Background: The enzyme that catalyzes O-linked β-N-acetylglucosamine glycosylation represses gene expression.
Results: Overexpression of this enzyme enhances gene repression by the glucocorticoid receptor whereas knockdown has an opposite effect.
Conclusion: O-GlcNAc transferase interacts with the glucocorticoid receptor to repress gene expression.
Significance: The discovery of a novel component of cortisol-induced gene repression enhances our understanding of hormone signaling and provides an attractive target for drug design.
Keywords: Gene Regulation, Glucocorticoid Receptor, NF-κB (NF-κB), O-GlcNAc, RNA Polymerase II
Abstract
Recruitment of O-GlcNAc transferase (OGT) to promoters plays an important role in gene repression. Glucocorticoid signaling represses the transcriptional activities of NF-κB and AP-1 through direct binding, yet the molecular mechanisms remain to be elucidated. Here we report that OGT is an important component of GR-mediated transrepression. OGT associates with ligand-bound GR in a multi-protein repression complex. Overexpression of OGT potentiates the GR transrepression pathway, whereas depletion of endogenous OGT by RNA interference abolishes the repression. The recruitment of OGT by GR leads to increased O-GlcNAcylation and decreased phosphorylation of RNA polymerase II on target genes. Functionally, overexpression of OGT enhances glucocorticoid-induced apoptosis in resistant cell lines while knockdown of OGT prevents sensitive cell lines from apoptosis. These studies identify a molecular mechanism of GR transrepression, and highlight the function of O-GlcNAc in hormone signaling.
Introduction
Glucocorticoids are commonly used to treat inflammation and blood cancer (1, 2). The glucocorticoid receptor (GR)3 mediates the anti-inflammatory and anti-cancer effects by directly binding to NF-κB and AP-1 to inhibit their transcriptional activity, referred to as “transrepression” (1). The molecular mechanism of transrepression has been subject to intense investigation. Earlier studies by De Bosscher and co-workers (3, 4) rule out the possibility that GR competes with nuclear coactivators for access to NF-κB and AP-1. It has been reported that GR can recruit the cofactor TIF2/GRIP1 to inhibit AP-1, yet how TIF2/GRIP1 repressses AP-1 is unclear (5). The large subunit of RNA Polymerase II (pol II) has a unique C-terminal domain (CTD) that comprises conserved YSPTSPS heptad repeats. Phosphorylation of the heptad repeats at Ser-2 is required for transcription. Yamamoto's group (6, 7) demonstrates that GR inhibits NF-κB by interfering with Ser-2 phosphorylation of pol II CTD at the promoter regions of interleukin-8 (IL-8) and intracellular adhesion molecule-1 (ICAM-1) genes. Nevertheless, the molecular details of GR repression of NF-κB and AP-1 signaling are largely unknown.
A broad variety of nuclear and cytoplasmic proteins are modified by O-linked β-N-acetylglucosamine (O-GlcNAc) monosaccharide at serine and threonine residues (8, 9). This highly dynamic and reversible modification involves two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), by which O-GlcNAc is attached to and removed from proteins, respectively. O-GlcNAcylation is conserved across metazoans and participates in a wide spectrum of cellular processes (10). Hundreds of O-GlcNAcylated proteins so far identified are involved in transcription, including RNA polymerase II and associated transcription factor Sp1 (9, 11, 12). The functions of O-GlcNAcylation on gene expression are conserved from round worms to mammals. In Caenorhabditis elegans, dynamic O-GlcNAc cycling occurs on the promoter regions of genes (13). Drosophila OGT is a Polycomb group protein that is essential to the maintenance of Polycomb transcriptional repression (14, 15). Burlingame and co-workers (16) further show that Polycomb repressive complex 2 modulates intracellular distribution of O-GlcNAc in mouse embryonic stem cells. Our previous work shows that mammalian OGT can inhibit the activity of transcription factor Sp1 (17). Importantly, OGT can function in parallel with histone deacetylation to promote gene silencing (18). These studies suggest that O-GlcNAc represents a novel molecular switch for gene regulation.
It has long been known that the tandem heptad repeats of pol II CTD are modified by O-GlcNAc, which is thought to antagonize CTD phosphorylation (11, 19). This prompted us to explore whether OGT mediates GR inhibition of NF-κB function. Here we demonstrate that OGT associates with ligand-bound GR in a multi-protein repression complex, which binds to the RelA subunit of NF-κB. Overexpression of OGT does not affect GR transactivation, but potentiates GR repression of NF-κB transcriptional activity. Depletion of endogenous OGT by RNA interference abolishes the repression. We further show that the recruitment of OGT by GR to a subset of NF-κB target genes leads to reduced phosphorylation and increased O-GlcNAcylation of pol II CTD. The cooperative action between OGT and GR in repressing NF-κB signaling is not restricted to anti-inflammation, since the NF-κB pathway is also involved in cell survival. Overexpression of OGT enhances glucocorticoid-induced apoptosis in resistant cell lines while knockdown of OGT prevents sensitive cell lines from apoptosis. These findings reveal a novel molecular mechanism of GR transrepression, and underscore the importance of O-GlcNAc in the regulation of hormonal signaling.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
The mammalian reporter construct κB3-luc (κB3DLO) was provided by K. Yamamoto (University of California, San Francisco) and AP-1-luc was purchased from Stratagenes. The mammalian expression plasmid pCMX-mRelA was provided by Inder Verma (The Salk Institute). Plasmids for bacterial expression of the full-length and deletion mutants of OGT in fusion with glutathione S-transferase (GST) were described previously (18). HA×3-OGT was subcloned into the pcDNA3.1 vector. The catalytically dead mutant OGT D925N was generated using QuickChange II Site-directed Mutagenesis kits (Stratagene). The sequences of RNAi oligos are as follows: scrambled siRNA, F-GAGGC AUGUC CGUUG AUUCG U, R-GAAUC AACGG ACAUG CCUCU U; hOGT siRNA, F-GAGGC AGUUC GCUUG UAUCG U, R-GAUAC AAGCG AACUG CCUCU U.
Cell Culture and Treatment
HEK293, CV-1and A549 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (GIBCO) containing 10% fetal bovine serum (FBS). CCRF-CEM and 697 cells were maintained in RPMI1640 medium (GIBCO) containing 10% FBS and 1% streptomycin and penicillin. U2OS.G cells were maintained in DMEM containing 10% FBS and 350 ng/ml G418. Cells were cultured at 37 °C and 5% CO2. 36 h after transfection, cells were treated with 1 μm dexamethasone, 10 ng/ml TNFα, or ethanol vehicle for 7 h, or cognate ligands for nuclear receptors overnight as described.
Luciferase Reporter Assays
Transient transfection of CV-1 cells was performed using Effectine Transfection Regents (Qiagen). Luciferase and β-galactosidase activity were assayed 48 h later. Transfection efficiencies were normalized to the activity of β-galactosidase. Each transfection was conducted in triplicate and repeated 3–5 times.
GST Pull-down Assays
All GST fusion proteins were expressed in E. coli and were purified as described previously (20). 35S-labeled nuclear receptor proteins rGRα, ERα, rTRα, hRARα, mPPARα, mPPARγ1, and mPPARδ and rGRα deletion mutants were synthesized in vitro using appropriate T7-based pCMX plasmids in a coupled transcription-translation system, TNT (Promega). 35S-labeled proteins were incubated with equal amounts of either immobilized GST or GST fusion proteins in binding buffer (50 mm Tris, pH 7.5, 10% glycerol, 100 mm NaCl, 0.1% Nonidet P-40, 1 mm EDTA, 1 mm DTT, 1 mm PMSF, and protease inhibitors) for 2 h at 4 °C. Beads were washed five times with the binding buffer. Bound proteins were eluted with 1× SDS-PAGE buffer, separated by SDS-PAGE, and visualized by fluorography. To preserve the physical interactions between ligand-bound nuclear receptors and OGT, cognate ligands (5 μm of estradiol (E2), triiodotyronine (T3), all-trans retinoid acid (atRA), and PPARδ agonist GW-501516, 125 μm PPARα agonist Wy-14643, and 25 μm PPARγ agonist Rosiglitazone, respectively) were added into buffers used in every step from in vitro translation to elution.
Co-immunoprecipitation Assay
HEK293 cells were transfected by Lipofectamine 2000 (Invitrogen) with 20 μg of expression vectors encoding HA epitope-tagged OGT, GR, RelA, and TIF2 proteins as indicated above. 36 h later, cells were treated with 1 μm dexamethasone (Dex) for 7 h and then extracted with lysis buffer (50 mm Tris, pH 8.0, 20% glycerol, 500 mm NaCl, 0.5% Nonidet P-40, 5 mm MgCl2, 0.2 mm EDTA, 1 mm DTT, 1 mm PMSF, 1× complete protease inhibitors (Roche)) with or without 1 μm Dex. Cellular debris was removed by centrifugation. Supernatants were diluted with 4× volume of dilution buffer (50 mm Tris, pH 7.5, 10% glycerol, 5 mm MgCl2, 0.2 mm EDTA, 1 mm DTT, 1 mm PMSF, and 1× complete protease inhibitors) and were pre-cleared with Protein A/G PLUS-agarose (Santa Cruz Biotechnology) plus 1 μg of normal mouse IgG and 1 μg of normal rabbit IgG, followed by immunoprecipitation at 4 °C with α-HA antibody (12CA5, Roche) overnight. Precipitates were collected by incubating with Protein A/G PLUS-agarose for 3 h and washed five times with LS buffer (50 mm Tris, pH 7.5, 10% glycerol, 100 mm NaCl, 0.1% Nonidet P-40, 1 mm EDTA) supplemented with 0.5 mm DTT, 0.5 mm PMSF, 1× complete protease inhibitors w/or w/o 1 μm Dex, resolved on SDS-PAGE, and analyzed by Western blotting using α-HA antibody (12CA5, Roche), α-GR antibody, and α-TIF2 antibody (BD Biosciences, 610984), respectively.
Sequential Chromatin Immunoprecipitation Assays
A549 cells were grown to 95% confluence in DMEM supplemented with 10% FBS followed by treatment with hormone or ethanol vehicle for 2 h. Cells were crosslinked with 1% formaldehyde at room temperature for 10 min. Cell lysates were prepared as described previously (18) and then were sonicated. Cell debris was removed by centrifugation. Supernatants were precleared with 20 μg of sheared salmon sperm DNA, 5 μg of normal IgG, and 50 μl of protein G-Sepharose for 2 h at 4 °C. Immunoprecipitations were enriched overnight at 4 °C with α-RNA pol II CTD antibody (8WG16, Abcam). Immunoprecipitates were washed and eluted, then immunoprecipitated with CTD phosphoserine-2 specific monoclonal antibody H5 (BAbCO) and O-GlcNAc monoclonal antibody CTD110.6, respectively. These secondary immunoprecipitates were washed and eluted, followed by heating at 65 °C for 6 h to reverse the formaldehyde crosslinking. DNA fragments were purified with DNA Clean & Concentrator-5 (Zymo Research). DNA signals were examined by PCR using the following PCR primer pairs: IL-8 (−121/+61)_F, GGGCCATCAGTTGCAAATC; IL-8 (−121/+61)_R, TTCCTTCCGGTGGTTTCTTC; IL-8 (−1042/−826)_F, AACAGTGGCTGAACCAGAG; IL-8 (−1042/−826)_R, AGGAGGGCTTCAATAGAGG; U6 (−245/+85)_F, GGC CTATTTCCCATGATTCC; U6 (−245/+85)_R, ATTTGCGTGTCATCCTTGC; ICAM-1 (−305/−91)_F, ACCTTAGCGCGGTGTAGACC-3′; ICAM-1 (−305/−91)_R, CTCCGGAACAAATGCTGC; HSP70 (+153/+423)_F, GGATCCAGTGTTCCGTTTCC; HSP70 (+153/+423)_R, GTCA AACACGGTGTTCTGCG; IκBα (−168/+21)_F, CTCATC GCAGGGAGTTTCT; IκBα (−168/+21)_R, ACTGCTGTGGGCTCTGCA.
TUNEL (Terminal Deoxynucleotidyl Transferase-mediated dUTP Nick Translation) Assay
In situ Cell Death Detection Kit, AP (Roche) was used according to the manufacturer's instructions. Briefly, A549 cells or U2OS.G cells were fixed with freshly made 4% paraformaldehyde, pH 7.4 for 1 h. Rinse cells with PBS between each step. Cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice, followed by incubation with TUNEL reaction mixture for 1 h at 37 °C in the dark, before examined by light microscopy. Average number of apoptotic cells was estimated by counting TUNEL-positive cells in four randomly selected medium-power fields (200×) in each of three independent experiments. Cells in areas with poor morphology or in the margins of slides were discarded.
Annexin-V Staining Assay
Annexin V-Fluor Staining kit (Roche) was used according to the manufacturer's instructions as described below. U2OS.G cells on the coverslips were rinsed with PBS twice. Cells were incubated with the labeling solution (prepared by diluting 20 μl of Annexin-V-Fluor labeling reagent and 20 μl of propidium iodide in 1 ml incubation buffer) for 12 min in the dark and analyzed by fluorescence microscopy.
Quantitative PCR
Total RNA was extracted from A549 or CCRF-CEM cells using TRIzol reagent (Invitrogen). Complementary DNA was synthesized from total RNA with Superscript II enzyme and random hexamer primers (Invitrogen). cDNAs were amplified with a SYBR green PCR kit and an ABIPRISM7700 detection system (Perkin Elmer). All data were normalized to the expression of 36B4 mRNA. Primer sequences are available from the authors on request.
RESULTS
Analysis of Functional Interactions between OGT and Nuclear Receptors
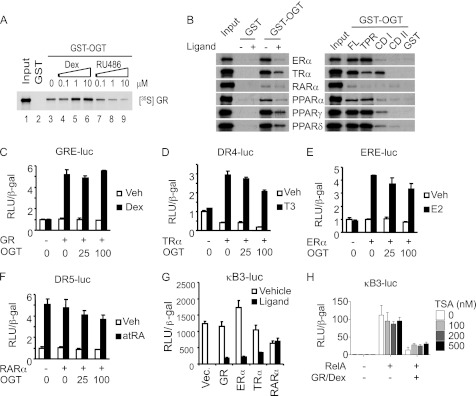
As a first step to address whether OGT is involved in nuclear receptor signaling, we used GST-OGT pull-down assays to assess physical interactions between OGT and nuclear receptors implicated in transrepression, including GR, estrogen receptor α (ERα), thyroid hormone receptor α (TRα), retinoic acid receptor α (RARα), and PPARs (21–23). [35S]Methionine-labeled nuclear receptor proteins synthesized in reticulocyte lysates were incubated with GST alone or GST-OGT fusion proteins immobilized on glutathione beads. The results showed that all the tested nuclear receptors bound to OGT, though RARα had only weak interaction with OGT (Fig. 1, A and B). We also examined whether ligands can modulate the interactions of nuclear receptors with OGT. Exposure to the synthetic glucocorticoid, Dex, increased GR binding to OGT in a dose-dependent manner, whereas the GR antagonist RU486 induced their dissociation (Fig. 1A). In contrast, the other nuclear receptors largely failed to bind to OGT in the presence of cognate ligands (Fig. 1B). OGT contains multiple tandem tetratricopeptide repeats (TPR) at the N terminus, and two catalytical domains (CD I and CD II) at the C terminus (24). Domain mapping analysis using different truncations of OGT suggested that TPR domain is the dominant region to interact with these nuclear receptors in vitro, except that TRα appears to preferably bind to CD I (Fig. 1B).
FIGURE 1.
Functional analysis of interactions between OGT and nuclear receptors. A and B, GST pull-down assays. [35S]methionine-labeled nuclear receptor proteins synthesized in reticulocyte lysates were incubated with GST alone or GST-OGT fusion proteins immobilized on glutathione beads. Inputs represent 25% of 35S-labeled proteins. C–F, luciferase reporter assays of CV-1 cells cotransfected with expression constructs for OGT in various amounts (displayed in ng), nuclear receptor, and the luciferase reporter construct containing cognate hormone response elements. Luciferase activity was normalized to β-galactosidase activity (β-gal). G, luciferase reporter assays of CV-1 cells cotransfected with the NF-κB-driven luciferase reporter (κB3-luc), together with expression vectors for GR, ERα, TRα, RARα, respectively. Cells were treated with cognate ligands (5 μm estradiol (E2), 5 μm triiodotyronine (T3), 5 μm all-trans retinoid acid (atRA), 5 μm PPARδ agonist GW-501516, 125 μm PPARα agonist Wy-14643, or 25 μm PPARγ agonist Rosiglitazone) overnight before assayed. H, luciferase reporter assays of CV-1 cells cotransfected with κB3-luc, β-gal, together with expression vectors for GR and RelA. Cells were treated with Dex and various concentrations of TSA overnight. Results are shown ± S.D.
Nuclear receptors primarily act as ligand-dependent transcription factors via hormone response elements (HREs). We examined the impact of OGT on nuclear receptor-induced transcription of HRE reporter plasmids. As shown in Fig. 1, C–F, forced expression of OGT did not affect the activity of GR but slightly inhibited the transactivation activity of liganded ERα, TRα, and RARα. It has been known that unliganded TRα is a potent repressor (25). Our data showed that OGT acts as a TRα corepressor (Fig. 1D), supporting the notion that O-GlcNAc is vital for transcriptional repression (18). Nevertheless, OGT appears not to be directly involved in the transactivation function of nuclear receptors.
We then assessed the ability of nuclear receptors to repress NF-κB. The results showed that, in the presence of their ligands, GR, ERα, and TRα strongly repressed NF-κB-driven expression of a luciferase reporter gene, whereas RARα had no effect (Fig. 1G). This is well correlated with their physical interactions with OGT, implying a possible role of OGT in mediating transrepression. Furthermore, we observed that a histone deacetylase (HDAC) inhibitor known as trichostatin A (TSA) was not able to relieve GR-mediated repression of NF-κB activity (Fig. 1H), which is consistent with previous reports, suggesting the existence of the HDAC-independent mechanism(s) of GR transrepression (26). Since OGT is known to be a HDAC-independent corepressor (18), we hypothesized that the physical interaction between GR and OGT might contribute to transrepression.
OGT Promotes GR-mediated Transrepression
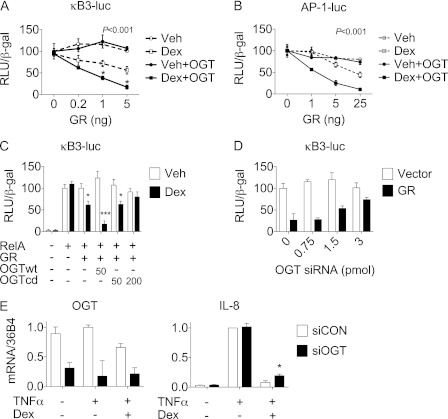
To test whether OGT is involved in GR-mediated transrepression, OGT, RelA, and different amounts of GR were co-expressed in CV-1 cells and their effects on the NF-κB-driven luciferase reporter (κB3-luc) were analyzed after incubation with either Dex or ethanol vehicle. As shown in Fig. 2A, overexpression of OGT markedly potentiated Dex-dependent, GR-mediated repression of NF-κB. Similarly, overexpression of OGT also potentiated GR-mediated inhibition on AP-1, suggesting that the recruitment of OGT by GR is part of a general mechanism of transrepression (Fig. 2B). In contrast to the wild-type OGT (OGTwt), a catalytically dead OGT mutant (OGTcd) had no synergistic effect with Dex-bound GR, indicating that the catalytic activity of OGT contributes to GR-mediated transrepression (Fig. 2C).
FIGURE 2.
OGT promotes GR-mediated transrepression. A–C, luciferase reporter assays of CV-1 cells cotransfected with κB3-luc or AP-1-driven reporter construct as indicated, together with (A–B) expression vectors for GR and OGT after Dex or ethanol vehicle treatment, p < 0.001, two-way ANOVA, followed by Bonferroni post-test, Dex+OGT versus Veh+OGT, *, p < 0.05; and (C) expression vectors for OGT and its catalytically dead mutant (OGTcd) after Dex or ethanol vehicle treatment; Dex versus Veh, *, p < 0.05; ***, p < 0.001, Student's t test. D, luciferase reporter assays of A549 cells cotransfected with κB3-luc and GR and OGT siRNA. Equal amount of nucleotides were ensured by compensation with vector and scrambled siRNA. E, quantitative PCR analysis of OGT and IL-8 expression in A549 cells transfected with OGT siRNA (siOGT) or scrambled siRNA (siCON) followed by TNFα and/or Dex treatment as described; siOGT versus siCON, *, p < 0.05, Student's t test.
To determine whether OGT is required for the transrepression, we analyzed the effect of OGT siRNA on the activity of the κB3-luc reporter construct. As shown in Fig. 2D, GR-mediated transrepression of NF-κB was markedly rescued in A549 cells exposed to an increasing amount of OGT siRNA, accompanied by a dose-dependent reduction in global O-GlcNAc levels (supplemental Fig. S1). It has been well characterized that NF-κB activates the proinflammatory gene IL-8 in A549 cells in response to TNFα signaling, which can be repressed by GR upon Dex addition (6). We observed that OGT knockdown did not affect TNFα-induced production of IL-8 mRNA but alleviated Dex-induced repression of IL-8 expression (Fig. 2E). These results indicate that the GR transrepression pathway is especially susceptible to changes in the level of intracellular OGT.
The GR/OGT/TIF2 Complex Targets NF-κB
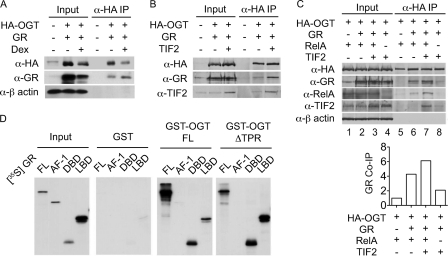
Considering the role of OGT in mediating GR transrepression, we tested whether OGT is a component of the GR inhibitory complex. HEK293 cells were co-transfected with expression vectors for HA epitope-tagged OGT (HA-OGT) and GR followed by treatment with Dex or ethanol vehicle. Western blotting analysis of the HA-OGT immunoprecipitates demonstrated that GR associated with OGT in living cells, which was enhanced by Dex treatment (Fig. 3A). The interaction between endogenous OGT and GR was observed in HeLa cells (supplemental Fig. S2). It has been reported that Dex induces the formation of an inhibitory complex containing GR and TIF2/GRIP1 (hereafter referred to as TIF2) that interferes with AP-1 activity (5). To explore whether OGT forms a complex with GR and TIF2, we co-transfected HEK293 cells with expression plasmids for HA-OGT, TIF2, and GR and analyzed the HA-OGT immunoprecipitates by Western blotting. The results showed that both GR and TIF2 were co-precipitated with HA-OGT, and TIF2 enhanced the association between GR and OGT (Fig. 3B). We then tested whether and how the GR/OGT/TIF2 complex physically interacts with NF-κB by assaying HEK293 cells co-transfected with expression plasmids for GR, HA-OGT, TIF2, and RelA. Western blotting analysis of the HA-OGT immunoprecipitates revealed that (1) OGT did not bind to RelA directly but did so in the presence of GR (Fig. 3C, lanes 5 and 6); (2) TIF2 enhanced the association between OGT, GR and RelA (Fig. 3C, lanes 6 and 7). These results identified OGT as a component of a GR-containing multi-protein complex that physically interacts with NF-κB.
FIGURE 3.
The GR/OGT/TIF2 complex interacts with NF-κB. A, co-immunoprecipitation analysis of A549 cells transfected with expression vectors for HA-OGT and GR in the presence or absence of Dex treatment. Immunoprecipitates were analyzed by Western blotting with α-HA, α-GR, and α-β-actin. B and C, co-immunoprecipitation analysis of A549 cells transfected with expression vectors for HA-OGT, GR, TIF2, and RelA, as described. Immunoprecipitates were analyzed by Western blotting with α-HA, α-GR, α-RelA, α-TIF2, and α-β-actin. Co-immunoprecipitated GR was quantified by densitometric analysis of α-GR blot (bottom panel). D, GST pull-down assays. In vitro synthesized [35S]methionine-labeled GR and its fragments were incubated with GST alone or GST-OGT fusion proteins immobilized on glutathione beads. Input represents 12.5% of [35S]-labeled GR.
We then asked which domains mediate the physical interaction between GR and OGT. In vitro synthesized GR protein fragments corresponding to full-length, the N-terminal activation domain (AF-1), the central DNA-binding domain (DBD), and the C-terminal ligand-binding domain (LBD) were examined. The results showed that 1) both the DBD and LBD of GR physically interacted with OGT (Fig. 3D), which supports Nissen and Yamamoto's (6) observation that both the DBD and LBD of GR are required for repressing NF-κB; 2) ΔTPR of OGT was sufficient to bind to GR and its fragments, respectively.
GR-mediated Repression Involves O-GlcNAcylation of the Pol II CTD
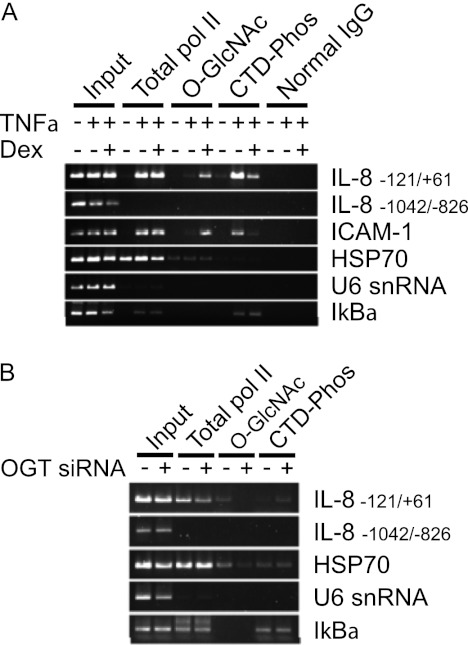
De Bosscher et al. (3) reported that GR-mediated transrepression of NF-κB targets the basal transcriptional machinery. In a more detailed study, Nissen and Yamamoto (6) demonstrated that GR suppresses TNFα-stimulated phosphorylation of the pol II CTD at the promoters of IL-8 and ICAM-1 (6). Because the pol II CTD can be modified by O-GlcNAc (16) and the catalytic activity of OGT is involved in the transrepression (Fig. 2C), we hypothesized that OGT mediates GR repression of NF-κB signaling by regulating the cross-talk between O-GlcNAcylation and phosphorylation of the pol II CTD. To test this hypothesis, we first asked the question whether Dex treatment alters O-GlcNAc levels of the pol II CTD at the promoters of IL-8 and ICAM-1 upon stimulation with TNFα. A549 cells were assayed by sequential chromatin immunoprecipitation (ChIP) using the antibody recognizing total RNA pol II at first, followed by antibodies recognizing phosphorylated CTD and O-GlcNAcylated CTD (CTD 110.6), respectively. Consistent with previous findings (6), TNFα induced the occupation of the IL-8 and ICAM-1 promoters by RNA pol II and the enrichment of the phospho-CTD (Fig. 4A and supplemental Fig. S3A). Importantly, Dex treatment resulted in increased O-GlcNAcylation and decreased phosphorylation of the CTD without affecting the promoter occupancy of total pol II (Fig. 4A and supplemental Fig. S3A).
FIGURE 4.
GR-mediated repression involves O-GlcNAcylation of the pol II CTD. A, sequential chromatin immunoprecipitation analysis of A549 cells. Cells treated with TNFα, Dex, or ethanol vehicle were subject to sequential chromatin immunoprecipitation using α-RNA pol II CTD antibody (Total pol II), followed by α-phos-Ser2 pol II CTD (CTD-Phos) or α-O-GlcNAc (O-GlcNAc). B, sequential chromatin immunoprecipitation analysis of A549 cells transfected with OGT siRNA or scrambled siRNA and then treated with TNFα and Dex.
TNFα also stimulates transcription of IκBα. However, neither the pol II recruitment nor the post-translational modifications of the CTD was affected by Dex (Fig. 4A and supplemental Fig. S3A). This is consistent with previous findings that IκB synthesis and inhibition of NF-κB activity are separable biochemical processes (6, 27). TNFα and Dex treatments had no notable effects on the pol II occupancy and the post-translational modifications of the CTD with regard to the following controls: a distal region that is 700 bp upstream of the NF-κB site of the IL-8 promoter, and promoters of HSP70 and U6 snRNA genes that do not contain NF-κB sites (Fig. 4A).
To ascertain that altered CTD phosphorylation is due to OGT action, the effects of OGT knockdown were analyzed after TNFα and Dex co-treatment. The results showed that transfection of OGT siRNA decreased the level of O-GlcNAcylated CTD and increased the level of phosphorylated CTD at the proximal promoter region of the IL-8 gene but not the other control promoter sequences (Fig. 4B and supplemental Fig. S3B). These results further indicate that OGT is an essential component for GR-mediated repression and acts through reduced phosphorylation of RNA-Pol II, which may reflect an enhanced O-GlcNAcylation status.
OGT-GR Participates in the Regulation of Apoptosis
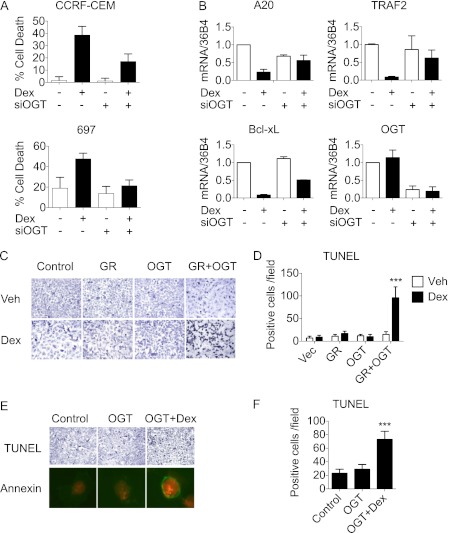
NF-κB not only acts as a central regulator of inflammation, but also plays a critical role in the regulation of cell proliferation and apoptosis (28). NF-κB is constitutively activated in many human cancers, which promotes cancer cell survival and resistance to apoptosis. Glucocorticoids are effective in treating blood cancer, such as acute lymphocytic leukemia (ALL), by repressing pro-survival signaling from NF-κB and inducing apoptotic cell death. AP-1 is also implicated in oncogenesis and metastasis of human cancers; however, alternations in AP-1 activity might not be involved in glucocorticoid-induced apoptosis, at least in human acute T-cell leukemia (29). To determine whether OGT is required for GR-mediated apoptosis, we performed OGT knockdown analysis using Dex-sensitive human ALL cell lines, such as T cell-derived CCRF-CEM and pre-B cell-derived 697 (30, 31). The cells were transfected with either OGT siRNA or scrambled siRNA, followed by Dex and ethanol vehicle treatment, respectively. Trypan blue viability tests revealed that the depletion of OGT led to a significant decrease in Dex-induced cell death in both CCRF-CEM and 697 cells (Fig. 5A). Quantitative PCR analysis of NF-κB-regulated anti-apoptotic genes in CCRF-CEM cells revealed that the depletion of OGT compromises the ability of Dex to suppress the expression of anti-apoptotic genes A20, Bcl-xL, and TRAF2 (Fig. 5B and supplemental Fig. S4) (32). These data suggest that OGT is an essential mediator of GR function in apoptotic cell death.
FIGURE 5.
OGT-GR interaction participates in the regulation of apoptosis. A, cell viability test in Dex-sensitive CCRF-CEM and 697 leukemia cells transfected with OGT siRNA or scrambled siRNA and then treated with Dex or ethanol vehicle. Results are shown in ± S.D. B, quantitative PCR analysis of indicated genes in CCRF-CEM cells transfected with OGT siRNA or scrambled siRNA and then treated with Dex or ethanol vehicle. Results are shown in ± S.D., n = 3. C, TUNEL assay for detecting apoptosis in A549 cells transfected with GR and OGT individually or in combination followed by Dex or ethanol vehicle treatment for 18 h. Representative results are shown. D, quantification of TUNEL-positive cells from experiments described in C. Dex versus Veh, ***, p < 0.001, Student's t test. E, TUNEL assay (upper panel) and Annexin V-Fluor staining (lower panel) of U2OS. G cells transfected with OGT followed by Dex or ethanol vehicle treatment for 18 h. F, quantification of TUNEL-positive cells from experiments described in E. OGT+Dex versus Control or OGT, ***, p < 0.001, Bonferroni's multiple comparison test.
Unlike blood cancer, solid tumors are usually resistant to glucocorticoid-induced cell death (33). To further test the functional involvement of OGT in GR-mediated repression of NF-κB, we assessed whether overexpression of OGT affects cell death in glucocorticoid-resistant cancer cell lines, including A549 lung cancer cells and U2OS osteosarcoma cells. A549 cells were transfected with expression vectors for GR and OGT, followed by Dex or ethanol vehicle treatment. TUNEL assays revealed that co-expression of GR and OGT produced a significant number of dead cells after Dex treatment, whereas individual expression of OGT and GR had no effect (Fig. 5, C and D). These results suggest that OGT can cooperate with GR to induce cancer cell death.
The induction of programmed cell death by OGT, through a concerted action with Dex-bound GR, was also observed in U2OS osteosarcoma cells (Fig. 5, E and F). U2OS cells stably expressing GR (U2OS.G) were transfected with OGT followed by Dex treatment (5). As shown in Fig. 5, E and F, overexpression of OGT increased the number of TUNEL-positive dead cells in a Dex-dependent manner. Annexin-5 staining confirmed that OGT induces U2OS.G cells to undergo apoptotic cell death (Fig. 5, E and F). Together with the data from A549 cells, these results clearly indicate that overexpression of OGT can enhance glucocorticoid-induced apoptosis in resistant cell lines.
DISCUSSION
GR Inhibition of NF-κB Involves O-GlcNAcylation
Although it has been known for two decades that GR suppresses NF-κB and AP-1 function by direct physical interaction, how this “tethered“ GR exerts its inhibitory effect remains elusive. We have now demonstrated that OGT is an essential mediator for GR inhibition of NF-κB through O-GlcNAc modification of pol II CTD (supplemental Fig. S5). There is a complex interplay between phosphorylation and O-GlcNAcylation at multiple sites of pol II CTD. Ser-2 and Ser-5 of the heptapeptide sequence, YSPTSPS, are phosphorylated by CTD kinases, whereas Thr-4 between the two phosphorylation sites seems to be a major O-GlcNAc site (19). The nature of the crosstalk between phosphorylation and O-GlcNAcylation of pol II CTD is not fully understood. A plausible hypothesis is that O-GlcNAc prevents phosphorylation at adjacent residues, resulting in a halt in transcription (8, 9). Previous studies suggest that GR can repress CTD phosphorylation by blocking the recruitment of the Ser-2 CTD kinase P-TEFb to the promoter of IL-8 and ICAM-1 genes (7). At least in in vitro assays, neither OGT nor CTD kinases has a distinct kinetic advantage toward CTD substrates (34). To this end, whether O-GlcNAcylation blocks the recruitment of P-TEFb or blockade of the kinase allows O-GlcNAcylation is unknown. Whether O-GlcNAcylation causes transcriptional pausing, arrest, or termination of pol II also warrants further investigation. Nevertheless, our findings confirm pol II as a crucial target of GR signaling and attest to the importance of the CTD phosphorylation/O-GlcNAcylation switch in transrepression.
GR/TIF2/OGT Serves as a Multifunctional Repression Complex
TIF2 acts as a corepressor of GR to inhibit AP-1 activity (5). Whether the GR/TIF2 complex affects NF-κB has not been addressed. The present study reveals the association of the GR/TIF2 complex with RelA (Fig. 3, A–C) and, more importantly, identifies OGT as an essential component. OGT suppresses the activity of both NF-κB and AP-1 in the presence of Dex (Fig. 2, A and B), supporting the involvement of the GR/TIF2/OGT complex in both the NF-κB and AP-1 pathways. An important question is whether other proteins are also present in this repression complex. Recent studies showed that TIF2 interacts with the histone methyltransferase Suv4–20h1 to modulate GR target genes, and the histone methyltransferase MLL5 is a direct target of OGT (35, 36). These observations raise an intriguing possibility that GR/TIF2 may recruit OGT and other chromatin-modifying enzymes that act in concert to antagonize NF-κB and AP-1 signaling. Further analysis is needed to define the components, mechanisms of action, and dynamics of the GR repression complex.
Biological Functions of Nuclear Receptor-OGT Interactions
A subset of nuclear receptors, including GR, ERα, TRα, RARα, LXRs, and PPARs, act as immune suppressors on binding a broad range of endogenous and synthetic ligands (1, 37). In this study, we have demonstrated an important role for OGT in GR inhibition of NF-κB and identified ligand-dependent physical interactions between OGT and several immunosuppressive nuclear receptors (Fig. 1B), implying that OGT might be a common mediator for nuclear receptor repression pathways. Emerging studies suggest an intrinsic link between metabolism and inflammation (38, 39). As a nutrient sensor, OGT might integrate systemic metabolism and immune responses via interactions with nuclear receptors.
In addition to the transcriptional control of inflammation, OGT-nuclear receptor interactions may also regulate other cellular processes affected by the NF-κB and AP-1 signaling pathways. This notion is supported by our findings that overexpression of OGT induces apoptosis in glucocorticoid-resistant cancer cell lines whereas depletion of endogenous OGT by small interfering RNA prevents glucocorticoid-sensitive cancer cell lines from cell death (Fig. 5, C–F and A). The proapoptotic effects of OGT are dependent on glucocorticoid-bound GR and are strongly associated with repression of NF-κB-inducible anti-apoptotic genes (Fig. 5B). These findings suggest that OGT is crucially important for the crosstalk between glucocorticoid and NF-κB signaling that balances the life and death of a cell. Additional studies are required to elucidate the molecular details of this process in specific cellular contexts.
Clinical Implications
Glucocorticoids are potent anti-inflammatory drugs that suppress proinflammatory NF-κB and AP-1 signaling. Yet their long-term administration causes undesirable or even detrimental side effects, primarily due to a pleiotropic role of GR in metabolic regulation. Other nuclear receptors also have this therapeutic caveat. To circumvent this, one strategy is to develop novel ligands that confer the selectivity of nuclear receptors to different cofactors (40). The identification of OGT as a cofactor essential for GR transrepression, therefore, provides an attractive target for drug design. Alternatively, simultaneous administration of multiple drugs at low doses is another strategy to minimize side effects (41–43). Investigation into potentially synergistic actions of glucocorticoids and OGT agonists may lead to novel strategies for combating inflammatory diseases and lymphoid malignancies.
Supplementary Material
Acknowledgments
We thank K. Yamamoto for the U2OS.G cell line and the κB3DLO reporter construct, I. Verma for the pCMX-p65 (RelA) expression vector, D. Green for the CCRF-CEM leukemia cell line, J. Reeds for the 697 pre-B leukemia cell line, and are grateful to Yao Wu and members of the Yang Laboratory for inspiring discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants DK066971 and DK089098, American Diabetes Association, and Ellison Medical Foundation (to X. Y.), National Institutes of Health Grants DK062434, CA014195-38, and HL105278, the Ellison Medical Foundation, and the Helmsley Charitable Trust (to R. M. E.), and a fellowship from the China Scholarship Council-Yale World Scholars in the Biomedical Sciences (to M. L.).

This article contains supplemental Figs. S1–S5.
- GR
- glucocorticoid receptor
- O-GlcNAc
- O-linked N-acetylglucosamine
- OGT
- O-GlcNAc transferase
- pol II
- RNA polymerase II
- CTD
- C-terminal domain
- TIF2
- transcriptional mediators/intermediary factor 2
- NF-κB
- nuclear factor κ-light-chain-enhancer of activated B cells
- AP-1
- activator protein 1.
REFERENCES
- 1. Glass C. K., Saijo K. (2010) Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376 [DOI] [PubMed] [Google Scholar]
- 2. Inaba H., Pui C. H. (2010) Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 11, 1096–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Bosscher K., Vanden Berghe W., Vermeulen L., Plaisance S., Boone E., Haegeman G. (2000) Glucocorticoids repress NF-κB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc. Natl. Acad. Sci. U.S.A. 97, 3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Bosscher K., Vanden Berghe W., Haegeman G. (2001) Glucocorticoid repression of AP-1 is not mediated by competition for nuclear coactivators. Mol. Endocrinol. 15, 219–227 [DOI] [PubMed] [Google Scholar]
- 5. Rogatsky I., Zarember K. A., Yamamoto K. R. (2001) Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J. 20, 6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nissen R. M., Yamamoto K. R. (2000) The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II C-terminal domain. Genes Dev. 14, 2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luecke H. F., Yamamoto K. R. (2005) The glucocorticoid receptor blocks P-TEFb recruitment by NFκB to effect promoter-specific transcriptional repression. Genes Dev. 19, 1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zachara N. E., Hart G. W. (2006) Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta 1761, 599–617 [DOI] [PubMed] [Google Scholar]
- 9. Hart G. W., Slawson C., Ramirez-Correa G., Lagerlof O. (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zachara N. E., Hart G. W. (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim. Biophys. Acta 1673, 13–28 [DOI] [PubMed] [Google Scholar]
- 11. Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 12. Jackson S. P., Tjian R. (1988) O-Glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell 55, 125–133 [DOI] [PubMed] [Google Scholar]
- 13. Love D. C., Ghosh S., Mondoux M. A., Fukushige T., Wang P., Wilson M. A., Iser W. B., Wolkow C. A., Krause M. W., Hanover J. A. (2010) Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 7413–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinclair D. A., Syrzycka M., Macauley M. S., Rastgardani T., Komljenovic I., Vocadlo D. J., Brock H. W., Honda B. M. (2009) Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc. Natl. Acad. Sci. U.S.A. 106, 13427–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gambetta M. C., Oktaba K., Müller J. (2009) Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 325, 93–96 [DOI] [PubMed] [Google Scholar]
- 16. Myers S. A., Panning B., Burlingame A. L. (2011) Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 108, 9490–9495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X., Su K., Roos M. D., Chang Q., Paterson A. J., Kudlow J. E. (2001) O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc. Natl. Acad. Sci. U.S.A. 98, 6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X., Zhang F., Kudlow J. E. (2002) Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110, 69–80 [DOI] [PubMed] [Google Scholar]
- 19. Kelly W. G., Dahmus M. E., Hart G. W. (1993) RNA polymerase II is a glycoprotein. Modification of the C-terminal domain by O-GlcNAc. J. Biol. Chem. 268, 10416–10424 [PubMed] [Google Scholar]
- 20. Su K., Yang X., Roos M. D., Paterson A. J., Kudlow J. E. (2000) Human Sug1/p45 is involved in the proteasome-dependent degradation of Sp1. Biochem. J. 348, 281–289 [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. (1991) Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol. Cell Biol. 11, 6016–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. (1991) Retinoic acid is a negative regulator of AP-1-responsive genes. Proc. Natl. Acad. Sci. U.S.A. 88, 6092–6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delerive P., De Bosscher K., Besnard S., Vanden Berghe W., Peters J. M., Gonzalez F. J., Fruchart J. C., Tedgui A., Haegeman G., Staels B. (1999) Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274, 32048–32054 [DOI] [PubMed] [Google Scholar]
- 24. Lazarus M. B., Nam Y., Jiang J., Sliz P., Walker S. (2011) Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469, 564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perissi V., Jepsen K., Glass C. K., Rosenfeld M. G. (2010) Deconstructing repression: evolving models of co-repressor action. Nat. Rev. Genet. 11, 109–123 [DOI] [PubMed] [Google Scholar]
- 26. De Bosscher K., Vanden Berghe W., Haegeman G. (2003) The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 24, 488–522 [DOI] [PubMed] [Google Scholar]
- 27. Heck S., Bender K., Kullmann M., Göttlicher M., Herrlich P., Cato A. C. (1997) I κBα-independent down-regulation of NF-κB activity by glucocorticoid receptor. EMBO J. 16, 4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He G., Karin M. (2011) NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 21, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartmann B. L., Geley S., Kofler R. (1999) Sequence-specific transcription factors during glucocorticoid-induced apoptosis in acute lymphoblastic leukemia cells. Wien. Klin. Wochenschr. 111, 360–367 [PubMed] [Google Scholar]
- 30. Norman M. R., Thompson E. B. (1977) Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 37, 3785–3791 [PubMed] [Google Scholar]
- 31. Harmon J. M., Norman M. R., Fowlkes B. J., Thompson E. B. (1979) Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J. Cell Physiol. 98, 267–278 [DOI] [PubMed] [Google Scholar]
- 32. Sionov R. V., Spokoini R., Kfir-Erenfeld S., Cohen O., Yefenof E. (2008) Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv. Cancer Res. 101, 127–248 [DOI] [PubMed] [Google Scholar]
- 33. Herr I., Büchler M. W., Mattern J. (2009) Glucocorticoid-mediated apoptosis resistance of solid tumors. Results Probl. Cell Differ. 49, 191–218 [DOI] [PubMed] [Google Scholar]
- 34. Comer F. I., Hart G. W. (2001) Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40, 7845–7852 [DOI] [PubMed] [Google Scholar]
- 35. Chinenov Y., Sacta M. A., Cruz A. R., Rogatsky I. (2008) GRIP1-associated SET-domain methyltransferase in glucocorticoid receptor target gene expression. Proc. Natl. Acad. Sci. U.S.A. 105, 20185–20190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujiki R., Chikanishi T., Hashiba W., Ito H., Takada I., Roeder R. G., Kitagawa H., Kato S. (2009) GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature 459, 455–459 [DOI] [PubMed] [Google Scholar]
- 37. Pascual G., Glass C. K. (2006) Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol. Metab. 17, 321–327 [DOI] [PubMed] [Google Scholar]
- 38. Donath M. Y., Shoelson S. E. (2011) Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 [DOI] [PubMed] [Google Scholar]
- 39. Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith C. L., O'Malley B. W. (2004) Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71 [DOI] [PubMed] [Google Scholar]
- 41. Beck I. M., Vanden Berghe W., Vermeulen L., Yamamoto K. R., Haegeman G., De Bosscher K. (2009) Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr. Rev. 30, 830–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bougarne N., Paumelle R., Haegeman G., Staels B., De Bosscher K. (2009) Circumventing glucocorticoid-mediated hyperinsulinemia via the activation of PPARα. Cell Cycle 8, 2311–2312 [DOI] [PubMed] [Google Scholar]
- 43. Bougarne N., Paumelle R., Caron S., Hennuyer N., Mansouri R., Gervois P., Staels B., Haegeman G., De Bosscher K. (2009) PPARα blocks glucocorticoid receptor α-mediated transactivation but cooperates with the activated glucocorticoid receptor α for transrepression on NF-κB. Proc. Natl. Acad. Sci. U.S.A. 106, 7397–7402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.