Abstract
Renin, a key enzyme controlling blood pressure, is produced mainly in the kidney. To identify the transcriptional regulatory elements of the mouse Ren-1c gene, the promoter regions were fused to the CAT reporter gene and transfected into embryonic kidney-derived 293 cells and four extrarenal cell lines, HeLa, HepG2, HT1080 and NIH3T3 cells. Transient transfection assay showed that sequences from -365 to +16 of the renin gene could direct transcription of the CAT hybrid gene only in 293 cells. Deletion analysis identified two transcriptionally active regions; the renin upstream-promoter element (RU-1 element; position -224 to -138) and the renin proximal-promoter element (RP-2 element; position -75 to -47). Although the RU-1 element functioned as an activator, depending on its orientation, it failed to trans-activate the renin promoter when the RP-2 element was deleted. By contrast, the proximal element alone exhibited a weak trans-activator property. Gel shift assay identified RU-1 element-binding factors in both 293 and HeLa cells, whereas 293 cell-dominant factors were shown to bind only to RP-2 element. Therefore, both RU-1 and RP-2 elements were found to be necessary for efficient CAT expression from the renin promoter in 293 cells, suggesting that activation of the Ren-1c promoter requires combined action between cell type-dominant and ubiquitous nuclear factors.
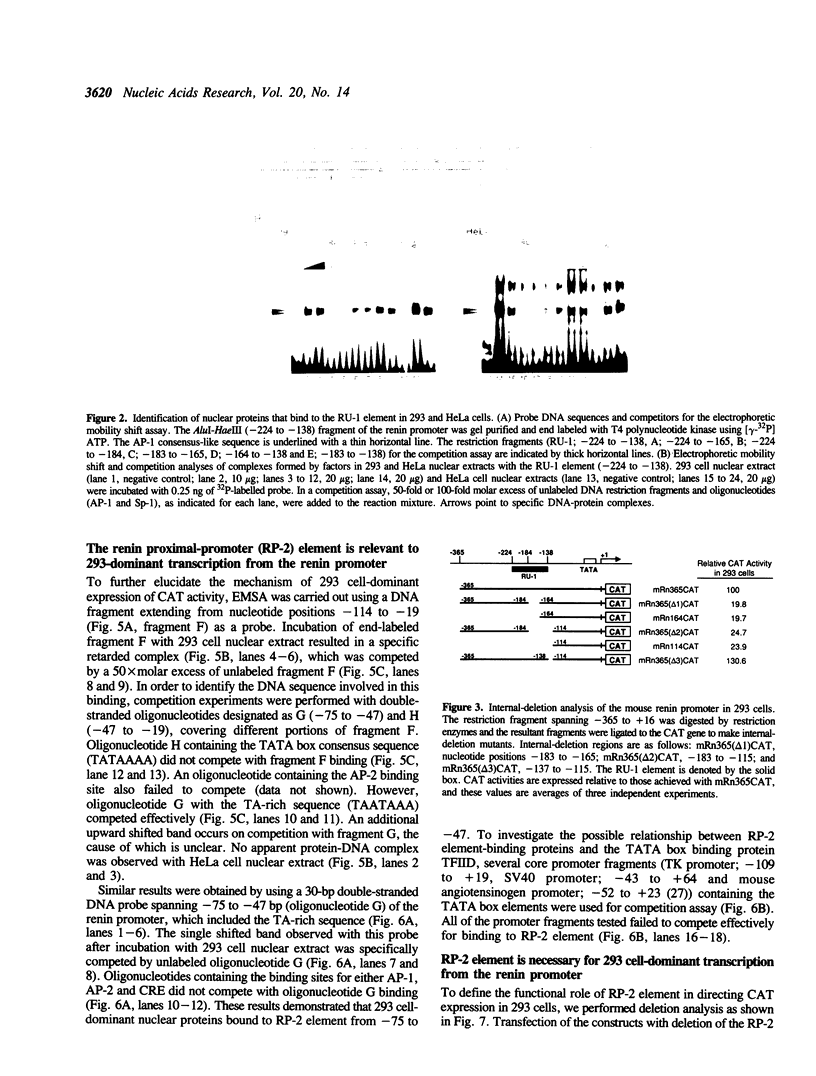
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett G., Horiuchi M., Paul M., Pratt R. E., Nakamura N., Dzau V. J. Identification of a negative regulatory element involved in tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):885–889. doi: 10.1073/pnas.89.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J., Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992 Jan;11(1):327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Mullins L. J., George H., Smith G., Brooks J., Pioli D., Brammar W. J. The nucleotide sequence of a mouse renin-encoding gene, Ren-1d, and its upstream region. Gene. 1989 Dec 7;84(1):91–104. doi: 10.1016/0378-1119(89)90143-1. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Nakamura N., Kelley P., Dzau V. J. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem. 1989 May 5;264(13):7357–7362. [PubMed] [Google Scholar]
- Buttice G., Quinones S., Kurkinen M. The AP-1 site is required for basal expression but is not necessary for TPA-response of the human stromelysin gene. Nucleic Acids Res. 1991 Jul 11;19(13):3723–3731. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin E., Medcalf R. L., Bergonzelli G. E., Kruithof E. K. Regulatory elements involved in constitutive and phorbol ester-inducible expression of the plasminogen activator inhibitor type 2 gene promoter. Nucleic Acids Res. 1991 Jul 25;19(14):3881–3886. doi: 10.1093/nar/19.14.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Jameson J. L., Habener J. F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987 Sep 5;262(25):12169–12174. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K. G., Haidar M. A., Baxter J. D., Reudelhuber T. L. Regulation of human renin expression in chorion cell primary cultures. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7588–7592. doi: 10.1073/pnas.87.19.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Field L. J., Gross K. W. Ren-1 and Ren-2 loci are expressed in mouse kidney. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6196–6200. doi: 10.1073/pnas.82.18.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Philbrick W. M., Howles P. N., Dickinson D. P., McGowan R. A., Gross K. W. Expression of tissue-specific Ren-1 and Ren-2 genes of mice: comparative analysis of 5'-proximal flanking regions. Mol Cell Biol. 1984 Nov;4(11):2321–2331. doi: 10.1128/mcb.4.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental C., Kanno M., Nomiyama H., Chambon P. Cooperativity and hierarchical levels of functional organization in the SV40 enhancer. Cell. 1988 Sep 23;54(7):943–953. doi: 10.1016/0092-8674(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Fukamizu A., Nishi K., Nishimatsu S., Miyazaki H., Hirose S., Murakami K. Human renin gene of renin-secreting tumor. Gene. 1986;49(1):139–145. doi: 10.1016/0378-1119(86)90393-8. [DOI] [PubMed] [Google Scholar]
- Fukamizu A., Takahashi S., Seo M. S., Tada M., Tanimoto K., Uehara S., Murakami K. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. J Biol Chem. 1990 May 5;265(13):7576–7582. [PubMed] [Google Scholar]
- Fukamizu A., Tanimoto K., Uehara S., Seo M. S., Handa S., Sagara M., Takahashi S., Imai T., Murakami K. Regulation of human renin and angiotensinogen genes. Biomed Biochim Acta. 1991;50(4-6):659–663. [PubMed] [Google Scholar]
- Fukamizu A., Uehara S., Sugimura K., Kon Y., Sugimura M., Hasegawa T., Yokoyama M., Nomura T., Katsuki M., Murakami K. Cell type-specific expression of the human renin gene. J Biol Regul Homeost Agents. 1991 Jul-Sep;5(3):112–116. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Wang C. K., Fujii H., Cromlish J. A., Weil P. A., Roeder R. G. Cloning and structure of a yeast gene encoding a general transcription initiation factor TFIID that binds to the TATA box. Nature. 1989 Sep 28;341(6240):299–303. doi: 10.1038/341299a0. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Nakamura N., Tang S. S., Barrett G., Dzau V. J. Molecular mechanism of tissue-specific regulation of mouse renin gene expression by cAMP. Identification of an inhibitory protein that binds nuclear transcriptional factor. J Biol Chem. 1991 Aug 25;266(24):16247–16254. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Itskovitz J., Sealey J. E., Glorioso N., Rosenwaks Z. Plasma prorenin response to human chorionic gonadotropin in ovarian-hyperstimulated women: correlation with the number of ovarian follicles and steroid hormone concentrations. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7285–7289. doi: 10.1073/pnas.84.20.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. A., Sigmund C. D., McGowan R. A., Kane-Haas C. M., Gross K. W. Expression of murine renin genes during fetal development. Mol Endocrinol. 1990 Mar;4(3):375–383. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lin H., Yutzey K. E., Konieczny S. F. Muscle-specific expression of the troponin I gene requires interactions between helix-loop-helix muscle regulatory factors and ubiquitous transcription factors. Mol Cell Biol. 1991 Jan;11(1):267–280. doi: 10.1128/mcb.11.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T., Murakami K., Inagami T. Rat renin: purification and characterization. Biochim Biophys Acta. 1978 Oct 12;526(2):560–571. doi: 10.1016/0005-2744(78)90146-8. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kawazoe S., Ichihara T., Shinyama H., Kageyama M., Morimoto S. Stimulatory effects of neuronally released norepinephrine on renin release in vitro. Am J Physiol. 1988 Oct;255(4 Pt 2):F614–F620. doi: 10.1152/ajprenal.1988.255.4.F614. [DOI] [PubMed] [Google Scholar]
- McCormick A., Brady H., Fukushima J., Karin M. The pituitary-specific regulatory gene GHF1 contains a minimal cell type-specific promoter centered around its TATA box. Genes Dev. 1991 Aug;5(8):1490–1503. doi: 10.1101/gad.5.8.1490. [DOI] [PubMed] [Google Scholar]
- Misseyanni A., Klug J., Suske G., Beato M. Novel upstream elements and the TATA-box region mediate preferential transcription from the uteroglobin promoter in endometrial cells. Nucleic Acids Res. 1991 Jun 11;19(11):2849–2859. doi: 10.1093/nar/19.11.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi J., Gardes J., Gonzalez M. F., Corvol P., Menard J. Angiotensinogen's role in ANG formation, renin release, and renal hemodynamics in isolated perfused kidney. Am J Physiol. 1989 Apr;256(4 Pt 2):F719–F727. doi: 10.1152/ajprenal.1989.256.4.F719. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Burt D. W., Paul M., Dzau V. J. Negative control elements and cAMP responsive sequences in the tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):56–59. doi: 10.1073/pnas.86.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondek B., Gloss L., Herr W. The SV40 enhancer contains two distinct levels of organization. Nature. 1988 May 5;333(6168):40–45. doi: 10.1038/333040a0. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Dreyfus M., Roux T. L., Rougeon F. Mouse kidney and submaxillary gland renin genes differ in their 5' putative regulatory sequences. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5489–5493. doi: 10.1073/pnas.81.17.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Sharp P. A. Identification of novel factors which bind specifically to the core promoter of the immunoglobulin heavy chain gene. J Biol Chem. 1991 Dec 5;266(34):22878–22886. [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Pinet F., Mizrahi J., Laboulandine I., Menard J., Corvol P. Regulation of prorenin secretion in cultured human transfected juxtaglomerular cells. J Clin Invest. 1987 Sep;80(3):724–731. doi: 10.1172/JCI113127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Pichard A. L., Gregori C., Ginot F., Kahn A. Interplay of an original combination of factors: C/EBP, NFY, HNF3, and HNF1 in the rat aldolase B gene promoter. Nucleic Acids Res. 1991 Nov 25;19(22):6145–6153. doi: 10.1093/nar/19.22.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E., Trask R. V., Fontanet H. L., Billadello J. J. Multiple positive and negative elements regulate human brain creatine kinase gene expression. Nucleic Acids Res. 1991 Nov 25;19(22):6231–6240. doi: 10.1093/nar/19.22.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Kao C. C., Pei R., Berk A. J. Yeast TATA-box transcription factor gene. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7785–7789. doi: 10.1073/pnas.86.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh S. Y., Tsai M. J. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem. 1991 Sep 5;266(25):16708–16714. [PubMed] [Google Scholar]
- Shimizu N., Dizon E., Zak R. Both muscle-specific and ubiquitous nuclear factors are required for muscle-specific expression of the myosin heavy-chain beta gene in cultured cells. Mol Cell Biol. 1992 Feb;12(2):619–630. doi: 10.1128/mcb.12.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Okuyama K., Ingelfinger J., Jones C. A., Mullins J. J., Kane C., Kim U., Wu C. Z., Kenny L., Rustum Y. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem. 1990 Nov 15;265(32):19916–19922. [PubMed] [Google Scholar]
- Tamura K., Tanimoto K., Takahashi S., Sagara M., Fukamizu A., Murakami K. Structure and expression of the mouse angiotensinogen gene. Jpn Heart J. 1992 Jan;33(1):113–124. doi: 10.1536/ihj.33.113. [DOI] [PubMed] [Google Scholar]
- Tanese N., Pugh B. F., Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991 Dec;5(12A):2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- Wang J., Oketani M., Watanabe T. Positive and negative regulation of immunoglobulin gene expression by a novel B-cell-specific enhancer element. Mol Cell Biol. 1991 Jan;11(1):75–83. doi: 10.1128/mcb.11.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Holladay L. A., Inagami T., Haas E., Murakami K. Human renal renin. Complete purification and characterization. J Biol Chem. 1980 Apr 25;255(8):3498–3502. [PubMed] [Google Scholar]
- Zhou D. X., Yen T. S. The ubiquitous transcription factor Oct-1 and the liver-specific factor HNF-1 are both required to activate transcription of a hepatitis B virus promoter. Mol Cell Biol. 1991 Mar;11(3):1353–1359. doi: 10.1128/mcb.11.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]