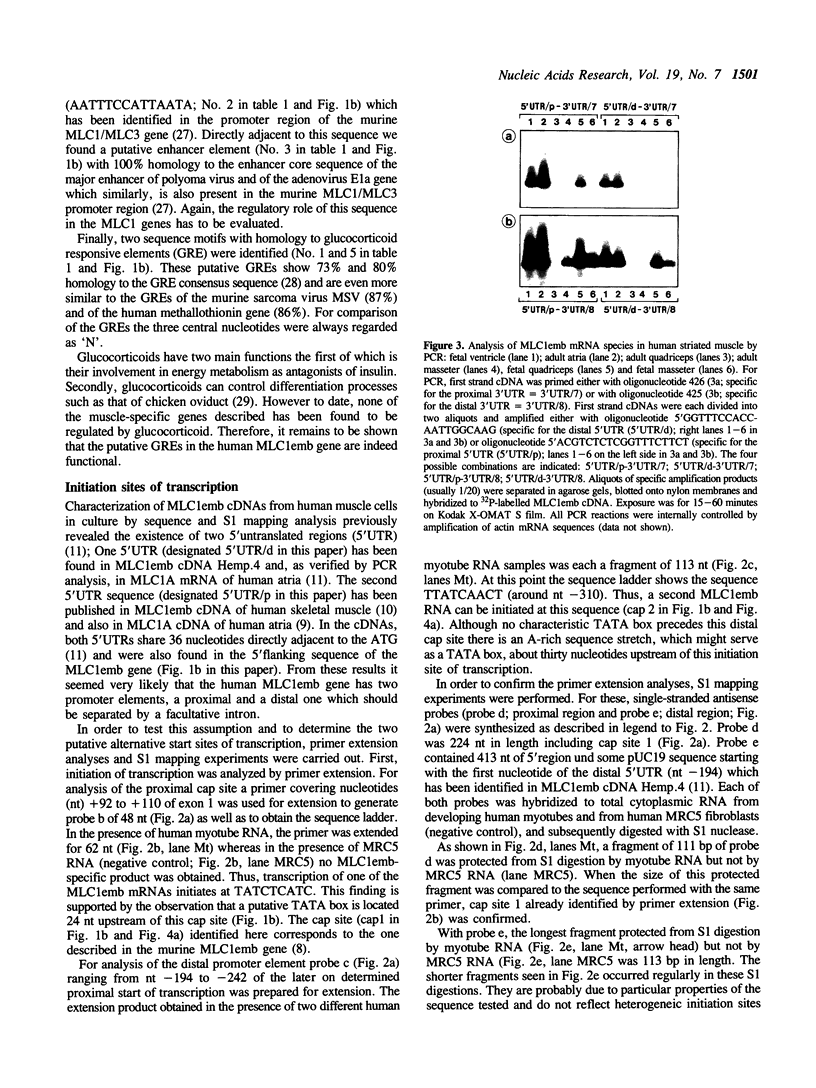
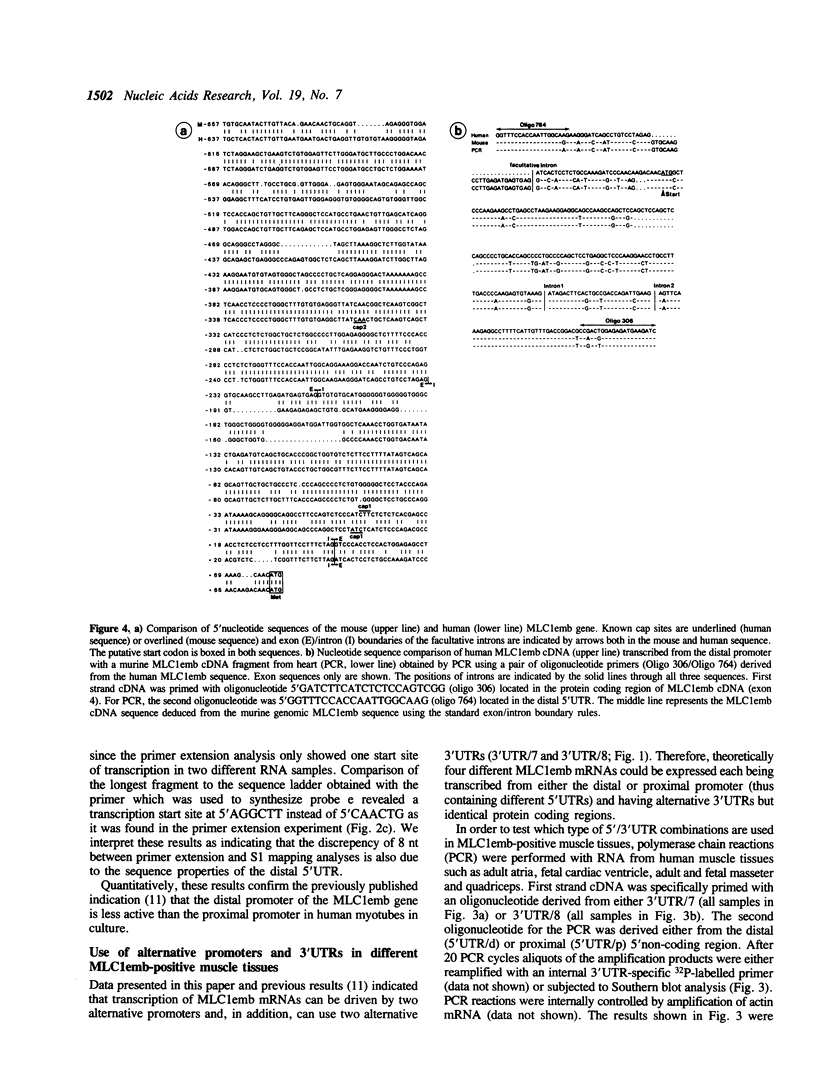


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H. H., Lohse P., Seidel U., Bober E. A novel human myosin alkali light chain is developmentally regulated. Expression in fetal cardiac and skeletal muscle and in adult atria. Eur J Biochem. 1988 Dec 1;178(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14428.x. [DOI] [PubMed] [Google Scholar]
- Barton P. J., Cohen A., Robert B., Fiszman M. Y., Bonhomme F., Guénet J. L., Leader D. P., Buckingham M. E. The myosin alkali light chains of mouse ventricular and slow skeletal muscle are indistinguishable and are encoded by the same gene. J Biol Chem. 1985 Jul 15;260(14):8578–8584. [PubMed] [Google Scholar]
- Barton P. J., Robert B., Cohen A., Garner I., Sassoon D., Weydert A., Buckingham M. E. Structure and sequence of the myosin alkali light chain gene expressed in adult cardiac atria and fetal striated muscle. J Biol Chem. 1988 Sep 5;263(25):12669–12676. [PubMed] [Google Scholar]
- Barton P. J., Robert B., Fiszman M. Y., Leader D. P., Buckingham M. E. The same myosin alkali light chain gene is expressed in adult cardiac atria and in fetal skeletal muscle. J Muscle Res Cell Motil. 1985 Aug;6(4):461–475. doi: 10.1007/BF00712583. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro U., Dalla Libera L., Catani C. Myosin light and heavy chains in muscle regenerating in absence of the nerve: transient appearance of the embryonic light chain. Exp Neurol. 1983 Jan;79(1):106–117. doi: 10.1016/0014-4886(83)90382-5. [DOI] [PubMed] [Google Scholar]
- Cohen A., Barton P. J., Robert B., Garner I., Alonso S., Buckingham M. E. Promoter analysis of myosin alkali light chain genes expressed in mouse striated muscle. Nucleic Acids Res. 1988 Nov 11;16(21):10037–10052. doi: 10.1093/nar/16.21.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubas P., Robert B., Garner I., Buckingham M. A comparison between mammalian and avian fast skeletal muscle alkali myosin light chain genes: regulatory implications. Nucleic Acids Res. 1985 Jul 11;13(13):4623–4643. doi: 10.1093/nar/13.13.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E., Craig A., Poustka A., Frischauf A. M., Lehrach H. A family of cosmid vectors with the multi-copy R6K replication origin. Gene. 1987;57(2-3):229–237. doi: 10.1016/0378-1119(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Hailstones D. L., Gunning P. W. Characterization of human myosin light chains 1sa and 3nm: implications for isoform evolution and function. Mol Cell Biol. 1990 Mar;10(3):1095–1104. doi: 10.1128/mcb.10.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Kurabayashi M., Komuro I., Tsuchimochi H., Takaku F., Yazaki Y. Molecular cloning and characterization of human atrial and ventricular myosin alkali light chain cDNA clones. J Biol Chem. 1988 Sep 25;263(27):13930–13936. [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Nabeshima Y., Kawashima M., Nakamura S., Nonomura Y., Fujii-Kuriyama Y. Isolation of the chick myosin alkali light chain gene expressed in embryonic gizzard muscle and transitional expression of the light chain gene family in vivo. J Mol Biol. 1988 Dec 5;204(3):497–505. doi: 10.1016/0022-2836(88)90350-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seharaseyon J., Bober E., Hsieh C. L., Fodor W. L., Francke U., Arnold H. H., Vanin E. F. Human embryonic/atrial myosin alkali light chain gene: characterization, sequence, and chromosomal location. Genomics. 1990 Jun;7(2):289–293. doi: 10.1016/0888-7543(90)90554-8. [DOI] [PubMed] [Google Scholar]
- Seidel U., Bober E., Winter B., Lenz S., Lohse P., Arnold H. H. The complete nucleotide sequences of cDNA clones coding for human myosin light chains 1 and 3. Nucleic Acids Res. 1987 Jun 25;15(12):4989–4989. doi: 10.1093/nar/15.12.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi-Yanicostas N., Barbet J. P., Laurent-Winter C., Barton P., Butler-Browne G. S. Transition of myosin isozymes during development of human masseter muscle. Persistence of developmental isoforms during postnatal stage. Development. 1990 Feb;108(2):239–249. doi: 10.1242/dev.108.2.239. [DOI] [PubMed] [Google Scholar]
- Wade R., Feldman D., Gunning P., Kedes L. Sequence and expression of human myosin alkali light chain isoforms. Mol Cell Biochem. 1989 Jun 1;87(2):119–136. doi: 10.1007/BF00219255. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M. Myosin from fetal hearts contains the skeletal muscle embryonic light chain. Nature. 1980 Aug 14;286(5774):731–733. doi: 10.1038/286731a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Kautz S., Hajdu G., Winter C., Whalen R. G., Starzinski-Powitz A. Heterogenic mRNAs with an identical protein-coding region of the human embryonic myosin alkali light chain in skeletal muscle cells. J Mol Biol. 1990 Feb 5;211(3):505–513. doi: 10.1016/0022-2836(90)90261-J. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Starzinski-Powitz A. A novel isoform of myosin alkali light chain isolated from human muscle cells. Nucleic Acids Res. 1989 Dec 25;17(24):10496–10496. doi: 10.1093/nar/17.24.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]