Background: Transport of phosphatidylserine (PS) analogs by the Drs2p flippase is regulated by PtdIns(4)P.
Results: PS stimulates dephosphorylation of the Drs2p·Cdc50p complex only in the presence of PtdIns(4)P.
Conclusion: The step at which PtdIns(4)P regulates lipid transport is identified.
Significance: Our coordinated overexpression system provides mechanistic insight into PS transport and will be useful for further Drs2p characterization and crystallization.
Keywords: Enzyme Mechanisms, Lipid Transport, Membrane Proteins, Membrane Transport, Phosphorylation, Cdc50 Proteins, P-type ATPases, Dephosphorylation, Flippases, Phosphoinositides
Abstract
Here, Drs2p, a yeast lipid translocase that belongs to the family of P4-type ATPases, was overexpressed in the yeast Saccharomyces cerevisiae together with Cdc50p, its glycosylated partner, as a result of the design of a novel co-expression vector. The resulting high yield allowed us, using crude membranes or detergent-solubilized membranes, to measure the formation from [γ-32P]ATP of a 32P-labeled transient phosphoenzyme at the catalytic site of Drs2p. Formation of this phosphoenzyme could be detected only if Cdc50p was co-expressed with Drs2p but was not dependent on full glycosylation of Cdc50p. It was inhibited by orthovanadate and fluoride compounds. In crude membranes, the phosphoenzyme formed at steady state at 4 °C displayed ADP-insensitive but temperature-sensitive decay. Solubilizing concentrations of dodecyl maltoside left this decay rate almost unaltered, whereas several other detergents accelerated it. Unexpectedly, the dephosphorylation rate for the solubilized Drs2p·Cdc50p complex was inhibited by the addition of phosphatidylserine. Phosphatidylserine exerted its anticipated accelerating effect on the dephosphorylation of Drs2p·Cdc50p complex only in the additional presence of phosphatidylinositol-4-phosphate. These results explain why phosphatidylinositol-4-phosphate tightly controls Drs2p-catalyzed lipid transport and establish the functional relevance of the Drs2p·Cdc50p complex overexpressed here.
Introduction
A hallmark of eukaryotic cells is the fact that plasma membranes as well as membranes of the late secretory pathway, including the trans-Golgi network (TGN),2 display asymmetric lipid distribution with the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine essentially confined in the cytosolic leaflets (1, 2). Maintenance of this asymmetry is critical for many physiological processes as PS externalization is associated, for example, with blood coagulation, macrophage-dependent clearance of apoptotic cells, and virus entry (3–5). The transbilayer distribution of aminophospholipids is largely determined by the presence of ATP-driven lipid translocases or “flippases” (6–8), and converging lines of evidence indicate that members of the P4 subfamily of P-type ATPases (9), hereafter referred to as P4-ATPases, constitute such flippases. There are five P4-ATPases in the yeast Saccharomyces cerevisiae among which Drs2p and Dnf3p are located in the TGN (10, 11) and Dnf1p and Dnf2p are located at the plasma membrane (12). Deletion of Dnf1p and Dnf2p inhibits ATP-dependent NBD-PC, -PS, and -phosphatidylethanolamine transport (12), whereas removal of Drs2p abolishes NBD-PS and -phosphatidylethanolamine transport (13, 14). The yeast P4-ATPase Drs2p, like the mammalian P4-ATPase ATP8A2, was recently purified and reconstituted into proteoliposomes, demonstrating that these P4-ATPases are directly involved in the translocation of PS analogs (15–17).
It is also clear now that the association of P4-ATPases with members of the Cdc50 protein family is of primary importance. Indeed, Cdc50 proteins have been shown to be required for stability and export of P4-ATPases from the endoplasmic reticulum in yeast and in mammalian cells (14, 18–21) as well as for proper functioning of the lipid-pumping complex (20, 22–24). Nevertheless, the molecular mechanism responsible for lipid translocation by P4-ATPase·Cdc50 protein complexes remains largely unknown.
Recent studies in yeast have shown that availability of a phosphoinositide, phosphatidylinositol-4-phosphate (PtdIns(4)P), is required for the Drs2p-dependent flip of a fluorescent analog of PS from the exoplasmic to the cytosolic leaflet of the TGN and that a cluster of basic amino acids in the C-terminal region of Drs2p forms a potential binding site for this phosphoinositide (25). In TGN membranes, PtdIns(4)P serves as a platform for the recruitment of effector proteins such as clathrin adaptor or lipid transfer proteins involved in membrane trafficking (for reviews, see Refs. 26 and 27). In addition, Drs2p has been shown to physically interact with the PtdIns(4)P phosphatase Sac1p, a negative regulator of secretory vesicle formation at the TGN (28). Collectively, these data highlight an important regulatory role for PtdIns(4)P in the control of Drs2p activity, but much remains to be elucidated on how this control is exerted.
For deeper characterization of the enzymatic basis for the lipid translocation machinery and because the preparation of sufficient amounts of high quality samples remains a major bottleneck in membrane protein functional and structural characterization, we devised a high yield co-expression system for the yeast P4-ATPase Drs2p and its associated subunit Cdc50p. This allowed us to measure the formation from [γ-32P]ATP of a transient phosphorylated intermediate during the catalytic cycle of Drs2p without the need for purification of this protein complex. Here we describe inhibitors of the formation of this 32P-labeled phosphoenzyme and characterize its time-dependent decay in crude yeast membranes or in the presence of detergents. Specifically, we reveal the dependence of the dephosphorylation kinetics on the presence of PtdIns(4)P: stimulation of Drs2p dephosphorylation by PS can be achieved only if PtdIns(4)P is present together with PS. Stimulation by PtdIns(4)P of the lipid flippase activity of Drs2p is therefore most probably exerted through acceleration of the fairly slow dephosphorylation step.
EXPERIMENTAL PROCEDURES
Materials
Products for culture of yeast and bacteria were from Difco (BD Biosciences). Restriction and modification enzymes as well as peptide-N-glycosidase F were purchased from New England Biolabs. Phusion® high fidelity polymerase was from Finnzymes. The Clone JETTM PCR cloning kit and the histidine probe (India HisprobeTM-HRP) were from Thermo Fischer Scientific. DDM was from Anatrace, C12E8 was from Nikkol Chemical, Triton X-100 was from Pierce, CHAPS was from Serva, octyl glucoside was from Calbiochem, Tween 20 was from Bio-Rad, digitonin was from Sigma, and Hecameg was a gift from H. Wroblewski and D. Plusquellec (CNRS, Rennes, France). POPC, DOPC, POPS, DMPS, lysophosphatidylcholine, egg lecithin, diC7PC, and PtdIns(4)P were from Avanti Polar Lipids. [γ-32P]ATP was from PerkinElmer Life Sciences (catalog number BLU002A). Chemicals were generally from Sigma (including desferrioxamine B and the avidin-peroxidase probe). Glass fiber A/E filters (1-μm porosity) were from Pall Corp. The EDTA-free protease inhibitor mixture was from Roche Diagnostics. BSA (albumin fraction V) was from Roth Sochiel. Precision Plus Protein Standards were from Bio-Rad. Rabbit polyclonal antibodies against Tlg2p were obtained as described (29). Mouse monoclonal anti-Dpm1p was from Molecular Probes. Rabbit sarcoplasmic reticulum membranes (30) and yeast-expressed SERCA1a-Bad (31) were prepared as described previously.
Yeast Strains
The S. cerevisiae W303.1b/GAL4 (a, leu2-3, his3-11, trp1-1::TRP1-GAL10-GAL4, ura3-1, ade2-1, canr, cir+) yeast strain was the same as described previously (32). The Δdrs2 and Δcdc50 deletion mutants were created in a W303.1b/GAL4 background using a loxP-HIS3-loxP cassette as described (12). Plasmid pYeDP60 was generously given by Denis Pompon (Laboratoire d'Ingénierie des Systèmes Biologiques et des Procédés, Toulouse, France).
Strategy for Coordinated Overexpression of Drs2p and Cdc50p in Yeast and Functionality of Constructs
The DRS2 open reading frame was supplemented either at its 3′- or 5′-end with a sequence coding for a biotin acceptor domain (Bad) and a sequence coding for a protease cleavage site (thrombin or TEV). The cleavage site was flanked by 2 glycines toward Bad and 4 glycines toward DRS2. Similarly, a sequence coding for a decahistidine tag was added at the 3′-end of CDC50. The fused genes were cloned independently in pYeDP60 expression plasmids (32–34), resulting in the generation of pYeDP60_DRS2-Bad and pYeDP60_CDC50-His10 vectors. In these vectors, DRS2 and CDC50 are both placed under the control of a strong galactose-inducible GAL10/CYC1 promoter. Starting from the pYeDP60_CDC50-His10 vector, a cassette containing this promoter, the CDC50 coding sequence, and the PGK terminator was then amplified by PCR and subsequently inserted in the pYeDP60_DRS2-Bad companion vector, resulting in the plasmid illustrated in Fig. 1. The complementation properties, growth curves, glycosylation properties, and phosphorylation abilities were essentially similar for the constructs bearing either a thrombin or a TEV cleavage site. Unless otherwise noted, the results illustrated in the various figures below were obtained with the thrombin constructs.
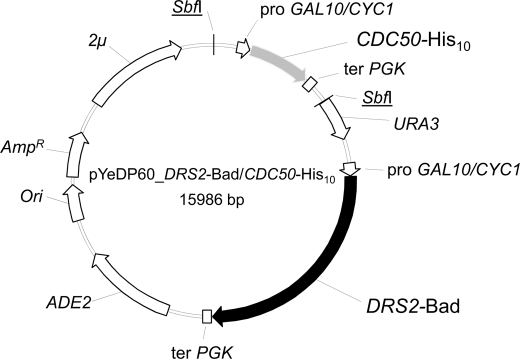
FIGURE 1.
Map of plasmid used for co-expression of Drs2p-Bad and Cdc50p-His10. The co-expression plasmid, derived from pYeDP60, contained sequences coding for CDC50 fused to a C-terminal His10 tag (gray arrow) and for DRS2 fused to a C-terminal Bad tag (black arrow) both under the control of an inducible GAL10/CYC1 hybrid promoter. A short sequence coding for a TEV or a thrombin cleavage site was also inserted between DRS2 and CDC50 open reading frames and their respective tags. ADE2, auxotrophy selection marker for adenine; Ori, bacterial replication origin; AmpR, gene conferring resistance to ampicillin; 2μ, yeast replication origin; URA3, auxotrophy selection marker for uracil; SbfI, restriction sites.
Expression of Drs2p-Bad and Cdc50p-His10 in Fernbach Flasks and Yeast Membrane Preparation
Yeast growth and induction of protein expression were performed essentially as described previously (31, 32). Yeast growth took place in a glucose-containing rich growth medium at 28 °C for 36 h, whereas expression of the proteins of interest took place during an additional 18 h in the presence of galactose at 18 °C. Membrane fractionation by differential centrifugation was performed essentially as described previously (31, 32). One modification consisted in using a “Pulverisette 6” planetary mill (Fritsch) for breaking yeast cells with glass beads before fractionation. The crude extract was then submitted to differential centrifugation, resulting in “P2” and “P3” membrane pellets recovered after intermediate and high speed centrifugation, respectively.
Protein Estimation and Immunodetection
Protein concentrations were measured using the bicinchoninic acid procedure (35) in the presence of 2% (w/v) SDS using bovine serum albumin as a standard. For electrophoretic separation and identification, proteins were loaded onto 8% Laemmli polyacrylamide gels and transferred to PVDF membranes using transfer buffer (30 mm Tris, 200 mm glycine, 10% (v/v) methanol). Detection of the biotinylated Drs2p-Bad was performed using a biotin probe (avidin conjugated to HRP), and detection of Cdc50p-His10 was performed using a His probe conjugated to HRP. When indicated, rabbit anti-Tlg2p was used as a Golgi marker, and mouse anti-Dpm1p was used as an endoplasmic reticulum marker (with goat anti-rabbit horseradish peroxidase- and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies, respectively). Blots were revealed by chemiluminescence with the ECL kit (GE Healthcare).
Solubilization of P3 Membranes with Detergent
D+ P3 membranes (i.e. P3 membranes obtained after co-expression of wild-type Drs2p-Bad and Cdc50p-His10) were diluted to 2 mg of total protein/ml in ice-cold solubilization buffer (SB) (10 mm Tris-HCl, pH 7.5, 300 mm NaCl, 20% (w/v) glycerol, 5 mm MgCl2) supplemented with 1 mm PMSF and an EDTA-free protease inhibitor mixture. Detergent was then added to membranes at the desired detergent-to-protein ratio, and the suspension was stirred gently on a wheel for 1 h at 4 °C. Insoluble material was pelleted by centrifugation at 100,000 × g for 1 h at 4 °C. The supernatant was withdrawn, and the pellet was resuspended in the same volume of SB as that of the total membrane fraction. Solubilization of Drs2p-Bad and Cdc50p-His10 was analyzed by Western blotting.
Glycosidase Assay
D+ P3 membranes were diluted to 2 mg/ml in SB supplemented with 1 mg/ml DDM, 1 mm PMSF, and an EDTA-free protease inhibitor mixture. Samples were incubated for 15 min on ice in the absence or presence of 10 μg/ml peptide-N-glycosidase F (i.e. 20,000 units/ml) before immunodetection and phosphoenzyme formation from [γ-32P]ATP.
Phosphorylation from [γ-32P]ATP
Formation (on ice) of the transient phosphoenzyme intermediate of the Drs2p catalytic cycle was measured after incubation with [γ-32P]ATP followed by acid quenching and filtration (see e.g. Ref. 36). For most experiments, 200-μl samples were preincubated on ice at 0.5 mg of total protein/ml in buffer A (100 mm KCl, 5 mm Mg2+, 50 mm Mops-Tris at pH 7) generally supplemented with 100 μm Ca2+, various amounts of EGTA, and detergent in the presence or absence of exogenous lipid. In some cases, 1 mm orthovanadate was added. Phosphorylation was triggered by addition of [γ-32P]ATP (at 0.25–1 mCi/μmol and generally at a final concentration of 0.5 μm) followed by acid quenching (typically 2 ml of 500 mm TCA + 30 mm H3PO4). After quenching, samples were left on ice for 15–20 min, a period sufficient for aggregation of the precipitated protein and therefore its retention by the filter (this aggregation period was critical in the presence of detergent). This was followed by filtration on an A/E glass fiber filter (or on two filters on top of each other) and careful rinsing with diluted acid (50 mm TCA + 3 mm H3PO4). Note that for yet unknown reasons mere preincubation of crude membranes in phosphorylation buffer before addition of [γ-32P]ATP allowed their vanadate-dependent phosphorylation level to slowly increase with time (see Fig. 5D, dotted line).
FIGURE 5.
Orthovanadate or fluoride compound inhibition of phosphorylation (from [γ-32P]ATP) of Drs2p-Bad in P3 membranes. D+ P3 membranes were incubated at 0.5 mg of protein/ml in buffer A supplemented with 0.1 mm CaCl2, and phosphorylation was subsequently measured 25 s after addition of 0.5 μm [γ-32P]ATP (on ice). A and B, incubation lasted 1 h and was performed either at 24 °C (open symbols) or on ice (gray symbols) followed in both cases by an additional 20–40 min on ice. A, incubation was performed in the presence of various concentrations of orthovanadate. B, incubation was performed in the absence or presence of KF (1 mm when present) and in the additional presence of either 50 μm BeCl2 (squares) or 50 μm AlCl3 (triangles) or in the presence of 5 mm MgCl2 only (diamonds). C, time dependence on ice of the inhibition by 100 μm VO4 (gray circles) or by 1 mm KF in the absence (diamonds) or presence of either 50 μm BeCl2 (squares) or 50 μm AlCl3 (triangles). Open circles, control incubation in the absence of any added compound. D, D+ P3 membranes were diluted on ice to 0.5 mg/ml at time 0 (open circles, dotted line), 50 μm AlCl3 and 1 mm KF were added at t = 20 min, and the residual phosphorylation level was assayed after various periods. To trigger recovery from inhibition, free aluminum was subsequently chelated at t = 95 min by adding either hydroxyl ions (Tris base) to bring the pH up to 8 (squares), 10 mm EGTA (upside down triangles), or 0.2 mm desferrioxamine (diamonds). For a control, Tris, EGTA, and desferrioxamine were also added to the membranes together with AlCl3 and KF at t = 20 min (open squares, triangles, or diamonds).
Turnover-dependent Dephosphorylation of Drs2p·Cdc50p
The kinetics of turnover-dependent dephosphorylation were measured by first phosphorylating the sample for 25 s on ice under the above conditions and then chasing 32P from the phosphoenzyme by adding excess non-radioactive nucleotide (usually 1 mm Mg2+-ATP) and simultaneously changing the temperature to 37 °C (if desired). Two slightly different protocols were used for that purpose.
In the experiments illustrated in Fig. 8, we used a protocol “with dilution” in which the phosphorylated sample (200 μl) was diluted 5-fold into a dephosphorylation medium (800 μl of buffer A supplemented with Ca2+ and EGTA) devoid of radioactive ATP but containing non-radioactive nucleotide and pre-equilibrated at 37 °C so that the temperature immediately after mixing was already close to the final temperature (at least 30 °C because of the 5-fold dilution of the initially ice-cold phosphorylation medium). Acid quenching was subsequently performed after various periods by adding concentrated acid (typically 1 ml of acid (1 m TCA + 60 mm H3PO4) was added to the 1-ml sample undergoing dephosphorylation).
FIGURE 8.
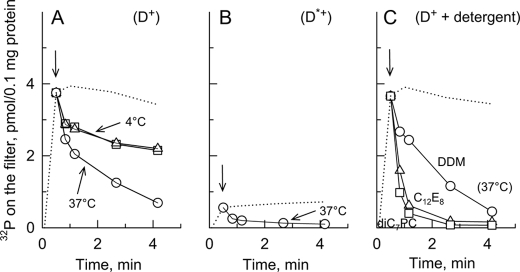
Turnover-dependent dephosphorylation of Drs2p-Bad either in membranes or in presence of various detergents. A, D+ P3 membranes were suspended at 0.5 mg of protein/ml in buffer A supplemented with 100 μm Ca2+ on ice. Phosphorylation was triggered by adding 0.5 μm [γ-32P]ATP and quenched after various periods (dotted line). After 25 s (arrow), aliquots were withdrawn and diluted 5-fold for dephosphorylation under three different conditions: first on ice in the presence of 1 mm non-radioactive Mg2+-ATP (squares; in this case, the Mg2+-ATP-containing dephosphorylation medium had previously been supplemented with 0.01 mg/ml pyruvate kinase and 0.2 mm phosphoenolpyruvate); second, on ice but in the presence of 1 mm ADP instead of Mg2+-ATP and without pyruvate kinase or phosphoenolpyruvate (triangles); and third, in the presence of 1 mm Mg2+-ATP but at 37 °C (circles). B, dephosphorylation in the presence of Mg2+-ATP at 37 °C (as in A) using D*+ P3 membranes. C, before triggering phosphorylation of D+ P3 membranes, the membrane suspension had been supplemented with 1 mg of DDM/ml, and dephosphorylation was measured after 5-fold dilution into a Mg2+-ATP-containing dephosphorylation medium at 37 °C that also contained a 1 mg/ml concentration of either DDM (circles), C12E8 (triangles), or diC7PC (squares). In all cases, the dotted line represents the time course for phosphorylation itself on ice (the actual data points were removed for clarity except for the data point at 25 s). This figure shows one representative experiment of several independent experiments with similar results.
In the experiments displayed in Fig. 9, we used an ATP chase protocol “without dilution.” The sample was first phosphorylated on ice and then transferred into a tube pre-equilibrated at 37 °C and containing a droplet of concentrated non-radioactive Mg2+-ATP for dephosphorylation during the desired time period. Buffer A had been supplemented with 0.1 mm Ca2+ and 1 mm EGTA to keep free Ca2+ close to “physiological” conditions. We checked that free Ca2+ had no effect on the dephosphorylation rate (data not shown).
FIGURE 9.
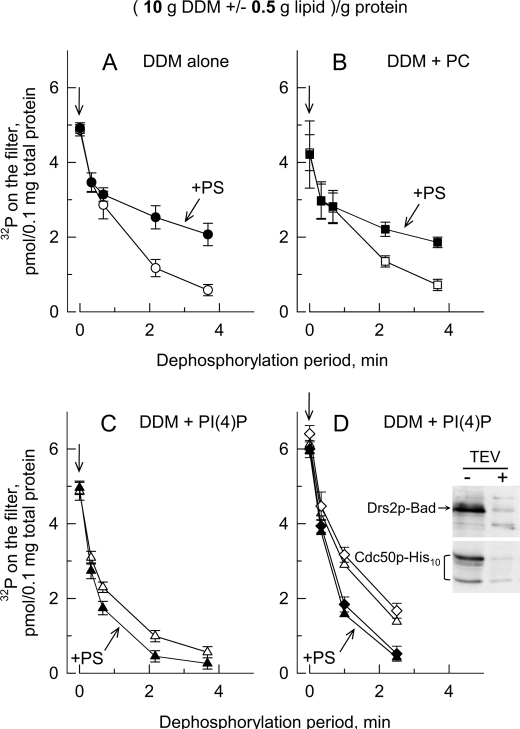
Presence of PtdIns(4)P is required to allow PS to stimulate turnover-dependent dephosphorylation of Drs2p in excess DDM. D+ P3 samples containing 0.5 mg of protein/ml were first solubilized with 5 mg of DDM/ml supplemented in some cases with various exogenous lipids (0.25 mg of each lipid/ml), resulting in detergent and exogenous lipid-to-total protein ratios of 10 g of DDM/g of protein and 0.5 g of each exogenous lipid/g of protein. After 25 s of phosphorylation from [γ-32P]ATP in buffer A supplemented with 0.1 mm Ca2+ and 1 mm EGTA, samples were either quenched with acid immediately or transferred into a tube pre-equilibrated at 37 °C and containing concentrated non-radioactive Mg2+-ATP (so that the final concentration of ATP was 1 mm) for dephosphorylation for various periods before acid quenching. A, open circles, DDM alone; closed circles, DDM + POPS. B, open squares, DDM + POPC; closed squares, DDM + POPC + POPS; C, open triangles, DDM + PtdIns(4)P (PI(4)P); closed triangles, DDM + PtdIns(4)P + POPS. D, dephosphorylation rates in the presence of PtdIns(4)P (open symbols) or PtdIns(4)P + POPS (closed symbols) were measured before (triangles) or after (diamonds) proteolytic excision of the Bad and His10 tags from constructs bearing a TEV cleavage site. Cleavage of the tag was performed by overnight incubation of membranes at 4 °C in the presence of TEV protease. Aliquots were analyzed by Western blotting for the residual tag contents using the biotin probe and the His probe (inset). Data from A and B are presented as the mean ±S.D. (error bars) of four independent experiments. Data from C are presented as means ± S.D. (error bars, n = 5 of three independent experiments). Data from D are presented as the mean ±S.D. (error bars) of duplicates.
Removal of Bad and His10 Tags
Removal of the tags from Drs2p-Bad and Cdc50p-His10 was performed by incubating yeast membranes at 2 mg of protein/ml in buffer A supplemented with 100 μm Ca2+ for 17 h at 8 °C (with mild stirring on a wheel) in the presence of a sufficient amount of TEV protease. The TEV protease was expressed in Escherichia coli and affinity-purified as described (37).
RESULTS
Large Scale Co(over)-expression of Drs2p-Bad and Cdc50p-His10, Cdc50p Glycosylation, and Vanadate-dependent Phosphorylation of Drs2p-Bad
We devised a pYeDP60_DRS2-Bad/CDC50-His10 plasmid (Fig. 1) appropriate for co-expression with high yield of Drs2p-Bad and Cdc50p-His10. Our various constructs proved functional as deduced (data not shown) from their ability to reverse the cold-sensitive growth phenotype of Δdrs2 and Δcdc50 yeast cells (38). After yeast growth, cells were broken, and “light” (P3) and “heavy” (P2) membrane fractions were prepared by differential centrifugation (31, 32).
The top gel of Fig. 2A compares the Drs2p-Bad contents of the P3 and P2 fractions obtained for wild-type Drs2p co-expressed with Cdc50p (hereafter referred to as “D+”) and shows for comparison the results obtained for an inactive Drs2p mutant co-expressed with Cdc50p as well (hereafter referred to as “D*+”). In D+ P3 membranes, comparison with yeast membranes expressing a known amount of SERCA1a-Bad (31) allowed us to estimate that the recombinant Drs2p protein accounted for about 3% of the total protein present in that fraction; this corresponds to about 10 mg of Drs2p/liter of culture (data not shown).
FIGURE 2.
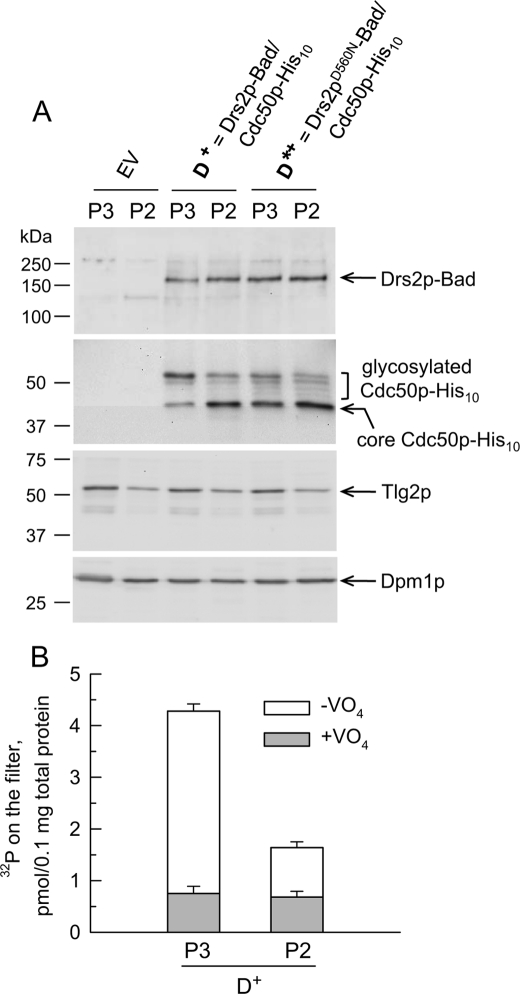
Characterization of P2 and P3 membranes from yeast co-expressing Drs2p-Bad and Cdc50p-His10. A, P3 and P2 membranes were obtained from yeast co-expressing Cdc50p-His10 and either wild-type Drs2p (D+) or the Drs2pD560N mutant (D*+), or from yeast transformed with an empty vector (EV). For detection of Drs2p-Bad and Cdc50p-His10 (using a biotin probe and a His probe, respectively), 0.4 μg of total P3 or P2 proteins was loaded for SDS-PAGE. For detection of Tlg2p (with α-Tlg2p polyclonal antibody), 5 μg of total P3 or P2 proteins was loaded for SDS-PAGE. For detection of Dpm1p (using an anti-Dpm1p monoclonal antibody), 20 μg of total P3 or P2 proteins was loaded. B, D+ P3 and P2 membrane fractions were tested for the ability of Drs2p to become phosphorylated from ATP (here at 0.5 μm [γ-32P]ATP) in the absence or presence of 1 mm orthovanadate (open bars or gray bars, respectively). Data are presented as the mean ±S.D. (error bars) of duplicates.
Detection of Cdc50p-His10 (Fig. 2A) revealed several bands: the slowest band ran just above the 50-kDa marker, and the fastest band ran between 37 and 50 kDa. In view of the fact that the expected molecular mass for Cdc50p-His10 is about 43 kDa and that Cdc50p contains four consensus sequences for N-linked glycosylation (Asn-Xaa-(Ser/Thr)) in its large lumenal loop, a likely hypothesis is that the slowest band corresponds to glycosylated Cdc50p-His10, whereas the fastest band corresponds to a less mature, unglycosylated form (with bands of intermediate mobility corresponding to intermediate levels of glycosylation). This hypothesis was supported by deglycosylation experiments using peptide-N-glycosidase F (see Fig. 3B below).
FIGURE 3.
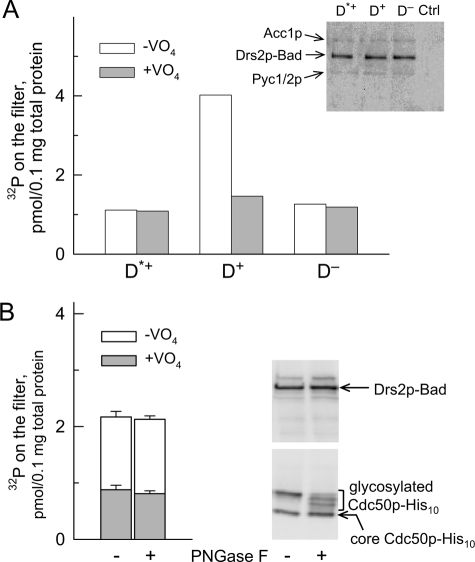
Co-expression of Cdc50p, but not its full glycosylation, is required for phosphorylation (from [γ-32P]ATP) of Drs2p-Bad expressed in P3 membranes. A, P3 membranes containing either Drs2p mutated at the catalytic site and co-expressed with Cdc50p (Drs2pD560N, D*+), wild-type Drs2p co-expressed with Cdc50p (D+), or wild-type Drs2p expressed alone (i.e. in the presence of endogenous Cdc50p only (D−)) were used. Samples (200 μl) were incubated on ice at 0.5 mg of total protein/ml in buffer A supplemented with 100 μm Ca2+ in the absence or presence of 1 mm orthovanadate. At time 0, phosphorylation was triggered by addition of a final concentration of 2 μm [γ-32P]ATP; after 25–30 s, this was followed by acid quenching and filtration. This graph shows one representative experiment of several independent experiments with similar results. Inset, after loading 0.25 μg of total protein for SDS-PAGE, Drs2p-Bad was detected using a biotin probe. In the range of molecular masses illustrated, the probe also weakly detected the biotinylated yeast proteins Acc1p (∼250 kDa) and Pyc1/2p (∼130 kDa). Ctrl, yeast membranes that were not transformed with DRS2- or CDC50-containing plasmid. B, D+ P3 membranes suspended at 2 mg/ml in SB containing 1 mg/ml DDM were incubated on ice for 15 min in the absence or presence of 10 μg/ml peptide-N-glycosidase F (PNGase F) (i.e. 20,000 units/ml). Membranes were subsequently diluted to 0.5 mg/ml in buffer A supplemented with 0.1 mm Ca2+ and 1 mm EGTA, and the ability of these samples to become phosphorylated was measured after addition of 0.5 μm [γ-32P]ATP in the absence (open bars) or presence (gray bars) of 1 mm orthovanadate. Data are presented as the mean ±S.D. (error bars) of duplicates. 1.5 μg of total protein was loaded for Western blot analysis. Detection of Drs2p was performed using a biotin probe, and detection of Cdc50p was performed using a His probe (gels on the right).
The pattern of Cdc50p-His10 glycosylation exhibited significant differences in P2 versus P3 membranes: in P3 membranes, but not in P2 membranes, the mature form of Cdc50p-His10 was predominant (see Fig. 2A, two central lanes). This suggests that Cdc50p in the P3 fraction has reached compartments where more complete maturation has occurred. In line with this observation, we found that the TGN marker Tlg2p (29) was more abundant in the P3 fraction than in the P2 fraction (Fig. 2A). Conversely, the endoplasmic reticulum marker Dpm1p was found distributed fairly equally between P3 and P2 membranes (Fig. 2A). Note that the glycosylation degree of Cdc50p in the D*+ sample (where the D560N mutant of Drs2p is expressed in combination with Cdc50p) was significantly reduced compared with that in the D+ sample even in P3 membranes.
Those members of the P-type ATPase family that have been studied extensively are known to transiently form a phosphorylated intermediate during their catalytic cycle. Therefore, our aim was to reveal the formation of such a phosphorylated intermediate by incubating P3 or P2 membranes with [γ-32P]ATP followed by acid quenching and filtration. In a previous work, vanadate-sensitive phosphorylation of overexpressed Drs2p had been detected only after membrane solubilization and Drs2p purification (23). Here, vanadate-sensitive phosphorylation was already observed in crude yeast membrane fractions. As displayed in Fig. 2B, phosphorylation of Drs2p in P2 membranes was 3–4-fold lower than that in P3 membranes (for the same amount of Drs2p or Cdc50p; Fig. 2A). This is consistent with the idea that proper trafficking is a prerequisite for recovery of a functional Drs2p·Cdc50p complex. We thus used P3 membranes for further functional characterization of Drs2p.
Cdc50p-dependent Phosphorylation from [γ-32P]ATP of Drs2p-Bad at Its Catalytic Aspartate and Effects of Cdc50p Deglycosylation
Because Cdc50 proteins have previously been shown to be required for phosphorylation of purified Drs2p or ATP8B1 (23, 39), we first checked whether this would still be the case in crude membranes obtained with our new overexpression system. As can be seen in Fig. 3A, vanadate-sensitive phosphorylation was indeed strictly dependent on the co-expression of Drs2p and Cdc50p (D+ sample as opposed to the D− sample where Drs2p was expressed in the presence of endogenous Cdc50p alone), whereas for the inactive D*+ mutant, vanadate-sensitive phosphorylation was insignificant. The virtual insensitivity to VO4 of D*+ and D− samples confirms that endogenous Drs2p and Cdc50p are present in negligible amounts compared with the overexpressed proteins.
To check whether the different phosphorylation levels measured in P3 or P2 membranes (see Fig. 2B) imply any direct role of the Cdc50p N-linked sugars on the Drs2p catalytic cycle, we subjected Cdc50p expressed in D+ P3 membranes to in vitro deglycosylation by peptide-N-glycosidase F. We found (Fig. 3B) that the ability of the initially correctly folded Drs2p to become phosphorylated from [γ-32P]ATP did not suffer from deglycosylation under incubation conditions (15 min at 4 °C) sufficient to allow the fully glycosylated Cdc50p to disappear.
We then looked for optimal conditions for measuring the steady-state phosphorylation level of D+ P3 membranes. The vanadate-dependent phosphoenzyme formed rapidly even at a low concentration of [γ-32P]ATP: it reached a maximal level after 20–30 s on ice in the presence of 0.5 μm [γ-32P]ATP (data not shown and Fig. 8, dotted lines), a low concentration that minimized vanadate-independent phosphorylation. We also found that the measured steady-state phosphorylation level for D+ P3 membranes was relatively insensitive to ionic conditions. Changes in pH (between 6 and 8.5; Fig. 4A) and in the KCl or NaCl concentration (between zero and 0.3 m; Fig. 4B) only had minor effects. Adding EGTA to Ca2+-containing medium ruled out any significant effect on the steady-state phosphorylation level of the free Ca2+ concentration (from 0.01 to 100 μm) or of the many (and possibly endogenous) metal species that can be chelated by EGTA. The phosphorylation level was also unaltered if the free Mg2+ concentration was reduced from 5 to 1 mm, but it decreased for much lower concentrations of free Mg2+ consistent with Mg2+-ATP being the substrate for phosphorylation as generally accepted for P-type ATPases (40–42). Moderate concentrations of Co2+, Zn2+, La3+, carbonyl cyanide m-chlorophenylhydrazone, sulfate, oxalate, or arsenate did not greatly affect the steady-state phosphorylation level (Fig. 4 and data not shown).
FIGURE 4.
Effect of various ionic conditions on phosphorylation (from [γ-32P]ATP) of Drs2p-Bad. A and B, the effect of pH and KCl was explored in the absence (open symbols) or presence (gray symbols) of 1 mm orthovanadate. C, the effect of manipulating the concentrations of a few metal ions was tested by adding either 0.6 or 5 mm EGTA (which reduced the free Ca2+ concentrations to 0.1 or 0.01 μm as indicated below the corresponding bars); by adding 4, 7.5, or 15 mm EDTA (which also lowered the free Ca2+ concentrations to various extents but in addition reduced the free Mg2+ concentrations); or by adding 50 or 500 μm Zn2+ or Co2+, or 50 μm La3+, or 10 μm H+ ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). Phosphorylation (on ice for 30 s) was triggered by addition of 0.5 μm [γ-32P]ATP followed by acid quenching. Data are presented as the mean ±SD (error bars) of duplicates.
Inhibition of Phosphorylation by Orthovanadate and Fluoride Compounds
We next studied in more detail the effect of putative inhibitors (Fig. 5). Micromolar concentrations of orthovanadate were sufficient to strongly inhibit Drs2p phosphorylation. The temperature of incubation did not affect the resulting inhibition (Fig. 5A), and the kinetics of inhibition by 0.1 mm VO4 were fairly rapid, being completed within 1 min on ice (Fig. 5C). It is well known that in the case of P2-type ATPases like SERCA1a VO4 binds rapidly to the Ca2+-free (“E2”) form of the pump only, not to its Ca2+-bound (“E1”) form (43). The fact that phosphorylation of Drs2p was inhibited by VO4 after only a short incubation suggests that in P3 membranes Drs2p “at rest” essentially resides in an E2-like conformation.
We also addressed the effect of fluoride compounds known to be reactive with E2-like states of other P-type ATPases (see e.g. Ref. 30). We found that Drs2p phosphorylation was severely inhibited by 1 mm fluoride combined with either 50 μm BeCl2 or 50 μm AlCl3 (Fig. 5B) and that inhibition was completed within 1 min in the presence of BeCl2 and within 5 min in the presence of AlCl3 (Fig. 5C). 1 mm fluoride alone only exerted very slow inhibition (Fig. 5C). Because of the presence of 5 mm Mg2+ in the medium, this slow inhibition could well correspond to inhibitory binding of a magnesium fluoride complex (the same order of reactivity for the various fluoride compounds was also found in the case of SERCA1a; see e.g. Ref. 30). In the absence of KF, BeCl2 or AlCl3 only had minor effects on the total phosphorylation level (Fig. 5B).
Inhibition of Drs2p phosphorylation by aluminum fluoride compounds was further characterized. At pH 7 in the presence of 1 mm KF, inhibition was virtually complete already in the presence of 5 μm total AlCl3, but the efficiency for inhibition strongly decreased under alkaline conditions (data not shown and Fig. 5D). The latter fact is consistent with the idea that, as for other P-type ATPases, the inhibitory complex could be AlF4− whose concentration strongly decreases under alkaline conditions due to increased formation of Al(OH)4− competitors (44). Similarly, at pH 7, inhibition by the aluminum fluoride complex could be minimized or prevented by including an aluminum chelator, either the classical Ca2+ chelator EGTA or the iron and aluminum chelator desferrioxamine B (Fig. 5D). If these chelators (or hydroxyl ions) were added after inhibition by aluminum fluoride had developed, Drs2p slowly recovered from its previous inhibition, showing that the inhibitory complex was not formed irreversibly (Fig. 5D).
Solubilization of D+ P3 Membranes by Detergents and Short or Long Term Effects of Detergents on Phosphorylation of Solubilized Drs2p·Cdc50p Complex
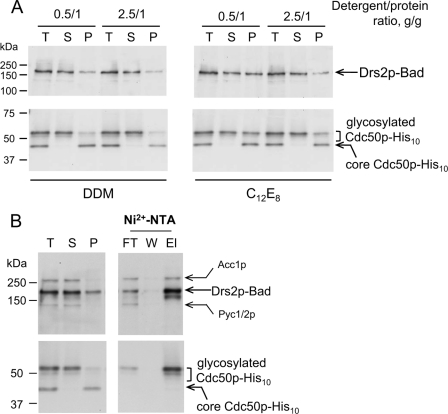
We then investigated the ability of various detergents to interact with Drs2p-Bad and Cdc50p-His10, solubilize these proteins, and possibly affect their function or stability. Fig. 6A shows that DDM allowed almost complete solubilization of the biotinylated Drs2p-Bad (compare total membrane (T), supernatant (S), and pellet (P) lanes) at DDM-to-protein ratios as low as 0.5 g/g. Solubilization of Drs2p-Bad with C12E8 proved possible too (Fig. 6A). In all cases, the unglycosylated portion of Cdc50p-His10 remained insoluble, whereas solubilization of the glycosylated, mature form displayed a similar dependence on detergent concentration as that of Drs2p-Bad (Fig. 6A). Triton X-100 or l-α-lysophosphatidylcholine as well as high critical micelle concentration detergents like CHAPS, Hecameg, or octyl glucoside also efficiently solubilized Drs2p-Bad and glycosylated Cdc50p-His10 (data not shown). When DDM-solubilized D+ P3 membranes (at 0.5 g/g DDM-to-protein ratio) were subjected to Ni2+-NTA affinity chromatography, a large fraction of both Cdc50p-His10 and Drs2p-Bad did bind to the resin (Fig. 6B, compare supernatant (S) and flow-through (FT) lanes), indicating that both proteins still interact with each other after solubilization with DDM. The two proteins could be co-eluted from the Ni2+-NTA beads using imidazole (elution buffer (El) lane).
FIGURE 6.
Detergent-induced solubilization of glycosylated Drs2p·Cdc50p complex. A, D+ P3 membranes were diluted to 2 mg of protein/ml in ice-cold SB supplemented with detergent (DDM or C12E8 with the indicated detergent-to-protein ratio) as well as with PMSF and a mixture of protease inhibitors and incubated as described under “Experimental Procedures” before high speed centrifugation. Pellet (P) fraction was resuspended in the same volume as that of the total membrane (T) fraction. 0.5 μg of the total membrane fraction was loaded for SDS-PAGE, and the same volumes of supernatant (S) and pellet fractions were loaded as well. Drs2p-Bad was detected using a biotin probe, and Cdc50p-His10 was detected using a His probe. B, D+ P3 membranes were solubilized with 0.5 g of DDM/g of protein (1 mg/ml DDM) and centrifuged as above. The supernatant was then added to Ni2+-NTA beads (400 μl of slurry for 10 ml of solubilized sample) and incubated overnight. Unbound proteins were collected in the flow-through (FT), the resin was washed with 5 ml of “wash” buffer (W) (SB supplemented with 0.2 mg/ml DDM), and proteins were eluted with 1.2 ml of “elution” buffer (El) (wash buffer supplemented with 250 mm imidazole). 0.8 μg of the total membrane fraction and the same volumes of supernatant, pellet, flow-through, wash buffer, and elution buffer fractions were analyzed by Western blotting.
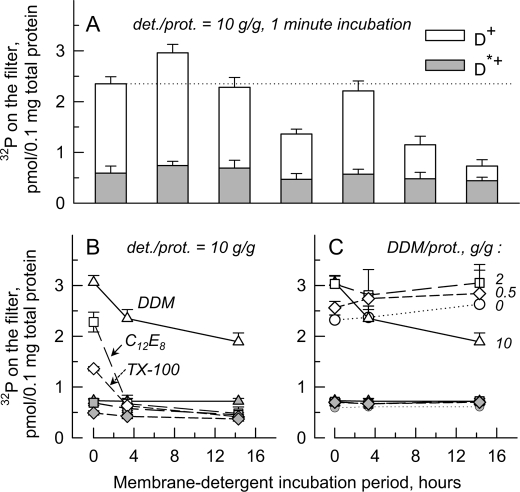
For testing the effect of various solubilizing detergents on Drs2p function, detergent at a relatively high detergent-to-protein ratio (10 g/g) was added to D+ or D*+ P3 membranes, and [γ-32P]ATP-dependent phosphorylation was measured. After short term incubation (1 min), the presence of various detergents in the phosphorylation assay resulted in various levels of phosphorylation for Drs2p (Fig. 7A). DDM led to phosphorylation levels slightly higher than in the absence of detergent. Some other detergents, including Triton X-100, digitonin (previously used in Ref. 23), or the short-chain lipid diC7PC (45), led to lower levels of phosphorylation. In similar experiments, Nonidet P-40, a close relative to Triton X-100, and n-dodecyl-N,N-dimethylamine-N-oxide were found to be rather poor (data not shown).
FIGURE 7.
Short and long term effects of detergents on phosphorylation (from [γ-32P]ATP) of Drs2p-Bad. P3 membranes, either D+ (open bars or open symbols) or D*+ (gray bars or gray symbols), were suspended at 2 mg/ml in SB supplemented with PMSF and a complete protease inhibitor mixture. A, samples were then incubated for 1 min in the presence of various detergents all at 20 mg/ml; the detergents used are indicated (Digit, digitonin; TX-100, Triton X-100). After such incubation, samples were diluted 4-fold into buffer A supplemented with 0.1 mm CaCl2, and phosphorylation was measured as described earlier using two superimposed A/E filters. Both filters were counted, and the sum of the radioactivities in the two filters was plotted. B, for selected detergents, similar measurements were repeated after incubation on ice for a few hours. The detergents tested were DDM (triangles), C12E8 (squares), and Triton X-100 (diamonds). C, DDM was tested at various concentrations, resulting in the various detergent (det.)-to-protein (prot.) ratios indicated. Data are presented as the mean ± S.D. (error bars, n = 3).
Because of the known ability of detergents in some cases to induce time-dependent inactivation of membrane proteins (see e.g. Ref. 46), the long term effects of incubation with DDM, C12E8, and Triton X-100 were also investigated. Of these three non-ionic detergents, DDM appears to be the least inhibitory after incubation for hours on ice (Fig. 7B). Compared with DDM, CHAPS either in the absence or in the presence of egg lecithin and DTT (16) did not lead to any gain in stability (data not shown). Similar experiments also demonstrated that α-DDM, C11-β-maltoside, or C10-β-maltoside did not preserve phosphorylation more effectively. Octyl thioglycoside and CYMAL-5 were not stabilizing detergents either (data not shown). Reducing the detergent-to-protein ratio helped to maintain Drs2p phosphorylation ability after long term incubation (Fig. 7C): at the low (but still solubilizing) DDM-to-protein ratio of 0.5 g/g, the D+ phosphorylation level even slightly increased over time as for membranes (Fig. 7C).
Turnover-dependent Dephosphorylation of Drs2p·Cdc50p Complex as Measured upon Isotopic Dilution of [γ-32P]ATP
We revealed turnover-dependent dephosphorylation of Drs2p by chasing the 32P-labeled phosphorylated species formed in Drs2p·Cdc50p-containing membranes with non-radioactive Mg2+-ATP. At 4 °C, a very significant fraction of Drs2p phosphoenzyme resisted ATP chase for minutes (Fig. 8A) in contrast with the much faster and complete phosphoenzyme decay that was observed for SERCA1a under identical conditions (data not shown). This decay was not sensitive to the presence of ADP (instead of Mg2+-ATP) in the dephosphorylation medium, but it was clearly sensitive to temperature as expected for an enzymatic process. At high temperature, however, this decay remained fairly slow: the half-time for the slow component of Drs2p dephosphorylation was of the order of 1–2 min at 37 °C (Fig. 8A). The corresponding results for the inactive D*+ sample (Fig. 8B) clearly show that most of this slow component of D+ dephosphorylation is indeed attributable to Drs2p phosphoenzyme decay, whereas at least part of the observed initial rapid phase of dephosphorylation in Fig. 8A can be accounted for by rapid decay of the background phosphorylation also observed for the inactive mutant.
Turnover-dependent decay of the Drs2p·Cdc50p phosphoenzyme was also explored in the presence of detergent. The presence of DDM did not much affect the kinetics of Drs2p·Cdc50p dephosphorylation at 37 °C (Fig. 8, A and C, compare circles), whereas phosphoenzyme decay was made significantly faster in the presence of C12E8 and even more so in the presence of the short-chain lipid diC7PC (Fig. 8C).
Effect of Exogenous PS and PtdIns(4)P on Dephosphorylation of Drs2p·Cdc50p Complex
Our ability to measure phosphorylation and dephosphorylation of Drs2p in the presence of detergent prompted us to test the effect of specific lipids in the environment of Drs2p. Unexpectedly, when yeast membranes were solubilized at a relatively high detergent-to-protein ratio (10 g of DDM/g of total protein), allowing a large dilution of proteins and endogenous protein-bound phospholipids within the solubilizing detergent micelles, the presence of exogenous PS during phosphorylation and dephosphorylation (here, 0.05 g of PS/g of DDM; hence, 0.5 g of PS/g of protein) slowed down Drs2p dephosphorylation (Fig. 9A). Control experiments ascertained that most of the non-catalytic phosphorylation dropped during the first rapid phase in all cases (data not shown). The slowing down effect of PS was apparently independent of the nature of the lipid fatty acid chains as it was observed for POPS (Fig. 9A), DMPS, and for a commercial mixture of E. coli lipids rich in various types of PS (data not shown). In similar experiments, PS also slowed down the dephosphorylation rate observed in the presence of C12E8 (data not shown).
Exogenous PS also exerted a slowing down effect on dephosphorylation of the solubilized Drs2p in the presence of exogenously added PC (Fig. 9B). PC alone only slightly slowed down dephosphorylation, and this effect was again apparently independent of the nature of the PC fatty acid chains as POPC and DOPC had similar slightly inhibitory effects (data not shown). The combination of PC and PS was as inhibitory as PS alone (Fig. 9, A and B). Remarkably, if exogenous PtdIns(4)P was added to DDM for the initial Drs2p solubilization, the additional presence of exogenous PS led to a clear acceleration of Drs2p dephosphorylation as expected for a transported phospholipid, whereas PtdIns(4)P alone already induced slight acceleration (Fig. 9C).
To rule out any possible artifactual role of the C-terminally located Bad tag in Drs2p-Bad, we investigated whether removing this tag would induce any change in the phosphorylation or dephosphorylation properties of Drs2p. Because of proteases cleavage sites inserted in the C terminus, we were able to remove Bad and His10 tags from Drs2p-Bad and Cdc50p-His10 (Fig. 9D, inset), respectively, and protease-treated membranes were solubilized with an excess of DDM. After tag removal, the effects of PS and PtdIns(4)P on phosphoenzyme decays were unchanged (Fig. 9D). This shows that the C-terminally located Bad sequence on Drs2p (as well as the His10 tag on Cdc50p) does not interfere with catalytic turnover and regulation of Drs2p function by PtdIns(4)P. Experiments similar to those illustrated in Fig. 9 but performed in the presence of lower relative amount of lipids showed weaker effects of POPC, POPS, and PtdIns(4)P (data not shown), suggesting that these lipids bind to their sites on Drs2p with relatively poor apparent affinity when they have to compete with an excess of DDM.
DISCUSSION
Co-expression of Drs2p and Cdc50p
Although several lines of evidence indicate that Drs2p is responsible for phospholipid flipping across membranes, much remains to be discovered about the translocation mechanism itself and the role of the Drs2p companion protein, Cdc50p. Toward this goal, we constructed a plasmid (Fig. 1) carrying both DRS2 and CDC50 genes and allowing for the coordinated overexpression of both proteins at high cell densities. From 1 liter of culture, 300 mg of protein was recovered in the light P3 membrane fraction, 3% of which (i.e. about 10 mg) was estimated to be biotinylated Drs2p-Bad accompanied by correctly glycosylated Cdc50p-His10, whereas the heavy P2 fraction was found to contain membranes expressing predominantly a non-glycosylated version of Cdc50p-His10 corresponding to immature Cdc50p that had probably not properly trafficked to Golgi membranes (Fig. 2). The poorer glycosylation observed for Cdc50p co-expressed with the inactive Drs2pD560N mutant (Fig. 2) is consistent with previous data suggesting that mutation of Asp-560 in Drs2p alters the interaction between Drs2p and Cdc50p as measured in vivo by the split ubiquitin assay (23).
Functionality of Overexpressed Drs2p·Cdc50p Complex
The quality of the complex formed was suitable for functional analysis without requiring any additional purification step. With Drs2p accounting for about 3% of total proteins in the P3 membrane fraction, the vanadate- or fluoride-sensitive phosphorylation level at steady state of about 3 pmol of 32P bound/100 μg of total protein (Figs. 3–9) would correspond to about 1 nmol of 32P bound/mg of Drs2p protein itself. This suggests that the stoichiometry for Drs2p phosphoenzyme amounts to at least a few tens of %, a value comparable with those reported for purified ATP8A2 in the absence of PS (17). This high phosphorylation level, which is much higher than in previous reports (23, 39), represents a significant improvement for future dissection of the molecular mechanism underlying Drs2p·Cdc50p-mediated lipid transport.
We found that after Drs2p has acquired an appropriately active conformation due to the chaperone role of fully glycosylated Cdc50p, maintenance of the complete glycosylation of Cdc50p is a prerequisite neither for Drs2p steady-state phosphorylation at its catalytic site (Fig. 3B) nor for its dephosphorylation (data not shown). This agrees with previous reports suggesting that glycosylation of only a few of the potential sites on Cdc50p is sufficient for its function (24).
For subsequent studies after solubilization, DDM was found to be a fair choice as this detergent allowed maintaining the formation of significant amounts of phosphoenzyme while minimizing the rate of detergent-induced irreversible inactivation and preserving the interaction between Drs2p and fully glycosylated Cdc50p (Figs. 6 and 7).
Measurement of Catalytic Turnover of Drs2p·Cdc50p Phosphoenzyme
In previous studies with either Drs2p·Cdc50p complex purified from yeast membranes or human ATP8B1·CDC50A complex purified from baculovirus-infected cells, only a very minor fraction of the phosphoenzyme formed was found capable of undergoing turnover-dependent dephosphorylation even in the presence of PS in the dephosphorylation medium (23, 39). Time-dependent decay of a 32P-labeled phosphorylated state of the purified ATP8A1 (ATPase II) has been reported previously (47), but in that study, the time dependence measured probably does not reflect the true kinetics of the catalytic cycle but merely the time needed for exhaustion of [γ-32P]ATP from the medium. In addition, the observed phosphoenzyme was formed in the absence of Mg2+, and it was detected after alkaline SDS-PAGE, an odd situation for the anticipated acylphosphate phosphoenzyme (48). Only very recently has it been possible to clearly observe the turnover-dependent phosphoenzyme decay of a P4-ATPase, namely human ATP8A2 (17).
Here, we were able for the first time to measure turnover-dependent decay of the 32P-labeled Drs2p·Cdc50p phosphoenzyme (Fig. 8). The kinetics of this decay were found to be ADP-insensitive (Fig. 8A), which according to classical rationales for P-type ATPases would characterize Drs2p phosphoenzyme formed at 4 °C as “mainly E2P.” In contrast, the ATP8A2 phosphoenzyme contains a significant proportion of the ADP-sensitive E1P form (17).
Effects of PS and PtdIns(4)P on Turnover-dependent Phosphoenzyme Decay
In the case of P2-ATPases like SERCA1a Ca2+-ATPase or Na+,K+-ATPase, it is the binding of countertransported H+ and K+ ions to the transport site in the E2P state that stimulates E2P dephosphorylation via the TGE motif of their A-domain (9, 49). Assuming that P4- and P2-ATPases share the same basic mechanism for transport, binding of the transported substrate (PS in the case of Drs2p) should therefore stimulate dephosphorylation of Drs2p, and this is indeed what is observed with ATP8A2 (17). However, when solubilization of yeast membranes was performed with a high DDM-to-protein ratio, the mere addition of PS to DDM slowed down Drs2p dephosphorylation. PS exerted its expected stimulatory effect on Drs2p dephosphorylation only in the presence of PtdIns(4)P (Fig. 9). Because the solubilized Drs2p was preincubated with PS in these experiments, there is no doubt that PS had ample time to reach its binding sites on Drs2p.
Note that when membrane solubilization was performed with a 10-fold lower detergent-to-protein ratio addition of PS no longer exerted any effect, and PtdIns(4)P was now stimulatory both in the absence and the presence of PS (data not shown). A likely reason for these results is that at such a modest DDM-to-protein ratio the PS-binding sites on Drs2p·Cdc50p remained occupied by endogenous PS derived from the yeast Golgi membranes so that addition of exogenous PS did not have any additional effect. But the PtdIns(4)P-binding sites on Drs2p·Cdc50p remained essentially unoccupied presumably because of the known instability of PtdIns(4)P and therefore the absence of any endogenous PtdIns(4)P in the final membrane fraction (hence the stimulating effect of added PtdIns(4)P).
If this interpretation is correct, the data at a high DDM-to-protein ratio are those that best reveal the intrinsic modulatory properties of the membrane lipids, namely the strict requirement for PtdIns(4)P for allowing PS to stimulate the catalytic cycle of Drs2p. Thus, our observations provide the enzymological basis for the recently established fact that PtdIns(4)P stimulates NBD-PS translocation in the TGN (25). This stimulation appears to be due to acceleration of the dephosphorylation step, a step at which the phospholipid ligand is expected to be loaded on the flippase.
From a physiological point of view, PtdIns(4)P is a crucial component of the signaling cascade for clathrin-coated vesicle formation at the TGN (26, 27). The dependence of Drs2p·Cdc50p phosphoenzyme decay on PtdIns(4)P is consistent with the need of enhanced phospholipid transport by Drs2p when vesicle budding is required (25).
It might appear as a paradox that the ligand to be transported by Drs2p (PS) does not stimulate pump dephosphorylation (and therefore completion of its catalytic cycle) in all cases and may even slow down this step. But this is only because in most classical cases coupling of P-type pump dephosphorylation to ion transport is quite tight (see e.g. Ref. 50). From a general enzymological point of view, this tight coupling is not strictly necessary. In the absence of PtdIns(4)P, the Drs2p enzyme might display an uncoupled catalytic cycle. The mitochondrial P450 enzyme is a classical example of an enzyme for which the balance between the desired oxidation of substrates and parallel abortive mechanisms is highly variable from one substrate to the other (51, 52). Even for P2-type ATPases (for instance Ca2+-ATPase), the existence of uncoupled pathways for dephosphorylation has been suggested, either for different isoforms or under different situations (53–56).
Phosphoenzyme Turnover Is Slower for Drs2p·Cdc50p Complex than for Mammalian Photoreceptor P4-ATPase, ATP8A2
For our complex, we found rather slow dephosphorylation rate constants (in the min−1 range) both in membranes and in DDM (see Figs. 8 and 9).
These slow dephosphorylation rate constants are not incompatible with the faster rate constants deduced from overall ATP hydrolysis rates measured previously for purified Drs2p (15). Indeed, those hydrolysis measurements were performed in the presence of C12E9, a detergent mixture related to the pure C12E8 detergent that in the present study strongly accelerated phosphoenzyme decay (Fig. 8C). Moreover, the rates of Drs2p-dependent NBD-PS translocation measured in proteoliposomes were much slower (15) than what would have been expected (assuming a coupling ratio close to 1) from the ATP hydrolysis rates measured in the presence of C12E9.
Nevertheless, the turnover rate constants measured for our complex are definitely slower than those deduced from the overall hydrolytic activity as well as from direct dephosphorylation measurements of human P4-ATPase ATP8A2 from photoreceptor outer segment disc membranes (16, 17). In addition, ATP8A2 ATPase activity has been described to be greatly stimulated by PS without any special requirement for PtdIns(4)P (16, 17). One possible interpretation for these apparent discrepancies between our results with Drs2p and those for ATP8A2 is that Drs2p-catalyzed lipid flip-flop in yeast TGN membranes does not need to be especially rapid. In contrast, ATP8A2 localized in retina might have to counterbalance rapid dissipation of lipid asymmetry by the “floppase” activity of opsin (57). From a mechanistic point of view, the fast turnover rate of ATP8A2 and its apparent lack of sensitivity to PtdIns(4)P might come from the fact that there is only poor conservation of the C-terminal tail (including the putative RMKKQR PtdIns(4)P-binding site (25)) between these two P4-ATPases.
Directly performing ATPase activity measurements on yeast P3 membranes with overexpressed Drs2p would of course be desirable. We made a few attempts along this line, but they remained unsuccessful because of the high background ATPase activity found in our crude yeast membrane fractions. Compared with this relatively high background activity, the expected activity resulting from dephosphorylation of 30–40 pmol/mg total protein with a dephosphorylation rate constant of 0.5–1 min−1 would indeed be 2 or 3 orders of magnitude lower. Reliable measurements must therefore await complete purification of the Drs2p·Cdc50p complex.
N- or C-terminal Location of Drs2p Tag?
A C-terminally TAP-tagged Drs2p was recently found to be functional in complementation experiments in vivo but not functional in vitro after affinity purification (15). In contrast, in the present work, C-terminally tagged Drs2p co-expressed with Cdc50p remained phosphorylatable from [γ-32P]ATP over long periods of time in the presence of moderate but solubilizing DDM concentrations, suggesting that Drs2p experiences only a small amount of time-dependent irreversible inactivation under these conditions. As time-dependent inactivation in our experiments was much more of concern in the presence of C12E8 (Fig. 7B), it is conceivable that C12E9, which was used in Ref. 15, led to lability of the C-terminally tagged Drs2p-TAP construct during its purification, whereas this lability was somehow not shared by N-terminally tagged TAP-Drs2p.
Beyond these speculations, we measured phosphorylation and dephosphorylation of the Drs2p·Cdc50p complex expressed in P3 membranes after removal of the Bad and His10 tags by proteolytic treatment. The fact that we did not detect any significant difference between treated and untreated membranes (Fig. 9D) indicates that the C-terminally located Bad tag does not perturb the catalytic cycle of Drs2p.
Fluoride Compounds and Perspectives for Purification of Drs2p·Cdc50p Complex
For future purification and structural characterization of the Drs2p·Cdc50p complex, the instability of Drs2p at a high detergent-to-protein ratio should probably be kept in mind. In the context of such time-dependent detergent-induced denaturation, our finding that fluoride compounds react with Drs2p (Fig. 5) might offer an interesting perspective similar to the one that turned out to be very useful for SERCA1a crystallization. Indeed, preliminary reaction of SERCA1a with these fluoride compounds was found to afford a strong protection of SERCA1a against irreversible denaturation during the subsequent lengthy process of purification and crystallization in the presence of detergent (58). Crystallization of aluminum or beryllium fluoride forms of Drs2p might therefore be the aim of studies in the near future.
Acknowledgments
We thank T. Graham for fruitful discussion and R. Kaback for critically reading the manuscript.
This work was supported by the CNRS and Commissariat à l'Energie Atomique, by a fellowship from the “Ministère de l'Enseignement Supérieur et de la Recherche” (to A. J.), and by a grant from the Agence Nationale pour la Recherche (to C. M., C. J., and M. l. M.).
- TGN
- trans-Golgi network
- P2- or P4-ATPase
- P-type ATPase of subtype 2 or 4
- P2 or P3 membranes
- membrane fraction obtained after intermediate or high speed centrifugation
- SERCA1a
- sarcoendoplasmic reticulum Ca2+-ATPase, isoform 1a
- Bad
- biotin acceptor domain
- TEV
- tobacco etch virus
- DDM
- n-dodecyl β-d-maltoside
- C12E8
- octaethylene glycol mono-n-dodecyl ether
- diC7PC
- 1,2-diheptanoyl-sn-phosphatidylcholine
- NBD
- 7-nitrobenz-2-oxa-1,3-diazol-4-yl
- PC
- phosphatidylcholine
- PtdIns(4)P
- phosphatidylinositol-4-phosphate
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine
- SB
- solubilization buffer
- NTA
- nitrilotriacetic acid
- C12E9
- nonaoxyethylene n-dodecyl ether
- TAP
- tandem affinity purification
- PS
- phosphatidylserine
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- DMPS
- 1,2-dimyristoyl-sn-glycero-3-phospho-l-serine.
REFERENCES
- 1. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alder-Baerens N., Lisman Q., Luong L., Pomorski T., Holthuis J. C. (2006) Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol. Biol. Cell 17, 1632–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mercer J., Helenius A. (2008) Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320, 531–535 [DOI] [PubMed] [Google Scholar]
- 4. Fadok V. A., Bratton D. L., Rose D. M., Pearson A., Ezekewitz R. A., Henson P. M. (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 [DOI] [PubMed] [Google Scholar]
- 5. Rosing J., Tans G., Govers-Riemslag J. W., Zwaal R. F., Hemker H. C. (1980) The role of phospholipids and factor Va in the prothrombinase complex. J. Biol. Chem. 255, 274–283 [PubMed] [Google Scholar]
- 6. Muthusamy B. P., Natarajan P., Zhou X., Graham T. R. (2009) Linking phospholipid flippases to vesicle-mediated protein transport. Biochim. Biophys. Acta 1791, 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devaux P. F. (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30, 1163–1173 [DOI] [PubMed] [Google Scholar]
- 8. Daleke D. L. (2007) Phospholipid flippases. J. Biol. Chem. 282, 821–825 [DOI] [PubMed] [Google Scholar]
- 9. Palmgren M. G., Nissen P. (2011) P-type ATPases. Annu. Rev. Biophys. 40, 243–266 [DOI] [PubMed] [Google Scholar]
- 10. Gall W. E., Geething N. C., Hua Z., Ingram M. F., Liu K., Chen S. I., Graham T. R. (2002) Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623–1627 [DOI] [PubMed] [Google Scholar]
- 11. Hua Z., Fatheddin P., Graham T. R. (2002) An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13, 3162–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., Holthuis J. C. (2003) Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang X., Halleck M. S., Schlegel R. A., Williamson P. (1996) A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272, 1495–1497 [DOI] [PubMed] [Google Scholar]
- 14. Chen S., Wang J., Muthusamy B. P., Liu K., Zare S., Andersen R. J., Graham T. R. (2006) Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic 7, 1503–1517 [DOI] [PubMed] [Google Scholar]
- 15. Zhou X., Graham T. R. (2009) Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc. Natl. Acad. Sci. U.S.A. 106, 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coleman J. A., Kwok M. C., Molday R. S. (2009) Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem. 284, 32670–32679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman J. A., Vestergaard A. L., Molday R. S., Vilsen B., Peter Andersen J. (2012) Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc. Natl. Acad. Sci. U.S.A. 109, 1449–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., Tanaka K. (2004) Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 3418–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furuta N., Fujimura-Kamada K., Saito K., Yamamoto T., Tanaka K. (2007) Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell 18, 295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulusma C. C., Folmer D. E., Ho-Mok K. S., de Waart D. R., Hilarius P. M., Verhoeven A. J., Oude Elferink R. P. (2008) ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology 47, 268–278 [DOI] [PubMed] [Google Scholar]
- 21. van der Velden L. M., Wichers C. G., van Breevoort A. E., Coleman J. A., Molday R. S., Berger R., Klomp L. W., van de Graaf S. F. (2010) Heteromeric interactions required for abundance and subcellular localization of human CDC50 proteins and class 1 P4-ATPases. J. Biol. Chem. 285, 40088–40096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poulsen L. R., López-Marqués R. L., McDowell S. C., Okkeri J., Licht D., Schulz A., Pomorski T., Harper J. F., Palmgren M. G. (2008) The Arabidopsis P4-ATPase ALA3 localizes to the Golgi and requires a β-subunit to function in lipid translocation and secretory vesicle formation. Plant Cell 20, 658–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenoir G., Williamson P., Puts C. F., Holthuis J. C. (2009) Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. J. Biol. Chem. 284, 17956–17967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coleman J. A., Molday R. S. (2011) Critical role of the β-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J. Biol. Chem. 286, 17205–17216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Natarajan P., Liu K., Patil D. V., Sciorra V. A., Jackson C. L., Graham T. R. (2009) Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat. Cell Biol. 11, 1421–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham T. R., Burd C. G. (2011) Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 21, 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vicinanza M., D'Angelo G., Di Campli A., De Matteis M. A. (2008) Phosphoinositides as regulators of membrane trafficking in health and disease. Cell. Mol. Life Sci. 65, 2833–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puts C. F., Lenoir G., Krijgsveld J., Williamson P., Holthuis J. C. (2010) A P4-ATPase protein interaction network reveals a link between aminophospholipid transport and phosphoinositide metabolism. J. Proteome Res. 9, 833–842 [DOI] [PubMed] [Google Scholar]
- 29. Holthuis J. C., Nichols B. J., Dhruvakumar S., Pelham H. R. (1998) Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picard M., Toyoshima C., Champeil P. (2006) Effects of inhibitors on luminal opening of Ca2+ binding sites in an E2P-like complex of sarcoplasmic reticulum Ca2+-ATPase with Be2+-fluoride. J. Biol. Chem. 281, 3360–3369 [DOI] [PubMed] [Google Scholar]
- 31. Jidenko M., Lenoir G., Fuentes J. M., le Maire M., Jaxel C. (2006) Expression in yeast and purification of a membrane protein, SERCA1a, using a biotinylated acceptor domain. Protein Expr. Purif. 48, 32–42 [DOI] [PubMed] [Google Scholar]
- 32. Lenoir G., Menguy T., Corre F., Montigny C., Pedersen P. A., Thinès D., le Maire M., Falson P. (2002) Overproduction in yeast and rapid and efficient purification of the rabbit SERCA1a Ca2+-ATPase. Biochim. Biophys. Acta 1560, 67–83 [DOI] [PubMed] [Google Scholar]
- 33. Pompon D., Louerat B., Bronine A., Urban P. (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272, 51–64 [DOI] [PubMed] [Google Scholar]
- 34. Cardi D., Montigny C., Arnou B., Jidenko M., Marchal E., le Maire M., Jaxel C. (2010) Heterologous expression and affinity purification of eukaryotic membrane proteins in view of functional and structural studies: the example of the sarcoplasmic reticulum Ca2+-ATPase. Methods Mol. Biol. 601, 247–267 [DOI] [PubMed] [Google Scholar]
- 35. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 36. Hatori Y., Hirata A., Toyoshima C., Lewis D., Pilankatta R., Inesi G. (2008) Intermediate phosphorylation reactions in the mechanism of ATP utilization by the copper ATPase (CopA) of Thermotoga maritima. J. Biol. Chem. 283, 22541–22549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parks T. D., Leuther K. K., Howard E. D., Johnston S. A., Dougherty W. G. (1994) Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal. Biochem. 216, 413–417 [DOI] [PubMed] [Google Scholar]
- 38. Chen C. Y., Ingram M. F., Rosal P. H., Graham T. R. (1999) Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147, 1223–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bryde S., Hennrich H., Verhulst P. M., Devaux P. F., Lenoir G., Holthuis J. C. (2010) CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J. Biol. Chem. 285, 40562–40572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Makinose M. (1969) The phosphorylation of the membranal protein of the sarcoplasmic vesicles during active calcium transport. Eur. J. Biochem. 10, 74–82 [PubMed] [Google Scholar]
- 41. Fukushima Y., Post R. L. (1978) Binding of divalent cation to phosphoenzyme of sodium- and potassium-transport adenosine triphosphatase. J. Biol. Chem. 253, 6853–6862 [PubMed] [Google Scholar]
- 42. Shigekawa M., Wakabayashi S., Nakamura H. (1983) Reaction mechanism of Ca2+-dependent adenosine triphosphatase of sarcoplasmic reticulum. ATP hydrolysis with CaATP as a substrate and role of divalent cation. J. Biol. Chem. 258, 8698–8707 [PubMed] [Google Scholar]
- 43. Pick U. (1982) The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 257, 6111–6119 [PubMed] [Google Scholar]
- 44. Macdonald T. L., Martin R. B. (1988) Aluminum ion in biological systems. Trends Biochem. Sci. 13, 15–19 [DOI] [PubMed] [Google Scholar]
- 45. Hauser H. (2000) Short-chain phospholipids as detergents. Biochim. Biophys. Acta 1508, 164–181 [DOI] [PubMed] [Google Scholar]
- 46. Lund S., Orlowski S., de Foresta B., Champeil P., le Maire M., Møller J. V. (1989) Detergent structure and associated lipid as determinants in the stabilization of solubilized Ca2+-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 264, 4907–4915 [PubMed] [Google Scholar]
- 47. Ding J., Wu Z., Crider B. P., Ma Y., Li X., Slaughter C., Gong L., Xie X. S. (2000) Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J. Biol. Chem. 275, 23378–23386 [DOI] [PubMed] [Google Scholar]
- 48. Hokin L. E., Sastry P. S., Galsworthy P. R., Yoda A. (1965) Evidence that a phosphorylated intermediate in a brain transport adenosine triphosphatase is an acyl phosphate. Proc. Natl. Acad. Sci. U.S.A. 54, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clausen J. D., Vilsen B., McIntosh D. B., Einholm A. P., Andersen J. P. (2004) Glutamate-183 in the conserved TGES motif of domain A of sarcoplasmic reticulum Ca2+-ATPase assists in catalysis of E2/E2P partial reactions. Proc. Natl. Acad. Sci. U.S.A. 101, 2776–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Møller J. V., Olesen C., Winther A. M., Nissen P. (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 [DOI] [PubMed] [Google Scholar]
- 51. Pompon D. (1987) Rabbit liver cytochrome P-450 LM2: roles of substrates, inhibitors, and cytochrome b5 in modulating the partition between productive and abortive mechanisms. Biochemistry 26, 6429–6435 [DOI] [PubMed] [Google Scholar]
- 52. Perret A., Pompon D. (1998) Electron shuttle between membrane-bound cytochrome P450 3A4 and b5 rules uncoupling mechanisms. Biochemistry 37, 11412–11424 [DOI] [PubMed] [Google Scholar]
- 53. Yu X., Inesi G. (1995) Variable stoichiometric efficiency of Ca2+ and Sr2+ transport by the sarcoplasmic reticulum ATPase. J. Biol. Chem. 270, 4361–4367 [DOI] [PubMed] [Google Scholar]
- 54. de Meis L. (2002) Ca2+-ATPases (SERCA): energy transduction and heat production in transport ATPases. J. Membr. Biol. 188, 1–9 [DOI] [PubMed] [Google Scholar]
- 55. Mall S., Broadbridge R., Harrison S. L., Gore M. G., Lee A. G., East J. M. (2006) The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J. Biol. Chem. 281, 36597–36602 [DOI] [PubMed] [Google Scholar]
- 56. de Meis L. (2003) Brown adipose tissue Ca2+-ATPase: uncoupled ATP hydrolysis and thermogenic activity. J. Biol. Chem. 278, 41856–41861 [DOI] [PubMed] [Google Scholar]
- 57. Menon I., Huber T., Sanyal S., Banerjee S., Barré P., Canis S., Warren J. D., Hwa J., Sakmar T. P., Menon A. K. (2011) Opsin is a phospholipid flippase. Curr. Biol. 21, 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamasaki K., Daiho T., Suzuki H. (2002) Remarkable stability of solubilized and delipidated sarcoplasmic reticulum Ca2+-ATPase with tightly bound fluoride and magnesium against detergent-induced denaturation. J. Biol. Chem. 277, 13615–13619 [DOI] [PubMed] [Google Scholar]