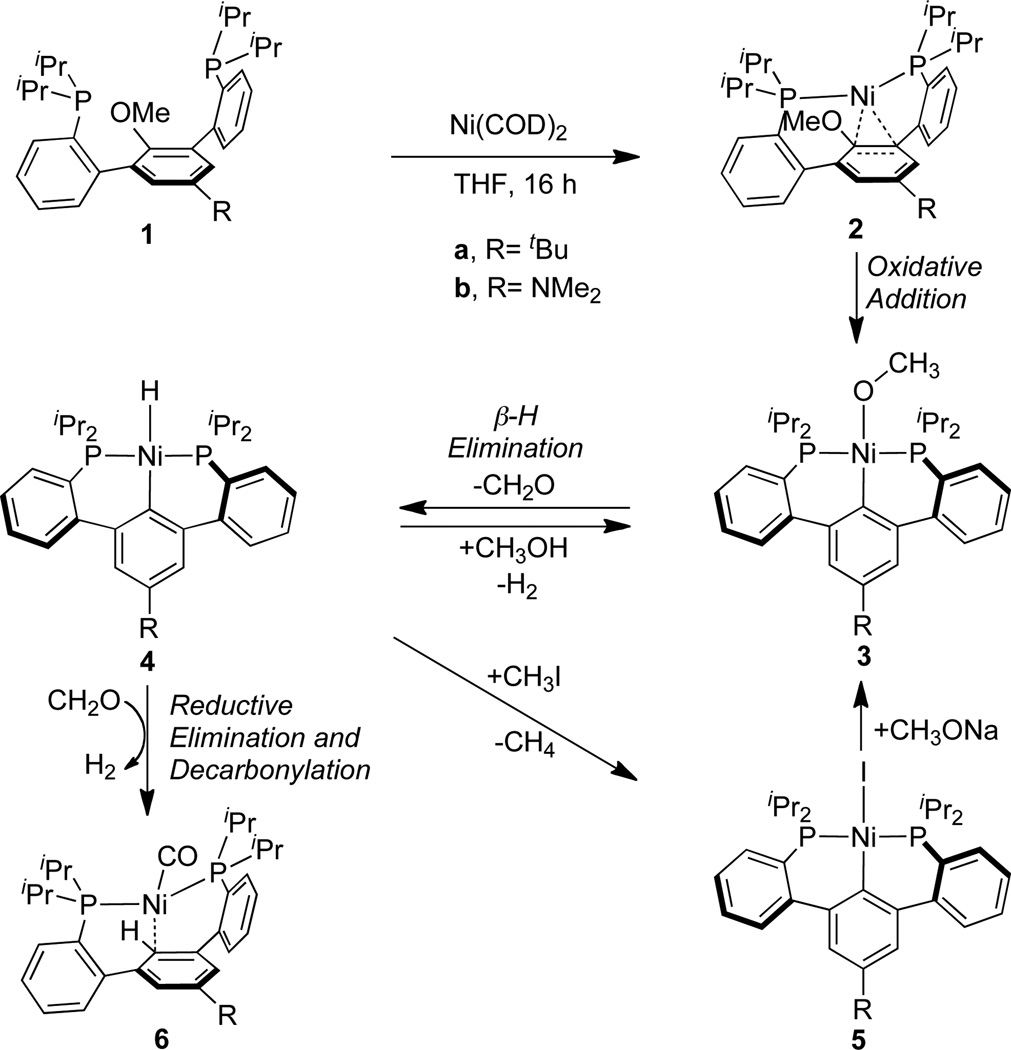
Abstract
Mechanistic studies of the hydrogenolysis of aryl ethers by nickel were undertaken with (diphosphine)aryl methyl ethers. A Ni(0) complex containing Ni-arene interactions adjacent to the aryl-O bond was isolated. Heating led to aryl-O bond activation and generation of a nickel-aryl-methoxide complex. Formal β-H elimination from this species produced a nickel-aryl-hydride which can undergo reductive elimination in the presence of formaldehyde to generate a carbon monoxide adduct of Ni(0). The reported complexes map out a plausible mechanism of aryl ether hydrogenolysis catalyzed by nickel. Investigations of a previously reported catalytic system using isotopically labeled substrates are consistent with the mechanism proposed in the stoichiometric system, involving β-H elimination from a nickel alkoxide rather than cleavage of the Ni-O bond by H2.
The elaboration of the aryl C–O bond to a variety of functional groups has emerged as a versatile synthetic tool in organic methodology,1 as phenol precursors are readily available and synthetic modification of the aromatic ring is facile. The strong aryl C–O bond, however is typically difficult to activate. Nickel-based catalysts have proven versatile in the conversion of substrates with aryl C–O2–13 or C–S14–16 bonds. Although cross-coupling of phenolic substrates tends to require prior conversion to the more reactive sulfonates,2 recent advances show that aryl phosphates, aryl esters, aryl carbamates, aryl ethers and even free phenols can be used as electrophiles in cross-coupling reactions.3–13 In a complementary approach, the conversion of aryl C–O to aryl-H bonds has been recognized as a valuable strategy for removing an oxygen-based directing group from an aryl ring. Silanes have been utilized as a hydride source for this transformation.10,11 Additionally, stoichiometric intramolecular aryl C-O activation has been reported with rhodium and palladium pincer complexes.17,18 In the context of biomass conversion to alternative fuels and chemicals, the depolymerization of lignin, a significant component of biomass containing aryl ether linkages, is a considerable challenge.19–21 Recently, an appealing strategy involving the cleavage of lignin-like aryl C–O bonds via nickel-catalyzed hydrogenolysis was reported by Hartwig et al.22 Given the general interest in the conversion of aryl C–O bonds, detailed mechanistic insight including the nature of the intermediates is instrumental in developing practical catalysts. Herein, we report detailed studies of the nickel-mediated reductive cleavage of an aryl-ether with pendant phosphines and extend the mechanistic implications of these studies to a catalytic system.
We recently reported that diphosphine-arene pincer ligands based on a p-terphenyl linker support mono- and dinuclear nickel complexes that exhibit strong nickel-arene interactions. 23,24 The m-terphenyl diphosphine motif was also found to predispose the metal center toward interaction with the carbon at the 2′-position of the central ring.25 Toward exploiting potential C-X activation chemistry, ligand variants with ether substitution at this position were prepared. The present study utilizes diphosphines with tert-butyl (1a) or dimethyl-amino (1b) substitution para to the ether on the central ring (Scheme 1); these two ligands lead to nickel complexes with similar reactivity but complementary crystallinity that allows for solid-state characterization of the resultant species.
Scheme 1.
Stoichiometric cleavage of an aryl C–O bond facilitated by nickel.
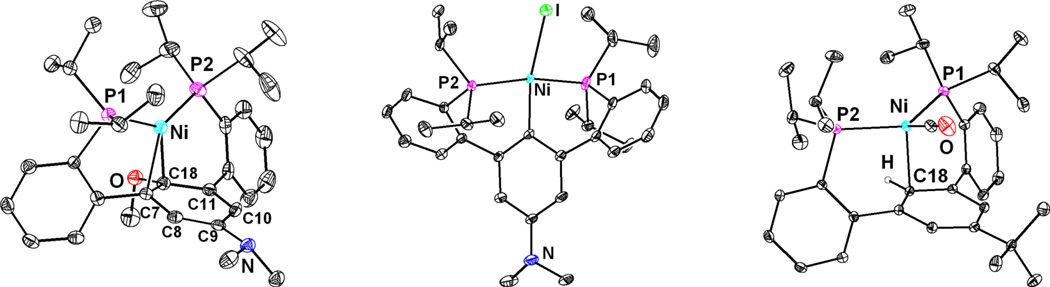
Addition of an equivalent of Ni(COD)2 to diphosphine at 20 °C (1a–b) led to generation of a new species (2a–b) according to NMR spectroscopy (Scheme 1). The 31P NMR chemical shifts of the resulting species (2a, 41.0 ppm; 2b, 40.7 ppm) are similar to those reported for the p-terphenyl diphosphine supported Ni(0) (40.4 ppm).23 Similarly, the protons assigned to the central arene resonate upfield compared with those of the free phosphine in the 1H NMR spectrum of 2a–b, whereas the ether OCH3 peak is only slightly shifted. These data are consistent with the formation of a Ni(0) species with interactions between the metal center and the aromatic π-system, but not the ether oxygen. A single-crystal X-ray diffraction (XRD) study of 2b confirmed the spectroscopic findings. In the solid state the metal center is bound by two phosphines and interacts with two carbon centers of the central arene (Figure 1). The short Ni-C distances (1.96–2.09 Å) indicate strong interactions between the nickel center and central arene. Consequently, the C-C distances of the central ring are consistent with partial localization of the double bonds. For 2b the C8–C9 and C10–C11 bonds (1.368(2) and 1.360(2) Å, respectively) are shorter than the rest of the central arene C-C bonds by >0.06 Å. The aryl C–O bond points away from the metal center, consistent with partial sp3 hybridization of the 2′-position of the central ring (2b, C18, Figure 1) due to the Ni-C interaction. Notably, an intermediate displaying η2-interactions between Ni(0) and the double bond adjacent to the oxygen was found computationally to precede C-O bond activation in the cross-coupling of phenol derivatives.8,26 Complex 2b is the only example of such an arrested intermediate characterized by crystallography, according to a Cambridge Structural Database search.27
Figure 1.
Solid-state structures of 2b (left), 5 (center), and 6a (right). Solvent molecules, anions, and select hydrogen atoms not shown for clarity.
Complexes 2a and 2b were found to convert to new species in solution at 45 °C. After the first 12 hours a single product (3a and 3b, respectively) was observed by NMR spectroscopy, in mixture with starting material. Monitoring by 1H NMR spectroscopy revealed that the peaks corresponding to the central arene ring shifted downfield. Additionally, the OCH3 resonance shifted nearly 0.5 ppm downfield relative to precursors 1a and 1b. Upon further heating, additional species, 4a and 4b, were observed. The central arene protons of these species are also shifted relative to 1a and 1b, respectively, in the aromatic region. Intriguingly, the OCH3 signal is absent, and an upfield triplet was observed in the hydride region of the 1H NMR spectrum (−2.85 ppm, 4a; −2.87 ppm, 4b). These upfield peaks are consistent with the formation of Ni(II) hydrides. Compound 4b was isolated in 55% yield by precipitation from THF upon stirring 2b at 20 °C for eight days; however efforts to obtain X-ray quality single crystals of 4b have been unsuccessful to date. Treatment with methyl iodide at 20 °C for 14 hours generated a new species assigned as a Ni(II) iodide (5); an XRD study of this species confirmed the above assignment (Figure 1). The nickel center is found in the same plane as the central arene. The Ni–C distance (1.919(1) Å) is consistent with an aromatic C–Ni bond. The diphosphine ligand framework, bound in pseudo-C2 fashion, acts as a classical tridentate diphosphine-aryl pincer,28 but with six-member chelates involving aryl-aryl linkages.29,30 The solid-state structure is consistent with the NMR spectroscopic data for 4 and 5 indicating the absence of the methoxy group and the lack of interaction with the π-system of the arene. Most importantly, the structure shows cleavage of the aryl C–O bond and displacement of oxygen by nickel.
Additional experiments were performed to confirm the nature of compound 3. The reaction of 4b with methanol at 20 °C led to the reformation of species 3b, albeit not quantitatively (1H NMR spectroscopy). Treatment of 5 with sodium methoxide at 20 °C led to the formation of 3b, 4b, and other unidentified species. Although species 3 could not be obtained by these routes without contamination from complex 4, the independent methods of generation are consistent with 3 being a Ni(II) aryl-methoxide. This is the product of oxidative addition of the aryl C–O bond to Ni(0).31 Similar stoichiometric reactions mediated by rhodium and palladium have been reported.17,18
The origin of the hydride in 4 was studied by using a version of the diphosphine ligand deuterated at the methoxy position (1b–d3). This precursor led to the formation of a species (4b–d1) with no signal in the hydride region of the 1H NMR spectrum, but otherwise displaying the same peaks as 4. This is consistent with the formation of a nickel-deuteride, which confirms the methoxy group as the source of hydrogen (deuterium). β-H elimination could occur via a five-coordinate intermediate, 32–34 although, in coordinatively saturated systems it has been proposed to occur via alkoxide dissociation35–37 or in bimolecular fashion.38,39 Given the strain of the chelates in complex 4, phosphine dissociation may also be possible,32,33,40,41 opening up a cis coordination site for β-H elimination.42–44 Further studies are necessary to determine the mechanism in the present system.
Although its generation does not require dihydrogen, hydride 4 is a potential intermediate in the hydrogenolysis of the aryl-O bond. Complexes 2a and 2b were heated separately to 100 °C, generating new species, 6a and 6b, in over 90% yield (1H NMR spectroscopy) within two hours. A Toepler pump experiment showed that ca. 0.9 equiv of combustible gas was generated in this reaction. The Ni-H peak is absent from the 1H NMR spectra of the products and a new triplet is present (6a, 6.82 ppm, 6b, 6.51 ppm) assigned to a new aryl ipso-C-H. Infrared (IR) spectroscopy revealed an absorption indicative of a terminal Ni(0)-CO (6a, 1929 cm−1; 6b, 1917 cm−1). An XRD study of 6a confirms the formation of a nickel carbonyl complex, with the metal center bound to the two phosphines and interacting with the π-system of the central aromatic ring. This binding mode is consistent with reductive elimination forming an aryl-H bond. The metal center is close to only one carbon atom from the central ring (6a, C18, Figure 1) and the Ni-C distance is longer (2.2763(7) Å) than in 2b. These structural parameters suggest that the metal center is less π-basic in 6 compared to 2, likely due to the electron withdrawing properties of the carbonyl ligand.
The formation of compound 6 indicates that in addition to reductive elimination of a C-H bond, decarbonylation of formaldehyde had occurred to generate H2. To determine the origin of the hydrogen involved in the C-H reductive elimination, deuterium labeled 2-d3 was investigated. Upon heating, the product does not display the aryl C-H peak in the 1H NMR spectrum, but otherwise displays the same spectroscopic features as 6, indicating that the deuterium originating from the methoxy group was transferred to the aryl. Performing the reaction in the presence of 1 atm H2, does not affect the isotope incorporation, indicating that Ni-methoxide cleavage by H2 does not compete with β-H elimination. No reaction was observed upon heating of isolated 4b for 6 hours at 100 °C. In contrast, in the presence of paraformaldehyde, conversion of 4b to 6b occurred within 4 hours at 60 °C. This behavior indicates that formaldehyde facilitates the reductive elimination step. π-Acidic ligands have been previously reported to facilitate reductive elimination.45,46 In the present case, isomerization to a cis-hydride-aryl species also must occur prior to reductive elimination. The reaction of 4b with carbon monoxide generated 6b as well, albeit more slowly and in a mixture with unidentified species.
Silanes have been used as hydride sources for the reductive cleavage of aryl C–O bonds.10,11 To test the effect of added silane, 2b was heated to 80 °C in benzene in the presence of two equivalents of Et3SiD and generated only less than 10% 6b-d1. A larger excess of Et3SiD (13 equiv) led to more deuterium incorporation (ca 20% 6b–d1). Treatment of 2b–d3 with Et3SiH (2 equiv) generated 6b–d1 and 6b in a 1:1 ratio.47 These results indicate that the mechanism involving β-H elimination (Scheme 1) is favored vs interception of the nickel methoxide by silane, at low concentration of silane. The increased isotopic incorporation from Et3SiH vs Et3SiD is consistent with normal isotope effects for β-H elimination (conversion of 3b to 4b) and transmetallation between 3b and silane to generate 4b.42–44,48,49
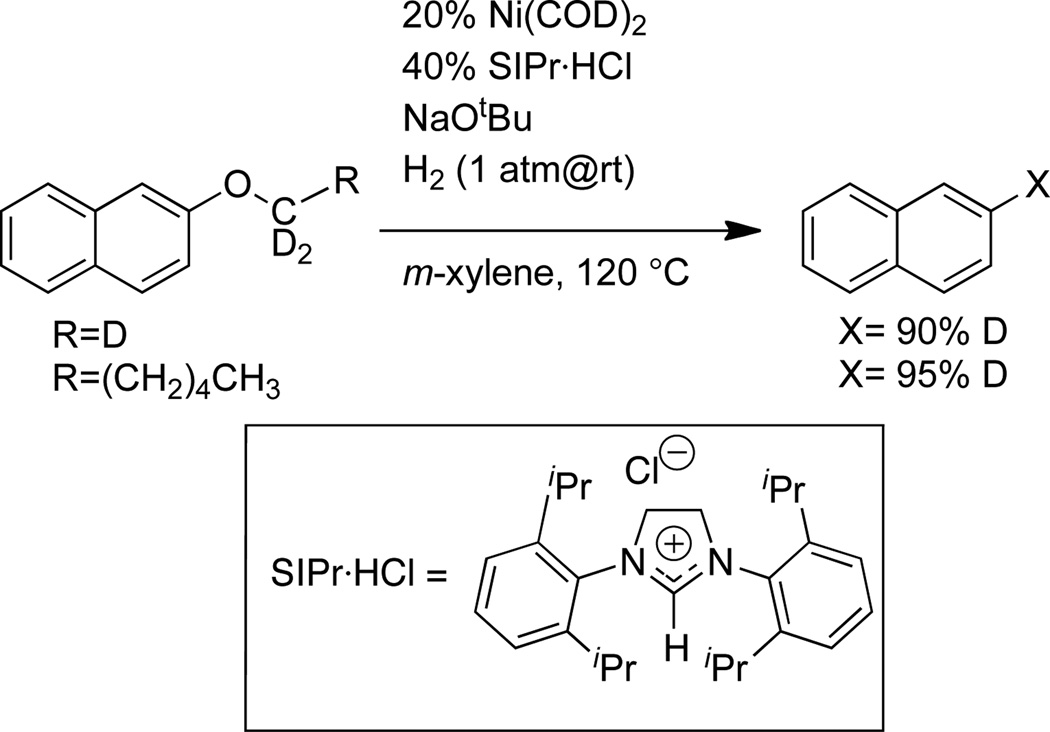
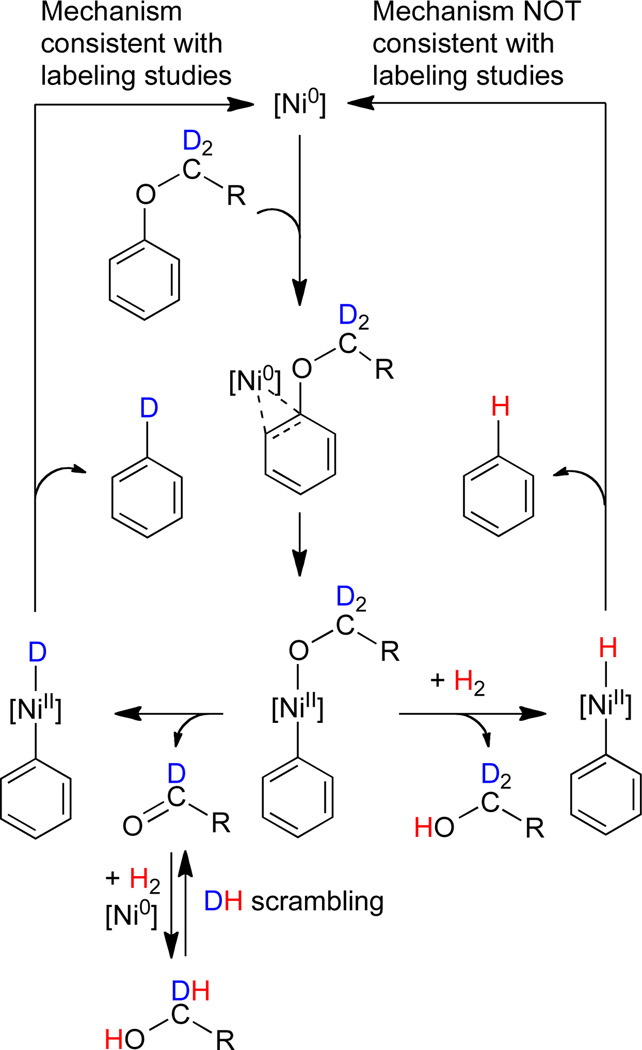
The generation of 6 and the intermediates characterized above map out a potential pathway for the cleavage of aryl alkyl ethers. Notably, this process does not require dihydrogen. To investigate the relevancy of this process for the catalytic reaction, aryl alkyl ethers were subjected to hydrogenolysis under reported reaction conditions (Scheme 2).22 2-Methoxynaphthalene and 2-hexyloxynaphthalene were selected as substrates. 1,3-Bis(2,6-diisopropylphenyl)-imidazolinium chloride (SIPr·HCl), NaOtBu, and Ni(COD)2 in m-xylene generate the catalytic species in situ. Heating to 120 °C for 16h under 1 atm of H2 leads to the formation of naphthalene and aliphatic alcohol (hexylsilylether analyzed upon derivatization). In order to elucidate the origin of the hydrogen delivered to the naphthyl group, substrates labeled with deuterium at the oxygen-bound carbon were prepared (NaphOCD3 and NaphOCD2(CH2)4CH3, Naph=2-naphthyl). Hydrogenolysis under H2 leads to the incorporation of deuterium in the resultant naphthalene, (> 90% Naph-D).50,51 The above results support a mechanism in which the aryl C–H is derived primarily from the ether substrate rather than dihydrogen. This finding is consistent with the mechanism observed for the stoichiometric reaction of the aryl ether with pendant phosphines (1): aryl C–O bond activation by nickel is followed by β-H elimination and reductive elimination (Scheme 3). Although the overall catalytic reaction is a hydrogenolysis of ethers, the reactive nickel hydride species may not result from the cleavage of a Ni-alkoxide bond by H2, but rather from β-H elimination from an alkoxide ligand. Isotopic labeling experiments with silanes as source of hydride (or deuteride) indicated isotopic scrambling and proved inconclusive (see SI).
Scheme 2.
Isotopic labeling studies of catalytic hydrogenolysis of arylalkylethers.
Scheme 3.
Mechanisms for the conversion of arylalkylethers to arenes.
Since the mechanism above does not require H2 for the conversion of arylether to arene, the catalytic trials were also performed in the absence of H2; less than 5% conversion was observed. In a separate experiment, a mixture of 1,3-bis(2,6-diisopropylphenyl)-imidazolinium chloride (SIPr·HCl), NaOtBu, and Ni(COD)2 in toluene was heated under H2, concentrated under vacuum to a dark red wax, reconstituted with m-xylene, and treated with aryl ether substrate under N2. Ether conversion to arene was observed, albeit in lower yields (ca. 50%) compared to standard conditions (ca. 75%). These experiments support the hypothesis that H2 is necessary for the formation of the active catalyst, but not for the actual catalytic transformation.
In summary, the mechanism of nickel-mediated aryl-ether hydrogenolysis was investigated. A substrate with pendant phosphines allowed the isolation and characterization of intermediates along the reaction pathway. These support a mechanism involving Ni(0) coordination to arene, oxidative addition of the aryl C–O bond, followed by β-H elimination, and formaldehyde-assisted reductive elimination of the aryl-H bond. Dihydrogen (1 atm) does not compete with the above processes. Isotopic labeling investigations of a catalytic system based on a nickel-NHC catalyst support a similar mechanism that does not require H2 for turnover. Overall the present studies provide mechanistic snapshots of a transformation of interest in organic methodology and with potential for biomass conversion. Future work will focus on detailed investigations of each step of this mechanism towards developing practical catalysts.
Supplementary Material
Acknowledgements
We thank Lawrence M. Henling for crystallographic assistance. We are grateful to Caltech, bp, and NSF GRFP (SL) for funding. The Bruker KAPPA APEXII X-ray diffractometer was purchased via an NSF CRIF:MU award to Caltech, CHE-0639094. The 400 MHz NMR spectrometer was purchased via an NIH award, RR027690.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and crystallographic details (CIF). This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2010;111:1346. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zim D, Lando VR, Dupont J, Monteiro AL. Org. Lett. 2001;3:3049. doi: 10.1021/ol016526l. [DOI] [PubMed] [Google Scholar]
- 3.Dankwardt JW. Angew. Chem. Int. Ed. 2004;43:2428. doi: 10.1002/anie.200453765. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone RAW, Neil McLean W. Tetrahedron Lett. 1988;29:5553. [Google Scholar]
- 5.Guan B-T, Xiang S-K, Wu T, Sun Z-P, Wang B-Q, Zhao K-Q, Shi Z-J. Chem. Commun. 2008:1437. doi: 10.1039/b718998b. [DOI] [PubMed] [Google Scholar]
- 6.Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866. doi: 10.1002/anie.200801447. [DOI] [PubMed] [Google Scholar]
- 7.Quasdorf KW, Tian X, Garg NK. J. Am. Chem. Soc. 2008;130:14422. doi: 10.1021/ja806244b. [DOI] [PubMed] [Google Scholar]
- 8.Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J. Am. Chem. Soc. 2011;133:6352. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. J. Am. Chem. Soc. 2008;130:14468. doi: 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]
- 10.Álvarez-Bercedo P, Martin R. J. Am. Chem. Soc. 2010;132:17352. doi: 10.1021/ja106943q. [DOI] [PubMed] [Google Scholar]
- 11.Tobisu M, Yamakawa K, Shimasaki T, Chatani N. Chem. Commun. 2011;47:2946. doi: 10.1039/c0cc05169a. [DOI] [PubMed] [Google Scholar]
- 12.Wenkert E, Michelotti EL, Swindell CS. J. Am. Chem. Soc. 1979;101:2246. [Google Scholar]
- 13.Antoft-Finch A, Blackburn T, Snieckus V. J. Am. Chem. Soc. 2009;131:17750. doi: 10.1021/ja907700e. [DOI] [PubMed] [Google Scholar]
- 14.Vicic DA, Jones WD. J. Am. Chem. Soc. 1997;119:10855. [Google Scholar]
- 15.Vicic DA, Jones WD. J. Am. Chem. Soc. 1999;121:7606. [Google Scholar]
- 16.Torres-Nieto J, Brennessel WW, Jones WD, García JJ. J. Am. Chem. Soc. 2009;131:4120. doi: 10.1021/ja809457s. [DOI] [PubMed] [Google Scholar]
- 17.van der Boom ME, Liou S-Y, Ben-David Y, Shimon LJW, Milstein D. J. Am. Chem. Soc. 1998;120:6531. [Google Scholar]
- 18.van der Boom ME, Liou S-Y, Ben-David Y, Vigalok A, Milstein D. Angew. Chem. Int. Ed. 1997;36:625. [Google Scholar]
- 19.Rinaldi R, Schuth F. Energy Environ. Sci. 2009;2:610. [Google Scholar]
- 20.Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM. Chem. Rev. 2010;110:3552. doi: 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- 21.Hicks JC. J. Phys. Chem. Lett. 2011;2:2280. [Google Scholar]
- 22.Sergeev AG, Hartwig JF. Science. 2011;332:439. doi: 10.1126/science.1200437. [DOI] [PubMed] [Google Scholar]
- 23.Velian A, Lin S, Miller AJM, Day MW, Agapie T. J. Am. Chem. Soc. 2010;132:6296. doi: 10.1021/ja101699a. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Day MW, Agapie T. J. Am. Chem. Soc. 2011;133:3828. doi: 10.1021/ja200368y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao ST, Lara NC, Lin S, Day MW, Agapie T. Angew. Chem. Int. Ed. 2011;50:7529. doi: 10.1002/anie.201102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Zhang S-L, Fu Y, Guo Q-X, Liu L. J. Am. Chem. Soc. 2009;131:8815. doi: 10.1021/ja810157e. [DOI] [PubMed] [Google Scholar]
- 27.An η6-aryl ether nickel complex has been characterized: Campora J, del Mar Conejo M, Reyes ML, Mereiter K, Passaglia E. Chem. Commun. 2003:78. doi: 10.1039/b209838e.
- 28.van der Boom ME, Milstein D. Chem. Rev. 2003;103:1759. doi: 10.1021/cr960118r. [DOI] [PubMed] [Google Scholar]
- 29.Steinke T, Shaw BK, Jong H, Patrick BO, Fryzuk MD. Organometallics. 2009;28:2830. doi: 10.1021/ja901346g. [DOI] [PubMed] [Google Scholar]
- 30.Kaufhold O, Stasch A, Pape T, Hepp A, Edwards PG, Newman PD, Hahn FE. J. Am. Chem. Soc. 2008;131:306. doi: 10.1021/ja807333f. [DOI] [PubMed] [Google Scholar]
- 31.Reaction of 1 with Ni(II) reagents results in stoichiometric cleavage of the ArO-CH3 bond. Investigations of this transformation will be reported in a future manuscript.
- 32.Ozawa F, Ito T, Yamamoto A. J. Am. Chem. Soc. 1980;102:6457. [Google Scholar]
- 33.Komiya S, Morimoto Y, Yamamoto A, Yamamoto T. Organometallics. 1982;1:1528. [Google Scholar]
- 34.Bryndza HE, Calabrese JC, Marsi M, Roe DC, Tam W, Bercaw JE. J. Am. Chem. Soc. 1986;108:4805. [Google Scholar]
- 35.Blum O, Milstein D. J. Organomet. Chem. 2000;593–594:479. [Google Scholar]
- 36.Smythe NA, Grice KA, Williams BS, Goldberg KI. Organometallics. 2008;28:277. [Google Scholar]
- 37.Fafard CM, Ozerov OV. Inorg. Chim. Acta. 2007;360:286. [Google Scholar]
- 38.Ritter JCM, Bergman RG. J. Am. Chem. Soc. 1998;120:6826. [Google Scholar]
- 39.Matas I, Cámpora J, Palma P, Álvarez E. Organometallics. 2009;28:6515. [Google Scholar]
- 40.McCarthy TJ, Nuzzo RG, Whitesides GM. J. Am. Chem. Soc. 1981;103:3396. [Google Scholar]
- 41.Alexanian EJ, Hartwig JF. J. Am. Chem. Soc. 2008;130:15627. doi: 10.1021/ja8056908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Hesslink H, Hartwig JF. J. Am. Chem. Soc. 2001;123:7220. doi: 10.1021/ja010417k. [DOI] [PubMed] [Google Scholar]
- 43.Saura-Llamas I, Gladysz JA. J. Am. Chem. Soc. 1992;114:2136. [Google Scholar]
- 44.Blum O, Milstein D. J. Am. Chem. Soc. 1995;117:4582. [Google Scholar]
- 45.Yamamoto T, Yamamoto A, Ikeda S. J. Am. Chem. Soc. 1971;93:3350. [Google Scholar]
- 46.Tatsumi K, Nakamura A, Komiya S, Yamamoto A, Yamamoto T. J. Am. Chem. Soc. 1984;106:8181. [Google Scholar]
- 47.An unidentified species was observed by 1H and 31P NMR spectroscopy, in amount roughly proportional to the label incorporation from SiX (X=D or H) into 6b. This is consistent with decreased generation of formaldehyde and hence lower formation of the nickel carbonyl species upon reductive elimination / decarbonylation..
- 48.Issenhuth J-T, Notter F-P, Dagorne S, Dedieu A, Bellemin-Laponnaz S. Eur. J. Inorg. Chem. 2010;2010:529. [Google Scholar]
- 49.Gómez-Gallego M, Sierra MA. Chem. Rev. 2011;111:4857. doi: 10.1021/cr100436k. [DOI] [PubMed] [Google Scholar]
- 50.Under hydrogenolysis conditions with D2, d0-naphthalene (m/z = 128) incorporates D atoms (up to m/z = 133 observed by GC-MS). Similarly, hydrogenolysis reactions of nondeuterated naphthyl alkyl ethers under D2 leads to scrambling of deuterium atoms into the substrates and resultant naphthalene, complicating analysis.
- 51.After subjecting the quenched reaction mixture of NaphOCD2(CH2)4CH3 under H2 to silyl derivatization, (n-hexyloxy)trimethylsilane was observed as >90% d0-isotopologue (m/z(−CH3) = 159). Conversely, (n-hexyloxy) trimethylsilane from reaction of NaphO(CH2)5CH3 under D2 returned a base peak of m/z = 161. Subjecting hexanol instead of arylalkylether to catalytic conditions under D2 and derivatization also yielded (n-hexyloxy)trimethylsilane with a base peak of m/z = 161.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.