Abstract
Research and clinical investigations in psychiatry largely rely on the de facto assumption that the diagnostic categories identified in the Diagnostic and Statistical Manual (DSM) represent homogeneous syndromes. However, the mechanistic heterogeneity that potentially underlies the existing classification scheme might limit discovery of etiology for most developmental psychiatric disorders. Another, perhaps less palpable, reality may also be interfering with progress—heterogeneity in typically developing populations. In this report we attempt to clarify neuropsychological heterogeneity in a large dataset of typically developing youth and youth with attention deficit/hyperactivity disorder (ADHD), using graph theory and community detection. We sought to determine whether data-driven neuropsychological subtypes could be discerned in children with and without the disorder. Because individual classification is the sine qua non for eventual clinical translation, we also apply support vector machine-based multivariate pattern analysis to identify how well ADHD status in individual children can be identified as defined by the community detection delineated subtypes. The analysis yielded several unique, but similar subtypes across both populations. Just as importantly, comparing typically developing children with ADHD children within each of these distinct subgroups increased diagnostic accuracy. Two important principles were identified that have the potential to advance our understanding of typical development and developmental neuropsychiatric disorders. The first tenet suggests that typically developing children can be classified into distinct neuropsychological subgroups with high precision. The second tenet proposes that some of the heterogeneity in individuals with ADHD might be “nested” in this normal variation.
Keywords: psychiatric disorders, research domain criteria, cognition, modularity, executive functions
In psychiatry, research and clinical investigation largely relies on the de facto assumption that the diagnostic categories identified in the Diagnostic and Statistical Manual of mental disorders (DSM-IV) represent etiologically homogeneous syndromes. However, there is considerable evidence that suggests the DSM does not necessarily describe homogenous conditions, but rather reflects the end result of multiple unique independent mechanistic pathways within a given disorder (1, 2). The mechanistic heterogeneity that potentially underlies the existing classification scheme might be limiting our ability to clarify etiology and identify novel therapeutics for several psychiatric illnesses (3).
A salient example, and our focus here, is attention deficit/hyperactivity disorder (ADHD). It is one of the earliest onset, most common, and costly neurodevelopmental disorders in child psychiatry (4, 5). Until recently, causal models of ADHD, as with other mental disorders, proposed a single core dysfunction (6). Investigators typically compare a group of children with ADHD defined by core symptoms (i.e., DSM) to a group of control children without the disorder. Statistical group differences based on psychometrics, functional brain imaging, or genetics are then used to inform models of the disorder.
This assumption of homogeneity in the case of ADHD has been questioned in numerous theoretical papers (7–13). For example, Nigg et al. (13) showed that several neuropsychological measures central to ADHD had substantial distributional overlap between ADHD and control samples. The data suggested that only a small minority of subjects with the disorder could be considered clinically “affected” on the basis of any one measure (13). Similar findings have been noted elsewhere (14, 15). In other words, whereas numerous unique neuropsychological measures have been proposed as related to ADHD, perhaps each of them applies to only a subset of those subjects with the disorder.
Although the role of heterogeneity in clinical populations has caught the collective attention of funding agencies (16) and the scientific community (8, 13, 15, 17), another, perhaps less palpable, reality may also be interfering with progress in understanding psychiatric illnesses—heterogeneity in typical populations. In the same way that investigators are often bound by the “cognitive box” (1) of the DSM when examining atypical development, they are also generally obliged to conduct their analysis as if typically developing comparison populations represent a monolithic group. Although consensus remains elusive on defining specific personality or cognitive “types” (18, 19), substantial evidence has accrued that individual differences in successful adaptive psychological styles are central to human development, functioning, social cohesion, and health outcomes (20–23). It may be that identifying a mechanism associated with a mental disorder requires comparing individuals to well-adjusted persons with the same cognitive style or profile.
Although it is easy to propose conceptually that there must be distinct subgroups within mental disorders (or typical populations), empirically demonstrating such subgroups is not straightforward. In the case of ADHD, emphasis has been on latent class analysis using symptom profiles (24), personality traits (25), and developmental trajectories of symptoms (26). These approaches are promising but appear to have mainly tended to identify severity classes rather than distinct categories (27). Efforts to identify types using neurocognitive measures—in theory, related to pathophysiological mechanisms—are still in the beginning stages. A key goal of this work is to identify procedures that are not prone to simply identifying severity groupings.
One approach, which may prove fruitful toward this goal, emanates from graph theory. Graph theory is a mathematical discipline about the study of networks, in which networks are simply sets of nodes or vertices joined in pairs by lines or edges (Fig. 1). Graph theory has been used to examine the organization of a number of relationships within systems (28). Importantly, many systems have well-defined internal structures and can be described or demarcated with graph theoretical analyses (28, 29). One area that has received considerable attention is the detection of community structure in networks. Community structure refers to the appearance of densely connected groups of nodes, with only sparse connections between the groups (Fig. 1) (29). The focus of this report is whether groups of children that share similar empirical neuropsychological features segregate to form specific data-driven phenotypic subtypes.
Fig. 1.
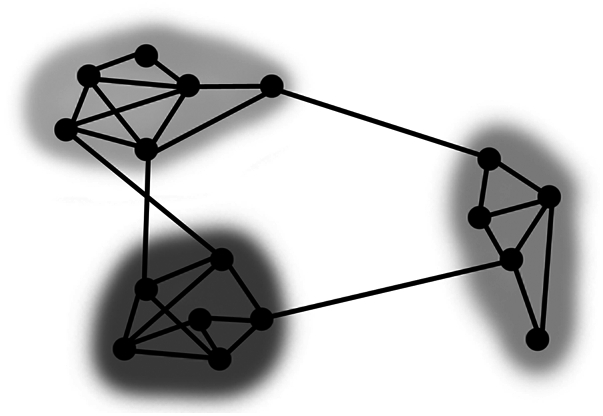
Graph theory and community detection. Displayed is a depiction of a network, where nodes (solid circles) are connected by edges (solid lines). In this paper nodes are participants and edges are correlations between participants’ neuropsychological scores. Community detection algorithms (29) can be applied to graph structures to identify clusters of nodes (shaded clouds) that share many edges within clusters relative to between clusters.
Because individual classification is the sine qua non for eventual translation to clinical use, we followed our community detection analysis with an investigation using support vector machine (SVM)-based multivariate pattern analysis (MVPA) (30, 31) to identify how well individual children can be identified as defined by the community detection delineated subtypes. SVMs are supervised classification algorithms rooted in statistical learning theory, capable of recognizing patterns for the purposes of categorization. Typically SVMs examine a set of training data for which each data point (e.g., person) has been assigned to a unique category with several defining features. On the basis of patterns among the features within each category, the training algorithm then builds a model capable of assigning new data points (e.g., individuals) into these specific categories. Here we use SVM-based MVPA to determine whether there is sufficient information in the neuropsychological scores to predict whether any individual can be accurately classified into a particular neuropsychological subgroup or profile defined by the community detection procedure. We also use the approach to determine whether ADHD status can be more accurately assigned after considering the community-based profiles.
Results
Feature Reduction Supported a Seven-Factor Model of Cognitive Abilities.
For the current investigation we apply community detection to a well-characterized dataset of 498 children who include both typically developing control youth (TDC) (n = 213) and youth with ADHD (n = 285) (Table S1). From these youth, some 20 neuropsychological measures were obtained that were intended to cover a wide domain of cognitive functions variously theorized to be involved in ADHD (Table S2 and Fig. 2).
Fig. 2.
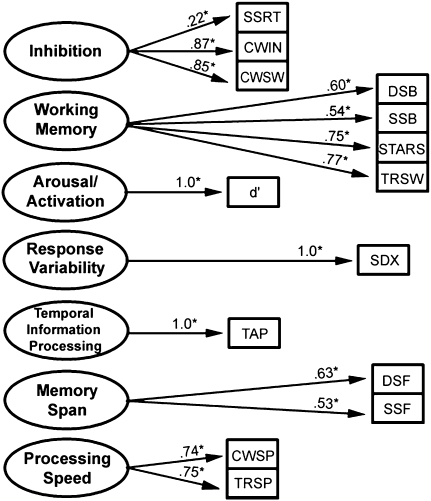
Data reduction for neuropsychological measures. Confirmatory factor analysis (CFA) was used to conduct rational reduction of the measures listed in Table S2. Shown is our conceptual model that depicts how we hypothesized that our measured variables relate to seven latent factors. It also displays the factor loadings for the seven-factor model. For ease of presentation, the figure does not display error terms, cross loadings, or correlations among latent factors. CWIN, color word inhibition; CWSP, color word speed; CWSW, color word switching; d′, D-prime; DSB, digit span backward; DSF, digit span forward; SDX, response variability; SSB, spatial span backward; SSF, spatial span forward; SSRT, stop signal reaction time; STARS, stars task; TAP, tapping task (temporal information processing task); TRSP, trails number and letter naming speed average; TRSW, trails-making task switching.
Our approach was to use a broad set of neuropsychological variables relevant to ADHD, while avoiding use of an excessive number of redundant indicators in our analysis. We therefore conducted a rational feature reduction using confirmatory factor analysis (CFA), according to the conceptual model that had guided our work (Fig. 2 and refs. 32 and 33). All measures were transformed such that higher scores were indicative of worse performance (e.g., slower speed or worse accuracy). Fig. 2 portrays our primary model with the empirical factor loadings (SI Text). Because we were sensitive to the possibility of equivalent models, we also tested several competing models that conformed in varying degrees to our theorized reasons for choosing these measures. Fit of all models was evaluated using several indexes, including the χ2-value, the comparative fit index (CFI), the Tucker Lewis index (TLI), and the root mean-square error of approximation (RMSEA) (SI Text).
Results for the feature reduction for the neuropsychological measures showed that a one-factor model fit inadequately [χ2 (70) = 386.2, CFI = 0.87, TLI = 0.84, RMSEA = 0.09], justifying our effort to create a multiconstruct model. Fit was satisfactory and comparable for the best-fitting five-, six-, and seven-factor models as follows: five-factor χ2 (63) = 103.4, CFI = 0.98, TLI = 0.97, RMSEA = 0.036; six-factor χ2 (58) = 95.1, CFI = 0.98, TLI = 0.98, RMSEA = 0.035; and seven-factor χ2 (52) = 89.9, CFI = 0.98, TLI = 0.97, RMSEA = 0.038. For our main analysis we present results for the seven-factor model because we viewed it as conceptually the most differentiated description of the abilities tested, the most consistent with our conceptual framework, and equivalent in fit to the other models. However, we conducted the modularity analysis using the best-fitting six- and five-factor solutions as well to see whether community assignments were conceptually similar with these slightly different indicators, and indeed they were (Fig. S1).
Comparing the Full ADHD and TDC Cohorts Replicated Previous Findings.
The second stage of our analysis began with a traditional comparison between the ADHD and TDC cohorts. This analysis was followed by our SVM pattern classifier to determine how well the neuropsychological scores can inform individual distinctions between ADHD and TDC.
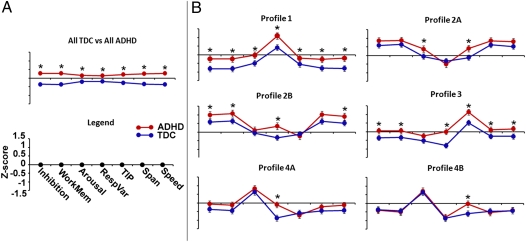
The comparisons yielded significant differences across all measures (Fig. 3A and Table S3). Importantly, despite these highly reliable differences, the SVM classifier was unable to make strong distinctions in individual cases with regard to ADHD status (65% accuracy, 86.3% sensitivity, and 38.5% specificity).
Fig. 3.
Atypical neuropsychological measures are specific to cognitive subgroup. Here we show the comparison of neuropsychological measures between ADHD and TDC. (A) Comparison between the entire TDC and ADHD samples. (B) ADHD vs. TDC comparison within each subgroup. Interestingly, atypical neuropsychological measures relative to the control population are not uniform across all subgroups. Rather, each subgroup has a unique pattern of atypical measures (*, significant differences between groups; details in Table S3).
This analysis served as a baseline to determine whether the subgroups identified by our community detection analysis (below) showed similar findings or whether the atypical nature of any given factor resided in specific profiles (i.e., groups of participants). In the same way, we also used this baseline to determine whether diagnostic classification could be improved when considering our community detection-derived profiles.
Community Detection Revealed Unique Subpopulations or Profiles in both the TDC and the ADHD Cohorts.
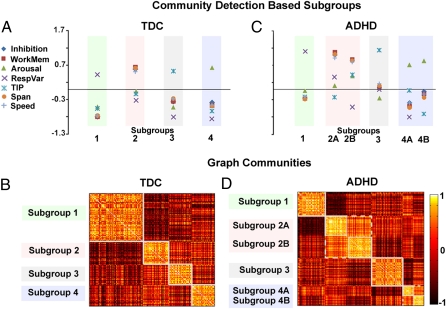
We first applied community detection to the TDC population. Although common practice would expect this investigation to yield one unitary group, the analysis instead yielded four unique communities or subgroups (Fig. 4). Importantly, the quality index (Q = 0.45) and variation of information (VOI) (two measures of community robustness), as well as a secondary randomization analysis (SI Text and Figs. S2 and S3), showed that the subgroups identified here were significantly different from random.
Fig. 4.
Community detection identified subgroups. (A) After applying the community detection procedure to the typically developing cohort, four unique subgroups (i.e., cognitive profiles) emerge (y axis = z score). The community structure is depicted by correlation matrices shown in B. These correlation matrices represent a 213 × 213 matrix (for TDC) and 285 × 285 matrix (for ADHD). On the grid, darker colors reveal lower or negative correlations between subjects, and lighter colors reveal positive correlations between subjects. Identified communities are outlined in white. (C) Independently applying the community detection algorithm to the ADHD cohort shows similar findings to those in A. The difference between the two appears to be split in subgroup 2 and subgroup 4. The correlation matrices of the ADHD cohort are presented in D.
Each of the four groups had unique patterns of factor scores. One group (43% of sample) appeared to have a pattern consistent with more response variability relative to their peers (subgroup 1). The second group (20% of sample) had reduced working memory, memory span, inhibition, and output speed (subgroup 2). The third group (18% of sample) had relatively inaccurate temporal information processing (subgroup 3). The last group (18% of sample) had relatively weak signal detection, suggesting suboptimal or altered arousal (subgroup 4). These groups show minimal differences in intelligence quotient (IQ), age, or sex ratios (with one exception, see Tables S4 and S5). This analysis shows that despite traditional assumptions that nonclinical control populations are uniform entities, our results fit better with the alternative supposition that there are unique neuropsychological profiles even in typically developing, well-adjusted samples.
We next applied the same community detection procedure to the ADHD sample. Independently testing the community structure in this sample revealed similar findings, albeit via six groups (Fig. 4). Again, Q (Q = 0.55) and the VOI analysis (Fig. S2) highlighted the robustness of the communities. Similar to the TDC, the first group (21% of sample) appeared to have a pattern consistent with high levels of reaction time variability relative to their peers (subgroup 1). As with TDC, the second group (17% of sample) appeared to have reduced working memory, memory span, and output speed (i.e., subgroup 2A). The third group (20% of sample) also had the same apparent weaknesses, but with a slightly modified profile in the remaining measures (i.e., subgroup 2B). The fourth group (25% of sample) had inaccurate temporal information processing (subgroup 3). The fifth group (8% of sample) was also similar to the fourth group of TDC youth in that they appeared to be characterized by suboptimal arousal (subgroup 4A). The sixth group (8% of sample) was similar to the fifth with regard to low arousal, however, with a slightly different profile in the remaining measures (subgroup 4B).
Strikingly, even though these groups were all distinct in their neuropsychological profiles, they again showed minimal differences in symptom scores, IQ, age, or sex ratios (Tables S4 and S5). Thus, these are not simply more or less severe ADHD groups, but rather unique cognitive profiles within children who all have similar severity of ADHD.
SVM-Based MVPA Highlights the Robustness of the Community Detection-Defined Profiles.
To further test the overall robustness of these defined groups we next arranged our SVM to classify individual subjects between the profiles identified within the TDC and ADHD cohorts. To do this we first split the ADHD and TDC samples into two: each cohort having a test set and a training set (SI Text). We independently reapplied our community detection procedure to both the ADHD and the TDC cohorts and reproduced the group assignments identified in the first analysis (Fig. S4). We then used our training set to train the SVM on the four group assignments for TDC and the six group assignments for the ADHD populations. The test evaluates how robustly any one individual can be classified into his/her neuropsychological profile.
Classification generally was quite respectable across the groups with 78% accuracy for the TDC population (subgroup 1, 93%; subgroup 2, 71%; subgroup 3, 71%; subgroup 4, 81.25%) and 77% accuracy for the ADHD population (subgroup 1, 78%; subgroup 2A, 75%; subgroup 2B, 66.25%; subgroup 3, 77%; subgroup 4A, 88%; note that subgroup 4B was unable to be examined as it was not reproduced in large enough numbers of participants within each split to do SVM testing) (SI Text).
Atypical Neuropsychological Measures Are Unique on the Basis of Profile and ADHD Classification Improves Within Profiles.
Considering the unique nature of the subclassifications, we next returned to our group comparisons between ADHD and TDC. However, instead of comparing all ADHD with all TDC, we now compared ADHD and TDC neuropsychological scores within their specific assigned neuropsychological “type” or group. The results are presented in Fig. 3 and Table S3. Whereas the ANOVA in six of the seven factors showed a main effect of diagnosis, the post hoc comparisons showed that not all subgroups had the same atypical neuropsychological scores.
Among ADHD subgroups, atypical inhibitory control, speed, and working memory were each found in three groups. Atypical arousal and span were each identified in two groups. Atypical response variability and temporal information priming were each found in four groups. Interestingly, the ADHD classification improved or remained the same for all but one of the groups, relative to the initial All ADHD vs. All TDC analysis. Group 4A had the maximal improvement at 84.1% classification of ADHD vs. TCD youth (83.3% sensitivity and 84.6% specificity). The remaining groups were as follows: group 1, 68% total, 49.2% sensitivity, 80.4% specificity; group 2A, 68.5%, 83.7%, 51.2%; group 2B, 64.7%, 80.4%, 44.2%; group 3, 73.6%, 88.73%, 46.2%; group 4A, 84.1%, 83.3%, 84.6%; and group 4B, 61.9%, 37%, 76.9%.
Discussion
Trait Variation in the Typically Developing Population Informs Heterogeneity in ADHD.
In this report we used graph theoretical tools to clarify a portion of the heterogeneity that exists within ADHD and typically developing control populations. Two important principles were identified that have the potential to advance our understanding of typical development and developmental neuropsychiatric disorders. The first tenet suggests that, on the basis of neuropsychological performance, typically developing children can be classified into distinct subgroups with high precision. The second tenet proposes that the heterogeneity in individuals with ADHD appears to be “nested” in this normal variation. As illustrated by our single subject classification procedures, comparing typically developing children with ADHD children within each of these distinct subgroups increases diagnostic accuracy (i.e., ADHD vs. non-ADHD classification) on the basis of the neuropsychological measures. This work highlights that illumination of such subgroups could potentially have significant practical importance for understanding the nature of typical development and identifying the etiologic underpinnings of complex disorders such as ADHD.
What Is the Role of Behavioral Variation in the Typically Developing Population and How Might It Arise?
For years evolutionary psychologists have argued that human behavior (and that of other animal species) is under the same selective pressures as the physical traits so elegantly described by Darwin (34). Indeed, Darwin himself predicted this likeness to be the case at the end of his work On the Origin of Species, noting “In the distant future I see open fields for far more important researches. Psychology will be based on a new foundation, that of the necessary acquirement of each mental power and capacity by gradation” (Darwin, 1859) (ref. 19, p. 399). As such, there are evolutionary arguments to be made with regard to how neuropsychological diversity might arise in the population.
The modeling literature related to adaptive complex social systems also provides significant evidence that suggests psychological heterogeneity is an important means by which to improve the robustness of the collective when faced with shifting environmental demands (35). It is a key feature in the stability of complex social systems and thus has likely been an important attribute of our evolving species.
With respect to our findings it should be noted that at times heritable diversity might form along a continuous dimension (i.e., unimodal distribution), whereas at others times it may form as multiple discrete strategies (i.e., multimodal distributions) (21, 36–38). In the latter proposal, average fitness would be about equal across the normal range of any given behavioral strategy, but individuals of different strategies might vary in the way they achieve fitness. Rapid and sizeable changes in environmental demands across time may have served as the driving force toward multiple “peaks” with regard to neurocognitive strategies or profiles in typical populations (21, 36), as found here.
For example, in the cognitive neuroscience literature some have recently argued that the single-nucleotide polymorphism (val158met) of the catechol O-methyltransferase (COMT) gene, which codes for an enzyme that degrades dopamine in prefrontal cortex, may relate to evolutionary trade-offs between efficient executive functioning (met) and improved emotional regulation (val) (21, 37). In this proposal each allele is maintained in the population because each provides an environment-specific selective advantage—one for cognitive efficiency under typical conditions and the other for emotional resilience under stressful circumstances (21, 37). Similar arguments have been made with regard to COMT and working memory, with an evolutionary trade-off between efficient working memory updating (val) and robust working memory maintenance (met) (22). It might be reasoned that the discrete subgroups observed here (Figs. 3 and 4) are a small representation of similar forms of neuropsychological diversity.
Just as important, these data suggest that heterogeneity within developmental neuropsychiatric disorders, such as ADHD, might be nested within this normal variation. This proposal provides a unique, and perhaps fruitful, way to conceptualize and study heterogeneity in developmental neuropsychiatric disorders.
Atypical Neuropsychological Patterns in ADHD Are Specific to Subgroup Membership.
In general all of the measures examined here have been previously reported as atypical in ADHD (ref. 13 and Fig. 3). The question then is, Are these measures equally atypical within all of the identified subgroups?
For the purposes of simplicity, after the community detection analysis, we labeled each identified subgroup on the basis of the “standout” factor(s) that was lower relative to the general cohort for the TDC population. This procedure left us with four groups, which we label as follows: a variability group (subgroup 1), a low executive group (subgroup 2), a low temporal information processing group (subgroup 3), and a low arousal group (subgroup 4).
Interestingly, independently applying Newman's community detection algorithm (29) on the ADHD cohort replicated this finding, showing similar subgroup patterns (Fig. 4). The main deviation from the finding in TDC was the revelation of an additional low arousal group (subgroup 4B) and an additional low executive group (subgroup 2B). This particular result, again, suggests that some of the heterogeneity within ADHD appears to be nested within the variation found in typically developing populations. If we begin to examine the cognitive deficits within these subgroups (Fig. 3 and Table S3), rather than comparing across the entire cohorts of ADHD and TDC (Fig. 3 and Table S3), we see unique defining patterns in the deficits based on specific profiles.
For example, poor response inhibition has been suggested to be a critical component of ADHD (39) and has been well established at the group level (40). Our initial analysis comparing the entire ADHD cohort to the TDC population replicates this particular finding (Fig. 3). However, a closer look at this effect based on the subgroups identified in our graph analysis shows that this measure is atypical in only three of the six ADHD subgroups relative to controls within the same profile.
A similar finding is reported for two other well-known deficits in ADHD—working memory and temporal information processing. Again, whereas the comparison of the full ADHD and TDC cohort replicated previous findings in this regard (41), the comparisons based on community structure yielded only a subset of profiles with working memory and temporal information processing deficits. Qualitatively stronger results appeared for response variability, which was atypical in four of the six communities, supporting theories of its importance in ADHD (8). Finally, weaker, although statistically significant, contributions toward ADHD status were also identified for spatial span and speed, but again in only a subset of the identified profiles (Fig. 3 and Table S3). These results support the claim outlined in the introductory section of this paper: Whereas numerous unique neuropsychological measures have been proposed as related to ADHD (13), each of them appears to apply to only a subset of those with the disorder.
Individual Classification Based on Neuropsychological Measures Improves After Community-Based Subtyping.
We note that the robustness of the identified communities was tested in several ways (e.g., Figs. S2 and S3), indicating that the group assignments identified here are highly deviant from random. One of these tests was the single-subject classification using the SVM-based MVPA. Here we demonstrated that there is enough information in the applied cognitive battery to make valid predictions of community assignment for individual subjects. In addition, we see that diagnostic classification of ADHD status for individuals in most of the subgroups, albeit modest in some, can be identified with higher accuracy when the community assignments were taken into account. The ability to characterize individual subjects and identify ADHD status empirically on the basis of a cognitive battery opens the door for tractable genetic, functional, and clinical applications. However, a crucial next step will be to evaluate the temporal stability of these cognitive types to see whether they improve on the temporal instability of DSM-IV clinical types.
Current Approach Can Be Extended Across Multiple Modalities for Other Neuropsychiatric Disorders.
Although we believe our reported findings are quite provocative, we do not claim that they are exhaustive. Repeating our analysis using additional or unique neuropsychological domains might further classify or characterize profiles. Likewise, extending the present approach to neuroimaging studies may accelerate such characterization and inform heterogeneity in the neurobiology underlying the typical developing and ADHD populations (42, 43). This approach would allow for a more streamlined procedure to help investigators target and test theories related to multiple unique pathways or circuits related to ADHD (42, 43). Indeed, the same methods may be well suited to categorize participants on the basis of the neural circuitry itself or genetic markers (or pathways) rather than neuropsychological domains. We hypothesize that each group identified here is represented by distinct, multiple brain profiles. Finally we note that the approach used here is not limited to the study of ADHD, but could be used to inform heterogeneity and perhaps etiology in other developmental or adult neuropsychiatric disorders, and thus has broad appeal.
Methods
Subjects and Demographics.
Children who completed a full research psychiatric evaluation aged 6–17 y participated in this study (TDC, n = 213; ADHD, n = 285) (SI Text). Demographic details are listed in Table S1.
Background Measures of Cognitive Functioning.
Youth completed a diagnostic screening along with other testing, which included a short form of the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (44). The age-adjusted standardized score was used as the estimate of full-scale IQ.
Neuropsychological Measures Theorized to Relate to ADHD.
The neuropsychological battery was designed to capture working memory (41), response inhibition (39, 45), response variability (8), temporal information processing (46), arousal and activation (47), interference control (48), and response speed (49). All of our measures are listed in Table S2. Detailed explanations of each measure are provided in SI Text.
Factor Analysis Data Reduction for Neuropsychological Measures.
Rational data reduction of our measures was accomplished via confirmatory factor analysis (Fig. 2). Fit for all of our models was evaluated using several indexes as noted in Results and SI Text, using the latent variable modeling program, MPLUS.
Identification of Subgroups via Community Detection.
To examine the strength of subject-to-subject relationships via graph theory, correlation matrices were created between subjects across the seven identified factor scores. This procedure created two square correlation matrices providing distance information (i.e., a correlation) between any given subject pair within the ADHD and TDC cohorts (Fig. 4). Subsequent community detection (29) was applied to these matrices separately. The threshold for connected vs. unconnected pairs in each cohort was based on the maximum threshold where reachability remained equal to 1 (SI Text). This reachability threshold for the TDC graph was at r = 0.56, and the threshold for the ADHD graph was r = 0.73. To ensure our analysis did not depend on threshold selection, we also ran our community detection across multiple thresholds. In addition, we applied a weight-conserving modularity algorithm not dependent on threshold selection (50). Both additional procedures yielded largely consistent results (Fig. S2 and SI Text). The strength of the modularity assignments was based on the quality index (Q), VOI (29, 51), and simulations created by iteratively repeating our analyses after randomizing the factor scores across participants (SI Text and Figs. S2 and S3). All of the preceding calculations were performed in MATLAB (Mathworks), using scripts generously provided by Olaf Sporns, Mikail Rubinov, and other collaborators (52) (Indiana University, Bloomington, IN).
Support Vector Machine-Based Multivariate Pattern Analysis.
For the SVM-based MVPA we use Spider (http://people.kyb.tuebingen.mpg.de/spider/main.html), an object-orientated environment for machine learning in MATLAB. Full details of this procedure are provided in SI Text.
Supplementary Material
Acknowledgments
We thank Bria L. Thurlow and Swathi Iyer for their helpful comments and figure preparation. We also thank the funding institutions and grants that supported this work: Grant R00 MH091238 (to D.A.F.), Oregon Clinical and Translational Research Institute (D.A.F.), Medical Research Foundation (D.A.F.), and Grants R01 MH086654 and R01 MH59105 (both to J.T.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115365109/-/DCSupplemental.
References
- 1.Hyman SE. The diagnosis of mental disorders: The problem of reification. Annu Rev Clin Psychol. 2010;6:155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532. [DOI] [PubMed] [Google Scholar]
- 2.Miller G. Psychiatry. Beyond DSM: Seeking a brain-based classification of mental illness. Science. 2010;327:1437. doi: 10.1126/science.327.5972.1437. [DOI] [PubMed] [Google Scholar]
- 3.Agid Y, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6:189–201. doi: 10.1038/nrd2217. [DOI] [PubMed] [Google Scholar]
- 4.Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol. 2007;32:711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- 5.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 6.Wakefield JC. Diagnosing DSM-IV—Part I: DSM-IV and the concept of disorder. Behav Res Ther. 1997;35:633–649. doi: 10.1016/s0005-7967(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 7.Berger A, Posner MI. Pathologies of brain attentional networks. Neurosci Biobehav Rev. 2000;24:3–5. doi: 10.1016/s0149-7634(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 9.Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child Adolesc Psychiatr Clin N Am. 2008;17:367–384, ix. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Sonuga-Barke EJ. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 11.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Nigg JT, Goldsmith HH, Sachek J. Temperament and attention deficit hyperactivity disorder: The development of a multiple pathway model. J Clin Child Adolesc Psychol. 2004;33(1):42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- 13.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Solanto MV, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- 15.Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Insel T, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 17.Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- 18.Lickliter R, Honeycutt H. Developmental dynamics: Toward a biologically plausible evolutionary psychology. Psychol Bull. 2003;129:819–835. doi: 10.1037/0033-2909.129.6.819. [DOI] [PubMed] [Google Scholar]
- 19.Buss DM, Reeve HK. Evolutionary psychology and developmental dynamics: Comment on Lickliter and Honeycutt (2003) Psychol Bull. 2003;129:848–853. doi: 10.1037/0033-2909.129.6.848. [DOI] [PubMed] [Google Scholar]
- 20.Buss DM. Evolutionary personality psychology. Annu Rev Psychol. 1991;42:459–491. doi: 10.1146/annurev.ps.42.020191.002331. [DOI] [PubMed] [Google Scholar]
- 21.Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 22.Braver TS, Cole MW, Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Curr Opin Neurobiol. 2010;20:242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman BP, Goldberg LR. Replicability and 40-year predictive power of childhood ARC types. J Pers Soc Psychol. 2011;101:593–606. doi: 10.1037/a0024289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd RD, et al. Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. Am J Psychiatry. 2001;158:1891–1898. doi: 10.1176/appi.ajp.158.11.1891. [DOI] [PubMed] [Google Scholar]
- 25.Martel MM, Goth-Owens T, Martinez-Torteya C, Nigg JT. A person-centered personality approach to heterogeneity in attention-deficit/hyperactivity disorder (ADHD) J Abnorm Psychol. 2010;119:186–196. doi: 10.1037/a0017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jester JM, et al. Trajectories of childhood aggression and inattention/hyperactivity: Differential effects on substance abuse in adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:1158–1165. doi: 10.1097/CHI.0b013e3181825a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubke GH, et al. Subtypes versus severity differences in attention-deficit/hyperactivity disorder in the Northern Finnish Birth Cohort. J Am Acad Child Adolesc Psychiatry. 2007;46:1584–1593. doi: 10.1097/chi.0b013e31815750dd. [DOI] [PubMed] [Google Scholar]
- 28.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 29.Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Rizk-Jackson A, et al. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington's disease using machine learning techniques. Neuroimage. 2011;56:788–796. doi: 10.1016/j.neuroimage.2010.04.273. [DOI] [PubMed] [Google Scholar]
- 32.Pennington BF. The Neuropsychology of Learning Disabilities. New York: Guilford; 1991. [Google Scholar]
- 33.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Darwin C. On the Origin of Species by Means of Natural selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JH, Page SE. Complex Adaptive Systems: An Introduction to Computational Models of Social Life. Princeton: Princeton Univ Press; 2007. [Google Scholar]
- 36.Nettle D. The evolution of personality variation in humans and other animals. Am Psychol. 2006;61:622–631. doi: 10.1037/0003-066X.61.6.622. [DOI] [PubMed] [Google Scholar]
- 37.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Mol Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 38.Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 40.Willcutt EG, et al. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46(10):1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 42.Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol. 2007;20:119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- 43.Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler Intelligence Scale for Children Technical and Interpretive Manual. 4th Ed. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 45.Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- 46.Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: Findings to date and new methods. J Neurosci Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Sergeant JA, Oosterlaan J, van der Meere JJ. Information Processing and Energetic Factors in Attention Deficit/Hyperactivity Disorder. New York: Kluwer/Plenum; 1999. [Google Scholar]
- 48.Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: Views from developmental psychology. Dev Neuropsychol. 1988;4:199–230. [Google Scholar]
- 49.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56:2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 51.Karrer B, Levina E, Newman ME. Robustness of community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:046119-1–046119-9. doi: 10.1103/PhysRevE.77.046119. [DOI] [PubMed] [Google Scholar]
- 52.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.