Abstract
Chemotherapy is one of the three most common treatment modalities for cancer. However, its efficacy is limited by multidrug resistant cancer cells. Drug metabolizing enzymes (DMEs) and efflux transporters promote the metabolism, elimination, and detoxification of chemotherapeutic agents. Consequently, elevated levels of DMEs and efflux transporters reduce the therapeutic effectiveness of chemotheraputics and, often, lead to treatment failure. Nuclear receptors, especially pregnane X receptor (PXR, NR1I2) and constitutive androstane activated receptor (CAR, NR1I3), are increasingly recognized for their role in xenobiotic metabolism and clearance as well as their role in the development of multidrug resistance (MDR) during chemotherapy. Promiscuous xenobiotic receptors, including PXR and CAR, govern the inducible expressions of a broad spectrum of target genes that encode phase I DMEs, phase II DMEs, and efflux transporters. Recent studies conducted by a number of groups, including ours, have revealed that PXR and CAR play pivotal roles in the development of MDR in various human carcinomas, including prostate, colon, ovarian, and esophageal squamous cell carcinomas. Accordingly, PXR/CAR expression levels and/or activation statuses may predict prognosis and identify the risk of drug resistance in patients subjected to chemotherapy. Further, PXR/CAR antagonists, when used in combination with existing chemotherapeutics that activate PXR/CAR, are feasible and promising options that could be utilized to overcome or, at least, attenuate MDR in cancer cells.
1. Introduction
With an annual economic burden of more than $150 billion, cancer is a major public health problem in the United States. Currently, one in four deaths in the United States can be attributed to cancer [1]. Chemotherapy (including hormone ablation therapy with chemical agents) is one of the three most common treatment modalities for cancer, but its efficacy is limited by drug resistant cancer cells [2–5]. Despite how selective the chemotherapeutic or how specific the intended target is, several barriers still lie between chemotherapeutics and their intended activities to kill tumor cells. One such barrier is the delivery of chemotherapeutics, at effective doses, to the tumor mass. After administration, the drug is first distributed, metabolized, and excreted by the human body. Then, after arriving at the tumor site, the chemotherapeutic agent(s) still need to permeate the tumor microenvironment and enter tumor cells. Several possible mechanisms and molecular alterations associated with tumors have been implicated in their resistance to chemotherapy, including hypoxia secondary to poor vascularization in tumors [6], activation of pro-surviving signals such as NF-κB [7, 8], overexpression of p-glycoprotein (P-gp) [9–11], presence of “side populations” of cancer stem cells that express active efflux transporters [12, 13], and defective apoptotic mechanisms [14–19]. Due to the limited therapeutic windows and steep toxicity curves associated with most chemotherapeutic agents, altered local metabolism and disposition of cancer drugs present challenges to treatment and may account for the variations in drug efficacy, as exemplified by multi-drug resistance (MDR).
Multi-drug resistance (MDR), a clinical phenomenon characterized by decreased intracellular drug retention and changed tumor response, is one of the primary factors that limit effective cancer therapy [20]. Much attention has been directed toward the mechanism behind drug resistance and many efforts have been invested to identify therapeutic approaches that mitigate drug resistance. A number of in vitro and in vivo models have been developed to study the development of MDR and assess the potential clinical application of MDR modulators [8, 12]. For instance, the differential induction of ATP binding cassette (ABC) transporters has been associated with MDR in many cancers [21, 22]. However, clinical applications have shown limited success, partially because MDR is a complex process and no single drug metabolizing enzyme (DME) [23] or ABC transporter [10] can induce MDR alone. Novel, multi-targeted strategies are needed to overcome the induction of MDR.
Several nuclear receptor families that regulate drug metabolism and disposition are increasingly recognized for their significance in this process, and treatments targeting them promise to open new avenues to alleviate, or even prevent, MDR. Among these nuclear receptors, pregnane X receptor (PXR) and constitutive androstane receptor (CAR) exhibit great flexibility in recognizing structurally diverse compounds, share significant similarities in ligand binding, and cross communicate during the transactional activation of their target gene promoters, which include cytochrome P450s (CYP) (e.g. CYP2B6, CYP3A4 and CYP2C9) [24, 25] and MDRs (e.g. P-gp) [26]. PXR and CAR have been speculated to play important roles in cancer MDR, because of their elevated expressions in breast [27], prostate [28], intestinal [29], colon [30] and endometrial cancers [31] and their roles as master transcription regulators of a broad spectrum of genes that encode phase I DMEs, phase II DMEs and efflux transporters [32–35].
In this review, we will highlight the recent findings regarding xenobiotic receptor regulation of DMEs and drug transporters and provide insight into nuclear receptor associated MDR during chemotherapy. We will first provide a brief background regarding the structural basis and biological functions of xenobiotic receptors. Then, we will focus on recent discoveries that encompass several key transcriptional regulators of xenobiotic enzyme and transporter expression, including PXR, CAR, proliferator-activated receptors (PPARs) and aryl hydrocarbon receptor (AHR). We will also describe the unique structural properties of PXR and CAR that enable them to recognize and accommodate a broad spectrum of xenobiotics and regulate multiple drug metabolism enzymes and transporters. This review will conclude with a discussion of the clinical significance of nuclear receptor activation mediated drug-drug interaction, as well as the potential uses and limitations of nuclear receptor antagonists that circumvent MDR and enhance the efficacy of existing cancer treatments.
2. Drug biotransformation, metabolism, and excretion systems
Accumulation of xenobiotics, including carcinogens, environmental pollutants, and therapeutic drugs, within the body can profoundly affect human health. The ability to clear these chemicals from our bodies is essential to survival, so naturally, mechanisms that metabolize and excrete these xenobiotics have evolved [36]. Xenobiotics metabolism and disposition are mediated by a large number of DMEs and transporters, and consist of four major stages: absorption/permeability, distribution, metabolism, and excretion. DMEs, which include phase I metabolizing enzymes, phase II metabolizing enzymes, and drug transporters, play an essential role in the metabolism, detoxification, and elimination of xenobiotics.
Phase I and phase II DMEs biotransform lipophilic xenobiotics into water-soluble metabolites that are more readily effluxed from the cell, and subsequently the body, by transporters. Phase I DMEs consist primarily of a number of dehydrogenases, reductases, and oxidases that detoxify xenobiotics by introducing a polar functional group into their target molecules. Among the phase I DMEs, the cytochrome P450s (CYPs; P450s) super family is the most important family of enzymes in the monooxygenation of lipophilic compounds. CYPs attach a reactive hydroxyl group, which can subsequently be utilized by phase II enzymes for further disposition, onto xenobiotics. These enzymes are found most abundantly in the liver, but their presence has also been observed in various organs including the lung, gastrointestinal tract, and kidney. Inside the cell, CYPs are membrane-bound enzymes that localize primarily to the endoplasmic reticulum (ER), where CYP catalyzed oxidation reactions are essential to cholesterol and sterol biosynthesis [37]. Certain CYPs are also present in other subcellular compartments, including the inner mitochondrial membranes and lysosomes [38]. Currently, 17 distinct families of CYPs have been identified in humans. CYP families 1–4 and 7, with approximately 17 total members, exhibit low substrate specificity and metabolize a diverse set of xenobiotics [39]; hence, they are believed to play crucial roles in both hepatic and extra-hepatic xenobiotic detoxification and elimination [40]. In fact, CYP2B6 and CYP2C enzymes are involved in the metabolism of approximately 25% and 20% of all xenobiotics, respectively [33]; and CYP3A4, alone, can metabolize approximately 50–60% of clinically used drugs [41–43] and is essential to the metabolism of an extensive range of endogenous substrates, including bile acid and steroid hormones.
Subsequently, phase II DMEs conjugate endogenous ligands with electrophilic xenobitoics or their phase I metabolites, primarily through methylation, esteration, acetylation, glucuronidation, sulfation, and glutathione and amino acid conjugation. Phase II products are usually more hydrophilic than their parental compounds and, thereby, are more readily excreted. Phase II xenobiotic-metabolizing enzymes include quinone reductases (QRs), NAD(P)H:menadione reductases (NMOs), methyltransferases, epoxide hydrolases (EPHs), N-acetyltransferases (NATs), glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and sulfotransferases (SULTs) [44]. Among these phase II DMEs, glucuronidation reactions, catalyzed by several UGT isoforms, play a principal role in phase II metabolism [45, 46]. Currently, 19 UGT isoforms have been identified in humans and are divided into three subfamilies, UGT1A, UGT2A, and UGT2B [47]. UGTs localize primarily to the ER membrane, where they catalyze the glucuronidation of a substantial array of endogenous substrates and exogenous compounds [45]. To properly coordinate with phase I DMEs, most phase II DMEs are also preferentially expressed in the liver, intestine, and kidney.
It should be noted that xenobiotic biotransformation does not always yield pharmacologically inactive metabolites, and can, instead, produce pharmacologically active, or even toxic, metabolites. For instance, the CYP3A4-mediated N-dechloroethylation of cyclophosphamide, a nitrogen mustard alkylating agent used to treat cancer and autoimmune diseases, produces a neurotoxic metabolite [48].
Ultimately, intracellular levels of both parental xenobiotics and their biotransformed metabolites are determined by membrane transporter proteins (sometimes called phase III enzymes) [49, 50]. Drug transporters modulate the absorption or excretion of a variety of structurally unrelated xenobiotics across the cell membrane and are preferentially expressed in several organs (including the liver and intestines) that are, predictably, important to the efflux of xenobiotics and their metabolites. Depending on the source of energy, these transporters can be divided into two classes: ATP binding cassette (ABC) transporters that utilize the ATP hydrolysis generated energy, and organic cation transporters (OCT) and organic anion-transporting polypeptides (OATP) that utilize proton gradients [51].
The importance of drug transporters in cancer chemotherapy is well recognized. Of the 48 known human ABC transporters, 11 have already been shown to play roles in MDR in cancer cells [52]. Multidrug resistance-associated protein (MRP) and P-glycoprotein (P-gp or MDR1) are perhaps the most notable ABC transporters, since their constitutive or inducible expression has been observed in a variety of human tumors and contributes to chemotherapeutic resistance, a phenomenon frequently referred to as the “MDR phenotype” [53]. For example, MCF-7 cells with higher multidrug resistance related protein 2 (MRP2) expression are more resistant toward tamoxifen [54], and newly invented ATP-binding cassette transporter-inhibiting peptides and heterocyclic or cyclic substituted amino derivatives that inhibit P-gp functions were found to sensitize cancer cells to chemotherapeutics.
In summary, phase I DMEs, phase II DMEs, and drug transporters orchestrate a defensive system that metabolize and eliminate xenobiotics. These DMEs and transporters are crucial to the protection of the human body from xenobiotics; however, dysregulation of their expression in tumor cells can compromise the efficacy of a variety of chemotherapeutics. For instance, the topoisomerase I inhibitor irinotecan (CPT-11) is commonly used to treat patients with metastatic colorectal cancer. Irinotecan does not exert anti-tumor effects in vivo until after it is hydrolyzed into its active metabolite, SN38, by carboxylesterases 1 and 2. Afterwards, SN38 is re-inactivated and metabolized into SN38G by UGTs [55, 56]. All the while, both irinotecan and its metabolites are subjected to immediate efflux through transporters, including P-gp and MRP2 [57, 58]. As such, the prevalence of certain DMEs and drug transporters directly affect the effectiveness of irinotecan, so manipulation of their expressions and activities has become an attractive strategy to circumvent MDR.
Further, phase I and phase II DME mediated drug disposition is frequently accompanied by drug-drug interactions – when one drug affects the pharmacokinetic or pharmacodynamic mechanisms of another, co-administered drug. Drug-drug interactions lead to either synergistic or antagonistic effects on one or both drugs, alter drug response, toxicity, and elimination, or produce new effects that cannot be observed when either drug is administered alone. For instance, the xanthine oxidase (XO) inhibitor allopuriol is often used to treat conditions associated with hyperuricemia, including gout. Allopuriol is also notorious for prolonging the effective durations of drugs that are metabolized by XO. When co-administered with the immunosuppressant mercaptopurine, which is used to treat acute lymphoblastic leukemia and various autoimmune disorders, the drugs can severely suppress bone marrow and induce pancytopenia and death [59].
Altogether, phase I DMEs, phase II DMEs, and drug transporters mediate the metabolism and elimination of various natural xenobiotics and therapeutic agents, sometimes leading drug-drug interactions. Alterations to their expressions or activities can profoundly impact both their ability to protect the human body against environmental xenobiotics and the effectiveness of therapeutic compounds.
3. The nuclear receptor superfamily
Nuclear receptors are important components of mammalian intercellular signaling mechanisms. The mammalian nuclear receptor superfamily comprises of more than 70 distinct members [60] that are divided into two general subclasses, based on their ligand binding requirement. The first subclass is comprised of ligand-dependent nuclear receptors that are regulated by a diverse group of exogenous compounds and endogenous substrates. These receptors include glucocorticoid receptor (GR), estrogen receptor (ER), androgen receptor (AR), and retinoic acid receptor (RAR). The second subclass of nuclear receptors includes the so-called orphan receptors. These receptors share sequence identity with nuclear receptors but their regulatory ligands still have not been identified [61]. Orphan receptors actually account for approximately 60% of known nuclear receptors [61, 62]. Several key orphan receptors, including PPARs, liver X receptors (LXRs), aryl hydrocarbon receptor (AHR), constitutive androstane receptor (CAR) and pregnane X receptor (PXR), are known to play crucial roles in development, homeostasis, and diseases [63]. So naturally, orphan receptors have become the focus of intense academic research and industrial targets for the development of novel therapeutic agents.
Most nuclear receptor share characteristic structural features, including a highly-conserved DNA-binding domain (DBD), a less conserved ligand binding domain (LBD), and two transactivation domains: activation function 1 (AF-1) and 2 (AF-2) [64]. Characterized by two C4-type zinc fingers, the DBDs of these receptors recognize receptor specific xenobiotic response elements (XREs) or hormone response elements (HREs), and guide the receptors to the promoter regions of specific target genes. Nuclear receptors bind to their response element as monomers, homodimers, or heterodimers with the retinoid X receptor (RXR), such as PXR and CAR [65]. The LBDs of these receptors can be extraordinary flexible in shape and size. Ligand binding at LBDs triggers conformational changes, within the LBD, to accommodate a spectrum of structurally distinct endogenous and xenobiotic ligands, and recruit co-activators and co-regulatory transcription factors, including transcriptional mediators/intermediary factor 2 (TIF2), steroid receptor co-activators (SRCs), and P300/CBP-associated factor (PCAF) [66–69]. The two transactivation domains guide transcription co-regulators to the target gene promoters. Typically, AF-1 domains are ligand-independent and localizes to the amino termini, while AF-2 domains are ligand-dependent and localizes to the carboxyl termini [70].
4. Regulation of drug biotransformation and metabolism by xenobiotic receptors
The significance of nuclear receptors in drug metabolism and disposition is evident from their ability to recognize a variety of structurally diverse compounds and their role in the regulation of many important DMEs [71, 72]. Expression of these DMEs and transporters, in response to endogenous and exogenous compounds, are subject to both transcriptional and post-transcriptional regulation. A limited number of studies have indicated that short term alterations to DME and transporter activities and cellular localization can be modulated at the post-transcriptional level. Several protein kinases have been shown to drastically alter the functional state of DMEs, mostly CYPs, through phosphorylation and dephosphorylation [73]. Modulation of these DMEs and drug transporters at the transcriptional level, however, is primarily regulated by nuclear receptor superfamily of transcription factors, together with co-activators and co-repressors [74]. As aforementioned, several different classes of xenobiotics are able to induce the transcription of genes that encode DMEs and transporters by binding directly to nuclear receptors – or alternatively, these nuclear receptors act as metabolic sensors that respond to both endogenous and exogenous chemicals, and thereby integrating the homeostatic biotransformation of endobiotics and xenobiotics. These nuclear receptors can target, either directly or indirectly, different regulatory sequences found in the promoter regions of DME and drug transporter genes.
4.1. Pregnane X receptor (PXR)
The pregnane X receptor (PXR; NR1I2; also termed as PAR or SXR) is a member of the nuclear receptor superfamily. Full-length PXR cDNAs was cloned by three independent groups in 1998 and was first found to respond to endogenous pregnanes (21-carbon) steroids that gave rise to its name. Ongoing research has, however, revealed that the biology of PXR is more complex than previously thought – PXR serves as a “molecular sentinel” that localizes to both the cytoplasm and nucleus and is able to bind to a wide spectrum of structurally distinct endobiotic substrates and xenobiotic compounds, including food additives, drugs, and environmental pollutants. It has since been shown to coordinate the detoxification of these endobiotics and xenobiotics by modulating the expression of DMEs, including CYP3A, CYP2B6, and various members of the UGTs superfamily [63, 75, 76], as well as drug efflux transporters, including P-gp and MRP2 [77]. In addition, PXR has been shown to be involved in the regulation of cholesterol homeostasis and bile acid metabolism, and, possibly, in the development of some cancers [78].
4.1.1. Mechanism of PXR activation
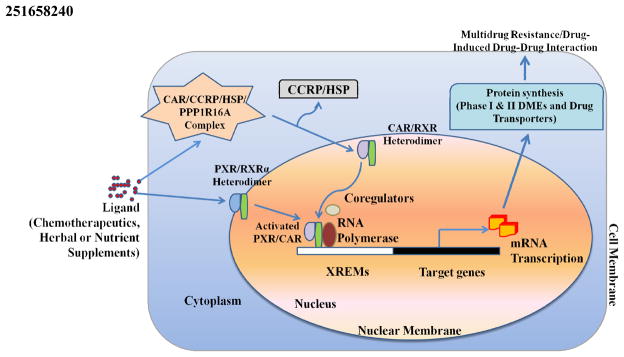
It is widely accepted that the induction of nuclear receptor target genes, by endobiotics or xenobiotics, is mediated through direct interactions between the receptors and their putative responsive elements, located in the promoter regions of these genes. PXR was originally believed to be localized to the nucleus, but later studies have revealed that it is also present in the cytoplasm. After ligand binding, PXR translocates to the nucleus to initiate gene transcription [79] (Figure 1). Similar to other nuclear receptors, PXR homologs from different species are all structurally related, with a conserved N-terminal DBD, a C-terminal LBD, and relatively short hinge region (amino acids 107–141) that separates the DBD and LBD. The DBD of human PXR (amino acids 41–107) contains two zinc fingers and forms a heterodimer with the retinoid X receptor (RXR) to bind to specific DNA response elements. The PXR DBD contains a bipartite nuclear localization sequence [80] and contacts specific (A/G)G(T/G)TCA sequences in the promoter regions of target genes [72, 75, 81]. These specific sequences are arranged as direct repeats separated by three- to five- nucleotide spacers (DR3, DR4, or DR5 elements), everted repeats separated by six or eight bases (ER6 or ER8), or inverted repeats with either no spacers or six-based spacers (IR0 or IR6) [72].
Figure 1.
Regulation of Phase I & II DMEs and drug transporter genes by nuclear receptors PXR and CAR. After ligand binding, cytoplasimc fractions of PXR translocates to the nucleus while CAR dissociates from its complex, comprised of tetratricopeptide repeat (TTR), cytoplasmic CAR retention protein (CCRP), 90-kDa heat shock protein (hsp90) and PPP1R16A, and translocates from the cytoplasm to the nucleus. Subsequently, both PXR and CAR form heterodimers with RXR and bind to their respective response elements to stimulate transcription of phase I & II DMEs and drug transporters.
Distinct from other nuclear receptors, PXR possesses flexible and spacious ligand binding pocket (LBP). This unique LBP enables PXR to ably accommodate a structurally diverse array of both endogenous and exogenous hydrophobic compounds [82]. Like other nuclear receptor LBDs, the PXR LBD contains three layers of α-helices arranged in a so-called “α-helical sandwich” that surrounds the receptor’s ligand binding pocket [66]. The PXR ligand-binding pocket is large, flexible, and capable of varying between 1,280, unbound, to more than 1,600 Å3 in volume [66, 83–85] when bound to, usually, lipophilic compounds with a limited number of polar groups [86]. Multiple structural studies have revealed that PXR’s LBD can be differentially triggered to expand in volume or adopt unique conformational structures that enable it to accommodate a variety of structurally diverse molecules [66, 83]. After ligand binding, PXR undergoes conformational changes before releasing its co-repressor complex and recruits various co-activators, including RXR [65].
Additionally, PXR ortholog sequences vary among species. Although the DBD regions of rabbit, rodent, and human PXRs are conserved and share approximately 95% sequence homology, the LBD are divergent and share only 75–80% amino acid homology. This feature is reflected by pronounced pharmacological divergence in species-specific PXR activation and target gene induction profiles. Accordingly, these species-specific variations compromise the use of animals for PXR ligand screenings.
4.1.2. PXR in DME and efflux transporter regulation
Originally identified as a steroid hormone receptor, PXR mediates the genomic effects of several steroid hormones, including progesterone, pregnenolone, and estrogen, in rodents and humans [75, 81]. However, its extreme flexibility and promiscuous in ligand recognition and target gene activation enable it serve as a unique xenobiotic sensor for drug metabolism [66, 87, 88]. There are a number of studies that have characterized endogenous and exogenous PXR agonists [87, 89]. To date, PXR has been observed to bind to a wide range of structurally distinct chemicals, including anticancer compounds [90–93], herbal components and plant extracts [94–96], cholesterol-lowering statins and SR12813 [97–99], the anti-tuberculoid antibiotic rifampicin [97], HIV protease inhibitors [100], vitamins [101], carotenoids [102, 103], endocrine disruptors [104, 105], pesticides [106, 107], plasticizers [104, 105, 108, 109], and PPAR and other nuclear receptor antagonists [110, 111]. In response to the aforementioned xenobiotics, PXR activates the transcription of a series of biologically crucial phase I and II DMEs, as well as drug transporters (Table 1) [112].
Table 1.
PXR and CAR regulations of DMEs and transporters that are associated with xenobiotic metabolism and transport and its co-expression analysis in human prostate tumors (r, correlation coefficient; NA, not available from the data set examined).
| Class | Gene | Receptor | References | Co-expression in human prostate tumor? |
|---|---|---|---|---|
| Phase I drug metabolism enzymes | CYP1A1 | CAR | [26, 241] | Yes(r=0.18, p=0.00084) |
| CYP1A2 | CAR | [162, 241] | Yes(r=0.12, p=0.028) | |
| CYP2A4 | CAR | [26] | NA | |
| CYP2A6 | PXR | [242, 243] | Yes(r=0.16, p=0.0029) | |
| CYP2B1/2 | CAR/PXR | [244] | NA | |
| CYP2B6 | CAR/PXR | [245] | Not for PXR or CAR | |
| CYP2B10 | PXR/CAR | [152, 246] | NA | |
| CYP2C8 | PXR/CAR | [122] | Yes for CAR (r=0.14, p=0.011) and PXR (r=0.13, p=0.016) | |
| CYP2C9 | PXR/CAR | [24, 121, 123] | Yes for PXR (r=0.12, p=0.023) but not for CAR (r=0.09, p=0.095) | |
| CYP2C19 | PXR/CAR | [247] | Yes for CAR (r=0.31, p=3.2e-08) and for PXR(r=0.47, p=0) | |
| CYP2C29 | CAR | [248] | NA | |
| CYP2C37 | CAR | [249] | NA | |
| CYP3A2 | PXR | [250] | NA | |
| CYP3A4 | PXR/CAR | [76] | Yes for PXR(r=0.24,p=8.7e-06) and CAR(r=0.18, p=0.00084) | |
| CYP3A7 | PXR | [25] | Yes (r=0.21, p=1e-04) | |
| CYP3A11 | PXR/CAR | [26] | NA | |
| CYP3A23 | PXR | [250] | NA | |
| CYP4F12 | PXR | [126] | Yes(r=0.34, p=7e-11) | |
| CYP7A1 | PXR | [125] | Yes (r=0.16, p=0.0024) | |
| AKR1C1/2 | PXR | [126] | Not | |
| ALDH1 | PXR/CAR | [129] | NA | |
| AKR1B7 | PXR/CAR | [127] | NA | |
| Phase II drug metabolism enzymes | UGT1A1 | CAR/PXR | [131, 132, 251, 252] | NA |
| UGT1A3 | PXR | [135] | NA | |
| UGT1A6 | PXR/CAR | [131, 136] | NA | |
| UGT1A9 | PXR/CAR | [136] | NA | |
| UGT2B1 | CAR | [136] | NA | |
| UGT2B5 | PXR | [132] | NA | |
| GSTA1 | PXR | [26, 253] | Not | |
| SULT2A1 | PXR/CAR | [142, 252] | Yes for PXR(r=0.13, p=0.017) but not for CAR (p>0.05) | |
| SULT1E1 | PXR/CAR | [254] | Not for PXR or CAR | |
| SULT2A2 | PXR/CAR | [26, 252] | NA | |
| SULT1A1 | PXR | [255] | Not for PXR or CAR | |
| SULT1B1 | PXR | [255] | Yes (r=0.33, p=1.6e-10) | |
| Drug transporters | MDR1 | PXR/CAR | [143, 256] | Yes for PXR(r=0.14, p=0.0082) but not for CAR (p>0.05) |
| MRP1 | CAR | [26, 257] | NA | |
| MRP2 | PXR/CAR | [144, 258] | NA | |
| MRP3 | PXR/CAR | [228, 258] | NA | |
| MRP4 | CAR | [149, 259] | NA | |
| SLCO1A4 | PXR | [26, 146, 260] | NA |
4.1.2.1. PXR in phase I DME regulation
Several phase I DMEs are regulated by PXR, including a number of CYPs, carboxylesterases, aldehyde and alcohol dehydrogenases, and enzymes involved in heme production and the P450 reaction cycle [26, 85, 112]. Particularly, PXR is a predominant regulator of the xenobiotic-responsive expression of CYP3A genes. Accordingly, PXR is highly expressed in human livers and intestines, where CYP3A is abundantly distributed and capable of metabolizing a broad range of structurally diverse xenobiotics [41, 42, 113, 114]. A large number of compounds that induce CYP3A expression are also PXR activators [115]. Analysis of the promoter regions of rodent and human CYP3A genes revealed that PXR regulates the xenobiotics-induced expression of CYP3A by binding directly to either direct repeats of TGAACT half-sites spaced by three base pairs (DR3) or everted or inverted repeats of TGAACT half-sites spaced by six base pairs (ER6 and IR6) [65, 81, 98, 116].
Because of the pronounced variances in PXR LBDs across different species, species-specific PXR activator profiles exist. For example, rifampicin, a potent inducer of CYP3A expression in human and rabbit, but not rodent, liver cells, is also a potent activator of human and rabbit PXR, but not rat or mouse PXR. Conversely, pregnenolone 16-alpha carbonitrile (PCN), a robust inducer of mouse, but not human or rabbit, CYP3A, is also a potent activator of rat PXR, but not human or rabbit PXR [98]. Studies using knockout and transgenic mouse models also show that PCN fails to induce CYP3A expression in PXR-null mice and that humanized mice that carry the human, instead of mouse, PXR ortholog respond to rifampicin. These studies further indicate that PXR-regulated induction of CYP3A gene expression is species-specific [117].
In addition to CYP3A4, rifampicin and phenytoin also induce CYP2B6 expression effectively through human PXR in a dose-dependent manner [118, 119]. Other human PXR agonists paclitaxel, SR-12813 and rifampicin are able to induce CYP2C8 and CYP2C9 expression in human primary hepatocytes [120–122]. Additional studies provided evidence that the several DR4 and DR5 elements were present within a 3500-base-pair sequence upstream from the CYP2C9 start site [24, 123]. Notably though, PXR activation was reported to repress, not activate, CYP7A1 expression in rodent models, which may function as a feedback mechanism to counteract bile acids induced stress responses [124, 125].
In addition to CYPs, PXR has been shown to regulate the expression of a number of other phase I DMEs that hydrolyze, reduce, and oxidize xenobiotics. Aldo-keto reductases (AKRs), including various NADPH-dependent oxidoreductases such as aldehyde reductase and aldose reductase, have been reported to be PXR target genes. In humans, both AKR1C1 and AKR1C2 genes contain PXR binding sites [126], and recently, Liu et al found that PXR recognizes and binds to multiple DR4 sites located in AKR1B7 promoter regions. Using mouse models, PCN was observed to induce AKR1B7 expression in wild-type, but not PXR knockout, mice [127]. Evans et al, using transgenic mice that express a constitutively active variant of human PXR (VP human PXR), further identified a plethora of other genes that encode phase I DMEs that are might be PXR targets. Constitutively active human hepatic VP-PXR appears to upregulate alcohol dehydrogenase 3A2 (ADH3A2), carboxylesterase 2, and aldehyde dehydrogenase 1A7 (ALDH1A7), and down-regulate hydroxysteroid dehydrogenases (HSDs) and betaine-homocysteine methyltransferase [112]. In agreement with these microarray analyses, xenobiotics were found to induce liver and intestine carboxylesterases in a PXR-dependent manner [128, 129].
4.1.2.2. PXR in phase II DME regulation
PXR also regulates the expression of several phase II (conjugation) DMEs that facilitate the excretion of phase I biotransformed xenobiotics. These phase II DMEs include UGTs, GSTs, and SULTs[130]. As mentioned above, UGT catalyzed glucuronidation reactions are essential to the clearance of bilirubin, drugs, and xenobiotics. Currently, a number of UGTs, including UGT1A1, UGT1A3, UGT1A4, UGT1A6 an UGT1A9, have been identified as PXR targets [131–136]. Using cultured HepG2 cells, Sugatani et al identified several potential PXR binding sites, including a DR-3 element, DR-4 element, and PXR response element (PXRE) [137]. In vivo experiments confirmed that PCN treatments enhance the transcription and activity of UGT1A1 and UGT1A9 in wild-type, but not PXR-null, mice [132]. And another study, conducted by Xie et al, further showed that UGT1A1 activity is markedly up-regulated in “humanized” PXR transgenic mice [131].
Glutathione S-transferases (GSTs) catalyze the conjugation of glutathione to electrophilic centers in xenobiotics. GSTs are an essential part of phase II detoxification and important to the development of MDR during chemotherapy [32]. PXR was first reported to be involved in the regulation of GSTs in rats [138]. In this study, high concentrations (μM level) of dexamethasone (DEX) induced GSTA2 gene expression via a PXR-dependent mechanism in primary adult rat hepatocytes. Although no canonical PXR-RXR responsive element can be found in the GST2A promoter, PXR appears to induce GSTA2 expression using a 20-bp region (−700 to −683) region containing the antioxidant response element (ARE) [138]. Using transgenic female mice with constitutively active humanized PXR, Gong et al found that, in response to mammalian oxidative stress, PXR induces the expression of several GST isoforms in a tissue and sex specific manner [139].
PXR is also involved in the regulation of sulfotransferase (SULT) gene expression. The SULT gene family encodes more than 10 distinct enzymes that catalyze the transfer of -SO3H groups from 3′-phosphoadenosine-5′-phosphosulfate donors to endogenous or exogenous substrates [140]. The sulfate conjugated substrates are more polar and more readily excreted and eliminated than their parental molecules. Among SULTs, hydroxysteroid sulfotransferase (SULT2A1) was the first to be identified as a PXR target [141]. Treatment with DEX significantly increases SULT2A1 mRNA and protein expressions in primary cultured rat, but not human, hepatocytes [141]. Importantly, an IR0 element found in the proximal promoter of SULT2A1, is speculated to be the PXR binding element [142]. Using animal models, subsequent studies have shown that PXR activation also induces the expression of a number of other SULT family members, including SULT1E1, SULT2A1 and SULT2A2 [140].
4.1.2.3. PXR in efflux transporter regulation
PXR activation has also been reported to regulate several efflux transporters, including ABC drug efflux transporters, multidrug resistance-associated proteins (MRPs), breast cancer resistance protein (BCRP), and P-glycoprotein (P-gp) [85, 92, 100, 112, 125, 143, 144].
P-glycoprotein, encoded by the ABCB1 gene, plays an important role in reducing drug absorption in the gut lumen. A DR-4 element, located in the upstream enhancer of the ABCB1 gene, has been identified as a direct PXR binding site and studies from different groups have confirmed that human PXR ligands, including SR-12813, rifampicin, clotrimazole, nifedipine, and mifepristone, potently promote P-gp expression in human primary hepatocyte and colon cancer cell lines [92, 143]. In addition, LS180 cells that express constitutively active PXR (VP-PXR) also express P-gp in the absence of ligands [92].
In addition, a novel ER-8 element has been found in the proximal promoter of ABCC2, the gene that encodes MRP2, and is speculated to provide binding sites for PXR/RXR heterodimers. PXR agonists DEX and PCN have been reported to induce MRP2 mRNA expression in rodent primary hepatocytes collected from wild-type, but not PXR-null, mice; and mutations to the ER-8 element is reported to abolish nuclear receptor responses [144]. Another PXR regulated drug transporter is OATP2. In response to bile salts or xenobiotics exposure, PXR/RXR heterodimers have been reported to bind to four potential PXR response elements (DR3-1, DR3-2, DR3-3 and DR3-4) located in the 5′-flanking regions of the rat OAPT2 gene [145, 146].
Altogether, this apparently ever increasing library of ligands and targets has distinguished PXR as a unique and integral mediator of endo- and xenobiotic metabolism and clearance.
4.2. Constitutive androstane/activated receptor (CAR)
Initially isolated as an orphan nuclear receptor and named MB67, CAR is predominantly expressed in the liver and maintains only a limited presence in certain extrahepatic tissues in humans [147]. Evident from its name, wild-type CAR does not require ligand binding to become activated. Instead, it readily forms heterodimers with RXR and targets retinoic acid response elements (RAREs) in target gene promoters. Much like PXR, CAR functions as a chemical sensor and regulates a broad range of hepatic and intestinal phase I DMEs (CYP3A4, CYP2Bs and CYP2Cs), phase II DMEs (UGTs and GSTs) [148], and drug transporters (MDR1, MRPs and OATP2) [144, 149]. Studies have shown that CAR deficient mice are more susceptible to hydrophobic bile acid and lithocholic acid (LCA) but are more resistant to acetaminophen induced hepatotoxicity [150, 151]. CAR also appears to cross-talk with PXR during xenobiotic response. These receptors recognize similar response elements and share a significant number of target genes [152]. Nevertheless, because CAR can induce target gene expression independent of ligand binding, it regulates xenobiotic metabolism in a way distinct from PXR.
4.2.1. Mechanism of CAR activation
CAR belongs to the same subgroup of the orphan nuclear receptor superfamily as PXR, LXR and farnesoid X receptor (FXR). Distinct from most other nuclear receptors, CAR exhibits strong basal transcriptional activity in the absence of ligands. CAR accumulates in the nucleus and can be constitutively active without xenobiotic stimulation [153]. However, CAR can also be activated via ligand binding. Inactivated CAR is retained in the cytoplasm, where it is attached to a complex comprised of tetratricopeptide repeat (TPR), cytoplasmic CAR retention protein (CCRP), 90-kDa heat shock protein (hsp90), and PPP1R16A (Figure 1) [154, 155]. Both ligand dependent and independent activation releases CAR from its cytoplasmic tethering complex and translocates the receptor into the nucleus, where CAR transactivates CAR-inducible genes. Heterodimerization with RXR triggers an allosteric transformation of the CAR LBD, which allows for ligand accommodation. Hence, CAR activation is a rather complex process and involves the binding of an agonist, recruitment of co-activators, dissociation of co-repressors, translocation to the nucleus, heterodimerization with RXR, binding with DNA before the receptor induces gene expression.
A number of CAR ligands, including androstenol, suppress the transcriptional activity of CAR by recruiting co-repressors instead of co-activators [156]. For example, two endogenous CAR ligands, the androstane metabolites androstanol (5α-androstan-3α-ol) and androstenol (5α-androstan-16-en-3α-ol), trigger the dissociation of CAR from its coactivators and, thereby, inhibit the transactivation of CAR. In this case, the inhibition CAR can be reversed after treatment with CAR inducers [156].
In spite of several common characteristics that human and rodent CAR share, such as nuclear translocation after phenobarbital (PB) treatment and PBREM binding, clear species-specific differences between human and rodent CAR exist. For example, 6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) is a potent human, but not mouse, CAR agonist [157]. Phenobarbital-like inducer, 1,4- bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), the most potent mouse CAR ligand known, does not activate human or rat CAR [115, 158]. In addition, mouse CAR inhibitors, including CaMK inhibitors, androstenol, and progesterone, do not suppress human CAR activity. In addition, several alternatively spliced human CAR variants, termed CAR2 and CAR3, have been identified [159]. These CAR isoforms are not constitutively active and must be activated via ligand binding. Further, because these CAR isoforms do not exist in rodents, data generated from standard rodent models may not reflect human CAR functions accurately.
4.2.2. CAR in DME and efflux transporter regulation
Many DMEs, most notably the CYPs including CYP3A, CYP2B, CYP2C, and CYP2H, that are regulated by CAR are also co-regulated by PXR (Table 1) [26, 160]; nevertheless, CAR is an important nuclear receptor that plays unique roles in xenobiotic and endobiotic metabolism. For example, PB and TCPOBOP treatments do not induce the expression of CYP1A1, CYP1A2, or CYP3A11 in CAR−/− mice [26, 148, 161, 162]. Further studies have identified a cis-element ER8 motif upstream of the CYP1A1 as a CAR-responsive element. This ER8 motif is highly conserved across the CYP1A1 promoters in various species and provides a binding site for CAR/RXRα heterodimer transactivation of both CYP1A1 and CYP1A2 genes in human hepatocytes [163].
UGT1A1 is the first UGT that was identified as a CAR target gene. CAR binds to a distal phenobarbital response enhancer module (gtPBREM) in the UGT1A1 promoter region [137]. In addition, several groups have established an essential and unique role of CAR in the regulation of the mammalian sulfation system. The CAR agonists PB and TCPOBOP both induce the expressions of SULT1C1, SULT1E1 and SULT2A1 in wild-type, but not CAR−/− mice [149, 164]. And further, SULT1A4 and SULT2A expressions, which are essential to the bile acid detoxification process, were elevated in transgenic mice with constitutively activated CAR (VP-CAR) [165]. Soon thereafter, a number of key phase II DMEs, including UGTs (UGT1A1, UGT1A3, UGT1A6, UGT1A9, UGT1A10 and UGT2B36) and GSTs (GSTA1 and GSTA2), were also found to be regulated by CAR [134, 164]. Altogether, these discoveries reinforce the notion that CAR serves as a global phase II DME regulator in both humans and many vertebrates (Table 1).
CAR also regulates a number of drug transporters, including MRP2 [144], MRP3, MRP5 [34] and MDR1. CAR is the key regulator of the metabolism of acetaminophen, a widely used analgesic (pain reliever) and antipyretic (fever reducer). Acetaminophen is hepatotoxic at high doses, and CAR regulates the expressions of many DMEs and drug transporters that both contributes to and alleviates acetaminophen induced hepatotoxicity, including the basolateral drug transporters MRP2, MRP3 and MRP4 that effluxes acetaminophen metabolites from the cell. Interestingly though, CAR−/− mice are more resistant toward acetaminophen induced hepatotoxicity than wild type mice. Acetaminophen itself is not hepatotoxic, but its phase I metabolite is – hepatotoxicity only occurs after cellular glutathione stores are depleted and phase I acetaminophen metabolites cannot be converted into inactive phase II metabolites. CAR−/− mice lack the xenobiotic sensor that initiates acetaminophen metabolism, so hepatotoxic acetaminophen metabolites do not accumulate [151, 166].
Altogether, CAR is an integral xenobiotic sensor that regulates the expressions of a wide spectrum of genes involved in the oxidative and conjugative metabolism and subsequent efflux of xenobiotics and endogenous substrates from cells.
4.3. PPARs
Like PXR and CAR, peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors involved in xenobiotic metabolism [167]. So far, three main PPAR families have been isolated: PPARα (NR1C1), PPARβ (also called PPARδ, NUC1, or NR1C2), and PPARγ (NR1C3), which is composed of two members, namely PPARγ1 and PPARγ2. These three families of PPARs have overlapping as well as distinct physiological functions, evident from their tissue- and developmental-specific expression patterns, and common and distinct ligands and target genes.
PPARα is predominantly expressed in the intestine, heart, liver, kidney and brown adipose tissues [168]. PPARβ is preferentially expressed in the small intestine, brain and kidney. PPARγ1 is abundantly expressed in liver, while PPARγ2 is expressed exclusively in adipose tissue for adipocyte differentiation [169]. While inactive PPAR-RXR heterodimers are bound to corepressor complexes, composed of nuclear receptor corepressors and silencing mediators for retinoid and thyroid hormone receptors (SMRTs) [170]. After ligand binding, PPARs undergo conformational changes that facilitate the release of corepressors and subsequent recruitment of transcription coactivators, including mediator complex subunit 1 (MED1), CREB-Binding Protein (CBP/p300), thyroid hormone receptor-associated protein 220 (TRAP220), PPAR-binding protein (PBP/PPARBP), and various coactivator-associated proteins including PRIP and PRIP-interacting proteins with methyltransferase domain (PIMT/NCoA6IP) and coactivators associated arginine methyltransferase/protein arginine N-methyltransferase 4 (CARM1/PRMT4) [171]. These PPAR complexes then modulate transcription by binding to peroxisome proliferator response elements (PPRE) located in the promoter regions of their target genes.
All the three PPAR isoforms are critical to maintaining energy homeostasis. PPARs regulate various aspects of lipid metabolism including adipogenesis [172]; nevertheless, each PPAR member plays a distinct role in energy metabolism [173]. Futher, PPARs are also involved in other, diverse biological processes such as nitrogen metabolism, cell differentiation and proliferation, inflammation, diabetes and cancer.
PPARs have also been proven to be pivotal in the regulation of phase I DMEs, phase II DMEs and efflux transporters. PPARα has been reported, by several different groups, to transactivate CYP4A gene expression [174–176]. Treatment with the PPARα agonist Wy-14643 led to transactivation of CYP4A1 and CYP4A3 in mouse liver, which cannot be observed in PPARα knockout mice livers [177]. In rabbits, the 5′-flanking sequence adjacent to the DR1 element upstream of the CYP4A6 gene was determined to be the PPARα binding site [178]. Further, several PPREs were found in the promoter regions of CYP4A1 and CYP4A6 genes [179]; however, PPARα failed to transactivate CYP4A expression in primary cultures of human hepatocytes [180]. PPARα is also able to regulate murine CYP8B1 expression [181]. Interestingly, PPARα and its agonists have been reported to repress CYP7A1 activity, by attenuating the transactivation of CYP7A1 through HNF-4 [182]. Moreover, recent application of whole-genome DNA microarrays revealed that PPARs modulate a broad variety of CYP genes in human hepatocytes, including CYP2A, CYP2A6, CYP2C8 and CYP2E [180].
PPARs also modulate the expression of several phase II conjugating enzymes. PPARα/RXR heterodimers can bind to PPREs and control the transcriptional activation of UGTs. PPARα activators, such as diethylhexyl phthalate and ciprofibrate, increase UGT isoenzyme mRNA expression in mice [136]. PPARα and PPARγ regulate UGT1A9 expression and activity, in response to arachidonic and linoleic acid metabolites, in human hepatocytes and macrophages and murine adipocytes by binding to a DR-1 response element located between −719 to −706 bp in the promoter region of UGT1A9 gene [183, 184]. Fibrates also induce the expression of the phase II DME SULT2A in a PPARα-dependent manner [185]. In addition, fibrates also activates PPARα and induces UGT2B4 expression in human hepatocytes, a phenomenon that cannot be observed in PPARα-null mice. Treatment with PPARα agonists fenofibric acid and Wy-14643 also leads to UGT2B4 mRNA transactivation in human HepG2 and Huh7 cells [186]. Other phase II conjugating enzymes, such as the GST enzymes GSTA1 and GSTM2 are also regulated by PPARα activators [180, 187].
Accumulating studies show that PPARs also regulate the genomic expression of drug transporters. In mice, PPARα promotes MDR2 synthesis [188]. In the presence of their agonists, overexpression of either PPARα or PPARγ upregulate ABCA1 gene expression [189]. PPARγ agonist rosiglitazone is promotes BCRP transcription and protein expression in monocyte-derived dendritic cells, while the PPARγ antagonist GW9662 blocks the induction of BCRP expression after rosiglitazone treatment [190].
In summary, the above studies show that PPARs are important regulators of DME and efflux transporter expressions. Clinical use of PPARs antagonists may profoundly affect the metabolism and clearance therapeutic molecules.
4.4. Aryl hydrocarbon receptor (AHR)
The aryl hydrocarbon receptor (AHR), also known as the dioxin receptor, is a member of the bHLH-PAS (basic Helix-Loop-Helix–Per-ARNT-Sim) family of transcriptional regulators. AHR resembles CAR and PXR in several ways, including intracellular localization, ligand binding/activation, nuclear localization and target gene activation [191]. In the absence of ligands, AHR is retained in the cytoplasm, where it is bound to a complex consisting of the chaperone HSP90 [192], AIP (Aryl Hydrocarbon Receptor-Interacting Protein), p23 [193], and hepatitis virus X protein-associated protein (XAP-2) [194], also known as ARA9 and AIP. The HSP90 and p23 protect the receptor from proteolysis [195], while XAP2 masks the nuclear localization sequence (NLS) of AHR, and thereby preventing its inappropriate transport into the nucleus [196, 197]. After ligand binding, HSP90 is released from the complex and AHR, still inside the complex, undergoes a conformational change that exposes the AHR NLS. The NLS then guide the receptor into the nucleus, where AHR forms a heterodimer with aryl hydrocarbon receptor nuclear translocator (ARNT). Then, the ligand-bound AHR-ARNT heterodimer binds to dioxin-responsive elements (DREs) in the promoter regions of a wide range of genes involved in carcinogen and drug metabolism. AHR is capable of recognizing a broad spectrum of non-aromatic and non-halogenated carcinogens and xenobiotic chemicals, including polycyclic aromatic hydrocarbons, aflatoxin benzo[a]pyrene, heterocyclic amines, 2,3,7,8-tetrachlorodibenzo-p-dioxin, omeprazole, and 3-methylcholanthrene [198, 199].
To date, a number of phase I/II and drug transporters, including CYP1As, CYP1Bs, UGT1As [133, 200–203] and ABCG2/BCRP [204], have been identified as AHR targets. Notably, by recognizing similar modules in the distal promoter region of target genes, AHR cross-talks with PXR/CAR to orchestrate xenobiotic detoxification [131, 205, 206]. Recently, Korzeniewski et al. revealed that AHR is also potentially involved in tumor progression, since it was highly expressed in hyperplasic breast cancer specimens and correlates with significantly lower survival rates [207]. In conclusion, AHR is involved in drug metabolism and may play an important role in tumor progression.
5. LXR and FXR
Two other nuclear receptors that regulate DMEs and efflux transporters are the Liver X receptor (LXR) and the Farnesol X receptor (FXR).
To date, two members of LXR family, LXRα and LXRβ, have been identified. LXRα is predominantly expressed in the liver and, to a lesser extent, the kidney, spleen and intestine [208]. LXRβ is expressed in nearly every tissue [209]. After ligand binding, LXR forms a heterodimer with RXR, which recognizes DR-4 motifs, termed “LXR response elements” (LXRE), in the proximal promoter regions of target genes [67]. As a cholesterol sensor, LXR recognizes several cholesterol metabolites, including (24S,25)-epoxy-cholesterol and (24S)-hydroxy-cholesterol [210, 211], and regulates cholesterol decomposition into hydrophilic bile acids through cholesterol 7α-hydroxylase (CYP7A) in the liver [212–214]. LXR also regulates the expression of several DMEs in humans, including CYP3A4 and CYP2B6 [215].
FXR is mainly expressed in the liver and intestine. As a bile acid receptor, FXR activation suppresses hepatic bile acid biosynthesis and bile acid transport from the intestinal lumen to the liver. Bile acids, including chenodeoxycholic acid, deoxycholic acid, lithocholic acid and cholic acid, are potent FXR activators. After ligand binding, FXR forms a heterodimer with RXR, which binds to FXR response elements (FXREs) in the xenobiotic responsive enhancer modules (XREMs) of various target genes. FXR has been reported to regulate the expression of cholesterol 7α-hydroxylase (CYP7A), intra-aortic balloon pump (IABP), phospholipid transfer protein (PLTP) and bile salt export pump (BSEP, ABCC11) [216–218].
Since both LXR and FXR are primarily responsible for the metabolism and homeostasis of endogenous substrates, such as cholesterol and bile acid, but not for the detoxification of xenobiotics, like chemotherapeutics, we will not expatiate on their activation and functions in this review.
6. Expression of xenobiotic receptors in cancer cells and implications in chemotherapy
6.1. Expression and activities of xenobiotic receptors in cancer cells
NR xenosensors, such as CAR and PXR, are expressed in cancer cells. Studies have shown that the CAR activator CITCO inhibits the proliferation and induces apoptosis of glioma stem cells [219]. Although PXR is mainly expressed in liver and intestinal tissues [76], its expression has been also detected in breast, prostate, and gastrointestinal cancers [28, 220–223], underscoring its clinical relevance in oncology. In a study conducted by Dotzlaw et al, PXR expression was detected in human breast cancer specimens and in T-47D, MCF-7, T-47D-5, and MDA-MB-231, but not in BT20 or MDA-MB-468, human breast cancer cells [222]. PXR activation in breast cancer cells stimulated the expression of organic anion transporter polypeptide 1A2 (OATP1A2), leading to enhanced estrogen effects [224]. Similar to CAR, PXR activation has been found to inhibit breast cancer cell proliferation [225]. Studies conducted by our lab have determiened that the PXR expressed in tumor cells is functional. PXR activation in breast and prostate cancer cells stimulates the expression of CYP3A4 and ABCB1 and increases cancer cell resistance toward chemotherapeutics [28, 226]. Using a web-based program (http://ist.genesapiens.org), we conducted an in silico analysis of the correlation between PXR/CAR expression and mRNA levels of selective DMEs and transporters in prostate tumor tissues. As shown in Table 1, CYP3A4 mRNA expression is strongly correlated with the expression of PXR and CAR, and ABCB1 mRNA expression is significantly correlated with PXR expression in prostate tumor tissues.
6.2. Activation of xenobiotic nuclear receptors by cancer chemotherapeutics
Furthermore, commonly used chemotherapeutic agents can activate human PXR, underscoring the importance of xenobiotic nuclear receptors, such as PXR, in cancer therapy [91, 227, 228]. Amoung the chemotherapeutics, both hydroxylated and non-hydroxylated tamoxifen activate PXR [229] and the anti-mitotic agent paclitaxel activates PXR and enhances P-gp mediated drug clearance [92]. Nevertheless, not all chemotherapeutics are subjected to PXR mediated drug metabolism. The semi-synthetic paclitaxel analog docetaxel is not a potent PXR activator and exhibits significantly longer plasma and intercellular half-lives [91].
6.3. PXR and the expression of ABC efflux transporters in cancer cells
The expression of functional PXR in cancer cells and activation of PXR by chemotherapeutics or other compounds can significantly impact tumor responses to chemotherapy [28, 226]. Enhanced expression of drug transporters, resulting from PXR activating, increases the severity of drug resistance exhibited by tumor cells. Many cancers acquire resistance to chemotherapeutics principally through MDR. Therapeutic agents that activate PXR may achieve less clinical efficacy in patients with high tumor PXR expression, since PXR may alter local concentrations of these anti-neoplastic agents. Therefore, untoward activation of PXR in tumor cells can lead to altered metabolism and disposition of chemotherapeutics within tumor tissues. Decreased concentrations of chemotherapeutics at the tumor site, in turn, substantially impact the intended efficacy of chemotherapy, especially in vivo – when the bioavailability of active chemotherapeutics is often a limiting factor.
6.4. Implications of PXR activation in drug-drug interactions during cancer chemotherapy
Another implication of untoward PXR activation is the potential drug-drug interactions involved in cancer therapeutics. Because of their role in the regulation of DMEs and drug transporters, nuclear receptors’ role in the drug resistance is of great clinical significance and scientific interest. Cancer patients often take other drugs, such as pain relievers, anti-depressants, anti-emetics, or alternative medicines including herbal supplements, in addition to the drugs that target cancer [230]. In many of these cases, PXR activation by drugs like rifampicin or the St. John’s Wort component hyperforin leads to upregulations of DMEs including CYP3A isoforms that metabolize concomitant medications. Rifampicin markedly reduces the serum level of bilirubin by enhancing UGT1A1 and MRP2 expressions [231]. St John’s Wort (SJW), a herbal anti-depressant, has been reported to trigger drug-drug interactions with various other drugs [232]. Coadministration of SJW compromises the bioavailability and toxicity of immunosuppressants (cyclosporine) [233] and HIV protease inhibitors (nevirapine) [234]. As shown by Kliewer and colleagues, SJW induced drug-drug interactions are mediated through the transactivation of human PXR and CYP3A4 [94]. Hyperforin, an important component of St. John’s Wort, induces upregulations of DMEs including CYP3A, which significantly impairs the anti-neoplastic efficacy, through decreased plasma levels, of irinotecan (CPT-11) [235]. Rifampicin, an important drug used during tuberculosis treatment, is a potent human PXR activator and impairs the efficacy of drugs that are metabolized by CYP3A4 and CYP2B6, including paclitaxel, midazolam and tamoxifen [236, 237]. Therefore, when designing combination therapy or when patients are provided with dietary supplements, potential activations of drug metabolism and resistance pathways must considered.
7. Summaries and perspective
PXR and other nuclear receptors are increasingly recognized as “master” xenosensors. These nuclear receptors regulate an extensive spectrum of genes involved in the metabolism and disposition of both endobiotics and xenobiotics. PXR and CAR share many response elements and crosstalk extensively during the transactivation of their target genes [238]. These “master” xenobiotic receptors are also promiscuous and can be activated by most chemotherapeutic agents. While undergoing treatment, cancer patients, especially those who are given several types of drugs, may experience loss of therapeutic efficacy or severe toxicity if PXR/CAR can become activated by anti-neoplastic agents or by other co-administered drugs. The activity of these nuclear receptors affects the pharmacokinetics, toxicity, and drug-drug interactions of many xenobiotic substances including chemotherapeutics.
Therapeutic agents that do not activate PXR/CAR may be preferentially adopted to circumvent the nuclear receptors that are involved drug resistance. Chemical structures can also be modified to prevent these drugs from acting as nuclear receptor ligands. For example, while paclitaxel is potent PXR activator and induces Pgp-mediated drug clearance, its semi-synthetic derivative docetaxel is a poor PXR activator and exhibits enhanced intracellular half-life and efficacy [92]. Targeted therapies that consider PXR or CAR activities/function should be pursued to avoid MDR or re-sensitize patients toward therapeutic agents.
Alternatively, ligands (agonists or antagonists) that modulate nuclear receptor activities have significant potentials in therapeutic applications. Currently, a growing number of PXR antagonists have been characterized. Enilconazole, ketoconazole, enilconazole, HIV protease inhibitor A-792611, coumestrol and sulforaphane have all been demonstrated to inhibit PXR activation (Table 2) [239, 240]. Suppression of PXR or CAR prior to chemotherapy may ameliorate drug resistance in patients that exhibit severe nuclear receptors mediated drug metabolism. Further studies that identify PXR antagonists are urgently needed, since these antagonists can potentially prevent PXR induced drug-drug interactions. The PXR antagonists should, however, be used with cautions. PXR plays conserved roles in the metabolism of endobiotics, including bile acids and steroid hormones, and long-term inhibition of PXR or CAR may also cause hypersensitivity to xenobiotics and other toxins. Therefore, development of tissue-specific PXR antagonists will provide the option to selectively disrupt PXR-mediated drug disposition in specific tissues or organs. Alternatively, chemotherapeutics that also do not activate PXR or other xenosensors can circumvent MDR and ensure treatment efficacy.
Table 2.
PXR antagonists
| Compound | Indications/Usage | IC50 | References |
|---|---|---|---|
| Et-743 | Antineoplastic Agent | 2 nM | [92] |
| Ketoconazole And Its Analogues (Fluconazole And Enilconazol) | Antifungal Agent | 20 μM | [239, 240] |
| Sulforaphane | Dietary Isothiocyanate | 12 μM | [261] |
| Coumestrol | Phytoestrogen | 12 μM | [262] |
| A-792611 | HIV Protease Inhibitor | 2 μM | [263] |
| Polychlorinated Biphenyls | Synthetic Hydrocarbon Compounds | 10uM | [264] |
| Camptothecin | Antineoplastic Agent | 0.58 μM | [90] |
| Ecteinascidin-743 (ET-743) | Antineoplastic Agent | 3 nM | [92] |
| Le unamide | Pyrimidine Synthesis Inhibitor | 6.8 μM | [265] |
| Itraconazole | Triazole Antifungal Agent | 8.96 μM | [265] |
| SPB03255 | Identified By Using Ligand-Based And Structure-Based (Docking) In Silica Methods | 5–25 μM | [265] |
ABBREVIATIONS
- ABC
ATP binding cassette
- AF
Activation function
- AhR
Aryl hydrocarbon receptor
- AHRE
AhR element
- AIP
Aryl hydrocarbon receptor-interacting protein
- AKRs
Aldo-keto reductases
- ARE
Antioxidant response element
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- BSEP
Bile salt export pump
- CAR
Constitutive androstane receptor
- CCRP
Cytoplasmic CAR retention protein
- CRE
CREB response element
- CYP
Cytochrome P450
- DBD
DNA-binding domain
- DEX
Dexamethasone
- DMEs
Drug metabolizing enzymes
- DRE
Dioxin-responsive element
- EPHs
Epoxide hydrolases
- ER
Endoplasmic reticulum
- FXR
Farnesoid X receptor
- FXRE
FXR response element
- GR
Glucocorticoid receptor
- GSTs
Glutathione S-transferases
- HREs
Hormone response elements
- HSP90
90-kDa heat shock protein
- IABP
Intra-aortic balloon pump
- LBD
Ligand binding domain
- LBP
Ligand binding pocket
- LXR
Liver X receptor
- LXRE
LXR response element
- MDR
Multidrug resistance
- MRP
Multidrug resistance-associated protein
- NATs
N-acetyltransferases
- NCoR
Nuclear receptor corepressor
- NLS
Nuclear localization sequence
- NMO
NAD(P)H: menadione reductase
- OATPs
Organic anion-transporting polypeptides
- OCTs
Organic cation transporters
- P-gp
P-glycoprotein
- PB
Phenobarbital
- PBP/PPARBP
PPAR-binding protein
- PCAF
P300/CBP-associated factor
- PCN
Pregnenolone 16-alpha carbonitrile
- PLTP
Phospholipid transfer protein
- PPAR
Peroxisome proliferator activated receptor
- PPRE
Peroxisome proliferator response element
- PXR
Pregnane X receptor
- QR
Quinone reductase
- RAREs
Retinoic acid response elements
- RXR
Retinoid X receptor
- SJW
St. John’s Wort
- SMRT
Silencing mediator for retinoid and thyroid hormone receptor
- SRCs
Steroid receptor co-activators
- SULTs
Sulfotransferases
- TCPOBOP
1,4- bis[2-(3,5-dichloropyridyloxy)]benzene
- TIF2
Transcriptional mediators/intermediary factor 2
- TPR
Tetratricopeptide repeat
- UGTs
UDP-glucuronosyltranferases
- XO
Xanthine oxidase
- XRE
Xenobiotic response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Bates SE, Regis JI, Robey RW, Zhan Z, Scala S, Meadows BJ. Chemoresistance in the clinic: overview 1994. Bull Cancer. 1994;81 (Suppl 2):55s–61s. [PubMed] [Google Scholar]
- 3.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–92. [PubMed] [Google Scholar]
- 4.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–56. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 5.Mansouri A, Henle KJ, Nagle WA. Tumor drug-resistance: a challenge to therapists and biologists. Am J Med Sci. 1994;307:438–44. doi: 10.1097/00000441-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9 (Suppl 5):31–40. doi: 10.1634/theoncologist.9-90005-31. [DOI] [PubMed] [Google Scholar]
- 7.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 8.Camp ER, Li J, Minnich DJ, Brank A, Moldawer LL, MacKay SL, et al. Inducible nuclear factor-kappaB activation contributes to chemotherapy resistance in gastric cancer. J Am Coll Surg. 2004;199:249–58. doi: 10.1016/j.jamcollsurg.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Johnson WW. P-glycoprotein-mediated efflux as a major factor in the variance of absorption and distribution of drugs: modulation of chemotherapy resistance. Methods Find Exp Clin Pharmacol. 2002;24:501–14. doi: 10.1358/mf.2002.24.8.705071. [DOI] [PubMed] [Google Scholar]
- 10.Haber M, Smith J, Bordow SB, Flemming C, Cohn SL, London WB, et al. Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J Clin Oncol. 2006;24:1546–53. doi: 10.1200/JCO.2005.01.6196. [DOI] [PubMed] [Google Scholar]
- 11.Hua J, Mutch DG, Herzog TJ. Stable suppression of MDR-1 gene using siRNA expression vector to reverse drug resistance in a human uterine sarcoma cell line. Gynecol Oncol. 2005;98:31–8. doi: 10.1016/j.ygyno.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–7. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 13.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–37. [PubMed] [Google Scholar]
- 14.Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 15.Ioffe ML, White E, Nelson DA, Dvorzhinski D, DiPaola RS. Epothilone induced cytotoxicity is dependent on p53 status in prostate cells. Prostate. 2004;61:243–7. doi: 10.1002/pros.20108. [DOI] [PubMed] [Google Scholar]
- 16.Murata T, Haisa M, Uetsuka H, Nobuhisa T, Ookawa T, Tabuchi Y, et al. Molecular mechanism of chemoresistance to cisplatin in ovarian cancer cell lines. Int J Mol Med. 2004;13:865–8. [PubMed] [Google Scholar]
- 17.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–49. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 18.Righetti SC, Della Torre G, Pilotti S, Menard S, Ottone F, Colnaghi MI, et al. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996;56:689–93. [PubMed] [Google Scholar]
- 19.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–92. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 20.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer. 2003;10:43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 21.Minemura M, Tanimura H, Tabor E. Overexpression of multidrug resistance genes MDR1 and cMOAT in human hepatocellular carcinoma and hepatoblastoma cell lines. Int J Oncol. 1999;15:559–63. doi: 10.3892/ijo.15.3.559. [DOI] [PubMed] [Google Scholar]
- 22.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Martin E, Pizarro RM, Martinez C, Gutierrez-Martin Y, Perez G, Jover R, et al. Acquired resistance to the anticancer drug paclitaxel is associated with induction of cytochrome P450 2C8. Pharmacogenomics. 2006;7:575–85. doi: 10.2217/14622416.7.4.575. [DOI] [PubMed] [Google Scholar]
- 24.Gerbal-Chaloin S, Daujat M, Pascussi JM, Pichard-Garcia L, Vilarem MJ, Maurel P. Transcriptional regulation of CYP2C9 gene. Role of glucocorticoid receptor and constitutive androstane receptor. J Biol Chem. 2002;277:209–17. doi: 10.1074/jbc.M107228200. [DOI] [PubMed] [Google Scholar]
- 25.Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annual review of pharmacology and toxicology. 2001;41:123–43. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- 26.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 27.Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, et al. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer research. 2006;66:535–42. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007;67:10361–7. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- 29.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annual review of pharmacology and toxicology. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Liu M, Zhai Y, Xie W. The Anti-Apoptotic Role of Pregnane X Receptor in Human Colon Cancer Cells. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuyama H, Hiramatsu Y, Kodama J, Kudo T. Expression and potential roles of pregnane X receptor in endometrial cancer. J Clin Endocrinol Metab. 2003;88:4446–54. doi: 10.1210/jc.2003-030203. [DOI] [PubMed] [Google Scholar]
- 32.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol. 2007;47:566–78. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 33.Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem. 2001;276:37739–42. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 34.Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–28. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- 35.Ramadoss P, Marcus C, Perdew GH. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin Drug Metab Toxicol. 2005;1:9–21. doi: 10.1517/17425255.1.1.9. [DOI] [PubMed] [Google Scholar]
- 36.Nebert DW. Drug Metabolism: Evolution. eLS: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 37.Gibbons GF. The role of cytochrome P450 in the regulation of cholesterol biosynthesis. Lipids. 2002;37:1163–70. doi: 10.1007/s11745-002-1016-x. [DOI] [PubMed] [Google Scholar]
- 38.Neve EP, Ingelman-Sundberg M. Intracellular transport and localization of microsomal cytochrome P450. Anal Bioanal Chem. 2008;392:1075–84. doi: 10.1007/s00216-008-2200-z. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, et al. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- 40.Lewis DF. P450 structures and oxidative metabolism of xenobiotics. Pharmacogenomics. 2003;4:387–95. doi: 10.1517/phgs.4.4.387.22752. [DOI] [PubMed] [Google Scholar]
- 41.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annual review of pharmacology and toxicology. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Maurel P. Cytochromes P450: Metabolic and Toxicological Aspects. 1996. pp. 241–70. [Google Scholar]
- 43.Quattrochi LC, Guzelian PS. Cyp3A regulation: from pharmacology to nuclear receptors. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:615–22. [PubMed] [Google Scholar]
- 44.Hinson JA, Forkert PG. Phase II enzymes and bioactivation. Can J Physiol Pharmacol. 1995;73:1407–13. doi: 10.1139/y95-196. [DOI] [PubMed] [Google Scholar]
- 45.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–61. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 46.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annual review of pharmacology and toxicology. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 47.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–85. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 48.Huang Z, Roy P, Waxman DJ. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol. 2000;59:961–72. doi: 10.1016/s0006-2952(99)00410-4. [DOI] [PubMed] [Google Scholar]
- 49.Bohan A, Boyer JL. Mechanisms of hepatic transport of drugs: implications for cholestatic drug reactions. Semin Liver Dis. 2002;22:123–36. doi: 10.1055/s-2002-30099. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H, Sugiyama Y. Transport of drugs across the hepatic sinusoidal membrane: sinusoidal drug influx and efflux in the liver. Semin Liver Dis. 2000;20:251–63. doi: 10.1055/s-2000-8408. [DOI] [PubMed] [Google Scholar]
- 51.Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther. 87:39–47. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 53.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 54.Choi HK, Yang JW, Roh SH, Han CY, Kang KW. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer. 2007;14:293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 55.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–94. [PubMed] [Google Scholar]
- 56.Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem. 2003;10:41–9. doi: 10.2174/0929867033368619. [DOI] [PubMed] [Google Scholar]
- 57.Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–54. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 58.Jansen WJ, Hulscher TM, van Ark-Otte J, Giaccone G, Pinedo HM, Boven E. CPT-11 sensitivity in relation to the expression of P170-glycoprotein and multidrug resistance-associated protein. British journal of cancer. 1998;77:359–65. doi: 10.1038/bjc.1998.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gearry RB, Day AS, Barclay ML, Leong RWL, Sparrow MP. Azathioprine and allopurinol: A two-edged interaction. Journal of Gastroenterology and Hepatology. 25:653–5. doi: 10.1111/j.1440-1746.2010.06254.x. [DOI] [PubMed] [Google Scholar]
- 60.Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–54. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 61.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 62.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumberg B, Evans RM. Orphan nuclear receptors--new ligands and new possibilities. Genes Dev. 1998;12:3149–55. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- 64.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature. 1995;375:377–82. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 65.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 66.Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–33. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42:1331–57. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 68.Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 69.Freedman LP. Increasing the complexity of coactivation in nuclear receptor signaling. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 70.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–41. [PubMed] [Google Scholar]
- 71.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Nie D. Pregnane X receptor and its potential role in drug resistance in cancer treatment. Recent Pat Anticancer Drug Discov. 2009;4:19–27. doi: 10.2174/157489209787002498. [DOI] [PubMed] [Google Scholar]
- 73.Oesch-Bartlomowicz B, Oesch F. Phosphorylation of cytochromes P450: First discovery of a posttranslational modification of a drug-metabolizing enzyme. Biochemical and Biophysical Research Communications. 2005;338:446–9. doi: 10.1016/j.bbrc.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 74.Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Advanced drug delivery reviews. 62:1238–49. doi: 10.1016/j.addr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 76.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–13. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dussault IFB. The nuclear receptor PXR: a master regulator of “homeland” defense. Crit Rev Eukaryot Gene Expr. 2002;12:53–64. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.30. [DOI] [PubMed] [Google Scholar]
- 78.Carnahan VE, Redinbo MR. Structure and function of the human nuclear xenobiotic receptor PXR. Curr Drug Metab. 2005;6:357–67. doi: 10.2174/1389200054633844. [DOI] [PubMed] [Google Scholar]
- 79.Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279:49307–14. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- 80.Kawana K, Ikuta T, Kobayashi Y, Gotoh O, Takeda K, Kawajiri K. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol Pharmacol. 2003;63:524–31. doi: 10.1124/mol.63.3.524. [DOI] [PubMed] [Google Scholar]
- 81.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekins S, Kortagere S, Iyer M, Reschly EJ, Lill MA, Redinbo MR, et al. Challenges predicting ligand-receptor interactions of promiscuous proteins: the nuclear receptor PXR. PLoS Comput Biol. 2009;5:e1000594. doi: 10.1371/journal.pcbi.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chrencik JE, Orans J, Moore LB, Xue Y, Peng L, Collins JL, et al. Structural disorder in the complex of human pregnane X receptor and the macrolide antibiotic rifampicin. Mol Endocrinol. 2005;19:1125–34. doi: 10.1210/me.2004-0346. [DOI] [PubMed] [Google Scholar]
- 84.Watkins RE, Davis-Searles PR, Lambert MH, Redinbo MR. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331:815–28. doi: 10.1016/s0022-2836(03)00795-2. [DOI] [PubMed] [Google Scholar]
- 85.Watkins RE, Maglich JM, Moore LB, Wisely GB, Noble SM, Davis-Searles PR, et al. 2.1 A crystal structure of human PXR in complex with the St. John’s wort compound hyperforin. Biochemistry. 2003;42:1430–8. doi: 10.1021/bi0268753. [DOI] [PubMed] [Google Scholar]
- 86.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 87.Ekins S, Erickson JA. A pharmacophore for human pregnane X receptor ligands. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:96–9. doi: 10.1124/dmd.30.1.96. [DOI] [PubMed] [Google Scholar]
- 88.Watkins RE, Noble SM, Redinbo MR. Structural insights into the promiscuity and function of the human pregnane X receptor. Curr Opin Drug Discov Devel. 2002;5:150–8. [PubMed] [Google Scholar]
- 89.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–66. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Tang Y, Robbins GT, Nie D. Camptothecin attenuates cytochrome P450 3A4 induction by blocking the activation of human pregnane X receptor. J Pharmacol Exp Ther. 334:999–1008. doi: 10.1124/jpet.110.168294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mani S, Huang H, Sundarababu S, Liu W, Kalpana G, Smith AB, et al. Activation of the steroid and xenobiotic receptor (human pregnane X receptor) by nontaxane microtubule-stabilizing agents. Clin Cancer Res. 2005;11:6359–69. doi: 10.1158/1078-0432.CCR-05-0252. [DOI] [PubMed] [Google Scholar]
- 92.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–90. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 93.Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, et al. Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:608–12. doi: 10.1124/dmd.30.5.608. [DOI] [PubMed] [Google Scholar]
- 94.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding XS, Staudinger JL. Induction of Drug Metabolism by Forskolin: The Role of the Pregnane X Receptor and the Protein Kinase A Signal Transduction Pathway. J Pharmacol Exp Ther. 2005;312:849–56. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- 96.Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–77. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 97.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–23. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 99.El-Sankary W, Gibson GG, Ayrton A, Plant N. Use of a reporter gene assay to predict and rank the potency and efficacy of CYP3A4 inducers. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:1499–504. [PubMed] [Google Scholar]
- 100.Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem. 2001;276:33309–12. doi: 10.1074/jbc.C100375200. [DOI] [PubMed] [Google Scholar]
- 101.Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:1075–82. doi: 10.1124/dmd.104.000299. [DOI] [PubMed] [Google Scholar]
- 102.Ruhl R, Sczech R, Landes N, Pfluger P, Kluth D, Schweigert FJ. Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane X receptor. Eur J Nutr. 2004;43:336–43. doi: 10.1007/s00394-004-0475-1. [DOI] [PubMed] [Google Scholar]
- 103.Ruhl R. Induction of PXR-mediated metabolism by beta-carotene. Biochim Biophys Acta. 2005;1740:162–9. doi: 10.1016/j.bbadis.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Takeshita A, Koibuchi N, Oka J, Taguchi M, Shishiba Y, Ozawa Y. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur J Endocrinol. 2001;145:513–7. doi: 10.1530/eje.0.1450513. [DOI] [PubMed] [Google Scholar]
- 105.Takeshita A, Inagaki K, Igarashi-Migitaka J, Ozawa Y, Koibuchi N. The endocrine disrupting chemical, diethylhexyl phthalate, activates MDR1 gene expression in human colon cancer LS174T cells. J Endocrinol. 2006;190:897–902. doi: 10.1677/joe.1.06664. [DOI] [PubMed] [Google Scholar]
- 106.Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002;64:1513–9. doi: 10.1016/s0006-2952(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 107.Lemaire G, de Sousa G, Rahmani R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 2004;68:2347–58. doi: 10.1016/j.bcp.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 108.Masuyama H, Hiramatsu Y, Kunitomi M, Kudo T, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X receptor-mediated transcription. Mol Endocrinol. 2000;14:421–8. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- 109.Masuyama H, Inoshita H, Hiramatsu Y, Kudo T. Ligands have various potential effects on the degradation of pregnane X receptor by proteasome. Endocrinology. 2002;143:55–61. doi: 10.1210/endo.143.1.8578. [DOI] [PubMed] [Google Scholar]
- 110.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–50. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 111.Xue Y, Moore LB, Orans J, Peng L, Bencharit S, Kliewer SA, et al. Crystal structure of the pregnane X receptor-estradiol complex provides insights into endobiotic recognition. Mol Endocrinol. 2007;21:1028–38. doi: 10.1210/me.2006-0323. [DOI] [PubMed] [Google Scholar]
- 112.Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–82. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- 113.Wrighton SA, Schuetz EG, Thummel KE, Shen DD, Korzekwa KR, Watkins PB. The human CYP3A subfamily: practical considerations. Drug Metab Rev. 2000;32:339–61. doi: 10.1081/dmr-100102338. [DOI] [PubMed] [Google Scholar]
- 114.Li AP, Kaminski DL, Rasmussen A. Substrates of human hepatic cytochrome P450 3A4. Toxicology. 1995;104:1–8. doi: 10.1016/0300-483x(95)03155-9. [DOI] [PubMed] [Google Scholar]
- 115.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–7. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 116.Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: a review of effectiveness and safety in health care settings. Am J Infect Control. 2000;28:184–96. [PubMed] [Google Scholar]
- 117.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–9. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 118.Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, et al. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:348–58. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 120.Rana R, Chen Y, Ferguson SS, Kissling GE, Surapureddi S, Goldstein JA. Hepatocyte nuclear factor 4{alpha} regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 38:591–9. doi: 10.1124/dmd.109.030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y, Ferguson SS, Negishi M, Goldstein JA. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther. 2004;308:495–501. doi: 10.1124/jpet.103.058818. [DOI] [PubMed] [Google Scholar]
- 122.Ferguson SS, Chen Y, LeCluyse EL, Negishi M, Goldstein JA. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol. 2005;68:747–57. doi: 10.1124/mol.105.013169. [DOI] [PubMed] [Google Scholar]
- 123.Ferguson SS, LeCluyse EL, Negishi M, Goldstein JA. Regulation of human CYP2C9 by the constitutive androstane receptor: discovery of a new distal binding site. Mol Pharmacol. 2002;62:737–46. doi: 10.1124/mol.62.3.737. [DOI] [PubMed] [Google Scholar]
- 124.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:1467–72. [PubMed] [Google Scholar]
- 126.Hariparsad N, Chu X, Yabut J, Labhart P, Hartley DP, Dai X, et al. Identification of pregnane-X receptor target genes and coactivator and corepressor binding to promoter elements in human hepatocytes. Nucleic Acids Res. 2009;37:1160–73. doi: 10.1093/nar/gkn1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu MJ, Takahashi Y, Wada T, He J, Gao J, Tian Y, et al. The aldo-keto reductase Akr1b7 gene is a common transcriptional target of xenobiotic receptors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2009;76:604–11. doi: 10.1124/mol.109.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Xu C, Wang X, Staudinger JL. Regulation of tissue-specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:1539–47. doi: 10.1124/dmd.109.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Runge-Morris M, Wu W, Kocarek TA. Regulation of rat hepatic hydroxysteroid sulfotransferase (SULT2-40/41) gene expression by glucocorticoids: evidence for a dual mechanism of transcriptional control. Mol Pharmacol. 1999;56:1198–206. doi: 10.1124/mol.56.6.1198. [DOI] [PubMed] [Google Scholar]
- 131.Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A. 2003;100:4150–5. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregname X receptor, is required for induction of UDP-glucuronosyltranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:908–15. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- 133.Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem. 2003;278:15001–6. doi: 10.1074/jbc.M300645200. [DOI] [PubMed] [Google Scholar]
- 134.Shelby MK, Klaassen CD. Induction of rat UDP-glucuronosyltransferases in liver and duodenum by microsomal enzyme inducers that activate various transcriptional pathways. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:1772–8. doi: 10.1124/dmd.106.010397. [DOI] [PubMed] [Google Scholar]
- 135.Vyhlidal CA, Rogan PK, Leeder JS. Development and refinement of pregnane X receptor (PXR) DNA binding site model using information theory: insights into PXR-mediated gene regulation. J Biol Chem. 2004;279:46779–86. doi: 10.1074/jbc.M408395200. [DOI] [PubMed] [Google Scholar]
- 136.Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:847–56. doi: 10.1124/dmd.108.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sugatani J, Nishitani S, Yamakawa K, Yoshinari K, Sueyoshi T, Negishi M, et al. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol Pharmacol. 2005;67:845–55. doi: 10.1124/mol.104.007161. [DOI] [PubMed] [Google Scholar]
- 138.Falkner KC, Pinaire JA, Xiao GH, Geoghegan TE, Prough RA. Regulation of the rat glutathione S-transferase A2 gene by glucocorticoids: involvement of both the glucocorticoid and pregnane X receptors. Mol Pharmacol. 2001;60:611–9. [PubMed] [Google Scholar]
- 139.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20:279–90. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 140.Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324:612–21. doi: 10.1124/jpet.107.129650. [DOI] [PubMed] [Google Scholar]
- 141.Duanmu Z, Locke D, Smigelski J, Wu W, Dahn MS, Falany CN, et al. Effects of dexamethasone on aryl (SULT1A1)-and hydroxysteroid (SULT2A1)-sulfotransferase gene expression in primary cultured human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:997–1004. doi: 10.1124/dmd.30.9.997. [DOI] [PubMed] [Google Scholar]
- 142.Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci U S A. 2002;99:13801–6. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–7. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- 144.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 145.Frank C, Makkonen H, Dunlop TW, Matilainen M, Vaisanen S, Carlberg C. Identification of pregnane X receptor binding sites in the regulatory regions of genes involved in bile acid homeostasis. J Mol Biol. 2005;346:505–19. doi: 10.1016/j.jmb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 146.Guo GL, Staudinger J, Ogura K, Klaassen CD. Induction of rat organic anion transporting polypeptide 2 by pregnenolone-16alpha-carbonitrile is via interaction with pregnane X receptor. Mol Pharmacol. 2002;61:832–9. doi: 10.1124/mol.61.4.832. [DOI] [PubMed] [Google Scholar]
- 147.Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–52. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 149.Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–7. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Huang W, Qatanani M, Evans RM, Moore DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517–22. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- 151.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–4. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 152.Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–23. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang H, Negishi M. Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab. 2003;4:515–25. doi: 10.2174/1389200033489262. [DOI] [PubMed] [Google Scholar]
- 154.Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol. 2003;64:1069–75. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- 155.Sueyoshi T, Moore R, Sugatani J, Matsumura Y, Negishi M. PPP1R16A, the membrane subunit of protein phosphatase 1beta, signals nuclear translocation of the nuclear receptor constitutive active/androstane receptor. Mol Pharmacol. 2008;73:1113–21. doi: 10.1124/mol.107.042960. [DOI] [PubMed] [Google Scholar]
- 156.Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, et al. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–5. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 157.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–83. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 158.Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–8. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Auerbach SS, Ramsden R, Stoner MA, Verlinde C, Hassett C, Omiecinski CJ. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res. 2003;31:3194–207. doi: 10.1093/nar/gkg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–37. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–26. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- 162.Lee CH, Ito Y, Yanagiba Y, Yamanoshita O, Kim H, Zhang SY, et al. Pyrene-induced CYP1A2 and SULT1A1 may be regulated by CAR and not by AhR. Toxicology. 2007;238:147–56. doi: 10.1016/j.tox.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 163.Yoshinari K, Yoda N, Toriyabe T, Yamazoe Y. Constitutive androstane receptor transcriptionally activates human CYP1A1 and CYP1A2 genes through a common regulatory element in the 5′-flanking region. Biochem Pharmacol. 79:261–9. doi: 10.1016/j.bcp.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 164.Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- 165.Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 166.Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, et al. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol. 2008;226:74–83. doi: 10.1016/j.taap.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 120(Suppl 1):S49–75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–66. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 169.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–73. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 170.Krogsdam AM, Nielsen CA, Neve S, Holst D, Helledie T, Thomsen B, et al. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor delta-mediated transactivation. Biochem J. 2002;363:157–65. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Viswakarma N, Jia Y, Bai L, Vluggens A, Borensztajn J, Xu J, et al. Coactivators in PPAR-Regulated Gene Expression. PPAR Res. 2010 doi: 10.1155/2010/250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 173.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–99. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Johnson EF, Palmer CN, Griffin KJ, Hsu MH. Role of the peroxisome proliferator-activated receptor in cytochrome P450 4A gene regulation. Faseb J. 1996;10:1241–8. doi: 10.1096/fasebj.10.11.8836037. [DOI] [PubMed] [Google Scholar]
- 175.Johnson EF, Palmer CN, Hsu MH. The peroxisome proliferator-activated receptor: transcriptional activation of the CYP4A6 gene. Ann N Y Acad Sci. 1996;804:373–86. doi: 10.1111/j.1749-6632.1996.tb18629.x. [DOI] [PubMed] [Google Scholar]
- 176.Aldridge TC, Tugwood JD, Green S. Identification and characterization of DNA elements implicated in the regulation of CYP4A1 transcription. Biochem J. 1995;306 (Pt 2):473–9. doi: 10.1042/bj3060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–22. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palmer CN, Hsu MH, Griffin HJ, Johnson EF. Novel sequence determinants in peroxisome proliferator signaling. J Biol Chem. 1995;270:16114–21. doi: 10.1074/jbc.270.27.16114. [DOI] [PubMed] [Google Scholar]
- 179.Johnson EF, Hsu MH, Savas U, Griffin KJ. Regulation of P450 4A expression by peroxisome proliferator activated receptors. Toxicology. 2002;181–182:203–6. doi: 10.1016/s0300-483x(02)00282-2. [DOI] [PubMed] [Google Scholar]
- 180.Richert L, Lamboley C, Viollon-Abadie C, Grass P, Hartmann N, Laurent S, et al. Effects of clofibric acid on mRNA expression profiles in primary cultures of rat, mouse and human hepatocytes. Toxicol Appl Pharmacol. 2003;191:130–46. doi: 10.1016/s0041-008x(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 181.Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gafvels M, Einarsson C, et al. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem. 2000;275:28947–53. doi: 10.1074/jbc.M002782200. [DOI] [PubMed] [Google Scholar]
- 182.Marrapodi M, Chiang JY. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Lipid Res. 2000;41:514–20. [PubMed] [Google Scholar]
- 183.Barbier O, Villeneuve L, Bocher V, Fontaine C, Torra IP, Duhem C, et al. The UDP-glucuronosyltransferase 1A9 enzyme is a peroxisome proliferator-activated receptor alpha and gamma target gene. J Biol Chem. 2003;278:13975–83. doi: 10.1074/jbc.M300749200. [DOI] [PubMed] [Google Scholar]
- 184.Turgeon D, Chouinard S, Belanger P, Picard S, Labbe JF, Borgeat P, et al. Glucuronidation of arachidonic and linoleic acid metabolites by human UDP-glucuronosyltransferases. J Lipid Res. 2003;44:1182–91. doi: 10.1194/jlr.M300010-JLR200. [DOI] [PubMed] [Google Scholar]
- 185.Fang HL, Strom SC, Cai H, Falany CN, Kocarek TA, Runge-Morris M. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol. 2005;67:1257–67. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- 186.Barbier O, Duran-Sandoval D, Pineda-Torra I, Kosykh V, Fruchart JC, Staels B. Peroxisome proliferator-activated receptor alpha induces hepatic expression of the human bile acid glucuronidating UDP-glucuronosyltransferase 2B4 enzyme. J Biol Chem. 2003;278:32852–60. doi: 10.1074/jbc.M305361200. [DOI] [PubMed] [Google Scholar]
- 187.Michel C, Desdouets C, Sacre-Salem B, Gautier JC, Roberts R, Boitier E. Liver gene expression profiles of rats treated with clofibric acid: comparison of whole liver and laser capture microdissected liver. Am J Pathol. 2003;163:2191–9. doi: 10.1016/S0002-9440(10)63577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kok T, Bloks VW, Wolters H, Havinga R, Jansen PL, Staels B, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem J. 2003;369:539–47. doi: 10.1042/BJ20020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barbier O, Fontaine C, Fruchart JC, Staels B. Genomic and non-genomic interactions of PPARalpha with xenobiotic-metabolizing enzymes. Trends Endocrinol Metab. 2004;15:324–30. doi: 10.1016/j.tem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 190.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, et al. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281:23812–23. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 191.Li H, Wang H. Activation of xenobiotic receptors: driving into the nucleus. Expert Opin Drug Metab Toxicol. 6:409–26. doi: 10.1517/17425251003598886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–5. [PubMed] [Google Scholar]
- 193.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem. 1999;274:13519–24. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 194.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18:978–88. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pongratz I, Mason GG, Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992;267:13728–34. [PubMed] [Google Scholar]
- 196.Petrulis JR, Hord NG, Perdew GH. Subcellular localization of the aryl hydrocarbon receptor is modulated by the immunophilin homolog hepatitis B virus X-associated protein 2. J Biol Chem. 2000;275:37448–53. doi: 10.1074/jbc.M006873200. [DOI] [PubMed] [Google Scholar]
- 197.Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH. The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem. 2003;278:2677–85. doi: 10.1074/jbc.M209331200. [DOI] [PubMed] [Google Scholar]
- 198.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–34. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 199.Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol Pharmacol. 1993;43:504–8. [PubMed] [Google Scholar]
- 200.Hankinson O, Brooks BA, Weir-Brown KI, Hoffman EC, Johnson BS, Nanthur J, et al. Genetic and molecular analysis of the Ah receptor and of Cyp1a1 gene expression. Biochimie. 1991;73:61–6. doi: 10.1016/0300-9084(91)90075-c. [DOI] [PubMed] [Google Scholar]
- 201.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annual review of pharmacology and toxicology. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 202.Emi Y, Ikushiro S, Iyanagi T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996;271:3952–8. doi: 10.1074/jbc.271.7.3952. [DOI] [PubMed] [Google Scholar]
- 203.Munzel PA, Schmohl S, Buckler F, Jaehrling J, Raschko FT, Kohle C, et al. Contribution of the Ah receptor to the phenolic antioxidant-mediated expression of human and rat UDP-glucuronosyltransferase UGT1A6 in Caco-2 and rat hepatoma 5L cells. Biochem Pharmacol. 2003;66:841–7. doi: 10.1016/s0006-2952(03)00389-7. [DOI] [PubMed] [Google Scholar]
- 204.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–63. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 205.Gerbal-Chaloin S, Pichard-Garcia L, Fabre JM, Sa-Cunha A, Poellinger L, Maurel P, et al. Role of CYP3A4 in the regulation of the aryl hydrocarbon receptor by omeprazole sulphide. Cell Signal. 2006;18:740–50. doi: 10.1016/j.cellsig.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 206.Patel RD, Hollingshead BD, Omiecinski CJ, Perdew GH. Aryl-hydrocarbon receptor activation regulates constitutive androstane receptor levels in murine and human liver. Hepatology. 2007;46:209–18. doi: 10.1002/hep.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Korzeniewski N, Wheeler S, Chatterjee P, Duensing A, Duensing S. A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification - implications for chemoprevention. Mol Cancer. 9:153. doi: 10.1186/1476-4598-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–45. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 209.Song C, Hiipakka RA, Kokontis JM, Liao S. Ubiquitous receptor: structures, immunocytochemical localization, and modulation of gene activation by receptors for retinoic acids and thyroid hormones. Ann N Y Acad Sci. 1995;761:38–49. doi: 10.1111/j.1749-6632.1995.tb31367.x. [DOI] [PubMed] [Google Scholar]
- 210.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 211.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 212.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 213.Jelinek DF, Russell DW. Structure of the rat gene encoding cholesterol 7 alpha-hydroxylase. Biochemistry. 1990;29:7781–5. doi: 10.1021/bi00486a001. [DOI] [PubMed] [Google Scholar]
- 214.Li YC, Wang DP, Chiang JY. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. J Biol Chem. 1990;265:12012–9. [PubMed] [Google Scholar]
- 215.Handschin C, Podvinec M, Amherd R, Looser R, Ourlin JC, Meyer UA. Cholesterol and bile acids regulate xenosensor signaling in drug-mediated induction of cytochromes P450. J Biol Chem. 2002;277:29561–7. doi: 10.1074/jbc.M202739200. [DOI] [PubMed] [Google Scholar]
- 216.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 217.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 218.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 219.Chakraborty S, Kanakasabai S, Bright JJ. Constitutive androstane receptor agonist CITCO inhibits growth and expansion of brain tumour stem cells. British journal of cancer. 104:448–59. doi: 10.1038/sj.bjc.6606064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Owen A, Chandler B, Back DJ, Khoo SH. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir Ther. 2004;9:819–21. [PubMed] [Google Scholar]
- 221.Albermann N, Schmitz-Winnenthal FH, Z’Graggen K, Volk C, Hoffmann MM, Haefeli WE, et al. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–58. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 222.Dotzlaw H, Leygue E, Watson P, Murphy LC. The human orphan receptor PXR messenger RNA is expressed in both normal and neoplastic breast tissue. Clin Cancer Res. 1999;5:2103–7. [PubMed] [Google Scholar]
- 223.Chen Y, Tang Y, Kodama J, Kudo T. [Google Scholar]
- 224.Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer research. 2008;68:9338–47. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC cancer. 2009;9:3. doi: 10.1186/1471-2407-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen Y, Tang Y, Chen S, Nie D. Regulation of drug resistance by human pregnane X receptor in breast cancer. Cancer biology & therapy. 2009;8:1265–72. doi: 10.4161/cbt.8.13.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Masuyama H, Suwaki N, Tateishi Y, Nakatsukasa H, Segawa T, Hiramatsu Y. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Mol Endocrinol. 2005;19:1170–80. doi: 10.1210/me.2004-0434. [DOI] [PubMed] [Google Scholar]
- 228.Huang R, Murry DJ, Kolwankar D, Hall SD, Foster DR. Vincristine transcriptional regulation of efflux drug transporters in carcinoma cell lines. Biochem Pharmacol. 2006;71:1695–704. doi: 10.1016/j.bcp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 229.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–33. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 230.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. Jama. 2008;300:2867–78. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–85. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 232.Fugh-Berman A, Ernst E. Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–95. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Moschella C, Jaber BL. Interaction between cyclosporine and Hypericum perforatum (St. John’s wort) after organ transplantation. Am J Kidney Dis. 2001;38:1105–7. doi: 10.1053/ajkd.2001.28617. [DOI] [PubMed] [Google Scholar]
- 234.de Maat MM, Hoetelmans RM, Math t RA, van Gorp EC, Meenhorst PL, Mulder JW, et al. Drug interaction between St John’s wort and nevirapine. Aids. 2001;15:420–1. doi: 10.1097/00002030-200102160-00019. [DOI] [PubMed] [Google Scholar]
- 235.Mathijssen RH, Verweij J, de Bruijn P, Loos WJ, Sparreboom A. Effects of St. John’s wort on irinotecan metabolism. J Natl Cancer Inst. 2002;94:1247–9. doi: 10.1093/jnci/94.16.1247. [DOI] [PubMed] [Google Scholar]
- 236.Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18:84–112. [PubMed] [Google Scholar]
- 237.Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59:7–13. doi: 10.1016/S0009-9236(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 238.Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1806–15. doi: 10.1124/dmd.107.015974. [DOI] [PubMed] [Google Scholar]
- 239.Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–68. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 240.Wang H, Huang H, Li H, Teotico DG, Sinz M, Baker SD, et al. Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin Cancer Res. 2007;13:2488–95. doi: 10.1158/1078-0432.CCR-06-1592. [DOI] [PubMed] [Google Scholar]
- 241.Sakuma T, Ohtake M, Katsurayama Y, Jarukamjorn K, Nemoto N. Induction of CYP1A2 by phenobarbital in the livers of aryl hydrocarbon-responsive and -nonresponsive mice. Drug metabolism and disposition: the biological fate of chemicals. 1999;27:379–84. [PubMed] [Google Scholar]
- 242.Kojima K, Nagata K, Matsubara T, Yamazoe Y. Broad but distinct role of pregnane x receptor on the expression of individual cytochrome p450s in human hepatocytes. Drug Metab Pharmacokinet. 2007;22:276–86. doi: 10.2133/dmpk.22.276. [DOI] [PubMed] [Google Scholar]
- 243.Itoh M, Nakajima M, Higashi E, Yoshida R, Nagata K, Yamazoe Y, et al. Induction of human CYP2A6 is mediated by the pregnane X receptor with peroxisome proliferator-activated receptor-gamma coactivator 1alpha. J Pharmacol Exp Ther. 2006;319:693–702. doi: 10.1124/jpet.106.107573. [DOI] [PubMed] [Google Scholar]
- 244.Xiong H, Yoshinari K, Brouwer KL, Negishi M. Role of constitutive androstane receptor in the in vivo induction of Mrp3 and CYP2B1/2 by phenobarbital. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:918–23. doi: 10.1124/dmd.30.8.918. [DOI] [PubMed] [Google Scholar]
- 245.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 246.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–8. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen Y, Ferguson SS, Negishi M, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol. 2003;64:316–24. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 248.Jackson JP, Ferguson SS, Moore R, Negishi M, Goldstein JA. The constitutive active/androstane receptor regulates phenytoin induction of Cyp2c29. Mol Pharmacol. 2004;65:1397–404. doi: 10.1124/mol.65.6.1397. [DOI] [PubMed] [Google Scholar]
- 249.Jackson JP, Ferguson SS, Negishi M, Goldstein JA. Phenytoin induction of the cyp2c37 gene is mediated by the constitutive androstane receptor. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:2003–10. doi: 10.1124/dmd.106.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang H, LeCulyse E, Liu L, Hu M, Matoney L, Zhu W, et al. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- 251.Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, et al. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–8. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 252.Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–8. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- 253.Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, et al. Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol. 2006;70:329–39. doi: 10.1124/mol.105.022046. [DOI] [PubMed] [Google Scholar]
- 254.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual review of pharmacology and toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 255.Runge-Morris M, Kocarek TA. Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab. 2005;6:299–307. doi: 10.2174/1389200054633871. [DOI] [PubMed] [Google Scholar]
- 256.Burk O, Arnold KA, Geick A, Tegude H, Eichelbaum M. A role for constitutive androstane receptor in the regulation of human intestinal MDR1 expression. Biol Chem. 2005;386:503–13. doi: 10.1515/BC.2005.060. [DOI] [PubMed] [Google Scholar]
- 257.Yu XQ, Xue CC, Wang G, Zhou SF. Multidrug resistance associated proteins as determining factors of pharmacokinetics and pharmacodynamics of drugs. Curr Drug Metab. 2007;8:787–802. doi: 10.2174/138920007782798171. [DOI] [PubMed] [Google Scholar]
- 258.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 259.Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–8. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 260.Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- 261.Zhou C, Poulton EJ, Grun F, Bammler TK, Blumberg B, Thummel KE, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–9. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 262.Wang H, Li H, Moore LB, Johnson MD, Maglich JM, Goodwin B, et al. The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Mol Endocrinol. 2008;22:838–57. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Healan-Greenberg C, Waring JF, Kempf DJ, Blomme EA, Tirona RG, Kim RB. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane X receptor. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:500–7. doi: 10.1124/dmd.107.019547. [DOI] [PubMed] [Google Scholar]
- 264.Tabb MM, Kholodovych V, Grun F, Zhou C, Welsh WJ, Blumberg B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR) Environ Health Perspect. 2004;112:163–9. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ekins S, Chang C, Mani S, Krasowski MD, Reschly EJ, Iyer M, et al. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]