Abstract
Vitamin D deficiency (VDD) is widespread and considered a risk factor for cardiovascular disease and stroke. Low vitamin D levels are predictive for stroke and more fatal strokes in humans, whereas vitamin D supplements are associated with decreased risk of all-cause mortality. Because VDD occurs with other comorbid conditions that are also independent risk factors for stroke, this study examined the specific effect of VDD on stroke severity in rats. Adult female rats were fed control or VDD diet for 8 wk and were subject to middle cerebral artery occlusion thereafter. The VDD diet reduced circulating vitamin D levels to one fifth (22%) of that observed in rats fed control chow. Cortical and striatal infarct volumes in animals fed VDD diet were significantly larger, and sensorimotor behavioral testing indicated that VDD animals had more severe poststroke behavioral impairment than controls. VDD animals were also found to have significantly lower levels of the neuroprotective hormone IGF-I in plasma and the ischemic hemisphere. Cytokine analysis indicated that VDD significantly reduced IL-1α, IL-1β, IL-2, IL-4, IFN-γ, and IL-10 expression in ischemic brain tissue. However, ischemia-induced IL-6 up-regulation was significantly higher in VDD animals. In a separate experiment, the therapeutic potential of acute vitamin D treatments was evaluated, where animals received vitamin D injections 4 h after stroke and every 24 h thereafter. Acute vitamin D treatment did not improve infarct volume or behavioral performance. Our data indicate that VDD exacerbates stroke severity, involving both a dysregulation of the inflammatory response as well as suppression of known neuroprotectants such as IGF-I.
Vitamin D is one of the oldest hormones, involved not only in calcium and bone homeostasis but also known to regulate genes involved with immunomodulation, proliferation, and regulation of cell growth and differentiation (1–4). Although the canonical synthetic pathway of activated vitamin D hormone, also known as calcitriol or 1,25-OH2-vitamin D3, ends in the kidney, many other cell types, such as vascular smooth muscle cells, microglia (5, 6), astrocytes (7), and cerebral neurons (7), synthesize 1-α-hydroxylase (CYP27B1), the final enzyme in the activation pathway, to elevate local levels of 1,25-OH2D3 (4, 5, 8, 9). Furthermore, vitamin D receptors (VDR) are widely expressed, including endothelium, activated monocytes and T cells (2, 10–12), astrocytes, cardiomyocytes (13), and neurons.
Although vitamin D research has increased remarkably in the last decade (2, 14–20), the putative benefits of this hormone remain to be definitively determined. Low vitamin D levels have been implicated in numerous chronic inflammatory conditions, including cardiovascular disease (CVD), cancer, autoimmune, and infectious diseases (2, 5, 16, 21–23). In the elderly population, vitamin D deficiency (VDD) is common and likely contributes to the increased pathogenesis and incidence of CVD and exacerbates these diseases once instated (24). Critical risk factors for cerebral infarction, such as age and hypertension, are associated with low serum 25-hydroxyvitamin D (25-OHD), the circulating form of vitamin D (25–27), and low serum 25-OHD is independently predictive of future strokes (24, 28). However, recent metaanalysis of randomized-controlled clinical trials investigating vitamin D supplements did not find a statistically significant benefit in reducing CVD risk or mortality (18, 29). Although multiple studies have found that vitamin D supplements may reduce CVD and mortality risk (30–32), evidence suggests that vitamin D supplementation is more important for at-risk populations than in generally healthy groups (33). For example, hypertensive subjects administered vitamin D in clinical trials experienced a blood pressure-lowering effect (27, 34–36). However, studies in which normotensive subjects were administered vitamin D saw no effects on blood pressure (18, 37).
Stroke is a leading cause of morbidity and mortality (38) and, with CVD, is the leading cause of death of both men and women (39) in the United States. Low vitamin D levels are associated with a greater risk for stroke, and experimental studies indicate that vitamin D can protect neurons from excitotoxic injury, similar to that seen in stroke. Cerebral ischemia has been shown to activate the VDR, resulting in increased nuclear translocation in an animal stroke model as well as elevation of VDR in human stroke patients (40). In vitro, vitamin D3 protects rat cortical neurons (41) and retinal ganglion cells (42) against glutamate-induced neurotoxicity and cortical neurons against cyanide-induced neurotoxicity (43). Pretreatment, but not concurrent treatment, with 1,25-OH2D3 also reduced cell death in rat mesencephalic neuronal cultures exposed to reactive oxygen species (44). Furthermore, vitamin D enhances endogenous antioxidant pathways, such as γ-glutamyl transpeptidase (45), and reduces inducible nitric oxide synthase (46), suggesting a mechanism for vitamin D-induced neuroprotection. Additionally, vitamin D up-regulates neurotrophic factors, such as nerve growth factor, neurotrophin-3, neurotrophin-4 (47, 48), IGF-I (49), and glial cell line-derived neurotrophic growth factor (50). Growth factors, such as IGF-I, contribute to central nervous system (CNS) repair by promoting axon and dendrite sprouting and regrowth after stroke, which is a critical component of recovery of function (51), and also support the growth and survival of neural progenitor cells and other support cells, such as astrocytes and microglia, that play an integral role poststroke recovery (52). Similarly, vitamin D3 has been shown to suppress inflammatory cytokines, such as TNF-α, IL-6, and nitric oxide in a microglial cell line (53), inhibit cluster of differentiation 4, major histocompatibility complex class III, and nitric oxide synthase in an experimental model of multiple sclerosis (46), and reduce ischemia-induced brain damage (54). Thus, vitamin D may promote cell survival through protrophic and antiinflammatory gene regulation.
Little is known about the role of VDD in the context of brain function and injury. Long-term vitamin D deprivation changes vasomotor reactivity in rats (55) and has profound effects on the anatomy of the developing brain (56), as well as the expression of critical growth factors and genes regulating oxidative phosphorylation, calcium homeostasis, synaptic plasticity, and neurotransmission (56, 57). In adult animals, mice maintained on a VDD diet were found to have impaired inflammatory cytokine production in activated macrophages (58). The effect of VDD on stroke severity, however, has not been studied. In the present study, we maintained animals on a VDD diet, and our data indicate that VDD increases stroke severity and sensory motor impairment, attenuates growth factor expression, and alters the profile of inflammatory cytokines.
Materials and Methods
Animals
Adult Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN), as mature adult females (4–5 months, 250 g) or middle-aged adult females (9–11 months, 280–350 g). Animals were maintained in a constant 12-h light, 12-h dark cycle, and food and water were available ad libitum. All procedures were in accordance with the National Institutes of Health and institutional guidelines governing animal welfare. For surgeries and tissue collection, the rats were deeply anesthetized (ketamine, 87 mg/kg; xylazine, 13 mg/kg).
Vitamin D treatment
Animals were assigned to VDD or vitamin D treatment groups, respectively, or a control group.
VDD studies
Animals were fed ad libitum control rat chow (diet no. 89124; Harlan Teklad, Madison, WI) or VDD chow (diet no. 89123, Harlan Teklad) for 8 wk. Control and VDD chow were otherwise equivalent in composition, including calcium and phosphorous levels. Individual body weight and diet consumption per cage were recorded weekly, and no differences were observed between groups.
Vitamin D treatment studies
To effectively solubilize and deliver vitamin D3 in vivo, 2-hydroxypropyl-β-cyclodextrin (Sigma, St. Louis, MO), an organic cyclic caging molecule, was used as a vehicle as described in Cekic et al. (59). Animals were injected with ip with 22.5% 2-hydroxypropyl-β-cyclodextrin solution alone (controls) or 22.5% 2-hydroxypropyl-β-cyclodextrin + 10 μg/kg vitamin D3 (Sigma), 4 h after middle cerebral artery occlusion (MCAo) and every 24 h thereafter for 5 d.
Middle cerebral artery occlusion
Animals were subjected to stereotaxic surgery to occlude the left MCA according to a previously established laboratory protocol (60). Briefly, anesthetized animals were placed in a stereotaxic frame, and a small hole was drilled in the skull at the following coordinates relative to bregma: +0.9 mm anterior posterior, +3.0 mm medial lateral. A needle, attached to syringe containing endothelin-1 (ET-1), was lowered to a depth of −8.0 mm relative to the dura. ET-1 was delivered slowly to the MCA at the rate of.02 μl/30 sec, for a total of 3 μl (0.5 μg/μl in PBS; American Peptide Co., Sunnyvale, CA). This experimental stroke model results in a prolonged ischemia and gradual reperfusion, which lasts approximately 7 h in the striatum and 16 h in the cortex (61). Optical imaging studies using a small fluorescent probe (Cy5.5; 1 kDa molecular mass) indicated that ET-1-induced vasoconstriction is clearly visible 5 h after ET-1, whereas reperfusion is apparent at 10 h in the midline (striatum) and is fully reestablished when imaged at 21 h after ET-1 (62). During surgery, the rats were maintained at 37 C throughout, and oxygen saturation and respiratory rate were constantly monitored using the Mouse Oximeter (STARR Life Sciences, Oakmont, PA). There were no differences in oxygen saturation (controls, 85.99 ± 1.477; VDD, 85.05 ± 1.95) and respiratory rate (control, 55.76 ± 3.78; VDD, 56.69 ± 4.78) between the treatment groups. At termination, the brain was rapidly removed, hand sectioned using a brain mold, and slices were processed for 2,3,5-triphenyl-tetrazolium chloride (TTC) (Sigma) staining and biochemical analysis.
Sensorimotor tests
The vibrissae-elicited forelimb placing test was performed before and after MCAo surgery to assess the functional effects of neurovascular injury, according to our published protocol (60, 62). Animals were subject to same-side placing trials and cross-midline placing trials elicited by stimulating the ipsi- and contralesional vibrissae. Ten trials each were performed for the ipsi- and contralesional vibrissae, and another experimenter recorded scoring during trials. Trials in which the animal seemed to struggle or make premature forelimb movements were not counted.
Tape test
The tape test is also a sensitive indicator of sensorimotor deficit in ischemic injury (63). Two pieces of adhesive-backed foam tape (1 × 1/2″) were used as bilateral tactile stimuli attached to the palmar surface of the paw of each forelimb. For each forelimb, the investigator recorded the time it took to remove each stimulus (tape) from the forelimbs during three trials per day for each forepaw. Animals were allowed to rest for 1 min between sessions, and each test session had a maximum time limit of 120 sec.
Infarct volume
Brain slices (2 mm thick) between −2.00 and +4.00 mm anterior posterior from bregma were incubated in a 2% TTC solution at 37 C for 30 min and later photographed using a digital camera attached to a dissecting microscope (Olympus, New York, NY). Infarct volume was determined from digitized images using the Quantity One software package (Bio-Rad, Hercules, CA). Typically, four such slices were used for analysis. The area of the cortical and striatal infarct was measured separately in all slices as well as the contralateral hemisphere. Details of volume determination are described in Ref. 60. The volume of the infarct was normalized to the volume of the contralateral (nonoccluded) hemisphere. To ensure reliable and consistent detection of the infarct zone, images were digitally converted to black-and-white and magnified, and all traces were performed by the same investigator (R.B.).
Tissue collection
For biochemical analyses, cortical and striatal tissue from the left and right hemispheres and splenic tissue was collected and stored at −80 C. Tissue was later processed for protein extraction, and protein concentrations were determined using the bicinchoninic acid method (Pierce, Rockford, IL) with BSA as a standard (64). Blood was centrifuged at 2500 rpm for 30 min to collect the plasma.
Vitamin D levels
Plasma concentrations of circulating 1,25-OH2 vitamin D, 25-OH vitamin D, and local 25-OHD concentrations in homogenized tissues were determined by ELISA (Immunodiagnostic Systems, Fountain Hills, AZ), according to manufacturer's instructions. For 1,25-OH2 vitamin D, briefly, controls and samples were delipidated and transferred to supplied immunocapsules containing monoclonal antibody to 1,25-dihydroxyvitamin D in suspension with vitamin D-binding protein inhibitor for immunoextraction. After eluting calcitriol from the immunocapsule gel, samples were evaporated in borosilicate glass tubes in a heating block for 30 min under nitrogen gas flow. Evaporated samples were reconstituted in assay buffer and incubated overnight with 100 μl of primary antibody solution. Standards, controls, and samples were then pipetted into a 96-well plate precoated with antibodies specific for 1,25-OH2D and incubated at room temperature for 90 min on an orbital plate shaker. After washes, the remainder of the assay was completed according to the same ELISA protocol as for 25-OHD. For 25-OHD, briefly, standards, controls, and samples were pipetted into a 96-well plate precoated with antibodies specific for 25-OH vitamin D and incubated at room temperature for 2 h. After washes, plates were incubated with 200 μl of streptavidin peroxidase for 30 min. After the next set of washes, 200 μl of stabilized chromogen were added to each well and incubated for an additional 30 min. The color reaction was stopped by an equal volume of stop solution and read at 450 nm (650-nm reference wavelength) in a microplate reader (Bio-Tek, Winooski, VT). Standard curves were established from optical densities of wells containing known dilutions of standard using KC3 software (Bio-Tek), and sample measurements were interpolated from standard curves. For homogenized tissues, the results of these assays were normalized for individual sample protein content. The lower detection limit of the ELISA is 6 nmol/liter as indicated by the manufacturer.
IGF-I levels
Concentrations of circulating IGF-I in plasma and local IGF-I in homogenized tissues was determined by ELISA (R&D Systems, Minneapolis, MN), according to manufacturer's instructions. Briefly, standards, controls, and samples were pipetted into a 96-well plate precoated with antibodies specific for IGF-I and incubated at room temperature for 2 h. After washes, plates were sequentially incubated with 100 μl of conjugate for 2 h. After the next set of washes, 100 μl of substrate solution were added to each well and incubated for an additional 30 min. The color reaction was stopped by an equal volume of stop solution and read at 450 nm (540-nm reference wavelength) in a microplate reader (Bio-Tek). Standard curves were established from optical densities of wells containing known dilutions of standard using KC3 software (Bio-Tek), and sample measurements were interpolated from standard curves. For homogenized tissues, the results of these assays were normalized for individual sample protein content.
Quantitative RT-PCR
Quantitative RT-PCR was performed for CYP27B1, 24-hydroxylase (CYP24A1), and IGF-I in brain tissue, and for IGF-I in liver tissue according to established laboratory protocol. Briefly, total RNA was extracted from homogenized tissues using an RNeasy kit (QIAGEN, Valencia, CA) according to manufacturer's directions. Total RNA for each sample was quantitated using NanoDrop technology (Thermo Scientific, Waltham, MA), and normalized total RNA samples were added to a solution containing qScript cDNA SuperMix (Quanta BioSciences, Gaithersburg, MD). SYBR Green PCR Master Mix (Molecular Probes, Inc., Eugene, OR)-based RT-PCR using MyiQ single-color RT-PCR detection system (Bio-Rad) was used to quantify the following: CYP27B1, CYP24A1, and IGF-I (Real Time Primers, Elkins Park, PA), and cyclophilin (Invitrogen, Carlsbad, CA). Forward and reverse primer sequences, respectively, for CYP24A1, 5′-TGGGTGAATACGCTCTACCC-3′ and 5′-TATCCAGCAGAGAGCCAGGT-3′; for CYP27B1, 5′-AAGGCAGCTGTCATCATCTC-3′ and 5′-TCTGAAGGCTTTGGATCTTG-3′; and for IGF-I, 5′-TCTGCTTGCTCACCTTTACC-3′ and 5′-TACATCTCCAGCCTCCTCAG-3′.
Cytokine assay
Cytokine expression in the brain and spleen was measured after MCAo using a cytokine multiplex ELISA (Milliplex rat MAP assay system; Millipore, Bedford, MA) in conjunction with the Bio-Plex Suspension Array Systems (Bio-Rad) with High-Throughput Fluidics, following procedures described in Lewis et al. (65). The results of these assays were normalized for individual sample protein content using data collected from the bicinchoninic acid procedure (described previously). Briefly, standards, controls, and samples were pipetted into a 96-well plate, followed by the addition of 25 μl of antibody-immobilized beads specific for rat granulocyte-macrophage colony stimulating factor, IL-1α, monocyte chemotactic protein-1, IL-4, IL-1β, IL-2, IL-6, IL-10, IL-12p70, IL-5, IFN-γ, IL-18, growth-related oncogene-KC, and TNF-α. After washes, plates were sequentially incubated with 25 μl of detection antibody, followed by the addition of 25 μl of streptavidin-phycoerythrin. Standard curves were established from median fluorescent intensities of wells containing known dilutions of standard, and sample measurements were interpolated from standard curves.
TGF-β1 levels
Concentrations of local TGF-β1 (which is not available as part of the multiplex assay) was determined by singleplex ELISA (Milliplex rat MAP assay system; Millipore), according to manufacturer's instructions and in conjunction with the Bio-Plex Suspension Array Systems (Bio-Rad) with High-Throughput Fluidics, following procedures described in Lewis et al. (65).
Statistical analysis
Statistical analysis was performed using a statistical software package (SPSS, Inc., Chicago, IL), and group differences were considered significant at P ≤ 0.05. For most measures, data were analyzed using a two-way ANOVA coded for diet or treatment as an independent variable (VDD and normal rat chow or 10 μg/kg vitamin D3 + vehicle and vehicle alone) and brain region as a repeated measure (cortex and striatum). The data are expressed as the mean ± sem for VDD and control mature adults or vitamin D treatment and control middle-aged adult females for each study. All experimental groups had an n = 4–7, which is indicated in each figure legend.
Results
VDD diet studies
Weight analysis
Average weight and diet consumption were similar in animals consuming VDD chow as compared with animals fed control chow over the 8-wk period. Starting weights were 262.06 ± 15.9 g for the control group and 264.8 ± 23.0 g in the VDD group. After the diet treatment period, final weights were 287.5 ± 19.1 and 278.45 ± 21.41 g, respectively, for control and VDD groups.
Local and circulating levels of 1,25-OH2 vitamin D and 25-OH vitamin D
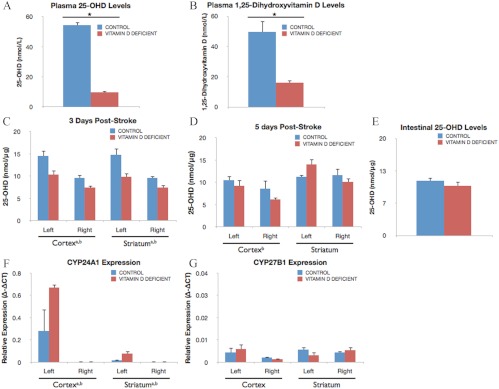
As shown in Fig. 1, A and B, VDD diet significantly reduced circulating vitamin D (25-OHD) levels in plasma to less than 22% of control levels (P < 0.05) and circulating activated vitamin D (1,25-OH2D) levels to less than 33% of control levels (P < 0.05), respectively. In the brain, 25-OHD levels were significantly and transiently suppressed in the cortex [F(1,11), 6.73; P < 0.05] and striatum [F(1,12), 11.84; P < 0.05] of VDD groups compared with chow controls, at 3 d after stroke (Fig. 1C). Furthermore, vitamin D levels were higher in the ischemic cortex [F(1,11), 38.15; P < 0.05] and striatum [F(1,11), 24.36; P < 0.05] compared with the contralateral side. At 5 d after stroke, vitamin D levels remained elevated in the ischemic cortex [F(1,7), 11.19; P < 0.05] but not in the striatum (Fig. 1D). For comparison, 25-OHD levels were measured in the small intestine, an unrelated tissue classically associated with vitamin D function. No group difference in intestinal tissue 25-OHD levels was observed, and local 25-OHD levels were similar in brain and intestinal tissue (Fig 1, D and E).
Fig. 1.
Effect of VDD diet on circulating and local 1,25-OH2D and 25-OH vitamin D levels. A and B, Vitamin D levels were measured in plasma from animals fed a VDD diet or a control diet for 8 wk. The VDD diet significantly reduced circulating vitamin D (25-OHD) levels to 22% of observed levels in rats fed control chow (*, P < 0.05) (A) and circulating activated vitamin D (1,25-OH2D) levels to less than 33% of control levels (*, P < 0.05) (B), respectively. Histograms depict mean ± sem. C, Brain levels of 25-OH vitamin D. Vitamin D was measured in lysates from the ischemic (left) and nonischemic (right) hemisphere at d 3 after MCAo surgery. Vitamin D levels were significantly higher in tissues from control diet-fed animals as compared with VDD diet animals (a, Main effect of diet). Ischemia elevated 25-OHD levels in both the cortex and striatum at 3 d (b, Main effect of ischemia). D, At 5 d after MCAo, there were no significant differences in vitamin D levels in brain tissue derived from VDD diet animals vs. controls. In the ischemic cortex, vitamin D levels remained elevated above nonischemic levels at 5 d after stroke but not in the striatum. E, Small intestine 25-OHD levels. Vitamin D levels were measured in the intestine, an unrelated tissue classically associated with vitamin D, for comparison with brain tissue measurements. As in the brain, VDD diet did not significantly affect intestinal 25-OHD levels. F and G, Expression of genes coding for vitamin D metabolic deactivating CYP24A1 and activating CYP27B1 enzymes was quantified in brain tissue at 5 d. F, CYP24A1 expression was significantly increased in animals fed VDD diet in the ischemic cortex and striatum (a, Main effect of diet). Injury further resulted in greater elevation of CYP24A1 in the ischemic hemisphere of both groups as compared with nonischemic tissues (b, Main effect of ischemia). G, No significant differences were found in CYP27B1 expression levels. Data were analyzed by two-way ANOVA for diet treatment and ischemia for each region; a, Main effect of diet; b, main effect of ischemia. Group differences were considered significant at P ≤ 0.05. Bars represent mean ± sem; n = 4–7 in each group.
Local expression of vitamin D-associated genes
Although circulating vitamin D was reduced by VDD diet, local vitamin D levels in brain tissue were not. To clarify the discrepancy between large group differences in circulating 25-OHD and 1,25-OH2D levels and virtually no diet-related differences in brain 25-OHD levels (Fig. 1D), expression of the activating CYP27B1 and inactivating CYP24A1 genes was analyzed in brain tissue. As shown in Fig. 1F, VDD diet significantly increased CYP24A1 expression in the ischemic cortex and striatum [F(1,10), 6.98; P < 0.05] compared with control diet at 5 d after stroke. Furthermore, ischemia led to a much greater elevation of CYP24A1 levels in the injured hemisphere as indicated by a significant effect of ischemia [F(1,10), 25.11; P < 0.05]. For CYP27B1, no significant differences were found (Fig. 1G).
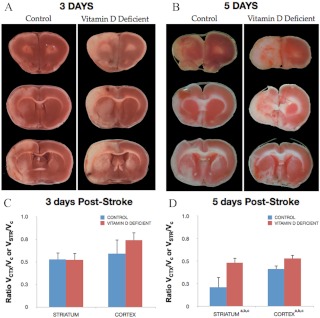
Infarct volume
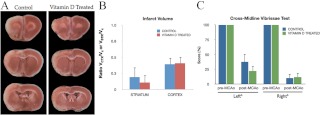
Animals were subject to ET-1-induced MCAo after 8 wk of specialized diets and were euthanized 3 or 5 d after stroke. A cortical-striatal infarct was observed in animals of both control and VDD groups, shown in TTC-stained sections in Fig. 2A (3 d after stroke) and Fig. 2B (5 d after stroke). At 3 d after stroke, infarct volume was not significantly different between the control diet and VDD groups (Fig. 2C). However, at 5 d after stroke, the volume of stroke tissue had partially improved in controls, whereas VDD animals had little or absent improvement. Thus, there was a significant increase in infarct volume [F(1,7), 5.85; P < 0.05] due to VDD, with greater cortical (28%) and striatal (126%) damage in the VDD group (Fig. 2D).
Fig. 2.
Effect of VDD diet on infarct volume. Animals maintained on a VDD diet or control diet were subject to MCAo and euthanized 3 or 5 d later. Representative slices of TTC-stained sections depict the rostrocaudal extent of the infarct in control and VDD animals 3 d (A) and 5 d (B) after ischemia. Unstained tissue is indicative of dead or infarcted tissue. Histograms depict mean ± sem of infarct volume in the cortex and striatum normalized to the contralateral side. C, At d 3, there were no significant differences in cortical or striatal infarct volume between the control diet and VDD animals. D, By 5 d after stroke, both cortical and striatal infarct volumes were significantly larger in the VDD group compared with the control diet-fed animals (a, Main effect of diet). Striatal volumes were more severely affected by VDD (c, Interaction effect of diet treatment and ischemia). Data were analyzed by two-way ANOVA for diet treatment and ischemia for each region; a, Main effect of diet; b, main effect of ischemia; c, interaction effect of diet treatment and ischemia. Group differences were considered significant at P ≤ 0.05. Bars represent mean ± sem; n = 4–7 in each group. Vctx, Volume cortex; Vstr, volume striatum; Vc, volume contralateral hemisphere.
Behavioral outcomes
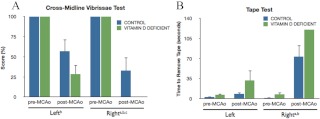
The vibrissae-elicited forelimb placement task evaluates functional capabilities dependent on the cortex and striatum and interhemispheric integration between these two regions. Forelimb placement was completely accurate before stroke surgery. At 3 d after stroke, significant functional deficits were evident in both diet groups. However, these deficits were more severe in the VDD animals vs. controls. In the cross-midline vibrissae-elicited forelimb placement test (Fig. 3A), VDD animals performed significantly worse than controls for the contralesional paw [F(1,11), 6.05; P < 0.05]. This finding was confirmed and extended by the tape test, a sensitive indicator of sensorimotor deficit in ischemic injury (52). As expected, no significant pre- and poststroke differences were seen in the amount of time needed to remove the tape from the ipsilesional paw. However, significant delays were seen in the amount of time needed to remove the tape from the contralesional paw after stroke [F(1,12), 84.79; P < 0.05] (Fig. 3B). Furthermore, VDD animals took longer to remove the tactile stimulus from the contralesional paw compared with controls before ischemic injury, and this was further amplified after stroke, where animals in the VDD group simply failed to remove the tape during the testing duration [F(1,12), 6.88; P < 0.05].
Fig. 3.
Effect of VDD diet on behavioral performance. All animals were assessed before and 3 d after stroke on two behavioral tasks. A, Histograms depict mean (±sem) percent correct responses on the cross-midline vibrissae-elicited forelimb placement task, pre- and postischemia. Performance on the left (ipsilesional) limb and right (contralesional) limb was significantly affected by ischemia (b, Main effect of ischemia). However, on the right limb, VDD diet treatment (green bar) significantly worsened performance after stroke compared with the control diet (blue bar) (c, Interaction effect of ischemia and diet). B, Tape test. Histograms depict mean (±sem) duration (in seconds) to remove tape from the palmar surface of the front limb. Performance on this task was significantly impaired after stroke compared with prestroke in both diet groups (b, Main effect of ischemia). Moreover, mean duration to remove the tape was also affected by diet, such that VDD diet groups took longer to remove the tape, or did not remove it at all during the allotted time, compared with the control diet animals (a, Main effect of diet). Data were analyzed by two-way ANOVA for pre-/postischemia and diet treatment for each limb; a, Main effect of diet; b, main effect of ischemia; c, interaction effect of diet treatment and ischemia. Group differences were considered significant at P ≤ 0.05. Bars represent mean ± sem; n = 6–7 in each group.
Local and circulating levels of IGF-I
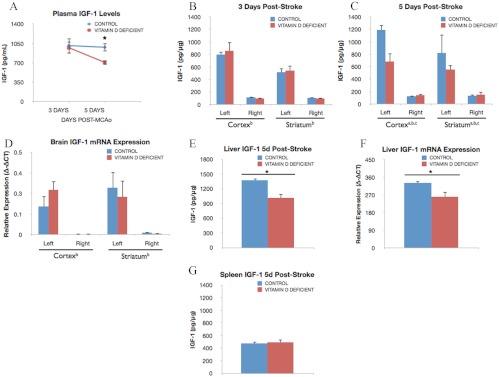
IGF-I expression was determined by ELISA in plasma and brain tissue. Plasma IGF-I is significantly higher in uninjured (prestroke) control animals (1435.1 ± 27.15 pg/ml) compared with VDD rats (746.18 ± 116.03 pg/ml). IGF-I levels were virtually identical in control and VDD groups 3 d after ischemia. However, by 5 d after ischemia, plasma IGF-I levels were 38.5% lower in the VDD group compared with controls (Fig. 4A). Brain IGF-I levels were elevated, as expected, in both the ischemic cortex and striatum as compared with nonischemic tissues at 3 d [F(1,12), 119.97; P < 0.05] and 5 d after ischemia [F(1,6), 67.24; P < 0.05]. No diet-induced differences were seen in IGF-I levels at 3 d after stroke (Fig. 4B). However, at 5 d after ischemia, IGF-I levels were further elevated in the control group, whereas VDD groups maintained a similar level of IGF-I seen at 3 d [F(1,6), 16.31; P < 0.05] (Fig. 4C), indicating that VDD attenuates elevated levels of this neuroprotectant. To determine whether the changes in serum and brain tissue IGF-I levels were due to a local or systemic effect, IGF-I levels were measured in the liver, the largest source of IGF-I. In keeping with circulating and brain tissue IGF-I levels, VDD liver tissue had significantly lower IGF-I expression as compared with control liver (P < 0.05) (Fig. 4E). However, IGF-I levels in the spleen were no different in VDD vs. controls, indicating differential tissue regulation of IGF-I by VDD (Fig. 4G). Additionally, IGF-I mRNA levels were also measured in the brain. As shown in Fig. 4D, IGF-I mRNA levels were significantly elevated on the ischemic side (both cortex and striatum). However, there was no difference in IGF-I gene expression between control and VDD groups. Collectively, these data indicate that poststroke brain IGF-I levels reflect a combination of local and systemic IGF-I levels and that diet-induced differences in IGF-I in the brain is likely due to influx of circulating peptide.
Fig. 4.
Effect of VDD diet on circulating and local IGF-I. A, Plasma IGF-I levels were measured at 3 and 5 d after ischemia in control and VDD female rats. At 3 d after stroke, plasma IGF-I levels were similar in VDD and control groups. However, by 5 d after stroke, IGF-I levels were sustained in control diet animals, whereas peptide levels were decreased in the VDD diet group. Thus, circulating IGF-I levels were reduced 38.5% of the levels observed in control chow-fed animals (*, P < 0.05). Data points represent mean ± sem. B, IGF-I levels in the brain 3 d after ischemia in VDD and control animals. Lysates from the ischemic (left) hemisphere had significantly greater IGF-I expression compared with the nonischemic (right) hemisphere (b, Main effect of ischemia). Diet did not affect IGF-I levels in either the cortex or striatum at 3 d after ischemia. C, At 5 d after ischemia, local IGF-I levels were still elevated in the ischemic hemisphere compared with the ischemic side (b). However, at 5 d after stroke, IGF-I levels were significantly elevated in the cortex and striatum of control animals compared with VDD animals (c, Interaction effect of diet treatment and ischemia). VDD attenuated IGF-I levels in ischemic cortical (75.5%) and striatal (47.2%) tissues. D, IGF-I mRNA expression in the brain 5 d after stroke. Similar to IGF-I protein, IGF-I mRNA levels were significantly greater in the ischemic hemisphere (b). However, diet did not affect IGF-I mRNA levels in the cortex or striatum. E and F, Liver expression of IGF-I. At 5 d after ischemia, IGF-I protein (E) and mRNA (F) levels were significantly reduced (35.14 and 28.0%, respectively) in VDD liver tissue as compared with liver from controls (*, P < 0.05). Data points represent mean ± sem. For comparison, IGF-I levels in the spleen, a peripheral lymphoid organ known to respond to hypoxia, are shown 5 d after stroke. G, Unlike liver tissue, splenic IGF-I levels were not affected by diet. Data were analyzed by three-way ANOVA for diet treatment, ischemia, and region as repeated measures; a, Main effect of diet; b, main effect of ischemia; c, interaction effect of diet treatment and ischemia. Group differences were considered significant at P ≤ 0.05. Bars represent mean ± sem; n = 4–7 in each group.
Effects of VDD on the central ischemic inflammatory response
The impact of the VDD diet on cerebral inflammation was assessed by measuring a panel of cytokines/chemokines in cortical and striatal brain tissue from VDD and control animals. In both groups, MCAo increased overall cytokine expression in the cortex and striatum of the ischemic hemisphere as compared with that of the nonischemic hemisphere (data not shown). However, baseline (nonischemic) and ischemia-induced cytokine expression was generally decreased in VDD animals as compared with controls. The table in Fig. 5A summarizes the differences in cytokine levels at 5 d after ischemia in VDD animals relative to controls. Specifically, VDD animals expressed significantly less IL-1α, IL-1β, IL-2, IL-4, and IFN-γ in response to cortical ischemia as compared with control animals, whereas reducing IL-1α and IL-10 in the striatum (P < 0.05). IL-6 was the only cytokine that was elevated in ischemic tissue in the VDD group compared with controls. As shown in Fig. 5B, ischemia elevated IL-6 levels in the cortex [F(1,6), 80.31; P < 0.05] and striatum [F(1,6), 5.97; P < 0.05] compared with the nonischemic side. Furthermore, in both regions, VDD led to a greater elevation of ischemia-induced IL-6 as indicated by a significant interaction effect of ischemia and diet in cortex [F(1,6), 9.08; P < 0.05] and striatum [F(1,6), 10.38; P < 0.05]. Ischemia also elevated TGF-β1 levels in the cortex [F(1,6), 58.35; P < 0.05] and striatum [F(1,6), 13.57; P < 0.05] compared with the nonischemic side (Fig. 5C). TGF-β1 levels were significantly correlated with IL-6 levels in the ischemic cortex (r = 0.81; P < 0.05) and striatum (r = 0.78; P < 0.05).
Fig. 5.
Postischemia brain and spleen cytokine expression in control and VDD rats. Brain samples from the ischemic (left) hemisphere and nonischemic (right) hemisphere were collected at d 5 after MCAo and analyzed for cytokine/chemokine expression. A, Arrows indicate the direction of significant changes in expression in specific cytokines in VDD animals relative to controls (P ≤ 0.05). B, At 5 d after stroke, IL-6 levels were significantly higher in the ischemic cortex and striatum compared with the nonischemic side (b, Main effect of ischemia). Furthermore, VDD resulted in a greater elevation of IL-6 in both the cortex (1.21-fold) and striatum (1.51-fold) compared with controls (c, Interaction effect of diet treatment and ischemia). C, At 5 d after stroke, TGF-β1 levels were significantly higher in the ischemic cortex and striatum compared with the nonischemic side (b, Main effect of ischemia). Data were analyzed by two-way ANOVA for diet treatment and ischemia; for each region; b, Main effect of ischemia; c, interaction effect of diet treatment of ischemia. Group differences were considered significant at P ≤ 0.05. Bars represent mean ± sem; n = 4–7 in each group. GM-CSF, Granulocyte-macrophage colony stimulating factor.
Effects of VDD on the peripheral ischemic inflammatory response
The spleen is known to mobilize its reservoirs of immune cells as part of the early response to inflammatory and hypoxic stimuli, such as cerebral ischemia (66–68), resulting in decreased splenic weight in the acute phase of stroke. This finding was confirmed in our model, where normalized splenic weights were inversely correlated with cortical infarct volume (r = −0.67, P < 0.05). Cytokine expression in the spleen was also assessed in VDD and control animals. Although cytokine levels in VDD ischemic brain tissue were generally decreased, specific cytokines in VDD splenic tissue were up-regulated as compared with levels in the control group. As Fig. 5A illustrates, IL-1α, IL-4, and IL-5 cytokine levels in splenic tissue from VDD animals were significantly higher than control levels by d 5 (P < 0.05). These data indicate that the spleen responds to ischemia by mounting peripheral immune mobilization.
Acute vitamin D treatment studies
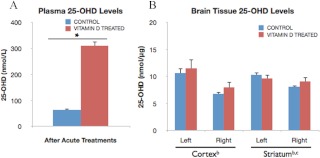
To determine the effect of acute vitamin D administration on ischemic injury, middle-aged female animals subject to ET-1-induced stroke were injected ip with 10 μg/kg vitamin D3 + vehicle or vehicle alone after stroke. As shown in Fig. 6A, vitamin D3 injections consistently elevated (3.9-fold) plasma 25-OHD levels significantly above those of animals injected with vehicle alone (P < 0.05). However, local 25-OHD levels in brain tissue (Fig. 6B) from vitamin D3-treated and control groups were not significantly regulated by vitamin D injections in the cortex [F(1,7), 0.45; P > 0.05] or striatum [F(1,7), 0.30; P > 0.05]. Furthermore, ischemia-induced up-regulation of local 25-OHD levels was seen in both the cortex [F(1,7), 7.65; P < 0.05] and striatum [F(1,7), 33.23; P < 0.05] as compared with contralateral nonischemic tissues, similar to that seen in the VDD study (Fig. 4B).
Fig. 6.
Levels of plasma and brain 25-OH vitamin D levels after vitamin D3 injections. Plasma and brain tissue was derived from control and vitamin D-treated female rats after five consecutive days of vehicle or vitamin D3 + vehicle ip injections, respectively. Samples were collected at 5 d after ischemia. A, Vitamin D treatments significantly increased circulating vitamin D, 25-OHD, levels (3.9-fold) as compared with levels in control rats injected with vehicle alone (*, P < 0.05). Bars represent mean ± sem. B, Brain 25-OHD levels, however, were not significantly affected by acute ip vitamin D3 treatments. There was a small but significant elevation of 25-OHD in ischemic tissues compared with nonischemic tissues. Additionally, in the striatum, 25-OHD levels were affected by an interaction of vitamin D treatment and ischemia, such that 25-OHD levels in the ischemic striatum were significantly higher in the control group compared with the vitamin D-treated animals. Data were analyzed by two-way ANOVA for hormone treatment and ischemia for each brain region; b, Main effect of ischemia; c, interaction effect of ischemia and hormone treatment. Bars represent mean ± sem; n = 4–5 in each group.
Effect of acute vitamin D3 treatment on infarct volume.
To determine the neuroprotective effects of vitamin D3 treatment, animals were subject to MCAo surgery followed by acute poststroke treatments of 10 μg/kg vitamin D3 + vehicle or vehicle alone. Hormone (or vehicle) treatment was initiated 4 h after stroke and then given every 24 h thereafter for 5 d. Animals were then terminated and assessed for infarct volume. Representative TTC-stained brain slices from control and vitamin D-treated animals are shown in Fig. 7A, and quantitative analysis of infarct volume, depicted in the histogram in Fig. 7B, indicated no significant differences between vehicle and vitamin D-injected groups.
Fig. 7.
Effect of vitamin D3 injections on infarct volume. Animals were subject to MCAo and euthanized 5 d later. A, Representative slices of TTC-stained sections depict the rostrocaudal extent of the infarct in control and vitamin D3-treated animals. B, Histograms depict infarct volume normalized to the contralateral side. Infarct volume was not affected by acute vitamin D3 treatment in the cortex or the striatum. Bars represent mean ± sem. C, Performance on sensory motor task before and after ischemia. Animals were tested before and after ischemia on the vibrissae-evoked forelimb placement task. Histogram depicts mean (±sem) correct responses on the cross-midline vibrissae-elicited forelimb placement task for each forelimb. Postischemic performance was significantly affected on the left and right limb. However, vitamin D3 treatment had no affect on the performance of either limb. Data were analyzed by two-way ANOVA for pre-/postischemia and vitamin D3 treatment; b, Main effect of ischemia. Group differences were considered significant at P ≤ 0.05; n = 4–5 in each group.Vctx, Volume cortex; Vstr, volume striatum; Vc, volume contralateral hemisphere.
Behavioral analysis
Consistent with the lack of vitamin D3 treatment effect on infarct volume, hormone treatment did not differentially affect forelimb placement as compared with control animals. The same-side paw-placing reflex task for the contralesional limb, in which both groups of animals performed 100% before MCAo, showed a significant loss (100%) of paw-placing behavior when the vibrissae contralateral to the lesion side was stimulated after MCAo (P < 0.05). The cross-midline task, which requires increased interhemispheric integration as compared with the same-side task, was significantly affected by MCAo in both forelimbs of vitamin D-treated and control animals [F(1,7), 91.53 for the left limb; F(1,7), 293.37 for the right limb; P < 0.05] (Fig. 7C). Vitamin D3 injections did not improve sensory motor function.
Local and circulating levels of IGF-I
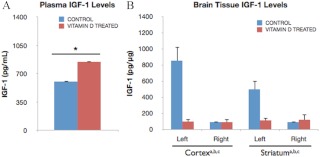
Acute poststroke vitamin D treatments differentially affected IGF-I levels in plasma and brain tissue. Analysis of plasma revealed significantly higher IGF-I levels (39.5%) in vitamin D-treated middle-aged animals as compared with controls (P < 0.05) (Fig. 8A). Thus, vitamin D availability is strongly associated with plasma IGF-I levels as indicated both by reduced circulating levels of IGF-I in VDD female animals and elevated IGF-I plasma levels in vitamin D-treated females as compared with control animals. Conversely, vitamin D treatments prevented ischemia-induced up-regulation of local IGF-I expression in ischemic brain tissue, as illustrated in Fig. 8B. Although IGF-I levels were elevated as expected in the ischemic cortex [F(1,7), 23.30; P < 0.05] and striatum [F(1,7), 13.92; P < 0.05], there was a strong interaction effect of ischemia and hormone treatment in both regions [F(1,7), 22.39; P < 0.05; F(1,7), 14.65; P < 0.05, respectively], indicating that hormone treatment inhibited ischemia-induced IGF-I up-regulation. Thus, IGF-I levels in the ischemic brain regions of hormone-treated animals were no different from their nonischemic counterparts.
Fig. 8.
Effect of vitamin D3 injections on plasma and brain IGF-I levels. Plasma and brain tissue derived from control and vitamin D-treated female rats 5 d after stroke were analyzed for IGF-I expression. A, Vitamin D treatments significantly increased circulating IGF-I levels (39.5%) as compared with levels in control rats injected with vehicle alone (*, P < 0.05). Bars represent mean ± sem. B, IGF-I levels were elevated in the ischemic cortex and striatum, but this was only seen in the controls and not the vitamin D3-treated animals. Thus, IGF-I levels were significantly lower in the ischemic cortex (11.5-fold) and striatum (3.4-fold) of hormone-treated animals. Data were analyzed by two-way ANOVA for hormone treatment and ischemia for each brain region; a, Main effect of hormone treatment; b, main effect of ischemia; c, interaction effect of hormone and ischemia. Group differences were considered significant at P < 0.05. Bars represent mean ± sem; n = 4–5 in each group.
Effects of acute vitamin D treatments on the central ischemic inflammatory response
In both vitamin D-treated and control animals, MCAo increased overall cytokine expression in the cortex and striatum of the ischemic hemisphere as compared with the nonischemic hemisphere. However, baseline cytokine expression was generally higher in vitamin D-treated females as compared with controls. This pattern contrasts with findings in VDD vs. control animals, wherein baseline cytokine expression was generally decreased in brain tissue from VDD animals. Vitamin D-treated females had significantly elevated cortical IL-2 and IL-5 (92.4% and 6.6-fold, respectively) and striatal IL-1β expression (22.1%) as compared with controls (P < 0.05) (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). A trend (P = 0.06) was also found for increased IL-1β expression (45.6%) in cortical tissue from vitamin D-treated animals.
Discussion
The present data indicate that VDD adversely affects stroke outcome, whereas poststroke vitamin D supplementation was ineffective. The VDD diet effectively reduced circulating vitamin D3 precursor (25-OHD) and active (1,25-OH2D3) forms of vitamin D compared with control chow. Reduced circulating 1,25-OH2D indicates an endocrine mechanism of biologically active vitamin D function in stroke injury. Levels of 25-OHD were also lower in brain tissue of VDD animals in the acute stroke phase, and the tissue availability of both 25-OHD and 1,25-OH2D was further compromised by elevated expression of the vitamin D catabolic enzyme, CYP24A1, in the same tissue at 5 d after stroke. Thus, dietary vitamin D restriction likely constrains availability and function of vitamin D metabolites. At 5 d after MCAo, VDD groups showed greater cortical and striatal infarction, whereas behavioral impairment was observed as early as 3 d after stroke. Circulating and local ischemia-induced IGF-I levels were also reduced in VDD groups at 5 d after stroke. Additionally, VDD suppressed antiinflammatory cytokines associated with T-helper cells and macrophages in the ischemic brain. Vitamin D3 injections significantly elevated circulating 25-OHD levels compared with controls. However, this treatment did not ameliorate infarct volume or behavioral deficits.
A growing body of data indicates that vitamin D levels are significantly lower in acute stroke patients vs. controls (69) and that low vitamin D levels increase the risk for future strokes (24) and are independently predictive for fatal strokes (28). Vitamin D is thought to modify the risk of CVD indirectly through chronic and inflammatory CV risk factors, such as diabetes, dyslipidemia, and metabolic syndrome (2, 14, 23, 70–72). Severely low vitamin D levels are reported in specific populations at risk for stroke, such as the elderly and nonwhite races (especially African Americans), whereas moderate deficiency is additionally seen in young and middle aged populations (25, 31, 73). The low levels of vitamin D in our VDD groups is comparable with those seen in stroke-prone human populations, and these low levels directly affect both brain tissue loss and exacerbate behavioral impairment. Furthermore, our data are also consistent with studies from other labs that report a neurotoxic role for VDD (59).
There are several mechanisms by which VDD could exacerbate stroke injury. VDD affects poststroke inflammatory responses, which play a critical role in the pathophysiology of cerebral ischemia (74–77). Cerebral ischemia results in a characteristic poststroke inflammatory response, involving activation of astrocytes and immune cells, increased vascular and blood brain barrier (BBB) permeability, influx of leukocytes, and cytokine production (38, 78, 79). Local immune cells (microglia) are activated initially, and subsequent peripheral immune cells gain access to the CNS as a result of a compromised BBB and increased adhesion molecule expression on cerebral vasculature and activated immune cells (78, 80, 81). Once activated, inflammatory cells can secrete cytotoxic substances, such as additional cytokines, that induce secondary damage and propagate immune cell activation and recruitment to the ischemic site (79, 81, 82). Although the poststroke inflammatory response is necessary to promote repair and regeneration (74, 83), infiltration of certain T-cell subsets may promote brain injury rather than facilitate recovery, whereas others are thought to provide neuroprotective benefits (74, 81, 84, 85). Additional cytokines released from recruited T cells at the ischemic site may play a key role in the pathogenesis of stroke, and the ratio of pro- to antiinflammatory T-cell subtypes is proposed to determine whether the poststroke inflammatory response will promote recovery or neurodegeneration. Although the adaptive immune response is generally suppressed by 1,25-OH2D3, the active form of vitamin D, this hormone nevertheless exerts direct effects on activation, phenotypic determinants, and secretions of T cells and antigen presenting cells (5, 86). Vitamin D enhances antiinflammatory Treg and Th2 development and cytokine production, whereas proinflammatory Th1 and Th17 subsets are suppressed (5, 87–90), suggesting a possible mechanism by which VDD may exacerbate ischemic cell loss. Although vitamin D has been shown to act as a potent immunomodulator capable of regulating cytokine secretion (2, 91–94), little is known of the effects of this hormone on inflammation in the context of ischemic stroke. The present study shows that IL-6 is specifically elevated in the ischemic brain of VDD animals. IL-6 and TGF-β have been shown to play an important role in regulating the Th17:Treg balance, and elevated IL-6 production inhibits Treg generation (95, 96). Our data and reports from other labs and clinical studies indicate that VDD promotes IL-6 expression (59, 97–99). We predict that VDD, directly or via regulation of cytokine intermediaries like IL-6 and TGF-β1, promotes an imbalance in T-cell subpopulations in the ischemic brain, which results in neurotoxicity.
The local inflammatory response in the brain is known to contribute to ischemic injury, yet the peripheral inflammatory response to stroke is only now becoming associated with neurological outcome (67, 77). Although antiinflammatory cytokine expression in brain tissue was reduced in VDD animals, cytokine levels in VDD splenic tissues were elevated vs. control levels, suggesting differential regulation between the central and peripheral components of the inflammatory response. The peripheral inflammatory response precedes brain inflammation in experimental stroke models and is characterized in part by increased IL-6, an established predictor of poor stroke outcome in humans (75, 77, 78). In vivo, depletion of macrophages from peripheral lymphoid organs before experimental stroke resulted in significant reduction of hematogenous (peripheral) macrophages at the ischemic site (66). Vitamin D is known to enhance innate immune responses, and microglia/macrophages mediate the intrinsic inflammatory response in the brain (66). Our experiments support vitamin D's role in both the central and peripheral ischemic inflammatory response.
In addition to its role as an immune modulator, VDD may also exacerbate ischemic cell loss by decreasing the availability of neuroprotective growth factors. Growth factors such as IGF-I contribute to CNS repair by promoting axon and dendrite sprouting and regrowth after stroke, which is a critical component of recovery of function (51), and also support the growth and survival of neural progenitor cells and other support cells, such as astrocytes and microglia, that play an integral role in poststroke recovery (52). Levels of IGF-I, a known vascular and neuroprotectant up-regulated in ischemia (100, 101), are positively correlated with vitamin D levels (49) and negatively correlated with ischemic stroke outcome in experimental and clinical studies (101–104). Furthermore, VDD is clinically associated with dysfunction or failure of the insulin/IGF-I axis in CVD states, such as diabetes and metabolic syndrome (49, 105, 106). Although IGF-I was up-regulated in the ischemic brain, VDD attenuated this peptide's expression, suggesting another mechanism by which VDD could exacerbate ischemic cell loss, because IGF-I provides support for local regrowth and repair mechanisms after stroke. Indeed, VDD animals had significantly lower IGF-I levels in ischemic tissues and also displayed more severe poststroke behavioral deficits. Although the liver is the single largest source, IGF-I is synthesized by diverse organs, including the brain (107), and exogenous IGF-I reduces ischemic injury in many species (103, 108–110). IGF-I has been shown to stimulate stroke-induced neurogenesis (111) and promote neuronal survival, neuronal myelination, and angiogenesis (112, 113). In stroke patients, lower levels of circulating IGF-I were predictive of a poorer outcome (114, 115, but see Ref. 116) and increased mortality (102, 117). Experimental studies have shown reciprocal regulation of the IGF-I and vitamin D systems. IGF-I promotes the hydroxylation of 25-OHD3 into the active 1,25-OH2D3 hormone by inducing renal CYP27B1, the enzyme responsible for vitamin D activation (118). Our results support such a role for IGF-I, because levels of this neuroprotective peptide were reduced in VDD serum, liver, and brain tissue, whereas circulating 1,25-OH2D3 was significantly reduced in VDD animals. In the present study, systemic vitamin D injections increased circulating (plasma) IGF-I levels but did not attenuate ischemic brain injury. However, this treatment did not elevate IGF-I in ischemic tissues, suggesting that local IGF-I elevation may be critical to attenuate ischemic injury in female rats.
Poststroke brain IGF-I levels represent a complex mix of locally synthesized IGF-I and peripherally derived peptide, which is largely synthesized by the liver. In normal conditions, very little IGF-I crosses the BBB, but after stroke (and concomitant changes in the BBB), systemic IGF-I enters the brain more easily. In the VDD experiments, for example, brain levels of IGF-I mRNA increase on the infarcted side but are no different between control and VDD groups, indicating the reduced IGF-I protein levels in the VDD group reflects differences in circulating IGF-I influx after stroke. Curiously, we did not observe elevated brain IGF-I levels in the vitamin D-treated stroke animals, and one possible explanation for this may be linked to vitamin D's well-known effects on elevating IGF-binding proteins (118, 119), which are thought to protect IGF-I from proteolytic cleavage but may also inhibit transfer of peptide across the BBB. Alternatively, interactions between VDR and the IGF-binding proteins have been reported (120), and in many cases, such binding suppresses VDR-mediated signaling (121) and may indirectly account for the absence of IGF-related neuroprotection in vitamin D-treated and/or VDD groups.
Although VDD exacerbated ischemic damage, supraphysiologic vitamin D treatment did not ameliorate injury in our experiments. This may be due to the fact that the dose was suboptimal or that the timing of vitamin D treatment was not effective. The 10 μg/kg dose of vitamin D was chosen in an effort to overcome early-stage inflammatory pathology already in place 4 h after ischemia (78). The majority of studies demonstrating neuroprotection by vitamin D exclusively focused on preemptive vitamin D administration (15, 54, 122), which does not adequately address the clinical reality that most stroke patients only receive medical attention several hours after stroke. Thus, in the present studies, animals were injected with vitamin D 4 h after ischemia, which did not promote neuroprotection. A more likely possibility is that vitamin D may act synergistically with other neuroprotectants but not independently (123). For example, acutely administered vitamin D in combination with progesterone, a neuroprotective hormone, reduced brain injury in female rats, where vitamin D alone was ineffective (59).
Collectively, the present studies indicate that VDD is an independent modulator of stroke severity. Our data also support the hypothesis that VDD-induced stroke severity may be due to the suppression of endogenous neuroprotectants, such as IGF-I, as well as dysregulation of the inflammatory response after ischemia. Besides its role as a neuroprotectant, IGF-I may also shape the immune environment of the ischemic brain. IGF-I exerts proliferative effects on T cells (124), and IGF-I signaling influences the antiproliferative action of IFN-γ on polarized Th1 cells (125). Thus, one intriguing possibility is that VDD in combination with reduced IGF-I expression may alter the ratio of cytotoxic (Th1, Th17) to cytoprotective (Th2, Treg) T-cell cohorts and thus increase tissue damage.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants AG027684 and AG028303 (to F.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BBB
- Blood brain barrier
- CNS
- central nervous system
- CVD
- cardiovascular disease
- CYP24A1
- 24-hydroxylase
- CYP27B1
- 1-α-hydroxylase
- ET-1
- endothelin-1
- MCAo
- middle cerebral artery occlusion
- 25-OHD
- 25-hydroxyvitamin D
- TTC
- 2,3,5-triphenyl-tetrazolium chloride
- VDD
- vitamin D deficiency
- VDR
- vitamin D receptor.
References
- 1. Baeke F, Gysemans C, Korf H, Mathieu C. 2010. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol 25:1597–1606 [DOI] [PubMed] [Google Scholar]
- 2. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. 2008. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. 2007. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647 [DOI] [PubMed] [Google Scholar]
- 4. Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, Bartik L, Egan JB, Wu Y, Kubicek JL, Lowmiller CL, Moffet EW, Forster RE, Jurutka PW. 2010. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “fountain of youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikle DD. 2009. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep 7:58–63 [DOI] [PubMed] [Google Scholar]
- 6. Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. 2006. Immune regulation of 25-hydroxyvitamin-D3–1α-hydroxylase in human monocytes. J Bone Miner Res 21:37–47 [DOI] [PubMed] [Google Scholar]
- 7. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. 2001. Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. J Clin Endocrinol Metab 86:888–894 [DOI] [PubMed] [Google Scholar]
- 8. Rizk-Rabin M, Zineb R, Zhor B, Michèle G, Jana P. 1994. Synthesis of and response to 1,25 dihydroxycholecalciferol by subpopulations of murine epidermal keratinocytes: existence of a paracrine system for 1,25 dihydroxycholecalciferol. J Cell Physiol 159:131–141 [DOI] [PubMed] [Google Scholar]
- 9. Mahon BD, Wittke A, Weaver V, Cantorna MT. 2003. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 89:922–932 [DOI] [PubMed] [Google Scholar]
- 10. Hewison M. 2010. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am 39:365–379, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. 2002. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest 109:1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. 2010. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 11:344–349 [DOI] [PubMed] [Google Scholar]
- 13. Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. 2008. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 149:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michos ED, Melamed ML. 2008. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care 11:7–12 [DOI] [PubMed] [Google Scholar]
- 15. Yasuhara T, Hara K, Maki M, Masuda T, Sanberg CD, Sanberg PR, Bickford PC, Borlongan CV. 2008. Dietary supplementation exerts neuroprotective effects in ischemic stroke model. Rejuvenation Res 11:201–214 [DOI] [PubMed] [Google Scholar]
- 16. Bouvard B, Annweiler C, Sallé A, Beauchet O, Chappard D, Audran M, Legrand E. 2011. Extraskeletal effects of vitamin D: facts, uncertainties, and controversies. Joint Bone Spine 78:10–16 [DOI] [PubMed] [Google Scholar]
- 17. Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM. 2009. Vitamin D and mortality in older men and women. Clin Endocrinol 71:666–672 [DOI] [PubMed] [Google Scholar]
- 18. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. 2010. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 152:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sood A, Arora R. 2010. Vitamin D deficiency and its correlations with increased cardiovascular incidences. Am J Ther 17:e105–9 [DOI] [PubMed] [Google Scholar]
- 20. Scragg R, Khaw KT, Murphy S. 1995. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr 49:640–646 [PubMed] [Google Scholar]
- 21. Bartley J. 2010. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther 8:1359–1369 [DOI] [PubMed] [Google Scholar]
- 22. Cantorna MT, Mahon BD. 2004. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med 229:1136–1142 [DOI] [PubMed] [Google Scholar]
- 23. Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. 2008. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 52:1949–1956 [DOI] [PubMed] [Google Scholar]
- 24. Marniemi J, Alanen E, Impivaara O, Seppänen R, Hakala P, Rajala T, Rönnemaa T. 2005. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis 15:188–197 [DOI] [PubMed] [Google Scholar]
- 25. Scragg R, Sowers M, Bell C. 2007. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am J Hypertens 20:713–719 [DOI] [PubMed] [Google Scholar]
- 26. Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. 2006. Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr 25:395–402 [DOI] [PubMed] [Google Scholar]
- 27. Pilz S, Tomaschitz A. 2010. Role of vitamin D in arterial hypertension. Expert Rev Cardiovasc Ther 8:1599–1608 [DOI] [PubMed] [Google Scholar]
- 28. Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, März W. 2008. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke 39:2611–2613 [DOI] [PubMed] [Google Scholar]
- 29. Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. 2011. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 96:1931–1942 [DOI] [PubMed] [Google Scholar]
- 30. Autier P, Gandini S. 2007. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- 31. Melamed ML, Michos ED, Post W, Astor B. 2008. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnard K, Colón-Emeric C. 2010. Extraskeletal effects of vitamin D in older adults: cardiovascular disease, mortality, mood, and cognition. Am J Geriatr Pharmacother 8:4–33 [DOI] [PubMed] [Google Scholar]
- 33. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. 2011. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 7:CD007470. [DOI] [PubMed] [Google Scholar]
- 34. Lind L, Lithell H, Skarfors E, Wide L, Ljunghall S. 1988. Reduction of blood pressure by treatment with αcalcidol. A double-blind, placebo-controlled study in subjects with impaired glucose tolerance. Acta Med Scand 223:211–217 [PubMed] [Google Scholar]
- 35. Pilz S, Tomaschitz A, Ritz E, Pieber TR. 2009. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 6:621–630 [DOI] [PubMed] [Google Scholar]
- 36. Zittermann A. 2003. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 89:552–572 [DOI] [PubMed] [Google Scholar]
- 37. Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, Beresford SA, Connelly SA, Curb JD, Grimm RH, Jr, Kotchen TA, Kuller LH, Wassertheil-Smoller S, Thomson CA, Torner JC. 2008. Effect of calcium and vitamin D supplementation on blood pressure: the women's health initiative randomized trial. Hypertension 52:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, Gladstone DJ, Ween J, Snaiderman A, Lanctôt KL. 2010. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol 23:199–205 [DOI] [PubMed] [Google Scholar]
- 39. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. 2011. Heart disease and stroke statistics—2011 update: a report from the American heart association. Circulation 123:e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ridder DA, Bulashevska S, Chaitanya GV, Babu PP, Brors B, Eils R, Schneider A, Schwaninger M. 2009. Discovery of transcriptional programs in cerebral ischemia by in silico promoter analysis. Brain Res 1272:3–13 [DOI] [PubMed] [Google Scholar]
- 41. Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N, Yoneda Y. 2006. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosc Res 83:1179–1189 [DOI] [PubMed] [Google Scholar]
- 42. Suemori S, Shimazawa M, Kawase K, Satoh M, Nagase H, Yamamoto T, Hara H. 2006. Metallothionein, an endogenous antioxidant, protects against retinal neuron damage in mice. Invest Ophthalmol Vis Sci 47:3975–3982 [DOI] [PubMed] [Google Scholar]
- 43. Li L, Prabhakaran K, Zhang X, Zhang L, Liu H, Borowitz JL, Isom GE. 2008. 1α,25-dihydroxyvitamin D3 attenuates cyanide-induced neurotoxicity by inhibiting uncoupling protein-2 up-regulation. J Neurosci Res 86:1397–1408 [DOI] [PubMed] [Google Scholar]
- 44. Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. 2001. Protective effects of 1 α,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 40:761–771 [DOI] [PubMed] [Google Scholar]
- 45. Garcion E, Thanh XD, Bled F, Teissier E, Dehouck MP, Rigault F, Brachet P, Girault A, Torpier G, Darcy F. 1996. 1,25-Dihydroxyvitamin D3 regulates γ1 transpeptidase activity in rat brain. Neurosci Lett 216:183–186 [DOI] [PubMed] [Google Scholar]
- 46. Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1997. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res 45:255–267 [DOI] [PubMed] [Google Scholar]
- 47. Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1994. 1,25-Dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 6:124–126 [DOI] [PubMed] [Google Scholar]
- 48. Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, De Luca HF, Brachet P. 1994. 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 24:70–76 [DOI] [PubMed] [Google Scholar]
- 49. Bogazzi F, Rossi G, Lombardi M, Tomisti L, Sardella C, Manetti L, Curzio O, Marcocci C, Grasso L, Gasperi M, Martino E. 2011. Vitamin D status may contribute to serum Igf1 concentrations in healthy subjects. J Endocrinol Invest 34:e200–e203 [DOI] [PubMed] [Google Scholar]
- 50. Naveilhan P, Neveu I, Wion D, Brachet P. 1996. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 7:2171–2175 [DOI] [PubMed] [Google Scholar]
- 51. Ron-Harel N, Schwartz M. 2009. Immune senescence and brain aging: can rejuvenation of immunity reverse memory loss? Trends Neurosci 32:367–375 [DOI] [PubMed] [Google Scholar]
- 52. Martino G, Pluchino S, Bonfanti L, Schwartz M. 2011. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 91:1281–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lefebvre d'Hellencourt C, Montero-Menei CN, Bernard R, Couez D. 2003. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res 71:575–582 [DOI] [PubMed] [Google Scholar]
- 54. Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ. 2000. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology 39:873–880 [DOI] [PubMed] [Google Scholar]
- 55. De Novellis V, Loffreda A, Vitagliano S, Stella L, Lampa E, Filippelli W, Vacca C, Guarino V, Rossi F. 1994. Effects of dietary vitamin D deficiency on the cardiovascular system. Res Commun Chem Pathol Pharmacol 83:125–144 [PubMed] [Google Scholar]
- 56. Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. 2003. Vitamin D3 and brain development. Neuroscience 118:641–653 [DOI] [PubMed] [Google Scholar]
- 57. Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Féron F. 2007. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 103:538–545 [DOI] [PubMed] [Google Scholar]
- 58. Kankova M, Luini W, Pedrazzoni M, Riganti F, Sironi M, Bottazzi B, Mantovani A, Vecchi A. 1991. Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology 73:466–471 [PMC free article] [PubMed] [Google Scholar]
- 59. Cekic M, Cutler SM, VanLandingham JW, Stein DG. 2011. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging 32:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Selvamani A, Sohrabji F. 2010. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging 31:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. 2001. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med 46:827–830 [DOI] [PubMed] [Google Scholar]
- 62. Selvamani A, Sohrabji F. 2010. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci 30:6852–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. 2006. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12:441–445 [DOI] [PubMed] [Google Scholar]
- 64. Sohrabji F, Peeples KW, Marroquin OA. 2000. Local and cortical effects of olfactory bulb lesions on trophic support and cholinergic function and their modulation by estrogen. J Neurobiol 45:61–74 [DOI] [PubMed] [Google Scholar]
- 65. Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. 2008. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol 195:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schroeter M, Jander S, Huitinga I, Stoll G. 2001. CD8+ phagocytes in focal ischemia of the rat brain: predominant origin from hematogenous macrophages and targeting to areas of pannecrosis. Acta Neuropathol 101:440–448 [DOI] [PubMed] [Google Scholar]
- 67. Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. 2006. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 26:654–665 [DOI] [PubMed] [Google Scholar]
- 68. Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. 2006. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol 176:6523–6531 [DOI] [PubMed] [Google Scholar]
- 69. Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. 2006. Reduced vitamin D in acute stroke. Stroke 37:243–245 [DOI] [PubMed] [Google Scholar]
- 70. Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P. 2003. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol 41:105–112 [DOI] [PubMed] [Google Scholar]
- 71. Zittermann A, Schleithoff SS, Koerfer R. 2007. Vitamin D and vascular calcification. Curr Opin Lipidol 18:41–46 [DOI] [PubMed] [Google Scholar]
- 72. Zittermann A, Schleithoff SS, Koerfer R. 2006. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 11:25–33 [DOI] [PubMed] [Google Scholar]
- 73. Ginde AA, Liu MC, Camargo CA., Jr 2009. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kreutzberg GW. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318 [DOI] [PubMed] [Google Scholar]
- 75. Vila N, Castillo J, Dávalos A, Chamorro A. 2000. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31:2325–2329 [DOI] [PubMed] [Google Scholar]
- 76. Hill JK, Gunion-Rinker L, Kulhanek D, Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP, Eckenstein FP. 1999. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res 820:45–54 [DOI] [PubMed] [Google Scholar]
- 77. Chapman KZ, Dale VQ, Dénes A, Bennett G, Rothwell NJ, Allan SM, McColl BW. 2009. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab 29:1764–1768 [DOI] [PubMed] [Google Scholar]
- 78. Becker KJ. 2001. Targeting the central nervous system inflammatory response in ischemic stroke. Curr Opin Neurol 14:349–353 [DOI] [PubMed] [Google Scholar]
- 79. del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. 2000. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 10:95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Engelhardt B. 2006. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ 43:259–280 [DOI] [PubMed] [Google Scholar]
- 81. Wang Q, Tang XN, Yenari MA. 2007. The inflammatory response in stroke. J Neuroimmunol 184:53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Danton GH, Dietrich WD. 2003. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136 [DOI] [PubMed] [Google Scholar]
- 83. Denes A, Thornton P, Rothwell NJ, Allan SM. 2010. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun 24:708–723 [DOI] [PubMed] [Google Scholar]
- 84. Yilmaz G, Granger DN. 2010. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med 12:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dénes A, Humphreys N, Lane TE, Grencis R, Rothwell N. 2010. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci 30:10086–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kamen DL, Tangpricha V. 2010. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med 88:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maruotti N, Cantatore FP. 2010. Vitamin D and the immune system. J Rheumatol 37:491–495 [DOI] [PubMed] [Google Scholar]
- 88. Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. 2008. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324:23–33 [DOI] [PubMed] [Google Scholar]
- 89. Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. 2002. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 195:603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. 2002. A 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 91. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. 2006. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83:754–759 [DOI] [PubMed] [Google Scholar]
- 92. Manolagas SC, Provvedini DM, Tsoukas CD. 1985. Interactions of 1,25-dihydroxyvitamin D3 and the immune system. Mol Cell Endocrinol 43:113–122 [DOI] [PubMed] [Google Scholar]
- 93. Cantorna MT, Humpal-Winter J, DeLuca HF. 2000. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3). Arch Biochem Biophys 377:135–138 [DOI] [PubMed] [Google Scholar]
- 94. Cantorna MT, Zhu Y, Froicu M, Wittke A. 2004. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 80(6 Suppl):1717S–1720S [DOI] [PubMed] [Google Scholar]
- 95. Kimura A, Kishimoto T. 2010. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40:1830–1835 [DOI] [PubMed] [Google Scholar]
- 96. Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A, Nomura S, Kishimoto T, Naka T. 2011. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol 186:32–40 [DOI] [PubMed] [Google Scholar]
- 97. Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. 2003. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab 88:4623–4632 [DOI] [PubMed] [Google Scholar]
- 98. Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. 2008. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 99. McCarty MF. 2005. Secondary hyperparathyroidism promotes the acute phase response—a rationale for supplemental vitamin D in prevention of vascular events in the elderly. Med Hypotheses 64:1022–1026 [DOI] [PubMed] [Google Scholar]
- 100. Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. 2004. Insulin-like growth factor-1 as a vascular protective factor. Circulation 110:2260–2265 [DOI] [PubMed] [Google Scholar]
- 101. Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. 1997. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke 28:1744–1748 [DOI] [PubMed] [Google Scholar]
- 102. Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, Corradi F, Ceresini G, Valenti G, Hoffman AR, Ceda GP. 2004. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med 117:312–317 [DOI] [PubMed] [Google Scholar]
- 103. Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. 1992. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun 182:593–599 [DOI] [PubMed] [Google Scholar]
- 104. Zhu W, Fan Y, Hao Q, Shen F, Hashimoto T, Yang GY, Gasmi M, Bartus RT, Young WL, Chen Y. 2009. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab 29:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alvarez JA, Ashraf A. 2010. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010:351–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. 2007. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr 26:573–580 [DOI] [PubMed] [Google Scholar]
- 107. Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. 1988. Insulin and insulin-like growth factors in the CNS. Trends Neurosci 11:107–111 [DOI] [PubMed] [Google Scholar]
- 108. Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. 2001. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience 105:299–306 [DOI] [PubMed] [Google Scholar]
- 109. Johnston BM, Mallard EC, Williams CE, Gluckman PD. 1996. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin Invest 97:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lee WH, Bondy CA. 1993. Ischemic injury induces brain glucose transporter gene expression. Endocrinology 133:2540–2544 [DOI] [PubMed] [Google Scholar]
- 111. Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. 2006. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci 24:45–54 [DOI] [PubMed] [Google Scholar]
- 112. Wang JM, Hayashi T, Zhang WR, Sakai K, Shiro Y, Abe K. 2000. Reduction of ischemic brain injury by topical application of insulin-like growth factor-I after transient middle cerebral artery occlusion in rats. Brain Res 859:381–385 [DOI] [PubMed] [Google Scholar]
- 113. Smith LE. 2005. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate 88:237–244 [DOI] [PubMed] [Google Scholar]
- 114. Bondanelli M, Ambrosio MR, Onofri A, Bergonzoni A, Lavezzi S, Zatelli MC, Valle D, Basaglia N, degli Uberti EC. 2006. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. J Clin Endocrinol Metab 91:3928–3934 [DOI] [PubMed] [Google Scholar]
- 115. Johnsen SP, Hundborg HH, Sørensen HT, Orskov H, Tjønneland A, Overvad K, Jørgensen JO. 2005. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab 90:5937–5941 [DOI] [PubMed] [Google Scholar]
- 116. Endres M, Piriz J, Gertz K, Harms C, Meisel A, Kronenberg G, Torres-Aleman I. 2007. Serum insulin-like growth factor I and ischemic brain injury. Brain Res 1185:328–335 [DOI] [PubMed] [Google Scholar]
- 117. van Rijn MJ, Slooter AJ, Bos MJ, Catarino CF, Koudstaal PJ, Hofman A, Breteler MM, van Duijn CM. 2006. Insulin-like growth factor I promoter polymorphism, risk of stroke, and survival after stroke: the Rotterdam study. J Neurol Neurosurg Psychiatry 77:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gómez JM. 2006. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol 7:125–132 [DOI] [PubMed] [Google Scholar]
- 119. Hyppönen E, Boucher BJ, Berry DJ, Power C. 2008. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British birth cohort. Diabetes 57:298–305 [DOI] [PubMed] [Google Scholar]
- 120. Cui J, Ma C, Qiu J, Ma X, Wang X, Chen H, Huang B. 2011. A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Mol Cell Endocrinol 338:84–92 [DOI] [PubMed] [Google Scholar]
- 121. Ikezoe T, Tanosaki S, Krug U, Liu B, Cohen P, Taguchi H, Koeffler HP. 2004. Insulin-like growth factor binding protein-3 antagonizes the effects of retinoids in myeloid leukemia cells. Blood 104:237–242 [DOI] [PubMed] [Google Scholar]
- 122. Ekici F, Ozyurt B, Erdogan H. 2009. The combination of vitamin D3 and dehydroascorbic acid administration attenuates brain damage in focal ischemia. Neurol Sci 30:207–212 [DOI] [PubMed] [Google Scholar]
- 123. Cekic M, Sayeed I, Stein DG. 2009. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrinol 30:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Clark R, Strasser J, McCabe S, Robbins K, Jardieu P. 1993. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest 92:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Conti L, Regis G, Longo A, Bernabei P, Chiarle R, Giovarelli M, Novelli F. 2007. In the absence of IGF-1 signaling, IFN-γ suppresses human malignant T-cell growth. Blood 109:2496–2504 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.