Abstract
Context:
Type 1 diabetes is known to be a state of insulin resistance; however, the tissues involved in whole-body insulin resistance are less well known. It is unclear whether insulin resistance is due to glucose toxicity in the post-Diabetes Control and Complications Trial era of tighter glucose control.
Objective:
We performed this study to determine muscle and liver insulin sensitivity individuals with type 1 diabetes after overnight insulin infusion to lower fasting glucose concentration.
Design, Patients, and Methods:
Fifty subjects [25 controls without and 25 individuals with type 1 diabetes (diabetes duration 22.9 ± 1.7 yr, without known end organ damage] were frequency matched on age and body mass index by group and studied. After 3 d of dietary control and overnight insulin infusion to normalize glucose, we performed a three-stage hyperinsulinemic/euglycemic clamp infusing insulin at 4, 8, and 40 mU/m2 · min. Glucose metabolism was quantified using an infusion of [6,6-2H2]glucose. Hepatic insulin sensitivity was measured using the insulin IC50 for glucose rate of appearance (Ra), whereas muscle insulin sensitivity was measured using the glucose rate of disappearance during the highest insulin dose.
Results:
Throughout the study, glucose Ra was significantly greater in individuals compared with those without type 1 diabetes. The concentration of insulin required for 50% suppression of glucose Ra was 2-fold higher in subjects with type 1 diabetes. Glucose rate of disappearance was significantly lower in individuals with type 1 diabetes during the 8- and 40-mU/m2 · min stages.
Conclusion:
Insulin resistance in liver and skeletal muscle was a significant feature in type 1 diabetes. Nevertheless, the etiology of insulin resistance was not explained by body mass index, percentage fat, plasma lipids, visceral fat, and physical activity and was also not fully explained by hyperglycemia.
Similar to type 2 diabetes, individuals with type 1 diabetes have higher cardiovascular disease (CVD) incidence and mortality compared with the general population (1). Unlike those with type 2 diabetes, however, people with type 1 diabetes have Framingham risk scores that do not predict increased CVD risk (2), nor are they more obese (centrally or otherwise) compared with age- and sex-matched peers without diabetes (3). Therefore, factors other than high blood levels of low-density lipoprotein cholesterol, low blood levels of high-density lipoprotein (HDL) cholesterol, smoking, elevated blood pressure, or obesity underlie CVD risk in this population. Additionally, the proposed major mechanisms responsible for microvascular disease in diabetes (polyol/sorbitol, reactive oxygen species, advanced glycation end products) have not been associated with macrovascular disease (4). In summary, individuals with type 1 diabetes have somewhat elusive risk factors predisposing them to CVD. Insulin resistance, an underappreciated feature of type 1 diabetes (5), is a possible culprit due to its known association with CVD and type 2 diabetes (6).
Much work has been conducted examining whole-body- and tissue-specific insulin resistance in type 2 diabetes (7–9). In type 2 diabetes, insulin resistance occurs in adipose tissue, liver, and skeletal muscle (10). In contrast, little is known about the site(s) of diminished insulin action in individuals with type 1 diabetes despite emerging evidence that it is also an insulin resistant state (5, 11–17). Only two studies reported tissue specific (hepatic) insulin sensitivity in individuals with type 1 diabetes with conflicting conclusions (7, 11). We previously reported insulin resistance in individuals with type 1 diabetes was related to coronary artery calcification and therefore cardiovascular disease risk (5). Given the potential for insulin resistance in individuals with type 1 diabetes as a potential target for clinical trials and CVD risk management, a more thorough understanding of the tissue distribution of insulin resistance is urgently needed. Furthermore, it is currently unknown whether insulin resistance in individuals with type 1 diabetes is similar to that in those with type 2 diabetes or unique in and of itself.
The aim of the current study was to quantify and compare peripheral and hepatic insulin sensitivity in a group of individuals with type 1 diabetes compared with a matched control group using state-of-the-art techniques. We hypothesized that individuals with type 1 diabetes would have decreased whole-body and hepatic insulin sensitivity compared with a matched nondiabetic control group. Confounding variables were controlled, where possible, and examined to determine whether tissue-specific insulin resistance could be attributed to factors previously described in individuals with type 1 or 2 diabetes or were unique to this group of individuals with type 1 diabetes.
Materials and Methods
Of the 87 individuals who completed the Coronary Artery Calcification in Type 1 diabetes (CACTI) insulin clamp substudy, isotope tracer data were analyzed from 50 subjects [25 people with type 1 diabetes and 25 matched people without diabetes (CON)]. The CACTI study enrolled 1416 adults between 19 and 56 yr of age, 652 with type 1 diabetes and 764 without any history of diagnosed diabetes. Study participants completed a fasting examination, including the measurement of lipids, blood pressure, glucose, hemoglobin A1C, and anthropometric measurements, including height, weight, and waist and hip circumference. All study participants had coronary artery calcium measured using electron beam computed tomography. All study participants were invited to return for follow-up visits 3 and 6 yr after the baseline examination, and all measures were repeated at each visit (3). Inclusion criteria for initial enrollment of type 1 diabetic subjects in the CACTI study were age 19–56 yr, no history of CVD, on insulin therapy within a year of diagnosis and current insulin therapy, diagnosed before age 30 yr and/or with positive antibodies, and diabetes duration 10 yr or longer. Within this substudy, inclusion criteria included those listed above and the following: hemoglobin A1C 9.5% or less, albumin excretion rate less than 200 μg/min, triglycerides less than 400 mg/dl, blood pressure less than 160/100 mm Hg, and a coronary artery calcium measurement at the 6 yr visit (visit 3) CACTI follow-up. All participants provided written informed consent and the study was approved by the Colorado Multiple Institutional Review Board.
Screening visit
After signing the informed consent, subjects performed a preliminary visit to the Clinical Translational Research Center (CTRC). This visit included a medical history questionnaire, medication inventory, diet questionnaire, insulin record, alcohol and tobacco use, and physical activity questionnaire. Body composition was determined using dual-energy x-ray absorptiometry analysis (Lunar DPX-IQ; Lunar Corp., Madison, WI).
Preliminary visit
After the screening visit, subjects arrived at the CTRC 3 d before the metabolic study day to pick up food for dietary control. In individuals with type 1 diabetes, a continuous glucose sensor was placed (CGMS; Medtronic MiniMed, Northridge, CA) to assess the relationship between short-term glycemic variability and measures of insulin sensitivity obtained during the clamp study.
Diet and activity control
Subjects were provided a diet with a standardized macronutrient composition (50% carbohydrate, 20% protein, 30% fat) for 3 d before their study day and were asked to refrain from vigorous physical activity, smoking, and alcohol. Daily energy requirement was estimated from the dual-energy x-ray absorptiometry measurement of fat-free mass using the equation: daily energy intake = 1.4 kcal/d × [372 + (23.9 × fat-free mass)] and analysis of dietary records (18). Premenopausal women were scheduled for their study during the early follicular phase of their menstrual cycle (d 2–10) to reduce this confounder of insulin sensitivity (19). Subjects withdrew from any medication other than insulin for 24 h before the clamp.
Hyperinsulinemic-euglycemic clamp visit
Subjects were admitted to the inpatient CTRC the evening before their study. Subjects with type 1 diabetes were instructed to take their last long-acting insulin injections at least 12 h before admission (24 h before the start of the clamp protocol). Dinner was provided on the unit and subjects then fasted overnight and through the clamp protocol. Subjects with type 1 diabetes were given bolus insulin for dinner per their usual regimen. All individuals with type 1 diabetes were maintained overnight on iv regular insulin with adjustments to achieve euglycemia by morning. Intravenous insulin was titrated to a goal of 100–150 mg/dl from 2000 to 0400 h and then 80–110 mg/dl from 0400 to 0700 h.
On the morning of the clamp study at approximately 0700 h, two antecubital catheters on the same arm were placed, one for infusions of stable isotopes of glucose and the other used to infuse dextrose, insulin, and potassium during the insulin clamp. Additionally, a hand vein was catheterized on the contralateral arm for blood draws during the study using the heated hand vein technique. At 0800 h, a primed continuous infusion of [6,6-2H2]glucose was initiated at 0.04 mg/kg · min and continued throughout a 2-h basal lead in period and the insulin clamp. For individuals with type 1 diabetes, the overnight insulin infusion was continued from 0700 h through the basal period until the first stage of the insulin clamp at 1000 h. Resting metabolic rate measurement and blood samples for determination of baseline hormones and substrates were performed over the final 30 min before the clamp. At 1000 h, a three-stage hyperinsulinemic euglycemic clamp was then initiated and continued for the next 4.5 h using the method of DeFronzo et al. (20). Briefly, a primed continuous infusion of insulin was administered at 4 mU/m2 · min for 1.5 h, 8 mU/m2 · min for 1.5 h, and then 40 mU/m2 · min for the final 1.5 h. A variable infusion of 20% dextrose, labeled with [6,6-2H2]glucose, was infused to maintain blood glucose approximately 90 mg/dl (21). Arterialized blood was sampled every 5 min for bedside determination of glucose concentration (Analox, Lunenberg, MA) and the dextrose infusion adjusted as necessary. During the last 30 min of each stage of the clamp, measurements of respiratory gas exchange were made via indirect calorimetry, and arterialized blood was taken for hormone for substrate measurements.
Computed tomography (CT)
Abdominal CT scans for calculation of abdominal visceral fat area and liver to spleen density ratios as a relative measure of liver fat content (22) were performed within 1 yr of the clamp study.
Isotope analysis
Glucose isotopic enrichment was measured using gas chromatography/mass spectrometry (GC model 6890 and MS model 5973A; Hewlett-Packard, Palo Alto, CA) using standard techniques (23).
Calculations
Rates of glucose appearance (Ra), disappearance (Rd), and metabolic clearance rate (MCR) were calculated using the Steele equation modified for stable isotopes during basal conditions (24), and as described by Finegood et al. (21) during the insulin clamp. Carbohydrate oxidation was calculated from indirect calorimetry using standard equations (25). Nonoxidative glucose disposal during the clamp was calculated from the difference in isotopically measured glucose Rd and carbohydrate oxidation. The concentration of insulin required for 50% inhibition of glucose Ra (IC50) was determined individually using linear curve fitting to describe the relationship between insulin concentration and glucose Ra.
Statistical analysis
Data are presented as mean ± sem. Differences between groups were analyzed using a one-way ANOVA (SPSS, Chicago, IL). Differences from basal to clamp stage within individuals were determined using a paired t test. Relationships between variables were determined using Pearson's correlation coefficient. Differences in gender distribution between groups were determined using a χ2 test for categorical variables. Linear regression models were used to examine associates of glucose Rd with hemoglobin A1C and whether hyperglycemia explained the differences in glucose Rd between study participants with and without type 1 diabetes. The β-coefficients for type 1 diabetes were compared between a model adjusted for hemoglobin A1C as well as age, sex, and diabetes status, and then a reduced model was fit adjusting only for age, sex, and diabetes status. An alpha level of 0.05 was used throughout the study.
Results
Demographic information for subjects is shown in Table 1. Groups were well matched and did not significantly differ by age, body mass index (BMI), race, ethnicity, percentage body fat, HDL cholesterol, estimated physical activity level, systolic or diastolic blood pressure, visceral fat content, liver density, liver to spleen density ratio, aspartate transaminase (AST), or alanine aminotransferase (ALT). Hemoglobin A1C was significantly higher and total cholesterol and triglyceride concentration significantly lower in individuals with type 1 diabetes compared with the CON. The mean duration of diabetes was 22.9 ± 1.7 yr (mean ± sem). The overnight insulin infusion resulted in a mean glucose concentration of 115 ± 9 mg/dl before starting the insulin clamp.
Table 1.
Demographics
| Controls (n = 25) | Type 1 diabetes (n = 25) | P value | |
|---|---|---|---|
| Age (yr) | 46.0 ± 1.5 | 45.0 ± 2.0 | 0.93 |
| Male gender (%) | 40 (10) | 56 (14) | 0.17 |
| BMI (kg/m2) | 25.9 ± 0.8 | 26.6 ± 0.9 | 0.53 |
| Race (% white) | 88 (22) | 96 (24) | 0.30 |
| Ethnicity (% non-Spanish origin) | 100 (25) | 96 (24) | 0.32 |
| Fat (%) | 30.0 ± 1.6 | 27.9 ± 1.4 | 0.27 |
| Hemoglobin A1C (%) | 5.4 ± 0.06 | 7.7 ± 0.16 ¥ | <0.0001 |
| Range (4.9–6.1) | Range (6.1–9.1) | ||
| Total cholesterol (mg/dl) | 166 ± 5 | 141 ± 7a | 0.005 |
| HDL cholesterol (mg/dl) | 55 ± 3 | 53 ± 3 | 0.68 |
| Triglycerides (mg/dl) | 103 ± 9 | 77 ± 8a | 0.03 |
| Estimated physical activity (kcal/wk) | 2705 ± 364 | 1786 ± 352 | 0.08 |
| Systolic blood pressure (mm Hg) | 111 ± 1.9 | 115 ± 2.0 | 0.16 |
| Diastolic blood pressure (mm Hg) | 75.1 ± 1.4 | 74.9 ± 1.5 | 0.92 |
| Visceral fat area (mm3) | 48716 ± 4992 | 48959 ± 5128 | 0.97 |
| Liver density (HU) | 62.4 ± 1.3 | 61.1 ± 1.4 | 0.49 |
| Liver/spleen density ratio | 1.3 ± 0.03 | 1.27 ± 0.02 | 0.40 |
| AST | 28 ± 3 | 26 ± 2 | 0.44 |
| ALT | 23 ± 2 | 24 ± 2 | 0.87 |
Values are means ± sem.
Significantly different from CON (P < 0.05).
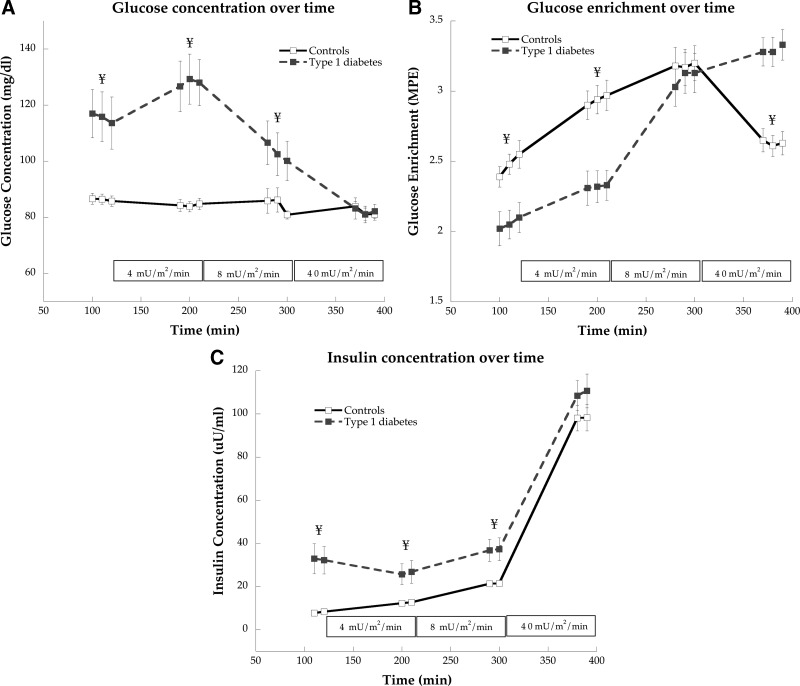
Despite overnight insulin infusion, glucose concentration was significantly higher in individuals with type 1 diabetes compared with controls under basal conditions (P = 0.001) and 4- (P < 0.0001) and 8 (P = 0.04)-mU/ m2 · min insulin infusions (Fig. 1A). Glucose concentrations were not significantly different between groups during the 40-mU/m2 · min insulin infusion (P = 0.95). In individuals with type 1 diabetes compared with CON, glucose enrichment was significantly lower under basal (P = 0.004, Fig. 1B) and 4 mU/m2 · min (P = 0.001), not different at 8 mU/m2 · min (P = 0.79), and significantly higher during the 40-mU/m2 · min (P < 0.0001) insulin infusions. We found significantly greater insulin concentrations in individuals with type 1 diabetes compared with control at basal (P = 0.001) and 4- (P = 0.01), and 8 (P = 0.005)-mU/m2 · min insulin infusions (Fig. 1C). Insulin concentration was not different between groups during the 40-mU/m2 · min infusion (P = 0.24).
Fig. 1.
Glucose concentration (A), enrichment (B), and insulin concentration (C) during basal and hyperinsulinemic/euglycemic clamp at 4, 8, and 40 mU/m2 · min insulin doses in control subjects and individuals with type 1 diabetes. Values are means ± sem.¥, Significantly different from control (P < 0.05).
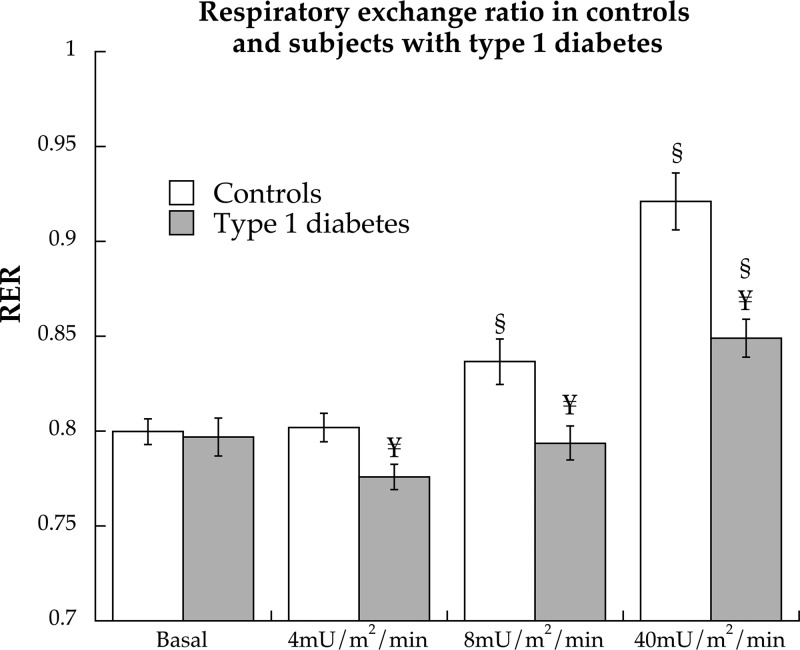
Basal respiratory exchange ratios were not different between groups (P = 0.94), but individuals with type 1 diabetes had lower respiratory exchange ratio values compared with CON during the 4- (P = 0.01), 8- (P = 0.008), and 40 (P = 0.0003)-mU/m2 · min insulin infusions (Fig. 2). Rates of fat oxidation were not different at rest (CON: 3.32 ± 0.25, type 1 diabetes: 3.83 ± 0.38 μmol/kg · min, P = 0.27) but were significantly higher in individuals with type 1 diabetes compared with controls during the 4- (CON: 3.32 ± 0.25, type 1 diabetes: 3.83 ± 0.38 μmol/kg · min, P = 0.27), 8- (CON: 3.24 ± 0.28, type 1 diabetes: 4.41 ± 0.29 μmol/kg · min, P = 0.006), and 40-mU/m2 · min insulin infusions (CON: 2.28 ± 0.32, type 1 diabetes: 3.92 ± 0.35 μmol/kg · min, P = 0.001).
Fig. 2.
Respiratory exchange ratio (RER) during basal period and hyperinsulinemic/euglycemic clamp at 4, 8, and 40 mU/m2 · min insulin doses in control subjects and individuals with type 1 diabetes. Values are means ± sem. §, Significantly different from basal (P < 0.05); ¥, significantly different from control (P < 0.05).
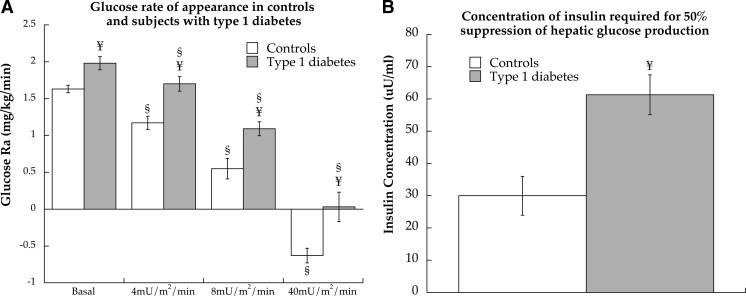
Whole-body insulin sensitivity has been previously reported for the entire CACTI substudy (5). During the 40-mU/m2 · min insulin clamp in this study population, glucose infusion rates were 9.40 ± 0.66 mg/kg · min for controls and were significantly lower at 4.23 ± 0.59 mg/kg · min for individuals with type 1 diabetes (P < 0.0001). Glucose Ra reflects endogenous glucose production with approximately 75% hepatic and 25% renal contribution in the postabsorptive state (26). Glucose Ra was significantly greater in individuals with type 1 diabetes compared with controls during the basal (P = 0.002) and 4- (P < 0.001), 8- (P = 0.002), and 40 (P = 0.002)-mU/ m2 · min insulin infusions (Fig. 3A). The concentration of insulin required to inhibit glucose Ra 50% (IC50) was significantly greater in individuals with type 1 diabetes compared with CON (P < 0.001, Fig. 3B).
Fig. 3.
Glucose Ra (A) during basal and hyperinsulinemic/euglycemic clamp at 4, 8, and 40 mU/m2 · min insulin doses in control subjects and individuals with type 1 diabetes. The concentration of insulin required for IC50 of hepatic glucose production in both groups (B). Values are means ± sem. §, Significantly different from basal (P < 0.05); ¥, significantly different from control (P < 0.05).
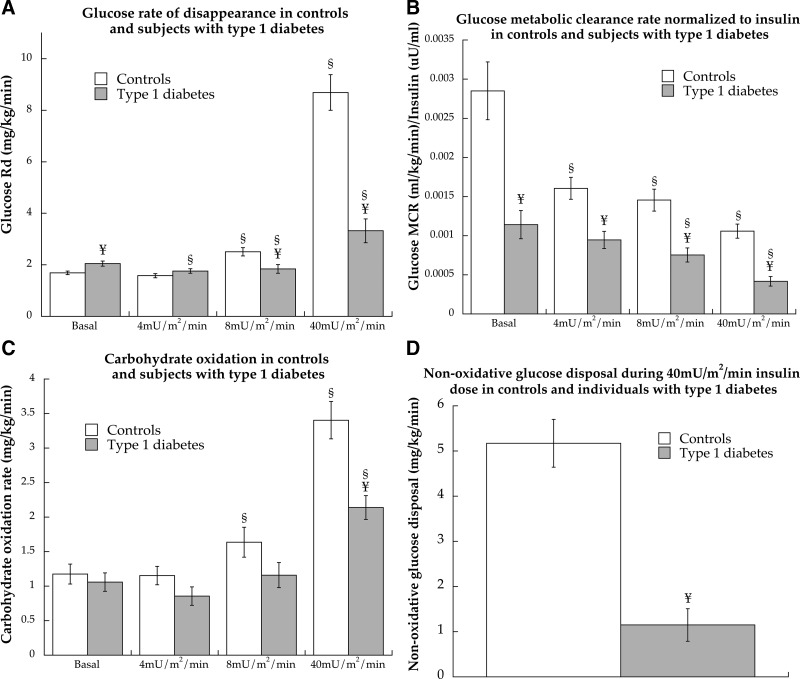
The glucose Rd reflects peripheral glucose uptake and was significantly greater during the basal period in individuals with type 1 diabetes compared with CON (P = 0.006, Fig. 4A). There were no differences between groups at 4 mU/m2 · min (P = 0.18), but Rd was significantly lower in individuals with type 1 diabetes compared with CON at 8 (P = 0.006) and 40 mU/m2 · min (P < 0.0001). The MCR was calculated to account for differences in glucose concentration and also normalized to insulin concentration to account for differences affecting glucose disposal. Normalized glucose MCR is shown in Fig. 4B and was significantly lower in individuals with type 1 diabetes compared with CON during every phase of the study (P < 0.001), showing decreased glucose transport. Carbohydrate oxidation was not different between individuals with type 1 diabetes and CON during basal (P = 0.56), 4 (P = 0.12) or 8 (P = 0.10)-mU/m2 · min insulin infusions but was significantly lower in individuals with type 1 diabetes (P = 0.0003) during the 40-mU/m2 · min infusion (Fig. 4C). However, the percent of glucose uptake oxidized was significantly lower (P < 0.0001) in CON (40.7 ± 5.1%) compared with individuals with type 1 diabetes (72.8 ± 5.2%). Nonoxidative glucose disposal, an estimate of glycogen storage, was significantly lower in individuals with type 1 diabetes compared with CON during the 40-mU/m2 · min insulin dose (P < 0.0001, Fig. 4D).
Fig. 4.
Glucose Rd (A), MCR (B), and MCR normalized to insulin concentration (C) for control subjects and individuals with type 1 diabetes during a hyperinsulinemic/euglycemic clamp at 4, 8, and 40 mU/m2 · min insulin doses and nonoxidative glucose disposal during the 40-mU/m2 · min insulin dose (D). §, Significantly different from basal (P < 0.05); ¥, significantly different from control (P < 0.05).
The IC50 for glucose Ra was not significantly related to liver density (P = 0.26), liver to spleen density ratio (0.98), AST (P = 0.27), ALT (P = 0.70), or basal glucose concentration (P = 0.13) but was positively related to hemoglobin A1C (r = 0.382, P = 0.01) in the overall study population as a whole. There was a significant inverse relationship between glucose MCR normalized to insulin concentration during the 40-mU/m2 · min insulin infusion and visceral fat content (r = −0.302, P = 0.03), basal glucose (r = −0.426, P = 0.002), hemoglobin A1C (r = −0.638, P < 0.0001), and log-transformed basal insulin concentration (r = −0.664, P < 0.0001). There was no relationship between glucose MCR normalized to insulin concentration during the high-dose insulin stage and estimates of physical activity as well as the amount and variability of glucose concentration during continuous glucose monitoring before the insulin clamp study. Linear regression analysis was performed to determine how much of the difference in glucose Rd during the 40-mU/m2 · min insulin infusion between diabetics and controls was due to differences in hemoglobin A1C. Table 2 shows that when adjusted for age and sex, the controls had a glucose Rd that was 5.10 mg/kg · min higher than those with type 1 diabetes. When hemoglobin A1C was included in this model, the difference between groups was 4.78 mg/kg · min, indicating only 0.32 mg/kg · min of the difference in glucose Rd between groups was due to hemoglobin A1C (P = 0.41 for a significant difference in the β-coefficients between the full and reduced models).
Table 2.
Statistical models for the importance of hemoglobin A1C in determining the difference in glucose Rd between controls and individuals with type 1 diabetes during the 40-mU/m2 · min insulin infusion
| Linear regression models | β-Coefficients [difference in glucose Rd associated with diabetes (mg/kg · min) and 95% CI] | P value | Model R2 |
|---|---|---|---|
| Model 1 adjusted for age and sex | 5.10 (3.56–6.65) | <0.0001 | 0.5681 |
| Model 2 adjusted for age, sex, and hemoglobin A1C | 4.78 (1.43–8.14) | 0.006 | 0.5685 |
CI, Confidence interval.
Discussion
It is well known that individuals with type 2 diabetes are insulin resistant compared with age-matched controls (7–9). However, insulin resistance in type 1 diabetes is less commonly appreciated (5, 27). Historically, most studies implicated hyperglycemia in the insulin resistance of type 1 diabetes (28, 29). It has been unclear whether insulin resistance is a feature of individuals with well-controlled type 1 diabetes in the post-Diabetes Control and Complications Trial era of tighter glucose control (30). To answer this question, subjects in our cohort were under better glucose control (hemoglobin A1C of 7.7%) compared with previously published studies (7, 11, 13, 14) (hemoglobin A1C 10.6–13.6%), glucose was normalized overnight via variable insulin infusion, and CGMS (i.e. glucose variability) for 3 d before the study showed no relationship to insulin sensitivity (5). Importantly, linear regression analysis revealed little influence of hemoglobin A1C on the difference in glucose disposal between groups. Therefore, hyperglycemia alone is not able to explain the dramatic insulin resistance observed in type 1 diabetes. Additionally, mechanisms of insulin resistance are unlike those found in other insulin resistant states because individuals with type 1 diabetes in this study were not obese and compared with insulin sensitive controls had similar percent body fat, physical activity levels, and visceral fat content. This finding may have tremendous relevance in the therapeutic approach and CVD prevention in people with type 1 diabetes.
Our data agree with two previous studies reporting hepatic insulin resistance in individuals with type 1 diabetes, but methodological differences warrant mention (7, 11). DeFronzo et al. (7) inferred hepatic insulin resistance by inappropriately elevated fasting glucose Ra in individuals with type 1 diabetes relative to circulating glucose and insulin concentrations, and in a separate study complete suppression of glucose Ra during a high dose insulin infusion (11). Similar to DeFronzo, we also found complete suppression of glucose Ra during the highest insulin infusion. However, we also used dynamic testing to determine the concentration of insulin required to suppress glucose Ra 50% (IC50), with state-of-the-art stable isotope techniques to confirm that hepatic insulin resistance is a key component of insulin resistance in individuals with type 1 diabetes. It is important to note that the underlying mechanism(s) responsible for the decrement in insulin sensitivity is unclear. Contrary to the etiology of hepatic insulin resistance in obesity and type 2 diabetes, hepatic insulin resistance was unrelated to steatosis as estimated by liver function tests and as measured by CT liver to spleen density ratios. Therefore, although prominent in type 1 diabetes, diminished hepatic insulin sensitivity is not due to hepatic steatosis and may implicate a pathway novel to type 1 diabetes.
Several previous studies reported whole-body insulin resistance in individuals with type 1 diabetes (7, 11, 13, 14) but must be interpreted in light of the subjects' poor glycemic control. For example, subjects in the previous studies with type 1 diabetes had poorly controlled diabetes, with mean hemoglobin A1C values of 10.6–13.6% (commensurate with average plasma glucose values of 250–340 mg/dl) when reported. Therefore, it has been difficult to extricate true insulin resistance from glucotoxicity to date. To minimize the confounding effect of glucotoxicity on insulin action, subjects in the current study had a mean hemoglobin A1C of 7.7 ± 0.16%, which is better than in previous studies. Our data are consistent with insulin resistance in individuals with type 1 diabetes as glucose metabolic clearance rate normalized to plasma insulin concentration was significantly lower in individuals with type 1 diabetes compared with CON during the 40-mU/m2 · min insulin infusion. Data from the indirect calorimetry are also consistent with decreased insulin sensitivity in individuals with type 1 diabetes because we found lower whole-body carbohydrate oxidation and nonoxidative glucose disposal in response to insulin compared with controls. Altogether these data indicate peripheral insulin resistance in individuals with type 1 diabetes. Similar to previous reports (17, 31), hyperglycemia explained only a small percentage (6.3%) of the decrease in insulin sensitivity in type 1 diabetes. Speculation exists regarding the role of chronic (32) and/or exogenous (i.e. nonpulsatile) (33) hyperinsulinemia in the insulin resistance of type 1 diabetes, which may be particularly relevant to skeletal muscle.
There are several mechanisms that may explain hyperinsulinemic-related skeletal muscle insulin resistance in individuals with type 1 diabetes. Blunted up-regulation of glucose transporter 4 (GLUT4) mRNA in response to insulin (34), deficits in insulin stimulated skeletal muscle ATP production (17), and similar to results in this study, decreased capacity for insulin stimulated glycogen storage (14, 29) have been described. The latter may be due to decreased glucose transport because basal and insulin stimulated glycogen synthase activity is not different in individuals with type 1 diabetes (35, 36), and glycogen storage can be normalized by forcing similar glucose disposal via mass action (35). Our data cannot rule out decreased capacity for glycogen synthesis in type 1 diabetes. Individuals with type 1 diabetes oxidized a greater proportion of their diminished glucose uptake, which would be consistent with decreased disposal of transported glucose into glycogen, making it available for oxidation. Nevertheless, decreased insulin-mediated skeletal muscle glucose transport appears to be the main limitation promoting insulin resistance in type 1 diabetes (14). Mechanisms promoting skeletal muscle insulin resistance in type 1 diabetes are unclear, but there is likely overlap with 2 diabetes, including increased intramuscular triglyceride content (15) and mitochondrial dysfunction (17). However, other defects in metabolism may be unique to type 1 diabetes. Similar mechanisms link both microvascular complications of type 1 diabetes and skeletal muscle insulin resistance, including increased reactive oxygen species, advanced glycation end products, and activation of protein kinase C (37–40). These mechanisms promoting microvascular complications may hold unique promise to direct targeted insulin sensitizing therapies in type 1 diabetes.
There are several limitations to the current study. First, fasting hyperglycemia was reduced in individuals with type 1 diabetes by an overnight insulin infusion to prevent measuring insulin resistance associated with acute glucose toxicity. Although a similar protocol has been used by others (14, 16), it is possible that the infusion was not long enough to normalize hyperglycemia-induced insulin resistance. Additionally, even though glucose concentration was controlled overnight, there was still a difference in basal glucose concentration between groups that could have influenced these data. Further lowering of basal glucose was not attempted in volunteers with type 1 diabetes to avoid hypoglycemia associated with intensive glucose control (41). Second, peripheral, not portal, insulin concentration was measured in this study. Control subjects may have continued to secrete insulin during the early clamp stages, resulting in higher portal insulin concentrations than was measured in the venous blood, which would result in an error in the calculation of hepatic IC50. Control subjects tended to be more physically active than individuals with type 1 diabetes, which may have influenced our results. We assumed that peripheral glucose disposal largely represents skeletal muscle but acknowledge that the splanchnic tissues dispose of a small proportion of glucose during a hyperinsulinemic/euglycemic clamp (42). Individuals with type 2 diabetes and maturity-onset diabetes of the young were excluded on clinical grounds, leaving a small chance of misclassification. Additionally, the first two stages of the insulin clamp were designed to show incremental decreased in glucose Ra to calculate the IC50 for this parameter. Unfortunately, for some of our subjects with type 1 diabetes, the 4-mU/m2 · min insulin infusion was lower than their basal insulin needs. This resulted in an increase in glucose Ra and glucose concentration in some individuals.
In summary, our results highlight significant insulin resistance in both liver and skeletal muscle in lean individuals with type 1 diabetes. Unlike previous studies that have noted whole-body insulin resistance in people with type 1 diabetes, our results were not attributable to acute glucotoxicity or glycemic control. Interestingly, insulin resistance did not appear to be explained by common predictors including BMI, percentage fat, plasma lipids, visceral fat, and physical activity. Together, these data support the notion that hepatic and skeletal muscle insulin resistance exist in a way unique to type 1 diabetes. Future studies are needed to determine whether targeting insulin resistance in this population improves their health through CVD prevention.
Acknowledgments
B.C.B. helped design the study, tested subjects, directed data analysis, and wrote the manuscript; D.H. analyzed all isotope data; I.E.S. tested subjects, contributed to the discussion, and edited the manuscript; D.M.M. tested subjects, contributed to the discussion, and edited the manuscript; J.K.S.-B. tested subjects, contributed to the discussion, provided statistical support, and edited the manuscript; R.H.E. helped design the study, contributed to the discussion, and edited the manuscript; L.P. contributed to the discussion and data interpretation and edited the manuscript; and M.R. helped design the study, contributed to the discussion, and edited the manuscript.
This work was supported by the National Institutes of Health's National Heart, Lung, and Blood Institute Grants R01 HL-61753 and R01 HL-079611, and Clinical Translational Research Center at the University of Colorado Denver Grants M01 RR000051 and RR-00036 as well as the Barbara Davis Center for Childhood Diabetes (Denver, CO). Support was also provided by the National Institutes of Health Diabetes Endocrinology Research Center Clinical Investigation Core Grant P30 DK57516; National Institute for Diabetes and Digestive and Kidney Diseases Grants K01 DK066219 (to B.C.B.) and K23 DK075360 (to D.M.M.); and the American Diabetes Association Junior Faculty Award 1-10-JF-50 (to J.K.S.-B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALT
- Alanine aminotransferase
- AST
- aspartate transaminase
- BMI
- body mass index
- CACTI
- Coronary Artery Calcification in Type 1 diabetes
- CON
- matched people without diabetes
- CT
- computed tomography
- CTRC
- Clinical Translational Research Center
- CVD
- cardiovascular disease
- HDL
- high-density lipoprotein
- MCR
- metabolic clearance rate
- Ra
- rate of glucose appearance
- Rd
- rate of glucose disappearance.
References
- 1. Kannel WB, McGee DL. 1979. Diabetes and cardiovascular disease. The Framingham study. JAMA 241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 2. Zgibor JC, Piatt GA, Ruppert K, Orchard TJ, Roberts MS. 2006. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care 29:1860–1865 [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M. 2003. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 52:2833–2839 [DOI] [PubMed] [Google Scholar]
- 4. Sheetz MJ, King GL. 2002. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA 288:2579–2588 [DOI] [PubMed] [Google Scholar]
- 5. Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. 2011. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes 60:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ginsberg HN. 2000. Insulin resistance and cardiovascular disease. J Clin Invest 106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFronzo RA, Simonson D, Ferrannini E. 1982. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23:313–319 [DOI] [PubMed] [Google Scholar]
- 8. Björnholm M, Zierath JR. 2005. Insulin signal transduction in human skeletal muscle: identifying the defects in type II diabetes. Biochem Soc Trans 33:354–357 [DOI] [PubMed] [Google Scholar]
- 9. Shulman GI. 2000. Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Defronzo RA. 2009. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeFronzo RA, Hendler R, Simonson D. 1982. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31:795–801 [DOI] [PubMed] [Google Scholar]
- 12. Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, Krishnan RK. 2000. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol 88:797–803 [DOI] [PubMed] [Google Scholar]
- 13. Yki-Järvinen H, Taskinen MR, Kiviluoto T, Hilden H, Helve E, Koivisto VA, Nikkilä EA. 1984. Site of insulin resistance in type 1 diabetes: insulin-mediated glucose disposal in vivo in relation to insulin binding and action in adipocytes in vitro. J Clin Endocrinol Metab 59:1183–1192 [DOI] [PubMed] [Google Scholar]
- 14. Cline GW, Magnusson I, Rothman DL, Petersen KF, Laurent D, Shulman GI. 1997. Mechanism of impaired insulin-stimulated muscle glucose metabolism in subjects with insulin-dependent diabetes mellitus. J Clin Invest 99:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, Battezzati A, Secchi A, Del Maschio A, Luzi L. 2003. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 285:E1174–E1181 [DOI] [PubMed] [Google Scholar]
- 16. Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. 2000. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 49:626–632 [DOI] [PubMed] [Google Scholar]
- 17. Kacerovsky M, Brehm A, Chmelik M, Schmid AI, Szendroedi J, Kacerovsky-Bielesz G, Nowotny P, Lettner A, Wolzt M, Jones JG, Roden M. 2011. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Intern Med 269:189–199 [DOI] [PubMed] [Google Scholar]
- 18. Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO. 2003. Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res 11:752–759 [DOI] [PubMed] [Google Scholar]
- 19. Widom B, Diamond MP, Simonson DC. 1992. Alterations in glucose metabolism during menstrual cycle in women with IDDM. Diabetes Care 15:213–220 [DOI] [PubMed] [Google Scholar]
- 20. DeFronzo RA, Tobin JD, Andres R. 1979. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21. Finegood DT, Bergman RN, Vranic M. 1987. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36:914–924 [DOI] [PubMed] [Google Scholar]
- 22. Joy D, Thava VR, Scott BB. 2003. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 15:539–543 [DOI] [PubMed] [Google Scholar]
- 23. Bergman BC, Cornier MA, Horton TJ, Bessesen D. 2007. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol 293:E1103–E1111 [DOI] [PubMed] [Google Scholar]
- 24. Wolfe R. 1992. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss [Google Scholar]
- 25. Frayn KN. 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Appl Physiol 55:628–634 [DOI] [PubMed] [Google Scholar]
- 26. Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. 1995. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest 96:2528–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenbaum CJ. 2002. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev 18:192–200 [DOI] [PubMed] [Google Scholar]
- 28. Yki-Järvinen H, Koivisto VA. 1986. Natural course of insulin resistance in type I diabetes. N Engl J Med 315:224–230 [DOI] [PubMed] [Google Scholar]
- 29. Vuorinen-Markkola H, Koivisto VA, Yki-Jarvinen H. 1992. Mechanisms of hyperglycemia-induced insulin resistance in whole body and skeletal muscle of type I diabetic patients. Diabetes 41:571–580 [DOI] [PubMed] [Google Scholar]
- 30. 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 31. Fasching P, Ratheiser K, Damjancic P, Schneider B, Nowotny P, Vierhapper H, Waldhäusl W. 1993. Both acute and chronic near-normoglycaemia are required to improve insulin resistance in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 36:346–351 [DOI] [PubMed] [Google Scholar]
- 32. Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. 2009. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem 284:27090–27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bratusch-Marrain PR, Komjati M, Waldhäusl WK. 1986. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes 35:922–926 [DOI] [PubMed] [Google Scholar]
- 34. Yki-Järvinen H, Vuorinen-Markkola H, Koranyi L, Bourey R, Tordjman K, Mueckler M, Permutt AM, Koivisto VA. 1992. Defect in insulin action on expression of the muscle/adipose tissue glucose transporter gene in skeletal muscle of type 1 diabetic patients. J Clin Endocrinol Metab 75:795–799 [DOI] [PubMed] [Google Scholar]
- 35. Yki-Järvinen H, Sahlin K, Ren JM, Koivisto VA. 1990. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes 39:157–167 [DOI] [PubMed] [Google Scholar]
- 36. Beck-Nielsen H. 1989. Insulin resistance in skeletal muscles of patients with diabetes mellitus. Diabetes Metab Rev 5:487–493 [DOI] [PubMed] [Google Scholar]
- 37. Cassese A, Esposito I, Fiory F, Barbagallo AP, Paturzo F, Mirra P, Ulianich L, Giacco F, Iadicicco C, Lombardi A, Oriente F, Van Obberghen E, Beguinot F, Formisano P, Miele C. 2008. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem 283:36088–36099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz-Peiffer C, Biden TJ. 2008. Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes 57:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. 2003. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52:1–8 [DOI] [PubMed] [Google Scholar]
- 40. Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. 2004. Diabetic retinopathy. Diabetes Care 27:2540–2553 [DOI] [PubMed] [Google Scholar]
- 41. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. 2009. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 42. DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. 1985. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]