SUMMARY
Progenitor cells in the cerebral cortex undergo dynamic cellular and molecular changes during development. Sall1 is a putative transcription factor that is highly expressed in progenitor cells during development. In humans, the autosomal dominant developmental disorder Townes-Brocks syndrome (TBS) is associated with mutations of the SALL1 gene. TBS is characterized by renal, anal, limb and auditory abnormalities. Although neural deficits have not been recognized as a diagnostic characteristic of the disease, ∼10% of patients exhibit neural or behavioral abnormalities. We demonstrate that, in addition to being expressed in peripheral organs, Sall1 is robustly expressed in progenitor cells of the central nervous system in mice. Both classical- and conditional-knockout mouse studies indicate that the cerebral cortex is particularly sensitive to loss of Sall1. In the absence of Sall1, both the surface area and depth of the cerebral cortex were decreased at embryonic day 18.5 (E18.5). These deficiencies are associated with changes in progenitor cell properties during development. In early cortical progenitor cells, Sall1 promotes proliferative over neurogenic division, whereas, at later developmental stages, Sall1 regulates the production and differentiation of intermediate progenitor cells. Furthermore, Sall1 influences the temporal specification of cortical laminae. These findings present novel insights into the function of Sall1 in the developing mouse cortex and provide avenues for future research into potential neural deficits in individuals with TBS.
INTRODUCTION
The mature cortex is a six-layered structure that controls complex functions, including motor coordination, and auditory, visual and somatosensory processing, as well as cognition (reviewed in Rosner, 1970). Appropriate regulation of cell number, cell-type specification, laminar positioning and circuit formation is essential for normal functioning of the mature nervous system. Dysregulation of cortical development can lead to a variety of gross cortical malformations and psychiatric disorders, including lissencephaly, periventricular heterotopia, microcephaly, epilepsy, autism and schizophrenia (reviewed in Arnold, 1999; Lian and Sheen, 2006; Pilz et al., 2002; Polleux and Lauder, 2004; Schwartzkroin and Walsh, 2000).
The type of division a progenitor cell (PC) makes is an important mechanism in regulating cell number and fate in the cortex. Early in development, the PC population expands by symmetric divisions, resulting in the production of two progeny radial glial cells (RGCs) (Noctor et al., 2008; Takahashi et al., 1996b). At the onset of neurogenesis (∼E10.5 in mice), RGC asymmetric neurogenic divisions result in the generation of a neuroblast and an RGC (Haubensak et al., 2004; Noctor et al., 2001; Noctor et al., 2008). By mid-neurogenesis (∼E14.5 in mice) these divisions represent the predominant division type in the ventricular zone (VZ) (Noctor et al., 2004). Subsequent asymmetric RGC divisions produce an RGC and an intermediate progenitor cell (IPC) (Haubensak et al., 2004; Miyata et al., 2004; Noctor et al., 2004; Noctor et al., 2008). IPCs (also referred to as basal progenitors) predominately undergo symmetric terminal neurogenic division at the basal side of the VZ or within the subventricular zone (SVZ), resulting in the production of two neurons (Attardo et al., 2008; Haubensak et al., 2004; Miyata et al., 2004; Noctor et al., 2004; Noctor et al., 2008). Although rare, symmetric proliferative IPC divisions have also been reported, resulting in the production of two daughter IPCs (Miyata et al., 2004; Noctor et al., 2004). Recent studies suggest that IPCs give rise to the majority of cortical neurons, so perturbing this population during development has the potential to impact neuronal organization and ultimately behavior (Haubensak et al., 2004; Martinez-Cerdeno et al., 2006; Miyata et al., 2004; Noctor et al., 2004; Noctor et al., 2008; Pontious et al., 2008; Sessa et al., 2008). The molecular mechanisms regulating specification, maintenance and fate of this population are just beginning to be understood.
This study investigated the role of a member of the Sall gene family, Sall1, in the developing brain and identifies a unique role for the Sall1 gene in regulating PCs in the cerebral cortex. Sall1 is a C2H2 zinc-finger-containing putative transcription factor that is highly expressed in the developing CNS and peripheral organs. Previous studies have shown that members of the Sall gene family play a role in cell cycle regulation, proliferation, neuronal differentiation, migration and cell adhesion in other species (Barembaum and Bronner-Fraser, 2004; Basson and Horvitz, 1996; Cantera et al., 2002; de Celis et al., 1999; Elling et al., 2006; Harrison et al., 2008a; Harrison et al., 2008b; Li et al., 2001; Sakaki-Yumoto et al., 2006; Toker et al., 2003). Mutation of SALL1 in humans results in the autosomal dominant developmental disorder Townes-Brocks syndrome (TBS). These mutations are predicted to produce a truncated protein retaining the transcriptional repression domain that is hypothesized to function in a transdominant negative manner (Kiefer et al., 2003; Kohlhase et al., 1998). TBS is characterized by multiple malformations with variable expression (Kohlhase et al., 1998; Townes and Brocks, 1972). The most common diagnostic features include imperforate anus, polydactyly, outer ear anomalies with hearing loss, and kidney abnormalities (Kohlhase et al., 1998; Townes and Brocks, 1972). Although cognitive alterations are only occasionally reported in TBS, it has not been investigated in depth; however, significant evidence has accumulated that indicates a role for Sall1 in neural development. First, a variety of neural and behavioral abnormalities have been described in individuals with TBS, including mental retardation, developmental delay, attention deficit hyperactivity disorder, hypoplasia of the corpus callosum, and seizures (Borozdin et al., 2006; Botzenhart et al., 2005; Cameron et al., 1991; Ishikiriyama et al., 1996). Also, studies in mice indicate that Sall1 is highly expressed in CNS PCs from early embryonic stages (Buck et al., 2000; Ott et al., 2001). Furthermore, mice expressing a truncated Sall1 protein that recapitulates the human mutation exhibit neural abnormalities, in addition to mimicking the human deficits observed in the gastrointestinal tract, limbs and kidneys (Kiefer et al., 2003). These studies suggest that Sall1 might also regulate neural development.
Sall1−/− mice die at birth because of kidney abnormalities (Nishinakamura et al., 2001). In light of the robust expression of Sall1 in neural PCs and its role in neural specification in other organisms (de Celis et al., 1999; Ott et al., 2001; Rusten et al., 2001), we investigated the role of Sall1 in the development of the CNS. Using classical- and conditional-knockout studies, we demonstrate a unique role for Sall1 in regulating cell cycle exit and neuronal specification in the cerebral cortex. Our studies indicate that, at early developmental stages, Sall1 promotes RGC cell cycle re-entry, whereas, from mid-neurogenesis, Sall1 promotes IPC cell cycle exit. Our analyses indicate that Sall1 promotes the terminal differentiation of IPCs and the production of superficial cortical layer neurons. These findings indicate that the cerebral cortex is particularly sensitive to loss of Sall1 and that Sall1 is required to regulate progenitor cell maturation and neuronal specification in the cerebral cortex. We have identified a cellular process that might be altered in individuals with TBS and could be used as a model system to evaluate the benefits of future therapeutic intervention.
RESULTS
Sall1 is expressed by cortical progenitor cells
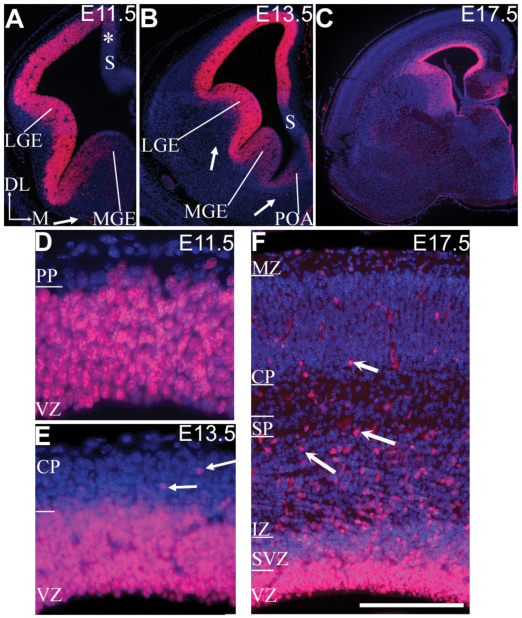
In Drosophila, Sall has been shown to play a role in cell fate specification and neuronal differentiation (de Celis et al., 1999; Rusten et al., 2001). We have previously shown that, in mice, Sall1 mRNA is expressed in the CNS, including in the telencephalon, diencephalon, hindbrain and spinal cord, from E8.5 (Buck et al., 2000; Ott et al., 2001) (note: sall1 is termed msal3 in Ott et al.). In light of the reported neural abnormalities in some individuals with TBS, a detailed expression study of the Sall1 protein in the developing forebrain of mice was performed. Expression of Sall1 protein in the early developing telencephalon mimicked the previously described Sall1 mRNA patterns. At E9.5 and E10.5, Sall1 protein is expressed in both dorsal and ventral PCs; expression is highest in lateral and ventral regions (our unpublished observations). By E11.5, Sall1 is robustly expressed by PCs in the VZ of the dorsal cortex, with weaker expression in the medial wall (Fig. 1A,D). Ventrally, Sall1 is expressed by cells in the lateral ganglionic eminence and the lateral-medial ganglionic sulcus, with weak expression in the medial ganglionic eminence (Fig. 1A). By E13.5, Sall1 expression extends medially into the medial cortical wall and ventrally into the medial ganglionic eminence and preoptic area (Fig. 1B,E). Sall1 was not expressed by cells in the subicular neural epithelium (Fig. 1A,B). At E17.5, expression persisted in PCs and Sall1 expression was found in cells in the cortical wall (Fig. 1C,F). Double labeling with neuronal markers indicated that these cells are not neurons and are most probably glial cells (data not shown).
Fig. 1.
Sall1 is expressed by cortical PCs. Sall1 immunohistochemistry (red) at E11.5 (A,D), E13.5 (B,E) and E17.5 (C,F). Sections are counterstained with DAPI (blue). At E11.5, E13.5 and E17.5, strong Sall1 expression is observed in the progenitor population in the dorsal cortex and lateral ganglionic eminence (AC). Weaker expression is observed in the medial cortex at E11.5 (*; A) and the medial ganglionic eminence at E11.5 and E13.5 (A,B). Sall1 is also expressed by a subpopulation of cells outside the progenitor population (arrows, A,B,E,F). Sall1 expression is absent from the subicular neural epithelium (S; A,B). High-power examination of the dorsal cortex indicates that Sall1 was expressed by PCs (D–F) and, at E17.5, by subsets of cells in the SVZ (F). Dorsal (DL) is up and medial (M) is right, as indicated (A). LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; VZ, ventricular zone; POA, pre-optic area; PP, preplate; CP, cortical plate; SVZ, subventricular zone; IZ, intermediate zone; SP, subplate; MZ, marginal zone. Scale bar: 75 μm (D); 125 μm (E); 200 μm (F); 300 μm (A); 600 μm (B); 1100 μm (C).
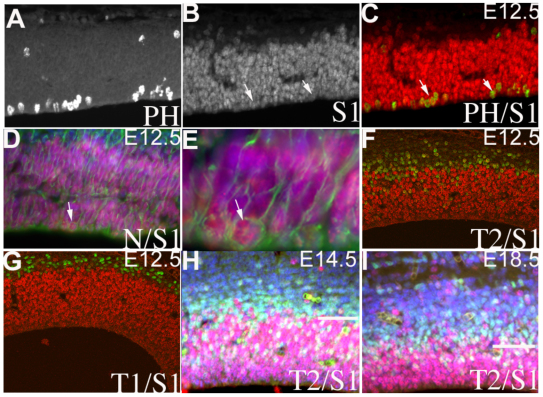
To determine whether Sall1 was expressed in all or in a subset of PCs, we examined the colocalization of Sall1 with cell- and stage-specific markers. Double labeling with Sall1 and either bromodeoxyuridine (BrdU; pulsed for 1 hour; data not shown) or phosopho-histone H3 (PH3) indicated that Sall1 is expressed in the S-phase and M-phase of the cell cycle in RGCs at all ages examined (Fig. 2A–C). Sall1 is coexpressed in close to 100% of nestin-positive (Fig. 2D,E) and of Pax6-positive (data not shown) cells in the VZ, and is absent from Tbr1-positive neurons from E12.5 (Fig. 2G) until birth (data not shown). IPCs can be identified from E12.5 as basal PH3-positive or Tbr2-positive cells. Sall1 is coexpressed in a subset of Tbr2-positive cells at the VZ-SVZ boundary, but is downregulated in Tbr2-positive cells in the SVZ (Fig. 2F,H,I). These findings suggest that Sall1 is expressed in RGCs and is downregulated once these cells differentiate into IPCs or neurons.
Fig. 2.
Sall1 is differentially expressed by RGCs versus IPCs. (A–G) Expression of phospho-histone H3 (PH), Sall1 (S1), nestin (N), Tbr2 (T2) and Tbr1 (T1) in E12.5 embryos. Sall1 is in red and other markers are in green. Double labeling (arrows) with Sall1 and PH3 (A–C) or nestin (D,E) indicates that these markers are coexpressed with Sall1 in PCs. White arrows in B and C indicate coexpression. The nestin-positive cell identified in D is magnified in E. At E12.5, Sall1 is absent from Tbr1-positive neurons (G) and present in a subset of Tbr2-positive cells (F). (H,I) Sall1 is expressed in a subset of Tbr2-positive cells at E14.5 (H) and E18.5 (I). The line in H,I represents the VZ-SVZ border.
The cerebral cortex is decreased in size at E18.5 in Sall1−/− animals
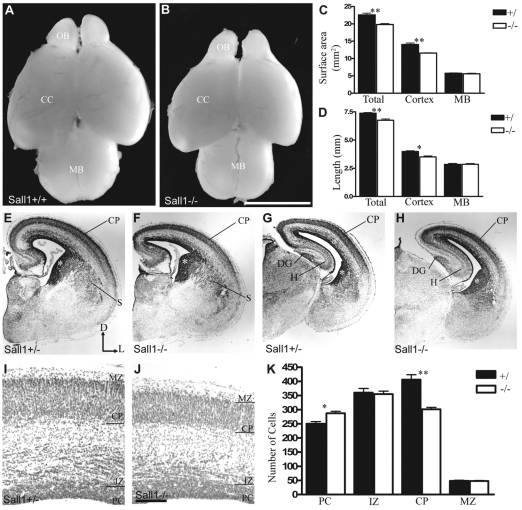
As mentioned, Sall1-deficient animals die at birth owing to kidney deficits (Nishinakamura et al., 2001). The gross anatomy of the brain and spinal cord of Sall1-mutant animals was therefore examined 1 day before birth at E18.5 (Fig. 3A,B and our unpublished observations). In Sall1-deficient animals, the total length and dorsal surface area of the brain was decreased by 8% (P<0.01, n=3) and 12% (P<0.01, n=3), respectively, compared with controls (Fig. 3A–D). This decrease was predominantly due to a decrease in the size of the olfactory bulbs (Harrison et al., 2008a) and a decrease in the length (by 12%; P<0.05, n=3) and dorsal surface area (by 17%; P<0.01, n=3) of the cerebral cortex, compared with controls (Fig. 3A–D). However, no alteration in the length (P=0.9, n=3) or dorsal surface area (P=0.5, n=3) of the midbrain (Fig. 3A–D) or gross anatomical abnormalities in the rest of the brain or spinal cord (our unpublished observations) were observed at all ages examined in Sall1-mutant animals, compared with controls. These data suggest that, although Sall1 is expressed in all CNS PCs, the developing cerebral cortex is particularly sensitive to the loss of Sall1.
Fig. 3.
The cerebral cortex is decreased in size at E18.5 in Sall1−/−mice. The cerebral cortex is decreased in size in Sall1-deficient animals (B) compared with controls (A) at E18.5. Measurements of brain surface area (C) and length (D) of control and Sall1−/− animals. (E–J) Nissl-stained coronal sections of the cortex at E18.5 (E–J) in control (E,G,I) and Sall1−/− animals (F,H,J). The cortical plate and striatum are decreased in size in Sall1-deficient animals (E–J). *, ventral progenitor population (E–H). (K) Quantification of dorsal cell number in control and Sall1-mutant animals. *, P<0.05; **, P<0.01 (C,D,K). OB, olfactory bulb; CC, cerebral cortex; MB, midbrain; CP, cortical plate; S, striatum; DG, dentate gyrus; H, hippocampus; PC, progenitor population; IZ, intermediate zone; CP, cortical plate; MZ: marginal zone; D, dorsal; L, lateral. Scale bars: 2500 μm (in B for A,B); 100 μm (in J for I,J); 500 μm (in J for E–H).
To assess the basis of the decrease in cortical size in Sall1-mutant animals, we histologically examined serial coronal sections of the cortex at E18.5 (n=4; Fig. 3E–J). Deficiencies were observed in both the dorsal and ventral telencephalon. The cortical plate was decreased in size in Sall1−/− animals at all rostral-caudal levels, compared with controls (Fig. 3E–K). In ventral regions, the striatum was smaller in Sall1-mutant animals compared with controls (Fig. 3F). In addition, an apparent increase in the number of darkly stained cells adjacent to the ventricles in both the dorsal cerebral wall and in the lateral and caudal ganglionic eminences was observed in Sall1-deficient animals compared with controls (Fig. 3F,H), which suggests an increase in the number of PCs in the telencephalon.
To verify these observations, the number of cells in each layer of the dorsal cerebral wall was quantified at E18.5. Total cell number was decreased by 9% in Sall1-deficient mice compared with controls at E18.5 (supplementary material Table S1, Fig. S1). Although the number of cells in the cortical plate was decreased by 26% (P<0.01, n=4), the number of PCs was increased by 14% (P<0.01, n=4; Fig. 3I–K). Together, these findings suggest that Sall1 is required for neurogenesis in both the dorsal and ventral telencephalon.
The decrease in the size of the cortical plate and changes in the proportion of different cell types could be due to abnormalities in proliferation, differentiation, migration or survival. We first determined whether alterations in cell survival could lead to the changes in cell number observed in the Sall1-deficient mouse cerebral cortex at E18.5. The number of activated-caspase-3-positive cells in the dorsal cerebral wall was quantified at E12.5, E14.5 and E18.5. No difference in the number of activated-caspase-3-positive cells was observed between Sall1−/− and control mice (data not shown; E12: n=2, P=0.86; E14.5: n=2, P=0.99; E18.5: n=4, P=0.15), which suggests that Sall1 is not required for cell survival.
Early born neuronal populations are overproduced in Sall1-mutant animals
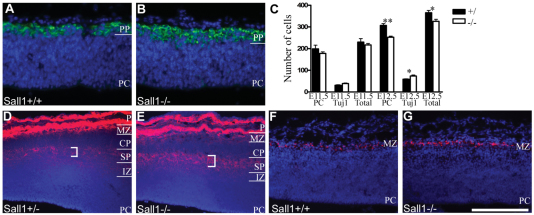
To identify the primary cellular deficit caused by loss of Sall1, the development of the dorsal cerebral wall from E11.5 until birth was examined. Cortical neurogenesis initiates at E9.5 when a subpopulation of RGCs in the dorsal cortex differentiate, producing neurons primarily destined for the preplate. Preplate neurogenesis continues until E13.5 (Price et al., 1997; Sheppard and Pearlman, 1997). To identify the onset of the deficits observed in Sall1−/−animals, the number of Tuj1-positive preplate neurons and Tuj1-negative RGCs was quantified in the dorsal cortex at E11.5 and E12.5 (Fig. 4). At E11.5, no significant difference in the number of RGCs or neurons was evident inSall1−/− mice compared with controls (n=3, Fig. 4C). However, by E12.5, a 17.6% decrease (P<0.01, n=4) in the number of RGCs and a 24.6% increase (P<0.05, n=4) in the number of neurons in the preplate was observed in Sall1−/− mice compared with controls (Fig. 4A–C), suggesting that early cortical PCs differentiate at the expense of re-entering the cell cycle.
Fig. 4.
Early born neuronal populations are overproduced in Sall1−/− mice. (A,B) Tuj1 (green) staining identified an increase in preplate size at E12.5 in Sall1-deficient animals (B) compared with controls (A). (C) Quantification of cortical cell number at E11.5 and E12.5. *, P<0.05; **, P<0.01. (D,E) CSPG (red) immunostaining of the subplate (bracketed region) and marginal zone identified an increase in these structures in Sall1−/− mice (E) compared with controls (D) at E14.5. Note: CSPG is also expressed by the meninges at the pial surface. (F,G) Reelin (red) immunostaining identified an increase in Cajal-Retzius cells in Sall1−/−mice (G) compared with controls (F) at E14.5. (A,B,D–G) Sections are counterstained with DAPI (blue). PP, preplate; PC, progenitor population; IZ, intermediate zone; SP, subplate; CP, cortical plate; MZ, marginal zone; P, pial surface. Scale bar: 100 μm (A,B); 200 μm (D,E); 400 μm (F,G).
The preplate subsequently splits to form the subplate and marginal zone. To determine whether structures derived from the enlarged preplate were altered in Sall1-mutant animals, we examined molecular markers of the marginal zone and subplate at E14.5 and E18.5. Chondroitin sulphate proteoglycan (CSPG) is an extracellular matrix protein expressed by cells in the pia, subplate and marginal zone (Sheppard et al., 1991). In Sall1-mutant mice, an increase in CSPG staining was observed in the subplate and marginal zone at E14.5 (n=3; Fig. 4D,E) and E18.5 (data not shown), compared with controls. Quantification of Tbr1-immunopositive subplate cells (see Methods) verified the increase in subplate cell number in Sall1-mutant animals at E18.5 (n=3, P<0.01; Fig. 5D). Intense staining for CSPG in pial cells makes it difficult to quantify CSPG-positive cells in the marginal zone; therefore, an independent marker of marginal zone cells was examined. Reelin is a secreted molecule expressed by Cajal-Retzius cells in the marginal zone (Ogawa et al., 1995; Schiffmann et al., 1997). In Sall1-deficient mice, more reelin-expressing cells were apparent in the marginal zone at E14.5 (Fig. 4F,G; n=4) and E18.5 (n=4; data not shown), compared with controls. Interestingly, these changes were accompanied by a 28.9% decrease in the number of cells in the cortical plate at E14.5 in Sall1-deficient animals compared with controls (supplementary material Table S1; n=3, P<0.05). This implies that, in the absence of Sall1, more cells are committed to early born structures, the preplate and its derivatives (the marginal zone and the subplate), and that fewer cells are committed to the later born structures such as the cortical plate.
Fig. 5.
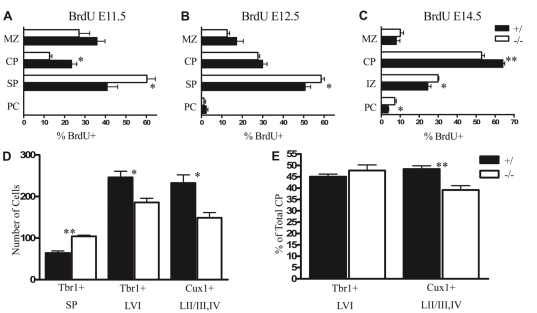
Sall1 regulates the number of cells committed to an early born fate. (A–C) Quantification of the proportion of total cells born (BrdU+) on E11.5 (A), E12.5 (B) or E14.5 (C) in defined cortical layers at E18.5. (D,E) Quantification of cell number in molecularly defined [Tbr1+: subplate and layer VI (LVI); Cux1+: layers II/III, IV (LII/III,IV)] cortical laminae at E18.5 (D) and the proportion of cells in cortical laminae as a percentage of total cortical plate cell number at E18.5 (E). *, P<0.05; **, P<0.01. PC, progenitor population; IZ, intermediate zone; SP, subplate; CP, cortical plate; MZ, marginal zone.
To verify this hypothesis, birth-dating studies were performed to determine whether the temporal order of neuronal specification was altered in the absence of Sall1. Pregnant dams were injected with BrdU on E11.5, E12.5 or E14.5, and the number and distribution of BrdU-labeled cells was examined at E18.5. The total number of cells born on E11.5 and E12.5 and present at E18.5 was increased in Sall1-mutant animals compared with controls (supplementary material Fig. S2, Table S1; E11.5: 41% increase, n=3, P<0.05; E12.5: 26.3% increase, n=4, P<0.01); however, the total number of cells born on E14.5 and present at E18.5 was similar between Sall1-deficient animals and controls (supplementary material Fig. S2, Table S1; n=3, P=0.1). Interestingly, the proportion of labeled cells in the cortical plate was decreased (E11.5: −45.7%, n=3, P<0.05; E14.5: −11.8%, n=3, P<0.01), with a concomitant increase in the subplate (E11.5: +47.8%, n=3, P<0.05; E12.5: +15.8%, P<0.05, n=4) or intermediate zone and PC populations (E14.5: +22.2%, n=3, P<0.05, and +98.5%, n=3, P<0.05, respectively) in Sall1−/− versus control animals (Fig. 5A–C). The majority of cells born on E14.5 that were located within the intermediate zone at E18.5 expressed neuronal markers and were observed below the histologically distinguished subplate in Sall1-deficient animals (supplementary material Fig. S2), which suggests that cells born on E14.5 are not continuously committed to a subplate fate past their normal birth-date in Sall1−/− animals but that neuronal migration might be altered.
Sall1 regulates deep versus superficial laminar fate
Given the decreased size of the cortical plate and increased number of cells committed to the subplate and marginal zone, and the changes in PC number, we investigated whether the inside-out pattern of cortical neurogenesis and layer specification was preserved in Sall1 mutant mice. The expression of four markers that are expressed in sequentially more superficial layers was examined at E18.5. No alteration was observed in the position of: the layer VI marker Tbr1 (supplementary material Fig. S1A,B) (Bulfone et al., 1995; Hevner, 2007; Hevner et al., 2001); the layer V marker Ctip2 (Arlotta et al., 2005; Avram et al., 2000) (supplementary material Fig. S1C,D); Brn2, expressed by layer V and layer II/III cells, as well as PCs and the intermediate zone (He et al., 1989; McEvilly et al., 2002) (supplementary material Fig. S1E,F); or Cux1, which is expressed by cells in upper cortical layers II/III and IV, as well as cells in PCs and the intermediate zone (supplementary material Fig. S1G,H) (Nieto et al., 2004). Taken together, these data suggest that loss of Sall1 does not alter cell-type specification of neurons destined for the cortical plate and that the inside-out pattern of neurogenesis is preserved.
In light of the decrease in size of the cortical plate and changes in the properties of cells born on E14.5, when upper cortical layers are being specified, we investigated whether Sall1 regulated the temporal production of deep versus superficial layer fate. The proportion of cells born on E14.5 that gave rise to deep (BrdU+Tbr1+; layer VI) or superficial (BrdU+Cux1+; layers II/III, IV) cortical layer neurons was quantified at E18.5. In Sall1−/−animals, the proportion of cells born on E14.5 specified as deep cortical layer neurons was increased by 213.6% (P<0.05, n=3; supplementary material Table S1), whereas the production of superficial cortical layer neurons was decreased by 28.6% (P<0.01, n=3; supplementary material Table S1), compared with controls. By E18.5, total cell number in layer VI and superficial cortical layers were decreased by 24.5% (P<0.05, n=3) and 35.9% (P<0.05, n=4), respectively, in Sall1−/− animals versus controls (Fig. 5D). Interestingly, when these values were expressed as a percent of total cortical plate cell number, no significant difference in the proportion of cells committed to layer VI was observed (n=3, P=0.4), whereas the proportion of cells committed to superficial cortical layers was decreased by 19.1% (n=4, P<0.01) in Sall1−/− animals compared with controls (Fig. 5E). Therefore, between E11.5 and E14.5, cells are selectively committed to a deep cortical layer fate at the expense of a more superficial cortical layer fate in Sall1−/− animals compared with controls.
Sall1 regulates cell cycle exit and IPC number during development
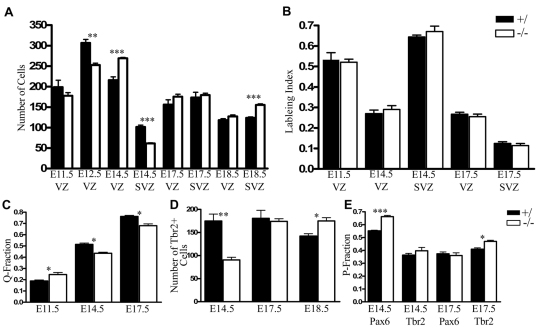
The changes in the number of neurons in different cortical regions could be a consequence of disruptions in PC proliferation, differentiation and/or neuronal migration. RGCs give rise to a second proliferative population, the SVZ, which consists of IPCs, a PC population characterized by basal mitotic divisions. Recent data suggest that the majority of neurons in the cortical plate are produced via terminal neurogenic divisions of IPCs (Haubensak et al., 2004; Miyata et al., 2004; Noctor et al., 2004; Noctor et al., 2008). In light of the alterations in PC and neuronal number in Sall1−/− animals, the proliferative properties of RGCs and IPCs were examined using short-term BrdU incorporation studies to determine whether Sall1 regulates PC cell cycle kinetics. These studies indicated that RGCs and IPCs were differentially altered over time in Sall1−/− mice compared with controls (Fig. 6A). Timed pregnant animals were injected with BrdU on E11.5, E14.5 or E17.5, and embryos were collected 60 minutes later. The number of cells in the VZ and SVZ was calculated and the proportion labeled with BrdU calculated. The labeling index was calculated for each age and progenitor region (BrdU+/total VZ PC or BrdU+/total SVZ PC; details of cell counts are provided in the Methods). These labeling indices were identical at all ages and regions examined in control and mutant animals, indicating that the proportion of cells in S-phase of the cell cycle is not altered in the VZ or SVZ in the absence of Sall1, and suggests that Sall1 does not regulate cell cycle kinetics or the rate of proliferation of cortical progenitors (Fig. 6B; E11.5: n=3, P=0.8; E14.5: n=4, VZ P=0.5, SVZ P=0.4; E17.5: n=4, VZ P=0.5, SVZ P=0.4). However, the total number of RGCs in the VZ was initially decreased at E12.5 (n=4, P<0.01), increased by 24.8% at E14.5 (n=3, P<0.01), and unchanged at E17.5 and E18.5 (n=3, P=0.7; n=4, P=0.2, respectively; supplementary material Table S1, Fig. S3; Fig. 6A) in Sall1−/− animals compared with controls. To determine whether early IPCs require Sall1 expression, we utilized PH3 staining to identify and quantify the number of basal mitotic divisions. No significant difference in the number of basal cortical mitotic divisions was observed at E12.5 between control and Sall1−/− mice (n=4, P=0.2; data not shown), which suggests that early cortical IPCs are not altered in the absence of Sall1. Expression of the transcription factor Tbr2 can be used to identify IPCs (Englund et al., 2005). Quantifying Tbr2-positive IPC number indicated that Tbr2+ IPCs were decreased by 48.1% by E14.5 (n=3, P<0.01), unchanged at E17.5 (n=3, P=0.7) and increased by 23% at E18.5 (n=3, P<0.05) in Sall1−/− mice compared with controls (Fig. 6D; supplementary material Fig. S4). These findings identify a role for Sall1 in regulating the production of the Tbr2+ IPC population from mid-neurogenesis.
Fig. 6.
Sall1 regulates cell cycle exit. Quantification of VZ and SVZ cell number at E11.5, E12.5, E14.5, E17.5 and E18.5 in control and Sall1−/− mice (A). The labeling index was not altered in Sall1-deficient animals at E11.5, E14.5 or E17.5 compared with controls (B). The rate of neuronal differentiation (Q fraction) was increased at E11.5 (E12.5: BrdU injected at E11.5), but decreased at E14.5 (E15.5: BrdU injected at E14.5) and E17.5 (E18.5: BrdU injected at E17.5) in Sall1−/− animals compared with controls (C). Quantification of Tbr2 cell number at E14.5, E17.5 and E18.5 (D). Alterations in the rate of VZ (Pax6+) and SVZ (Tbr2+) cell cycle re-entry in Sall1−/−animals compared with controls at E14.5 and E17.5 (E). *, P<0.05; **, P<0.01; ***, P<0.001.
The dramatic changes identified in PCs in Sall1-deficient animals prompted us to examine the rate of neuronal differentiation. The proportion of cells that re-enter versus exit the cell cycle in a 24-hour period was quantified using long-term BrdU incorporation studies. Pregnant dams were injected with BrdU on E11.5, E14.5 or E17.5, and embryos were collected 24 hours later. The proportion of cells that differentiated into neurons was quantified (BrdU+Tuj1+/total BrdU+) and termed the quiescent-fraction (Q). Q gradually increases from 0 to 1 in control animals, whereas the proliferative fraction (P) decreases from 1 to 0 from E9.5 to E18.5 (Takahashi et al., 1994). At E11, Q was 0.19±0.01 in control animals and 0.24±0.02 in Sall1-deficient animals, which represents a 26.3% increase over controls (Fig. 6C; n=4, P<0.05). This finding verifies our previous results that showed increased numbers of neurons at E12.5 in Sall1-mutant animals. By E14.5, Q was 0.51±0.01 in control animals versus 0.44±0.01 in Sall1 mutants, which represents a 13.7% decrease compared with controls (Fig. 6C; n=3, P<0.05). This suggests that more cells are re-entering the cell cycle in mutants than in controls. Interestingly, the decrease in Q at E14.5 seemed to be in part due to a decrease in the previously described rapidly exiting subpopulation of cells born on E14.5 (supplementary material Fig. S3) (Takahashi et al., 1996a). By E17.5, Q increased to 0.76±0.01 in control animals and to 0.68±0.02 in Sall1-deficient animals (Fig. 6C), which represents a 10.5% decrease in Q in the absence of Sall1 compared with controls (n=4, P<0.05). Thus, in the absence of Sall1, early PCs differentiate rather than re-enter the cell cycle, whereas, later in neurogenesis, PCs preferentially re-enter the cell cycle rather than differentiate, compared with controls.
These findings indicate that E14.5 is a critical developmental point, when Sall1 switches from promoting a proliferative fate to a differentiative fate. The early decrease (E14.5) and later increase (E18.5) in Tbr2-positive cells and the changes observed in neuronal differentiation in Sall1-mutant animals suggested that changes in the type of division that a PC makes might contribute to the observed phenotype. The proportion of E14.5 and E17.5 proliferating cells that re-entered the cell cycle and gave rise to RGCs (Pax6+) or IPCs (Tbr2+) within a 24-hour period was quantified using BrdU labeling. Although Pax6 expression does not exclusively identify RGC populations, because its expression is maintained in ∼25% of Tuj1+ newly differentiated neurons up to 20 hours after cell division, (Kawaguchi et al., 2004) and because coexpression of Pax6 and Tbr2 has been observed in cells at the VZ-SVZ border (Englund et al., 2005), intense Pax6 staining (Pax6+++) can be used to identify RGCs and their immediate progeny. We observed a 21.0% increase in the proportion of BrdU-labeled Pax6+++ RGCs (n=3, P<0.001) with no change in the proportion of Tbr2+ IPCs (n=4, P=0.3) in Sall1−/− compared to control animals at E14.5 (Fig. 6E). This suggests that, in the absence of Sall1, proportionately more PCs are undergoing proliferative RGC divisions at mid-neurogenesis. By E17.5, a 14.6% increase in the proportion of cells that re-entered the cell cycle as Tbr2+ IPCs was observed in Sall1−/− animals compared with controls (P<0.05, n=3; Fig. 6E). The fact that the proportion of cells re-entering the cell cycle as Pax6+++ RGCs was unchanged (P=0.6, n=3; Fig. 6E) suggests that, in the absence of Sall1, proportionately more IPCs are undergoing self-renewing divisions, rather than terminal neurogenic divisions, at this age. Together, these findings indicate that Sall1 is a crucial regulator of PC maturation during development.
Sall1 expression in dorsal progenitor cells regulates progenitor cell maturation
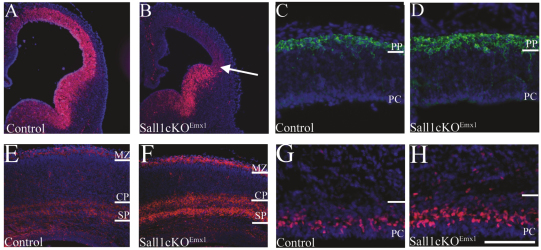
Our results point to a role for Sall1 in regulating neuronal differentiation in the dorsal cortex. However, alterations in the ventral forebrain were histologically observed in Sall1-deficient mice. Interneurons derived from the ventral telencephalon tangentially migrate to their final laminar cortical position through the SVZ, intermediate zone and marginal zone (reviewed in Marin and Rubenstein, 2001). Furthermore, dorsal progenitors have been shown to molecularly interact with ventrally derived migrating interneurons (Tiveron et al., 2006) and it is hypothesized that migrating interneurons can influence dorsal PC differentiation (Haydar et al., 2000). To determine the contribution of ventrally derived cells to the observed phenotype, we generated mice with a floxed Sall1 allele (Inoue et al., 2010; Yuri et al., 2009) and restricted deletion of Sall1 to dorsal PCs using Emx1-Cre (mutant animals are termed Sall1-cKOEmx1). Emx1 expression initiates at E9.5, and Emx1-driven Cre recombination is evident from E10.5 (Gorski et al., 2002). Recombination is observed in the majority of excitatory neurons in the cortex, but not in interneurons (Gorski et al., 2002). In order to determine whether recombination of the floxed Sall1 allele had occurred, we examined Sall1 expression in the dorsal cortex at E12.5 (Fig. 7A,B). In control animals, Sall1 expression extended into the dorsal cortex at E12.5 (Fig. 7A); however, in Sall1-cKOEmx1 embryos, strong Sall1 expression was limited to the ventral telencephalon and did not extend beyond the cortico-striatial boundary (n=3; arrow, Fig. 7B). Weak Sall1 expression was observed in the dorsal cortex at E12.5 in Sall1-cKOEmx1 animals, which might represent residual Sall1 protein present in dorsal PCs produced prior to the recombination event. However, functional deletion of Sall1 was confirmed by E12.5 because the Sall1-cKOEmx1 cortical phenotype was identical to Sall1-null animals. An increase in the number of Tuj1-positive cells present in the dorsal cerebral cortical wall was observed in Sall1-cKOEmx1 mice compared with controls (Fig. 7C,D). Also, similar to the Sall1-null animals, CSPG staining revealed an increase in the number of cells in the subplate by E18.5 in Sall1-cKOEmx1 animals compared with controls (Fig. 7E,F). Deficits in the ventral telencephalon were not observed, as expected.
Fig. 7.
Conditional deletion of Sall1 in dorsal PCs indicates that Sall1 regulates PC fate progression. Sall1 (red) expression is localized to the ventral telencephalon and does not extend into the dorsal cortex in Sall1-cKOEmx1 animals (arrow, B), unlike controls (A) at E12.5. Tuj1 staining (green) of cortical neurons at E12.5 identified an increase in preplate cell number in Sall1-cKOEmx1 (D) animals compared with controls (C). CSPG (red) immunostaining of the subplate and marginal zone identified an increase in these structures in Sall1-cKOEmx1 (F) animals compared with controls (E) at E18.5. Tbr2 (red) immunostaining at E18.5 identified an increase in IPCs in Sall1-cKOEmx1 (H) animals compared with controls (G). PC, progenitor population; PP, preplate; SP, subplate; CP, cortical plate; MZ, marginal zone. Scale bar: 100 μm (C,D,G,H); 350 μm (E,F); 400 μm (A,B).
Similar to null animals, the total length and dorsal surface area of the brain was decreased by 3.6% (P<0.05, n=3) and 5.8% (P<0.05, n=3) at E18.5, respectively, in Sall1-cKOEmx1 animals compared with controls (supplementary material Fig. S5). Specifically, the cerebral hemispheres were decreased in length and dorsal surface area by 12.8% (P<0.05, n=3) and 12.8% (P<0.05, n=3), respectively, in Sall1-cKOEmx1 animals compared with controls. As expected, no alteration in the length (P=0.9, n=3) or dorsal surface area (P=0.9, n=3) of the midbrain or other brain regions were observed (supplementary material Fig. S5). We quantified the number of cells in the dorsal cortex in Sall1-cKOEmx1 animals and, similar to Sall1-deficient animals, observed an increase in the number of PCs (17.6%; n=3, P<0.05) and a decrease in the number of cells in the cortical plate (23.3%; n=3, P<0.01) at E18.5 in Sall1-cKOEmx1 animals compared with controls (supplementary material Fig. S5). To determine whether the increase in the number of PCs was due to an increase in the number of IPCs, we examined Tbr2 staining at E18.5. In Sall1-cKOEmx1 animals, an increase in the number of Tbr2-positive cells was observed in the dorsal cortex at E18.5 compared with controls (n=3; Fig. 7G,H). These findings suggest a requirement for Sall1 expression in dorsal PCs to regulate PC maturation.
Cells born in the ventral telencephalon migrate through the intermediate zone and marginal zone during development en route to the cortex. Although we observed an increase in subplate cell number (in the intermediate zone) and reelin-positive Cajal-Retzius cell number (in the marginal zone) in Sall1−/− animals, we observed no alteration in total cell number in the intermediate zone or marginal zone in Sall1-deficient animals compared with controls. In Sall1-cKOEmx1 animals, we also observed an increase in subplate and marginal zone populations; however, total cell number was increased by 16.2% in the intermediate zone (16.2%; n=3, P<0.01) and by 25.5% in the marginal zone (28.5%; n=3, P<0.001) compared with controls at E18.5 (supplementary material Fig. S5). These data suggest that a population of ventrally derived migrating cells is decreased in Sall1−/− animals and that this deficit is rescued in Sall1-cKOEmx1 animals.
DISCUSSION
These studies demonstrate that Sall1 regulates development of the cerebral cortex in mice and provide insight into neural abnormalities that might be present in individuals with TBS. Sall1 is expressed by CNS PCs throughout neurogenesis, as well as in peripheral organs. Using classical knockout studies, we identified a unique requirement for Sall1 expression in dorsal cortical PCs. At E18.5, both the surface area and depth of the cerebral cortex were decreased in size in Sall1−/− animals compared with controls. These alterations are a consequence of changes in the type of division that cortical PCs undergo. Early in development, Sall1−/−PCs preferentially exit the cell cycle, whereas later in development IPCs preferentially re-enter the cell cycle. These changes are associated with an increased commitment of neurons to an early cortical fate and a decrease in the number of neurons committed to a later cortical fate, compared with controls. These findings suggest that Sall1 temporally regulates cortical neurogenesis in cortical PCs.
Sall1 regulates neuronal output in deep versus superficial cortical layers
Although the inside-out pattern of laminar specification was normal in the absence of Sall1, the temporal order of deep to superficial laminar fate specification was altered. The first neuronal layer to be specified is the preplate, born from E11.5 to E12.5 in control animals, which splits to form the subplate and marginal zone at ∼E13.5 (Gupta et al., 2002; Price et al., 1997; Sheppard and Pearlman, 1997). Deep cortical layers (layers VI and V) are subsequently specified from E12.5 to E13.5, with production of superficial cortical layers (layers II, III, IV) predominating from E14.5 (Angevine and Sidman, 1961; Caviness, 1982; Gillies and Price, 1993; Levers et al., 2001; Price et al., 1997; Takahashi et al., 1999). From E11.5 to E12.5, Sall1-deficient PCs preferentially exit the cell cycle at the expense of re-entering. Birth-dating studies indicate that, early in development, proportionately more of these differentiating neurons gave rise to subplate cells at the expense of committing cells to a cortical layer fate in Sall1−/− animals. These findings suggest that early in development Sall1 promotes proliferative symmetric divisions over asymmetric differentiative divisions in RGCs and restricts neurogenesis of preplate neurons in early cortical PCs.
Interestingly, increased early neurogenesis is often associated with depletion of the PC population. As a consequence, the cortical plate is decreased in size owing to the lack of available PCs later in development (Bedford et al., 2005; Caviness et al., 2003; Handler et al., 2000; Roy et al., 2004; Wines-Samuelson et al., 2005). Our findings suggest that the decrease in cortical plate size in Sall1-mutant animals is not simply due to depletion of the PC population. Although Sall1 promotes cell cycle re-entry in early cortical PCs from E11.5 until E13.5, it facilitates cell cycle exit in late PCs. The paradoxical role of Sall1 in early versus late PCs is mediated by changes in the properties of RGCs versus IPCs and occurs as PCs transition from producing neurons with a deep layer fate to those with a superficial layer fate. We demonstrated that proportionately more PCs re-entered the cell cycle as Pax6+++ RGCs and fewer exited the cell cycle and differentiated into neurons in Sall1-mutant animals at E14.5, compared with controls. These findings indicate that, from E14.5, differentiative divisions were decreased and that symmetric VZ proliferative divisions were increased in Sall1−/−animals compared with controls. Interestingly, these changes were associated with a decrease in the rapidly exiting subpopulation of cells born on E14.5 (Takahashi et al., 1996a). Birth-dating of Sall1-deficient PCs at E14.5 indicated that proportionally more PCs gave rise to deep cortical layers (Tbr1+ layer VI neurons) and proportionately fewer PCs gave rise to superficial cortical layers (Cux1+ layer II/III, IV neurons) compared with controls. Coincident with these changes in laminar specification, RGCs were increased and IPCs were decreased in Sall1-deficient animals at E14.5, compared with controls. By E17.5, however, an increase in the proportion of cells that re-entered the cell cycle as Tbr2+ IPCs was observed in Sall1−/− animals compared with controls, accompanied by a decrease in the proportion of cells that differentiated into neurons, with no change in the proportion of cells that re-entered the cell cycle as Pax6+++ RGCs. This suggests that Sall1−/− IPCs are undergoing symmetric self-renewing divisions at the expense of neuronal differentiation. Because early (E12.5) IPC number was normal in Sall1-deficient animals, these observations support the hypothesis that Sall1 might influence the transition from deep to superficial neuronal specification by regulating the terminal differentiation of IPCs.
A role for Sall1 in intermediate progenitor cell function
It is interesting that Sall1 is highly expressed in RGCs and downregulated in IPCs yet it has a major phenotype in regulating IPC properties. What are the molecular mechanisms that could mediate the actions of Sall1 in RGCs versus IPCs? Recently, several molecules have been implicated in regulating IPC proliferation and differentiation. Tbr2 is sufficient to suppress a radial glial cell phenotype and promote an IPC phenotype (Sessa et al., 2008). Disruption of Notch or Fragile-X mental retardation protein signaling promotes the transition of RGCs to IPCs (Mizutani et al., 2007; Saffary and Xie, 2011; Yoon et al., 2008). IPC number is decreased in the absence of either Frs2α, Foxg1 or insulinoma associated 1, which indicates that these proteins regulate the expansion of IPCs (Siegenthaler et al., 2008; Yamamoto et al., 2005). By contrast, Cux2 has been shown to limit the proliferation of IPCs. In the absence of Cux2, an increased number of IPCs was observed at E15.5, similar to the phenotype observed in Sall1-deficient mice (Cubelos et al., 2008). One of the most likely signaling pathways that Sall1 could interact with is the Wnt pathway. Similar to Sall1, analysis of Wnt signaling in cortical PCs has shown that the Wnt–β-catenin pathway has differential effects on RGCs versus IPCs (Wrobel et al., 2007; Munji et al., 2011). A downstream mediator of the Wnt pathway, β-catenin, is predominantly expressed in RGCs and its expression must be downregulated to induce the production of IPCs (Mutch et al., 2010). Interestingly, numerous studies have shown that Sall1 is activated in response to Wnt signaling and can interact with β-catenin (de Celis et al., 1996; de Celis et al., 1999; Farrell and Munsterberg, 2000; Sato et al., 2004). Sall1 might therefore mediate some of the effects of Wnt signaling in cortical PCs to control the transition from an RGC to an IPC.
Sall1- and Cux2-deficient animals both have an increased number of IPCs; however, in Cux2-deficient animals this increase was associated with an increase in the number of cells in superficial cortical layers, whereas, in Sall1-deficient mice, superficial cortical neuron number is decreased. This decrease in Sall1-mutant animals is a consequence of decreased neuronal differentiation from E14.5, as well as a decrease in the commitment of neurons to a superficial cortical layer fate. This does not exclude the possibility that the increased number of IPCs present in Sall1−/− mice at E18.5 might be continuously committed to the production of superficial cortical layers postnatally, leading to normal neuronal number in superficial layers in adults. Also, because Sall1 is predominantly expressed in radial glial cells, it is possible that Sall1 regulates migration of neurons in a non-cell-autonomous manner. These potentials could be examined by analyzing the postnatal maturation of the cerebral cortex and the final organization of cortical laminae. Alternatively, gliogenesis might be altered in Sall1-deficient mice, because glial cells are born at late prenatal and early postnatal stages. However, we have not seen any differences in glial or oligodendrocyte markers in Sall1-null or conditional knockout animals (our unpublished observations). In summary, our findings support the hypothesis that, from mid-neurogenesis, Sall1 promotes terminal neurogenesis of PCs, initially by controlling the transition from an RGC to an IPC and later by regulating terminal neurogenesis of IPCs.
The SALL family in human disease
In summary, we identified a previously unknown role for Sall1 in regulating PC fate progression in the developing cortex. In early cortical PCs Sall1 promotes proliferative over neurogenic divisions in RGCs, whereas later in development, Sall1 promotes differentiative over proliferative divisions in IPCs. Other members of the Sall gene family have also been shown to restrict PC proliferation and must be eliminated in a cell to allow cellular differentiation in other systems. Sall1 is required for the proliferation or survival of embryonic stem cells (Karantzali et al., 2011) and in multipotent renal PCs in mice, but is not required for their generation or differentiation (Nishinakamura, 2008; Nishinakamura et al., 2011). In Drosophila, a Sall1 homolog, the Spalt gene, has been shown to restrict the number of sensory organ precursor cells and inhibit their neuronal differentiation (de Celis et al., 1999; Rusten et al., 2001). The related Sall family member Sall4 is required to maintain cells in a proliferative undifferentiated state (Barembaum and Bronner-Fraser, 2004; Elling et al., 2006; Sakaki-Yumoto et al., 2006). Of the four mammalian Sall genes identified, expression studies indicate that they are expressed in cortical PCs as well as in distinct and overlapping structures in the developing nervous system and peripheral organs (Harrison et al., 2008a; Harrison et al., 2008b; Kohlhase et al., 2000; Kohlhase et al., 2002a; Ott et al., 2001) (and our unpublished observations). Mutations in three of these genes are associated with known human disorders with neural abnormalities and overlapping phenotypes in peripheral organs, including TBS (SALL1), Okihiro syndrome/Duane radial ray syndrome, Holt-Oram syndrome, acro-renal-ocular syndrome (SALL4) and 18q deletion syndrome (SALL3) (Al-Baradie et al., 2002; Kohlhase et al., 1999a; Kohlhase et al., 2002b; Kohlhase et al., 1998). Many of the mutations identified in individuals with TBS are located in exons 2 and 3 and in intron 2 of Sall1, and are predicted to lead to premature termination of transcription (Blanck et al., 2000; Engels et al., 2000; Kohlhase et al., 1999b; Kohlhase et al., 2003; Kohlhase et al., 1999c; Kohlhase et al., 1998; Marlin et al., 1999; Salerno et al., 2000; Surka et al., 2001; Walter et al., 2006). Although it is thought that many of these transcripts will be degraded by nonsense-mediated RNA decay, recent evidence has suggested that the truncated protein can function in a transdominant negative manner (Kiefer et al., 2003; Kiefer et al., 2008). These findings indicate that, in humans, TBS could be caused by a gain of function. However, deletions of SALL1 have also been identified in some individuals with a milder form of TBS (Borozdin et al., 2006), indicating that haploinsufficiency can also lead to the disorder. It is therefore likely that the differences seen in the two phenotypes observed in humans and mice with either a deletion or truncation of the Sall1 gene are due to the cell-type-specific protein interactions on target gene promoters. Previous studies demonstrated that Sall family members could interact with each other via their N-terminal region (Kiefer et al., 2003; Sakaki-Yumoto et al., 2006; Sweetman et al., 2003). Our investigations and those of other researchers have found that the related family members Sall2, Sall3 and Sall4 are also expressed in cortical neural progenitor cells (Magdaleno et al., 2006; Ott et al., 2001; Parrish et al., 2004), suggesting that Sall family members might functionally compensate for each other and/or functionally interact to regulate neurogenesis in the developing cerebral cortex. The rarity of TBS in humans and the variable penetrance of this disorder make it difficult to assign a concise neural phenotype in individuals with TBS. The findings presented here provide important insights into the role of Sall1 in neural precursors, with relevance to human neural precursors, and will provide a framework for future studies aimed at examining neural function in individuals with TBS.
METHODS
Animals
Embryos were obtained from the mating of Sall1 heterozygote (Sall1+/−) animals, maintained on a C57BL/6J background, and genotyped as previously described (Nishinakamura et al., 2001). No alterations in cortical development were observed in Sall1+/−animals in our study (unpublished observations) and therefore these embryos were also used as controls. Controls (Sall1+/+ or Sall1+/−mice) are jointly referred to as +/. Emx1-Cre animals (Gorski et al., 2002) were genotyped by PCR for the Cre gene, using previously described conditions (Furuta et al., 2000). For Sall1 expression studies, embryos were obtained from timed pregnant CD1 mice from Charles Rivers Laboratories (Wilmington, MA). The day of vaginal plug was designated as embryonic day 0.5 (E0.5). For birth-dating studies, pregnant dams were injected with BrdU (50 μg/g of body weight) at the indicated times before embryo collection. For proliferation studies, BrdU was injected 60 minutes prior to embryo collection. Embryos were collected via cesarean section from E11.5 to E18.5. Brains from E18.5 embryos were dissected from the skull prior to fixation. Embryos were either fixed in 4% paraformaldehyde (PFA; pH 7.4) and processed through increasing sucrose gradients for cryosectioning, or fixed in Carnoys solution (1:3:6 acetic acid: chloroform: 100% ethanol) and then processed through a butanol series for paraffin sectioning. PFA-fixed brains were embedded in Tissue-Tek Optimal Cutting Temperature (O.C.T.) compound (EMS, Hatfield, PA) and sectioned at 20 μm. Paraffin-embedded embryos were sectioned at 10 μm. Animal protocols and procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and adhered to the National Institutes of Health guidelines.
Measurement of cortical size
Brains from E18.5 embryos were dissected from the skull and fixed overnight in 4% PFA. Brains were visualized on a Nikon (Melville, NY) dissecting microscope, photographed with a Photometrics (North Reading, MA) CoolSNAP digital camera and IP Lab software (Biovision Technologies, Exton, PA). Images were subsequently imported into Photoshop 7.0 (Adobe Systems, San Jose, CA). Analyses were performed by an observer blind to the genotype. Total brain length was measured as the distance from the rostral extent of the olfactory bulb to the caudal extent of the inferior colliculus. Cortical length was measured as the distance from the rostral extent of the cortex to the caudal extent of the cortex, at the lateral edge of the olfactory bulb. Midbrain length was measured as the distance from the rostral extent of the superior colliculus to the caudal extent of the inferior colliculus. Surface area was analyzed in similarly defined regions. Statistical analysis was performed using an unpaired t-test with InStat 3 software (GraphPad Software, San Diego, CA).
Immunohistochemistry
Immunohistochemistry and Nissl staining was performed as previously described (Harrison et al., 2008a; Roy et al., 2004), with the following antibodies: mouse anti-BrdU (1:25, Amersham Biosciences, Piscataway, NJ); rabbit anti-Brn2 (1:500, Santa Crux Biotechnology, Santa Cruz, CA); rabbit anti-activated-caspase-3 (1:200, Promega, Madison, WI); mouse anti-CSPG (1:5, Developmental Studies Hybridoma Bank, The University of Iowa, IA); rat anti-Ctip2 (1:500, Abcam, Cambridge, MA); rabbit anti-Cux1 (Santa Crux Biotechnology); chicken anti-nestin (1:500, Thermo Scientific); rabbit anti-Pax6 (1:500, Covance, Berkeley, CA); rabbit anti-PH3 (1:200, US Biological, Swampscott, MA); mouse anti-reelin (1:500, Abcam); mouse anti-Sall1 (1:500, PPMX Perseus Proteomics, Tokyo, Japan); rabbit anti-Tbr1 (1:1000, Chemicon, Temecula, CA); rabbit anti-Tbr2 (1:1000, Chemicon); rabbit anti-Tuj1 (1:1000, Sigma, St Louis, MO). Sections were visualized on a Nikon fluorescent microscope, photographed with a Photometrics CoolSNAP digital camera and IP Lab software. Composite images were prepared using Photoshop 7.0. Contrast, color and brightness were adjusted in Photoshop 7.0.
Histological identification of cortical layers
To assign cellular subtypes and boundaries to cortical layers, sections were examined at 40× magnification. The VZ was identified as the cellular region that is adjacent to the ventricle and contains organized parallel arrays of cells with elongated nuclei arranged perpendicular to the ventricular surface. The SVZ was composed of a compact layer of cells with round nuclei arranged in a disorganized manner, adjacent to the VZ. In this study the progenitor population was defined to include the VZ and SVZ. The intermediate zone was cell sparse compared with the progenitor population and contained a mixture of cells with round and elongated nuclei arranged both parallel and perpendicular to the ventricular surface. The subplate was identifiable within the intermediate zone as a layer of large round nuclei, deep to the cortical plate. The cortical plate was evident from E14.5 and identifiable owing to an increased packing density of round cell nuclei arranged in rows. The marginal zone was located superficial to the cortical plate, adjacent to the pial surface. The marginal zone contained a mixture of cells with round nuclei and elongated nuclei arranged parallel to the ventricular surface.
Analysis and cell counts
For quantification of cell number in the dorsal cortex, stained sections were imaged as described above. Sections were histologically matched for rostral-caudal level between genotype. For cell counts, matched sections through the presumptive somatosensory 1 hindlimb and forelimb region or through the presumptive somatosensory 1 trunk region were quantified (Franklin and Paxinos, 1997). Cortical layers were histologically distinguished as described above. For all analyses a 150 μm window in the medial-lateral dimension spanning from the ventricular to pial surface of the dorsal cortex from three non-adjacent sections per animal were counted, and the mean of the three sections per animal was calculated. The means from three or four animals per genotype were compared i.e. three or four mutant animals were compared with three or four wild-type animals. The analyses were performed by an observer blind to the genotype. Cell counts were performed and quantified using a cell counting program, as previously described (Roy et al., 2004). The data were subsequently decoded and statistical analysis of the results was performed using an unpaired t-test with InStat 3 software. Graphs were prepared using Prism 4 software (GraphPad Software). Values are represented as mean ± s.e.m. Significance was set at P<0.05.
Cell death studies
For studies of cell survival, the number of activated-caspase-3-positive cells spanning the ventricular to pial surface was quantified.
Proliferation studies
Pregnant animals were injected with a single dose of BrdU (on E11.5, E14.5 or E17.5), and embryos were collected 60 minutes later and processed for immunohistochemistry. Sections were stained with an anti-BrdU antibody and counterstained with DAPI. To calculate the labeling index, the total number of DAPI-positive cells within the VZ and SVZ was counted per window. The number of BrdU-positive cells in the same window was counted and the percent labeled calculated to determine the labeling index (number BrdU positive cells/total number of DAPI-positive cells). E11.5 sections were also counterstained with the neuronal marker Tuj1 and the E11.5 VZ population was defined as the Tuj1-negative population; E14.5 and E17.5 VZ and SVZ populations were histologically distinguished using DAPI staining.
Laminar cell counts
To determine the number of cells in each cortical layer, individual markers were used to identify distinct laminae. Cells expressing a high level of Tbr1 identify layer VI, and Cux1 expression identifies layers II/III and IV (Bulfone et al., 1995; Nieto et al., 2004). Tbr1-positive subplate cells, located in the intermediate zone, were histologically distinguished from deep cortical plate cells by DAPI staining. The amount of Tbr1-positive or Cux1-positive cells was expressed as a percentage of total cortical plate cell number to identify whether alterations in the proportion of cells committed to deep (Tbr1+/cortical plate cell number) versus superficial (Cux1+/cortical plate cell number) layers were present in Sall1−/−mice.
Birth-dating studies
For birth-dating studies (E18.5 embryos injected with BrdU on E11.5, E12.5 or E14.5), BrdU-stained sections were examined and only heavily labeled BrdU-positive cells were quantified. Heavily labeled cells were assumed to be cells that incorporated BrdU and subsequently exited the cell cycle. Those cells that re-entered the cell cycle will dilute the BrdU label in the nucleus and have a weaker BrdU staining. For laminar birth-dating studies, deep cortical layer VI cells were defined by high expression of Tbr1, and superficial cortical layers (layers II/III, IV) were defined by Cux1 expression. The proportion of BrdU+ cells within the cortical plate committed to a deep versus superficial layer fate was quantified: deep (Tbr1+BrdU+/BrdU+) and superficial (Cux1+Brdu+/BrdU+).
Cell cycle exit versus cell cycle re-entry studies
The proportion of cells that exit the cell cycle in a defined period is referred to as the quiescent fraction (Q). To identify the proportion of cells that exit the cell cycle, embryos were injected with BrdU on either E11.5, E14.5 or E17.5 and collected 24 hours later. Cells that exited the cell cycle to become neurons were identified as BrdU-positive and Tuj1-positive. Tuj1 is a pan neural marker that is expressed by neurons approximately 1 hour after they exit the cell cycle, and is maintained in migrating and mature neurons (Lee et al., 1990a; Lee et al., 1990b; Menezes and Luskin, 1994; Hammerle and Tejedor, 2002). This marker is present in cells in the VZ and SVZ and can be used to identify cells differentiating into neurons (Menezes and Luskin, 1994; Hammerle and Tejedor, 2002). The quiescent fraction was determined as the proportion of cells that were double positive for BrdU and Tuj1 divided by the total BrdU-positive population (BrdU+Tuj1+/total BrdU).
TRANSLATIONAL IMPACT.
Clinical issue
Mutations in the human SALL1 gene cause Townes Brocks syndrome (TBS). This condition is variably penetrant, and patients exhibit abnormalities primarily in the limbs, kidneys, ears, heart, bone, esophagus and/or anus. Various neural deficits, including mental retardation, have been identified in a small proportion of patients, although most patients are not formally examined for cognitive, emotional or behavioral alterations. Additionally, the rarity of the disorder, coupled with variable penetrance and phenotype even in individuals with similar mutations, has made it difficult to assess the impact of genetic lesions in SALL1 on cognitive or behavioral function in humans.
Results
This paper identifies and characterizes a distinct phenotype involving cerebral cortical neural precursor cells in a mouse model of Sall1 deficiency. The authors demonstrate that, although Sall1 is expressed in all central nervous system progenitor cells, the cerebral cortex is particularly sensitive to Sall1 deficiency in developing mice. In the absence of Sall1, early cortical progenitor cells exhibit enhanced neuronal differentiation, whereas later progenitor cells proliferate rather than differentiate. In addition, deficiency of Sall1 leads to a prolonged production of neurons during development that are destined for early cortical structures such as deep cortical layers.
Implications and future directions
Although neural deficits are not commonly reported in individuals with TBS, these data indicate a key role for Sall1 in regulating the rate of neural cell production in the cerebral hemispheres during mouse development. Because the cerebral cortex mediates higher-order thought processing, reasoning, memory and emotion, these findings suggest that individuals with TBS have previously unrecognized neurodevelopmental abnormalities, potentially affecting neural processing, emotion and/or behavior. Future studies should examine the cognitive and behavioral consequences of either Sall1 deficiency or expression of a disease-associated human SALL1 allele in the mouse cerebrum, and aim to identify the associated cellular alterations. Identifying the cellular processes affected by Sall1 deficiency or mutation might provide potential therapeutic targets in humans.
The proportion of cells that re-enter the cell cycle in a defined period is referred to as the proliferative fraction. To identify the proportion of cells that re-entered the cell cycle, embryos were injected with BrdU on either E14.5 or E17.5 and collected 24 hours later. PC cycle re-entry was determined as the proportion of cells that were double positive for BrdU and the RGC marker Pax6 or IPC marker Tbr2 divided by the total BrdU-positive population (BrdU+Pax6+/total BrdU or BrdU+Tbr2+/total BrdU). To quantify the VZ and SVZ using molecular markers, the number of Pax6- or Tbr2-positive cells within the PC population was quantified. To quantify the IPC cell number at E12.5, the number of PH3-positive cells in abventricular regions in the progenitor domain was quantified (Haubensak et al., 2004).
Supplementary Material
Acknowledgments
We thank K. Mauro, E. Drill, W. Halfter, M. Parrish, C. Lagenaur, E. Thiels, L. Lillien and C. Lance-Jones for technical help and discussion. The chondroitin sulfate proteoglycan antibody developed by W. Halfter was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences.
Footnotes
FUNDING
This work was supported by the National Institutes of Health [NIMH MH60774 to A.P.M.]; the National Institute on Drug Abuse [NIDA grant AA13004 to A.P.M.]; the Ministry of Education, Culture, Sports, Science, and Technology of Japan [Grant-in-Aid for Scientific Research B23390228 to R.N.]; and the National Eye Institute (NEI) [NIH R01 EY014998 to K.R.J.].
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
S.J.H. and A.P.M. designed the research and interpreted the results; S.J.H. performed the research and analyses; R.N. generated the Sall1-null and Sall1-cKO animals; K.R.J. provided the Emx1-Cre mice; S.J.H. and A.P.M. wrote the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.002873/-/DC1
REFERENCES
- Al-Baradie R., Yamada K., St Hilaire C., Chan W. M., Andrews C., McIntosh N., Nakano M., Martonyi E. J., Raymond W. R., Okumura S., et al. (2002). Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am. J. Hum. Genet. 71, 1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine J. B., Jr, Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 [DOI] [PubMed] [Google Scholar]
- Arlotta P., Molyneaux B. J., Chen J., Inoue J., Kominami R., Macklis J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 [DOI] [PubMed] [Google Scholar]
- Arnold S. E. (1999). Neurodevelopmental abnormalities in schizophrenia: insights from neuropathology. Dev. Psychopathol. 11, 439–456 [DOI] [PubMed] [Google Scholar]
- Attardo A., Calegari F., Haubensak W., Wilsch-Brauninger M., Huttner W. B. (2008). Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE 3, e2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram D., Fields A., Pretty On Top K., Nevrivy D. J., Ishmael J. E., Leid M. (2000). Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J. Biol. Chem. 275, 10315–10322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barembaum M., Bronner-Fraser M. (2004). A novel spalt gene expressed in branchial arches affects the ability of cranial neural crest cells to populate sensory ganglia. Neuron Glia Biol. 1, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M., Horvitz H. R. (1996). The Caenorhabditis elegans gene sem-4 controls neuronal and mesodermal cell development and encodes a zinc finger protein. Genes Dev. 10, 1953–1965 [DOI] [PubMed] [Google Scholar]
- Bedford L., Walker R., Kondo T., van Cruchten I., King E. R., Sablitzky F. (2005). Id4 is required for the correct timing of neural differentiation. Dev. Biol. 280, 386–395 [DOI] [PubMed] [Google Scholar]
- Blanck C., Kohlhase J., Engels S., Burfeind P., Engel W., Bottani A., Patel M. S., Kroes H. Y., Cobben J. M. (2000). Three novel SALL1 mutations extend the mutational spectrum in Townes-Brocks syndrome. J. Med. Genet. 37, 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borozdin W., Steinmann K., Albrecht B., Bottani A., Devriendt K., Leipoldt M., Kohlhase J. (2006). Detection of heterozygous SALL1 deletions by quantitative real time PCR proves the contribution of a SALL1 dosage effect in the pathogenesis of Townes-Brocks syndrome. Hum. Mutat. 27, 211–212 [DOI] [PubMed] [Google Scholar]
- Botzenhart E. M., Green A., Ilyina H., Konig R., Lowry R. B., Lo I. F., Shohat M., Burke L., McGaughran J., Chafai R., et al. (2005). SALL1 mutation analysis in Townes-Brocks syndrome: twelve novel mutations and expansion of the phenotype. Hum. Mutat. 26, 282. [DOI] [PubMed] [Google Scholar]
- Buck A., Archangelo L., Dixkens C., Kohlhase J. (2000). Molecular cloning, chromosomal localization, and expression of the murine SALL1 ortholog Sall1. Cytogenet. Cell Genet. 89, 150–153 [DOI] [PubMed] [Google Scholar]
- Bulfone A., Smiga S. M., Shimamura K., Peterson A., Puelles L., Rubenstein J. L. (1995). T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron 15, 63–78 [DOI] [PubMed] [Google Scholar]
- Cameron T. H., Lachiewicz A. M., Aylsworth A. S. (1991). Townes-Brocks syndrome in two mentally retarded youngsters. Am. J. Med. Genet. 41, 1–4 [DOI] [PubMed] [Google Scholar]
- Cantera R., Luer K., Rusten T. E., Barrio R., Kafatos F. C., Technau G. M. (2002). Mutations in spalt cause a severe but reversible neurodegenerative phenotype in the embryonic central nervous system of Drosophila melanogaster. Development 129, 5577–5586 [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr (1982). Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 256, 293–302 [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Goto T., Tarui T., Takahashi T., Bhide P. G., Nowakowski R. S. (2003). Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb. Cortex 13, 592–598 [DOI] [PubMed] [Google Scholar]
- Cubelos B., Sebastian-Serrano A., Kim S., Moreno-Ortiz C., Redondo J. M., Walsh C. A., Nieto M. (2008). Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb. Cortex 18, 1758–1770 [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Barrio R., Kafatos F. C. (1996). A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature 381, 421–424 [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Barrio R., Kafatos F. C. (1999). Regulation of the spalt/spalt-related gene complex and its function during sensory organ development in the Drosophila thorax. Development 126, 2653–2662 [DOI] [PubMed] [Google Scholar]
- Elling U., Klasen C., Eisenberger T., Anlag K., Treier M. (2006). Murine inner cell mass-derived lineages depend on Sall4 function. Proc. Natl. Acad. Sci. USA 103, 16319–16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels S., Kohlhase J., McGaughran J. (2000). A SALL1 mutation causes a branchio-oto-renal syndrome-like phenotype. J. Med. Genet. 37, 458–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R. A., Bulfone A., Kowalczyk T., Hevner R. F. (2005). Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell E. R., Munsterberg A. E. (2000). csal1 is controlled by a combination of FGF and Wnt signals in developing limb buds. Dev. Biol. 225, 447–458 [DOI] [PubMed] [Google Scholar]
- Franklin K. B. J., Paxinos G. (1997). The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press [Google Scholar]
- Furuta Y., Lagutin O., Hogan B. L., Oliver G. C. (2000). Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132 [PubMed] [Google Scholar]
- Gillies K., Price D. J. (1993). The fates of cells in the developing cerebral cortex of normal and methylazoxymethanol acetate-lesioned mice. Eur. J. Neurosci. 5, 73–84 [DOI] [PubMed] [Google Scholar]
- Gorski J. A., Talley T., Qiu M., Puelles L., Rubenstein J. L., Jones K. R. (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Tsai L. H., Wynshaw-Boris A. (2002). Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet. 3, 342–355 [DOI] [PubMed] [Google Scholar]
- Hammerle B., Tejedor F. J. (2002). A method for pulse and chase BrdU-labeling of early chick embryos. J. Neurosci. Methods 122, 59–64 [DOI] [PubMed] [Google Scholar]
- Handler M., Yang X., Shen J. (2000). Presenilin-1 regulates neuronal differentiation during neurogenesis. Development 127, 2593–2606 [DOI] [PubMed] [Google Scholar]
- Harrison S. J., Nishinakamura R., Monaghan A. P. (2008a). Sall1 regulates mitral cell development and olfactory nerve extension in the developing olfactory bulb. Cereb. Cortex 18, 1604–1617 [DOI] [PubMed] [Google Scholar]
- Harrison S. J., Parrish M., Monaghan A. P. (2008b). Sall3 is required for the terminal maturation of olfactory glomerular interneurons. J. Comp. Neurol. 507, 1780–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W., Attardo A., Denk W., Huttner W. B. (2004). Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar T. F., Wang F., Schwartz M. L., Rakic P. (2000). Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J. Neurosci. 20, 5764–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. (1989). Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature 340, 35–41 [DOI] [PubMed] [Google Scholar]
- Hevner R. F. (2007). Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J. Neuropathol. Exp. Neurol. 66, 101–109 [DOI] [PubMed] [Google Scholar]
- Hevner R. F., Shi L., Justice N., Hsueh Y., Sheng M., Smiga S., Bulfone A., Goffinet A. M., Campagnoni A. T., Rubenstein J. L. (2001). Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29, 353–366 [DOI] [PubMed] [Google Scholar]
- Inoue S., Inoue M., Fujimura S., Nishinakamura R. (2010). A mouse line expressing Sall1-driven inducible Cre recombinase in the kidney mesenchyme. Genesis 48, 207–212 [DOI] [PubMed] [Google Scholar]
- Ishikiriyama S., Kudoh F., Shimojo N., Iwai J., Inoue T. (1996). Townes-Brocks syndrome associated with mental retardation. Am. J. Med. Genet. 61, 191–192 [DOI] [PubMed] [Google Scholar]
- Karantzali E., Lekakis V., Ioannou M., Hadjimichael C., Papamatheakis J., Kretsovali A. (2011). Sall1 regulates embryonic stem cell differentiation in association with nanog. J. Biol. Chem. 286, 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A., Ogawa M., Saito K., Matsuzaki F., Okano H., Miyata T. (2004). Differential expression of Pax6 and Ngn2 between pair-generated cortical neurons. J. Neurosci. Res. 78, 784–795 [DOI] [PubMed] [Google Scholar]
- Kiefer S. M., Ohlemiller K. K., Yang J., McDill B. W., Kohlhase J., Rauchman M. (2003). Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum. Mol. Genet. 12, 2221–2227 [DOI] [PubMed] [Google Scholar]
- Kiefer S. M., Robbins L., Barina A., Zhang Z., Rauchman M. (2008). SALL1 truncated protein expression in Townes-Brocks syndrome leads to ectopic expression of downstream genes. Hum. Mutat. 29, 1133–1140 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Wischermann A., Reichenbach H., Froster U., Engel W. (1998). Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat. Genet. 18, 81–83 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Hausmann S., Stojmenovic G., Dixkens C., Bink K., Schulz-Schaeffer W., Altmann M., Engel W. (1999a). SALL3, a new member of the human spalt-like gene family, maps to 18q23. Genomics 62, 216–222 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Kohler A., Jackle H., Engel W., Stick R. (1999b). Molecular cloning of a SALL1-related pseudogene and mapping to chromosome Xp11.2. Cytogenet. Cell Genet. 84, 31–34 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Taschner P. E., Burfeind P., Pasche B., Newman B., Blanck C., Breuning M. H., ten Kate L. P., Maaswinkel-Mooy P., Mitulla B., et al. (1999c). Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am. J. Hum. Genet. 64, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J., Altmann M., Archangelo L., Dixkens C., Engel W. (2000). Genomic cloning, chromosomal mapping, and expression analysis of msal-2. Mamm. Genome 11, 64–68 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Heinrich M., Liebers M., Frohlich Archangelo L., Reardon W., Kispert A. (2002a). Cloning and expression analysis of SALL4, the murine homologue of the gene mutated in Okihiro syndrome. Cytogenet. Genome Res. 98, 274–277 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Heinrich M., Schubert L., Liebers M., Kispert A., Laccone F., Turnpenny P., Winter R. M., Reardon W. (2002b). Okihiro syndrome is caused by SALL4 mutations. Hum. Mol. Genet. 11, 2979–2987 [DOI] [PubMed] [Google Scholar]
- Kohlhase J., Liebers M., Backe J., Baumann-Muller A., Bembea M., Destree A., Gattas M., Grussner S., Muller T., Mortier G., et al. (2003). High incidence of the R276X SALL1 mutation in sporadic but not familial Townes-Brocks syndrome and report of the first familial case. J. Med. Genet. 40, e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K., Rebhun L. I., Frankfurter A. (1990a). Posttranslational modification of class III beta-tubulin. Proc. Natl. Acad. Sci. USA 87, 7195–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K., Tuttle J. B., Rebhun L. I., Cleveland D. W., Frankfurter A. (1990b). The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil. Cytoskeleton 17, 118–132 [DOI] [PubMed] [Google Scholar]
- Levers T. E., Edgar J. M., Price D. J. (2001). The fates of cells generated at the end of neurogenesis in developing mouse cortex. J. Neurobiol. 48, 265–277 [DOI] [PubMed] [Google Scholar]
- Li D., Dower K., Ma Y., Tian Y., Benjamin T. L. (2001). A tumor host range selection procedure identifies p150(sal2) as a target of polyoma virus large T antigen. Proc. Natl. Acad. Sci. USA 98, 14619–14624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian G., Sheen V. (2006). Cerebral developmental disorders. Curr. Opin. Pediatr. 18, 614–620 [DOI] [PubMed] [Google Scholar]
- Magdaleno S., Jensen P., Brumwell C. L., Seal A., Lehman K., Asbury A., Cheung T., Cornelius T., Batten D. M., Eden C., et al. (2006). BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 4, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O., Rubenstein J. L. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790 [DOI] [PubMed] [Google Scholar]
- Marlin S., Blanchard S., Slim R., Lacombe D., Denoyelle F., Alessandri J. L., Calzolari E., Drouin-Garraud V., Ferraz F. G., Fourmaintraux A., et al. (1999). Townes-Brocks syndrome: detection of a SALL1 mutation hot spot and evidence for a position effect in one patient. Hum. Mutat. 14, 377–386 [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V., Noctor S. C., Kriegstein A. R. (2006). The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 16 Suppl. 1, i152–i161 [DOI] [PubMed] [Google Scholar]
- McEvilly R. J., de Diaz M. O., Schonemann M. D., Hooshmand F., Rosenfeld M. G. (2002). Transcriptional regulation of cortical neuron migration by POU domain factors. Science 295, 1528–1532 [DOI] [PubMed] [Google Scholar]
- Menezes J. R., Luskin M. B. (1994). Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J. Neurosci. 14, 5399–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Saito K., Kawano M., Muto T., Ogawa M. (2004). Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145 [DOI] [PubMed] [Google Scholar]
- Mizutani K., Yoon K., Dang L., Tokunaga A., Gaiano N. (2007). Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 [DOI] [PubMed] [Google Scholar]
- Munji R. N., Choe Y., Li G., Siegenthaler J. A., Pleasure S. J. (2011). Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J. Neurosci. 31, 1676–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch C. A., Schulte J. D., Olson E., Chenn A. (2010). Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS ONE 5, e12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Monuki E. S., Tang H., Imitola J., Haubst N., Khoury S. J., Cunningham J., Gotz M., Walsh C. A. (2004). Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 479, 168–180 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R. (2008). Stem cells in the embryonic kidney. Kidney Int. 73, 913–917 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R., Matsumoto Y., Nakao K., Nakamura K., Sato A., Copeland N. G., Gilbert D. J., Jenkins N. A., Scully S., Lacey D. L., et al. (2001). Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128, 3105–3115 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R., Uchiyama Y., Sakaguchi M., Fujimura S. (2011). Nephron progenitors in the metanephric mesenchyme. Pediatr. Nephrol. 26, 1463–1467 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S., Kriegstein A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martinez-Cerdeno V., Ivic L., Kriegstein A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martinez-Cerdeno V., Kriegstein A. R. (2008). Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J. Comp. Neurol. 508, 28–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Miyata T., Nakajima K., Yagyu K., Seike M., Ikenaka K., Yamamoto H., Mikoshiba K. (1995). The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899–912 [DOI] [PubMed] [Google Scholar]
- Ott T., Parrish M., Bond K., Schwaeger-Nickolenko A., Monaghan A. (2001). A new member of the spalt-like zinc finger protein family, Msal3, is expressed in the CNS and sites of epithelial/mesenchymal interaction. Mech. Dev. 101, 203–207 [DOI] [PubMed] [Google Scholar]
- Parrish M., Ott T., Lance-Jones C., Schuetz G., Schwaeger-Nickolenko A., Monaghan A. P. (2004). Loss of the Sall3 gene leads to palate deficiency, abnormalities in cranial nerves, and perinatal lethality. Mol. Cell. Biol. 24, 7102–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz D., Stoodley N., Golden J. A. (2002). Neuronal migration, cerebral cortical development, and cerebral cortical anomalies. J. Neuropathol. Exp. Neurol. 61, 1–11 [DOI] [PubMed] [Google Scholar]
- Polleux F., Lauder J. M. (2004). Toward a developmental neurobiology of autism. Ment. Retard. Dev. Disabil. Res. Rev. 10, 303–317 [DOI] [PubMed] [Google Scholar]
- Pontious A., Kowalczyk T., Englund C., Hevner R. F. (2008). Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 30, 24–32 [DOI] [PubMed] [Google Scholar]
- Price D. J., Aslam S., Tasker L., Gillies K. (1997). Fates of the earliest generated cells in the developing murine neocortex. J. Comp. Neurol. 377, 414–422 [PubMed] [Google Scholar]
- Rosner B. S. (1970). Brain functions. Annu. Rev. Psychol. 21, 555–594 [DOI] [PubMed] [Google Scholar]
- Roy K., Kuznicki K., Wu Q., Sun Z., Bock D., Schutz G., Vranich N., Monaghan A. P. (2004). The Tlx gene regulates the timing of neurogenesis in the cortex. J. Neurosci. 24, 8333–8345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten T. E., Cantera R., Urban J., Technau G., Kafatos F. C., Barrio R. (2001). Spalt modifies EGFR-mediated induction of chordotonal precursors in the embryonic PNS of Drosophila promoting the development of oenocytes. Development 128, 711–722 [DOI] [PubMed] [Google Scholar]
- Saffary R., Xie Z. (2011). FMRP regulates the transition from radial glial cells to intermediate progenitor cells during neocortical development. J. Neurosci. 31, 1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki-Yumoto M., Kobayashi C., Sato A., Fujimura S., Matsumoto Y., Takasato M., Kodama T., Aburatani H., Asashima M., Yoshida N., et al. (2006). The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development 133, 3005–3013 [DOI] [PubMed] [Google Scholar]
- Salerno A., Kohlhase J., Kaplan B. S. (2000). Townes-Brocks syndrome and renal dysplasia: a novel mutation in the SALL1 gene. Pediatr. Nephrol. 14, 25–28 [DOI] [PubMed] [Google Scholar]
- Sato A., Kishida S., Tanaka T., Kikuchi A., Kodama T., Asashima M., Nishinakamura R. (2004). Sall1, a causative gene for Townes-Brocks syndrome, enhances the canonical Wnt signaling by localizing to heterochromatin. Biochem. Biophys. Res. Commun. 319, 103–113 [DOI] [PubMed] [Google Scholar]
- Schiffmann S. N., Bernier B., Goffinet A. M. (1997). Reelin mRNA expression during mouse brain development. Eur. J. Neurosci. 9, 1055–1071 [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Walsh C. A. (2000). Cortical malformations and epilepsy. Ment. Retard. Dev. Disabil. Res. Rev. 6, 268–280 [DOI] [PubMed] [Google Scholar]
- Sessa A., Mao C. A., Hadjantonakis A. K., Klein W. H., Broccoli V. (2008). Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 60, 56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A. M., Pearlman A. L. (1997). Abnormal reorganization of preplate neurons and their associated extracellular matrix: an early manifestation of altered neocortical development in the reeler mutant mouse. J. Comp. Neurol. 378, 173–179 [DOI] [PubMed] [Google Scholar]
- Sheppard A. M., Hamilton S. K., Pearlman A. L. (1991). Changes in the distribution of extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J. Neurosci. 11, 3928–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler J. A., Tremper-Wells B. A., Miller M. W. (2008). Foxg1 haploinsufficiency reduces the population of cortical intermediate progenitor cells: effect of increased p21 expression. Cereb. Cortex. 18, 1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surka W. S., Kohlhase J., Neunert C. E., Schneider D. S., Proud V. K. (2001). Unique family with Townes-Brocks syndrome, SALL1 mutation, and cardiac defects. Am. J. Med. Genet. 102, 250–257 [DOI] [PubMed] [Google Scholar]
- Sweetman D., Smith T., Farrell E. R., Chantry A., Munsterberg A. (2003). The conserved glutamine-rich region of chick csal1 and csal3 mediates protein interactions with other spalt family members. Implications for Townes-Brocks syndrome. J. Biol. Chem. 278, 6560–6566 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr (1994). Mode of cell proliferation in the developing mouse neocortex. Proc. Natl. Acad. Sci. USA 91, 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr (1996a). Interkinetic and migratory behavior of a cohort of neocortical neurons arising in the early embryonic murine cerebral wall. J. Neurosci. 16, 5762–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr (1996b). The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J. Neurosci. 16, 6183–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Goto T., Miyama S., Nowakowski R. S., Caviness V. S., Jr (1999). Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J. Neurosci. 19, 10357–10371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron M. C., Rossel M., Moepps B., Zhang Y. L., Seidenfaden R., Favor J., Konig N., Cremer H. (2006). Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J. Neurosci. 26, 13273–13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. S., Teng Y., Ferreira H. B., Emmons S. W., Chalfie M. (2003). The Caenorhabditis elegans spalt-like gene sem-4 restricts touch cell fate by repressing the selector Hox gene egl-5 and the effector gene mec-3. Development 130, 3831–3840 [DOI] [PubMed] [Google Scholar]
- Townes P. L., Brocks E. R. (1972). Hereditary syndrome of imperforate anus with hand, foot, and ear anomalies. J. Pediatr. 81, 321–326 [DOI] [PubMed] [Google Scholar]
- Walter K. N., Greenhalgh K. L., Newbury-Ecob R. A., Kohlhase J. (2006). Mosaic trisomy 8 and Townes-Brocks syndrome due to a novel SALL1 mutation in the same patient. Am. J. Med. Genet. A 140, 649–651 [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M., Handler M., Shen J. (2005). Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev. Biol. 277, 332–346 [DOI] [PubMed] [Google Scholar]
- Wrobel C. N., Mutch C. A., Swaminathan S., Taketo M. M., Chenn A. (2007). Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev. Biol. 309, 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Yoshino I., Shimazaki T., Murohashi M., Hevner R. F., Lax I., Okano H., Shibuya M., Schlessinger J., Gotoh N. (2005). Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proc. Natl. Acad. Sci. USA 102, 15983–15988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. J., Koo B. K., Im S. K., Jeong H. W., Ghim J., Kwon M. C., Moon J. S., Miyata T., Kong Y. Y. (2008). Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 58, 519–531 [DOI] [PubMed] [Google Scholar]
- Yuri S., Fujimura S., Nimura K., Takeda N., Toyooka Y., Fujimura Y., Aburatani H., Ura K., Koseki H., Niwa H., Nishinakamura R. (2009). Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells 27, 796–805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.