Abstract
Metabolic syndrome is characterized by a combination of obesity, hypertension, insulin resistance, dyslipidemia, and impaired glucose tolerance. This multifaceted syndrome is often accompanied by a hyperdynamic circulatory state characterized by increased blood pressure, total blood volume, cardiac output, and metabolic tissue demand. Experimental, epidemiological, and clinical studies have demonstrated that patients with metabolic syndrome have significantly elevated cardiovascular morbidity and mortality rates. One of the main and frequent complications seen in metabolic syndrome is cardiovascular disease. The primary endpoints of cardiometabolic risk are coronary and peripheral arterial disease, myocardial infarction, congestive heart failure, arrhythmia, and stroke. Alterations in expression and/or functioning of several key proteins involved in regulating and maintaining ionic homeostasis can cause cardiac disturbances. One such group of proteins is known as ryanodine receptors (intracellular calcium release channels), which are the major channels through which Ca2+ ions leave the sarcoplasmic reticulum, leading to cardiac muscle contraction. The economic cost of metabolic syndrome and its associated complications has a significant effect on health care budgets. Improvements in body weight, blood lipid profile, and hyperglycemia can reduce cardiometabolic risk. However, constant hyperadrenergic stimulation still contributes to the burden of disease. Normalization of the hyperdynamic circulatory state with conventional therapies is the most reasonable therapeutic strategy to date. JTV519 (K201) is a newly developed 1,4-benzothiazepine drug with antiarrhythmic and cardioprotective properties. It appears to be very effective in not only preventing but also in reversing the characteristic myocardial changes and preventing lethal arrhythmias. It is also a unique candidate to improve diastolic heart failure in metabolic syndrome.
Keywords: ryanodine receptors, metabolic syndrome, JTV519, K201
Metabolic syndrome
Metabolic syndrome is characterized by a combination of obesity, hypertension, insulin resistance, dyslipidemia, and impaired glucose tolerance.1 The mechanisms responsible appear to be multifactorial, and include family history, physical inactivity, and a sedentary lifestyle. Key market players spend millions of dollars developing new therapeutic strategies against components of metabolic syndrome and its related complications. The challenge in this area is that the emerging therapeutic agents seem not to be very effective in treating obesity and insulin resistance or reducing further cardiometabolic risk.2 This multifaceted syndrome is often accompanied by a hyperdynamic circulatory state characterized by increased blood pressure, total blood volume, cardiac output, and metabolic tissue demand.3–10 Hypertension generally amplifies the high cardiovascular risk if the disease remains uncontrolled for a long time.11–15
Experimental, epidemiological, and clinical studies have demonstrated that patients with metabolic syndrome have significantly elevated cardiovascular morbidity and mortality.10–17 Hypertension and changes in heart rate generally appear early on, with the risk of developing coronary artery disease, arteriosclerosis, and heart failure increasing at a later stage.3–10,16 In the end, making a decision regarding “which aggravates which first” is complicated. All these assessments aim to generate the best treatment modalities which provide a better health care strategy in a cost-effective manner. The basic therapeutic approach still focuses on decreasing body weight (adipose tissue mass) and hepatic fat deposition. Diet, exercise, and lifestyle modification are out of professional control in most cases, because they depend on the patient’s intellectual capacity and their economic situation. Coping with all these strategies requires good patient monitoring with conventional and/or new therapeutic agents.
Newly identified compounds should aid in the management of body weight with improvement in blood glucose in patients with diabetes.18 Randomized controlled clinical trials have shown that exenatide, a glucagon-like peptide analog, is effective in reducing glycemic events and assisting with beneficial weight loss.2,19 Nausea is a known side effect of this drug, along with rare cases of pancreatitis, but its side effect profile still lies within the acceptable range.19 The other newer agents, ie, the dipeptidyl peptidase-4 (DPP-4) inhibitors (sitagliptin and vildagliptin) combined with metformin, glitazone, and sulfonylurea in clinical trials have been shown to be very effective for blood glucose control.2,18,20 The trials indicate that these agents are associated with fewer hypoglycemia episodes and less weight gain, and that they are also an effective intervention to decrease obesity. On the other hand, less information is available about whether they have any beneficial effects on cardiovascular complications. However, some knowledge already exists showing that they reduce glycosylated hemoglobin (HbA1c) levels by a couple of percentage points and decrease hyperglycemic/hypoglycemic episodes, especially nocturnal ones.18,20 However, further data are required to assess their long-term efficacy and tolerability in clinical trials to determine their exact cardiovascular benefits in metabolic syndrome.
Cardiovascular dysfunction in metabolic syndrome
Obesity and metabolic syndrome can cause cardiovascular complications.1,3–10,13,16,21–24 The underlying molecular mechanisms responsible could be related to the development of a hyperdynamic circulatory state, which may trigger a variety of cardiac and hemodynamic changes. To date, from metabolic syndrome through diabetes, a new treatment paradigm is emerging. To cope with the complications associated with metabolic syndrome, early diagnosis of the disease is essential. Despite an increase in the diagnosed/ undiagnosed ratio in recent years, they have to treat with new updated therapeutic guidelines to protect against complications.21,23–25 Under these circumstances, a poor diet secondary to a sedentary lifestyle without appropriate treatment increases cardiometabolic risk. The basic treatment strategies still appear as simple as ever, and include control of body weight, blood pressure management, and normalization of blood lipids, with maintenance of normoglycemia. The link between metabolic syndrome and cardiovascular risk is clear, but the underlying molecular mechanisms need to be investigated to assist our treatment decisions.
The main consequences of the cardiometabolic risk profile are coronary and peripheral arterial disease, myocardial infarction, congestive heart failure, arrhythmia, and stroke.10 However, because overweight and obesity are independent risk factors, insulin resistance and lipid disturbances appear to be integral components of the disease.10,14,18 Reduced HbA1c, low-density lipoprotein cholesterol and triglycerides and increased high-density lipoproteins are already well known to lower cardiometabolic risk.
Cardiac ryanodine receptors
Alterations in the expression and/or function of several key proteins involved in regulating and maintaining ionic homeostasis can cause cardiac disturbances. One such group of proteins is called the ryanodine receptors (RyRs), which are a component of the intracellular Ca2+ release channels located in the membrane of the sarcoplasmic reticulum.4,26,27 Three isoforms have been identified in the human heart, and are referred to as type 1, 2, and 3 (RyR1, RyR2, and RyR3, respectively).27,28 Type 2 ryanodine receptors are the major release channels through which Ca2+ leaves the sarcoplasmic reticulum and leads to cardiac muscle contraction.28 Rapid depolarization of the sarcoplasmic reticulum membrane in the heart by Ca2+ via the RyR2 receptor is an important step in cardiac contractility. However, the physiological role of the RyR1 and RyR3 isoforms also expressed in the human heart remains unclear.27
RyR2 is a homotetramer comprising four 565 kDa monomers, each containing a transmembrane segment.28 The RyR2 receptor normally closes at low cytosolic Ca2+ levels during diastole. However, submicromolar cytosolic Ca2+ levels increase the probability of the channel being open with high-affinity binding sites available.4,28–32 A plant alkaloid ryanodine isolate, Ryania speciosa, found in central and South America, locks the RyR2channel in a subconductance state and induces paralysis, and was previously tested as an insecticide.28 Subsequently, ryanodine was used to purify RyR2 from the sarcoplasmic reticulum as a high-affinity ligand.28,33 Beyond that, no therapeutic indication has been identified as yet which would warrant its development as a pharmaceutical product.
The calcium channel binding protein, FKBP12.6 (12.6 kDa FK506-binding protein) binds to RyR2 and stabilizes the closed state of the channel, and has recently been renamed as calstabin 2.34–36 Dissociation of FKBP12.6 from RyR2 increases the open probability of the channel.28 Furthermore, genetic deletion of calstabin 2 enhances Ca2+ release from the sarcoplasmic reticulum and causes leaky RyR2.28 Protein kinase A and calcium/calmodulin dependent kinase A (CaMKII) phosphorylate serine 2809 (Ser2809) and Ser2814, respectively, on RyR2.37–39 However, many different phosphorylation sites (Ser2808, Ser2809, Ser2814, Ser2815, and Ser2030) have now been reported for both these enzymes across different species.37–39 Phosphorylation of RyR2 by protein kinase A causes increased sensitivity of RyR2 to intracellular Ca2+, dissociation of calstabin 2 from the channel, and enhanced RyR2 activity,40 leading to an increase in the open probability of the channel. Recent evidence has demonstrated that nitrosylation and/or oxidation of RyR2 also alters the binding affinity of calstabin 2, which affects channel activity.41,42 CaMKII is also a holoenzyme which sensitizes the channel to cytosolic Ca2+ and increases the open probability of the channel, but does not dissociate calstabin 2 from the channel.39
Other modulatory proteins, ie, phosphodiesterase 4D3 (PDE4D3), calmodulin, protein phosphatase 1 and 2a, and sorcin, modulate the N terminal cytoplasmic domain of RyR2, which includes calstabin 2, protein kinase A, and CaMKII.28 PDE4D3 binds to kinase-anchoring protein, which is part of the RyR2 macromolecular complex and degrades cyclic adenosine monophosphate (cAMP). Protein kinase A also binds kinase-anchoring protein to regulate local cAMP levels near the channel, as does PDE4D3. Protein phosphatase 1 and 2a dephosphorylate phosphorylated channels via spinophilin and PR130, respectively, and indirectly regulate channel activity. Protein phosphatase 1 predominantly dephosphorylates Ser2808 and Ser2809, while protein phosphatase 2a dephosphorylates Ser2814. Many of these modulatory proteins can prevent or ameliorate the probability of the channel being open, thereby contributing directly to cardiac contraction.28
Nevertheless, calmodulin, a 17 kDa protein, assists in the closing state of RyR2, binding 3583–3603 amino acids after Ca2+ release from the sarcoplasmic reticulum during excitation- contraction coupling, which basically inhibits RyR2.43–45 Sorcin, a 22 kDa Ca+ binding protein, also reduces the open probability of RyR2, which decreases the amplitude of Ca2+ release from the sarcoplasmic reticulum without affecting L-type Ca2+ current when Ca2+ levels are elevated.46,47 Calsequestrin, junctin, and triadin are modulatory proteins related to the C terminus which form a complex with RyR2 in the lumen of the sarcoplasmic reticulum.28,48 Calsequestrin sequesters Ca2+ in the sarcoplasmic reticulum, causing an increase in the open probability of RyR2.28 Triadin and junctin link calsequestrin to the channel and normalize the Ca2+ load in the sarcoplasmic reticulum.28 Current strategies for the management of cardiac disturbances do not focus directly on RyR2 and no relevant molecule has been approved as yet anywhere in the world. Several attempts at developing RyR2 as a therapeutic molecule have been made in the past, but were unable to demonstrate efficacy and safety.
Ryanodine receptor dysfunction in metabolic syndrome
Obesity and its associated comorbidities affect almost one third of the population of the western world, and their prevalence tends to increase each year. The economic cost of metabolic syndrome and its complications have a significant impact on health care budgets worldwide. The most common complications seen in metabolic syndrome are cardiovascular in nature, and include arrhythmias, hypertension, and coronary artery disease. While improvements in body weight, blood lipid profile, and hyperglycemia can reduce the cardiometabolic risk, constant hyperadrenergic stimulation still contributes to the burden of the disease.
One of the mechanisms underpinning the increased cardiometabolic risk in metabolic syndrome is impaired Ca2+ storage in the sarcoplasmic reticulum and diastolic Ca2+ leak during hypersympathetic stimulation.4,16 It is assumed that treatment should aim to restore the normal hyperphosphorylation state involving excitation-contraction coupling proteins, such as RyR2.3,4,9,16 Many laboratories have found basal catecholamine levels to be elevated in metabolic syndrome.3,4,9,10,12,16,25,49 We have also previously demonstrated that induction of metabolic syndrome by chronic high-fat feeding increases circulating plasma epinephrine levels by 55% and norepinephrine levels by 31%.3 Chronic activation of the sympathetic nervous system, as occurs in metabolic syndrome, leads to increased protein kinase A-mediated phosphorylation of RyR2 at Ser2809 and Ser2808 (see Figure 1).3–5,16 Recent experimental evidence suggests that chronic hyperphosphorylation of RyR2 contributes to impaired contraction, generation of lethal ventricular arrhythmias, and development of heart failure via leaky RyR2 channels.50
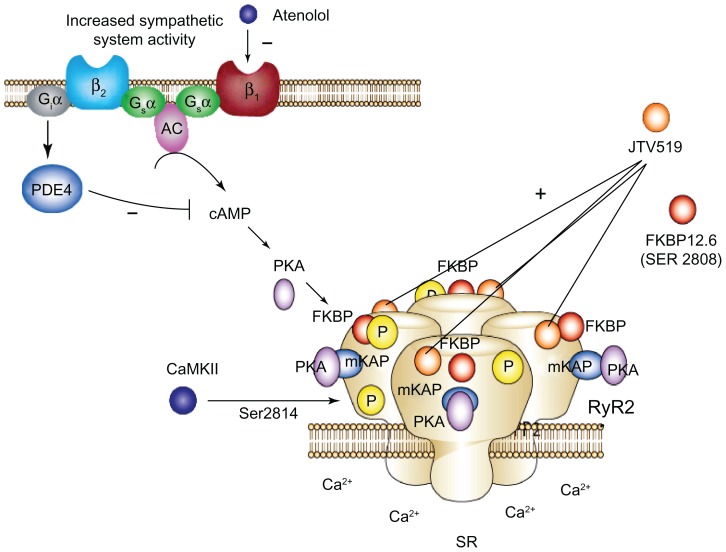
Figure 1.
Schematic diagram of RyR2 dysfunction in metabolic syndrome. Cardiac RyR2 dysfunction seen in metabolic syndrome (MetS) could be related to the cAMP/PKA-dependent pathway under constant hyper adrenergic stimulation. Increased Ser2809 phosphorylation of cardiac RyR2 in MetS is possibly mediated by PKA activation. Increase in circulating catecholamine stimulates G-protein-coupled β-ARs thereby activating intracellular cyclic adenosine monophosphate (cAMP) and PKA. JTV-519 (K201) increases binding affinity of FKBP12.6 to RyR2, which stabilizes the closes state of RyR2 channels and prevents Ca++ leak which protects from ventricular arrhythmias, contractile dysfunction and reduce Ca++ overload.
Abbreviations: SR, sarcoplasmic reticulum; β1–AR, adrenoreceptor; β2–AR, adrenoreceptor; Gsα, stimulatory protein G alpha; Giα, Inhibitory protein alpha; AC, adenyle cyclase; PDE4, phosphodiesterase 4; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; mKAP, a kinase anchoring protein; CaMKII, calcium/calmodulin dependent kinase II; Ser 2808, Serin2808; Ser 2814, Serine2814.
We have recently demonstrated that the functional integrity of RyR2 is compromised by metabolic syndrome, as evidenced by augmented RyR2 phosphorylation together with diminished RyR2 binding affinity.4 RyR2 Ser2809 and RyR2 Ser2808 phosphorylation sites have been used widely as an index of RyR2 phosphorylation by protein kinase A. Other studies have demonstrated that CaMKII also phosphorylates the RyR2 (Ser2814 or Ser2815) molecule, and that CaMKII can activate RyR2 gating but not at protein kinase A (Ser2808 or Ser2809) sites, preventing dissociation of FKBP12.6 from RyR2 (see Figure 1). Protein kinases C and G can also phosphorylate the RyR2 molecule.28,39,40,51–53 Protein kinase C-mediated and protein kinase G-mediated pathways are mainly responsible for phosphorylation of the Ser2030 RyR2 molecule, which can also be phosphorylated by protein kinase A but is certainly a poor substrate for CaMKII.28,35,51,54 Increased sympathetic nervous system activity could be a mechanism by which metabolic syndrome leads to impaired RyR2 function. The possibility of phosphorylation of RyR2 sites by different pathways in different species has not been clearly defined as yet, and needs to be investigated.
The cardiac RyR2 dysfunction seen in metabolic syndrome could be related to a cAMP/protein kinase A-dependent pathway under constant hyperadrenergic stimulation (see Figure 1).2–5,16 Increased Ser2809 phosphorylation of cardiac RyR2 in metabolic syndrome is possibly mediated by activation of protein kinase A, whereby there is an increase in circulating catecholamines which stimulate G protein-coupled β-adrenoceptors, thereby activating intracellular cAMP and protein kinase A (see Figure 1). This hypothesis is supported by earlier studies in which chronic sympathetic activation resulted in protein kinase A-mediated hyperphosphorylation of RyR2.28,54,55 Earlier studies indicate that constant sympathetic nervous system activity can switch the predominant cardiac signaling pathway from protein kinase A to CaMKII.28,37,51,56 Whether metabolic syndrome alters signaling pathway through the protein kinase A or CaMKII is presently unknown; however, Xiao et al recently demonstrated that constant β-adrenoreceptor stimulation switches the signaling pathway from being predominantly protein kinase A-driven to being mainly CaMKII-driven.50,53
We have also demonstrated that metabolic syndrome compromises only the functional integrity of the cardiac RyR2 receptor without downregulation, as evidenced by diminished binding affinity and no change in mRNA or protein expression.4 Changes in modulatory proteins that could also contribute to impaired functional integrity of RyR2 include altered dissociation of FKBP12.6 and/or PDE4D3 deficiency. However, whether these modulatory proteins contribute to impaired functional integrity of RyR2 in metabolic syndrome is unknown.
The majority of patients with metabolic syndrome have absolute or relative insulin deficiency (diabetes), including hyperinsulinemia, dyslipidemia, and dysregulation of the renin-angiotensin system. These parameters compromise the molecular levels of myocytes, including RyR2 and sarco(endo)plasmic reticulum Ca2+-ATPase. Direct and indirect effects of glucotoxicity trigger overproduction of reactive oxygen species, poly(ADP-ribose) polymerase, and advanced glycation end-products, and induce apoptosis. 57 They cause altered expression and function of RyR2 via post-translational modifications of extracellular matrix components, which contribute to systolic and diastolic dysfunction.57,58 Hyperglycemia causes two types of post-translation modifications on RyR2 and sarco(endo)plasmic reticulum Ca2+-ATPase; first, it increases production of reactive oxygen species (superoxide anions, hydroxyl radicals, lipid peroxides, hydrogen peroxide) and, second, it increases reactive nitrogen species (nitrosonium cation, nitroxyl anion, peroxynitrite).32,59 These free radical and nonradical species alter the tertiary structure of RyR2, causing RyR2 to become sensitive to endogenous ligands, such as Ca2+ and adenosine triphosphate (ATP).32,59 We have previously demonstrated that advanced glycation end-products are formed on intracellular RyR2 during diabetes; however, treatment with insulin minimizes these nonenzymatic products, which attenuate protein activity. 32 RyR2 is a large transmembrane and long-lived protein (approximately eight days). It localizes in the sarcoplasmic reticulum and plays a critical role in cardiac excitation-contraction coupling. When advanced glycation end-product complexes attenuate the RyR2 protein, the changes are permanent.31,32,59 For that reason, an oral hypoglycemic or insulin treatment-focused therapeutic strategy is not able to restore cardiac contraction.
Dyslipidemia is the other major component of metabolic syndrome and characterized by increased circulating fatty acids and triglycerides,57 causing cardiac lipotoxicity and accumulation of fatty acids in cardiomyocytes which, in turn, leads to increased shortening of the action potential in the K-ATP channel and dysregulation of the open probability in the RyR2 channel. It also causes diminished cycling of the L-type Ca2+ channel and reduction of Ca2+ stores in the sarcoplasmic reticulum.57 Chronic lipid accumulation in cardiomyocytes also contributes to apoptosis via inhibition of the mitochondrial respiratory chain if fatty acids are not completely metabolized.57,60 However, intracardiac fatty acid accumulation enhances oxygen demand and generation of reactive oxygen species, and diminishes ATP synthesis because of mitochondrial dysfunction. 60 In the normal subject, the heart obtains energy for cardiac contractility from fatty acid oxidation, but in a hyperinsulinemic state, the need for myocardial glucose decreases significantly and shifts fatty acid synthesis to the β-oxidation pathway, causing secondary dysregulation of open probability of the RyR2 channel.61
The chronic hyperinsulinemia seen in metabolic syndrome also activates the sympathetic nervous system and the renin angiotensin system indirectly through crosstalk between insulin-dependent signaling and cardiac progrowth pathways, via similar common elements in their molecules.57 Insulin activates a signaling cascade with neurohormonal growth agonists, eg, insulin like growth factor 1 and angiotensin II, which coordinate cell growth and protein synthesis activated by extracellular signal-regulated kinase, and the phosphoinositol-3 kinase/protein-kinase B cascade associated with physiological cardiac hypertrophy.62 Both these pathways also activate the sympathetic nervous system and the renin- angiotensin system via trigger adrenergic and AT1 receptors, as well as the angiotensin II pathway in the hyperinsulinemic state.57 Constant hyperadrenergic stimulation further activates intracellular cAMP and protein kinase A, thereby causing hyperphosphorylation of RyR2 and mild to moderate leaky channels (see Figure 1).28,35,54,55 Decreasing the binding affinity of FKBP12.6 to RyR2 increases the probability of open RyR2 channels, and Ca2+ leak is seen during diastole. Ventricular arrhythmias, contractile dysfunction, and Ca2+ overload is triggered as part of that pathology.35,37,54,55,57
Cardiac hypertrophy and heart failure have been reported in patients with hypertension, diabetes, insulin resistance, and obesity, and increased sympathetic nervous system and renin-angiotensin system activity is well demonstrated in these patients. Indeed, the main complexity in therapeutic disadvantages is the indirect contribution of chronic hyperinsulinemia to already overstimulated adrenergic and angiotensin II pathways, and requires long-term follow-up studies. Intensive glucose control has demonstrated an indirect correlation with decreased cardiovascular complications, including hypertension, myocardial infarction, and stroke. This clearly answers the important question of which comes first, ie, whether overinsulinization triggers the central nervous system indirectly or whether the central nervous system could operate as a main driver of hyperinsulinemia.
Dysregulation of intracellular Ca2+ homeostasis has been reported previously in situations of abnormal membrane lipid content, glucotoxicity, and hyperinsulinemia, as well as in hyperadrenergic states.49,57,58 Alterations in the expression and function of sarco(endo)plasmic reticulum Ca2+-ATPase, the Na-K-ATPase Na+/Ca2+ exchanger, and RyR2 have been identified in these patient groups, and associated with hypertension, type 1 and 2 diabetes mellitus, obesity, and dyslipidemia.28,57,58 Although metabolic syndrome does not always coexist with type 2 diabetes, obesity alone can increase the risk of developing type 2 diabetes and this in turn increases diabetes-associated cardiovascular complications.49
Future therapeutic concepts
Until recent years, normalization of the hyperdynamic circulatory state in metabolic syndrome using conventional therapies, such as adrenergic receptor blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers, was the most reasonable therapeutic strategy (see Figure 1).2,4,5 Other important aims were to reduce body weight, increase exercise capacity, restore a normal plasma lipid profile, and improve metabolic biomarkers. However, in spite of normalizing these parameters, cardiometabolic risk remains mostly irreversible if the disease continues in the long term.2–5 Cardioselective β1-adrenoreceptor blockers control heart rate and aortic pressure, and decrease the cardiac index, especially during exercise (Figure 1). We recently documented that basal catecholamine levels are elevated in metabolic syndrome.3 It is very clear that adrenergic receptor blockers can normalize the increased sympathetic nervous system activity, thereby at least being able to protect the RyR2 channel against Ca2+ leak during diastole.
The β1-adrenoceptor blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers suppress the hyperadrenergic state, thereby restoring the stoichiometry of the RyR2 channel. Many studies, including CONSENSUS, SOLVD-T, Val-HeFT, and CHARM, have demonstrated that angiotensin receptor blockers are more effective than angiotensin-converting enzyme inhibitors in congestive heart failure.63–65 In the Val-HeFT trial, angiotensin receptor blockers reduced the heart failure hospitalization rate when added to conventional therapy, including an angiotensin-converting enzyme inhibitor in most patients, but had no effect on mortality.66,67 The CHARM trial demonstrated that an angiotensin receptor blocker reduced morbidity and mortality as a result of reduced systolic function with or without combination with an angiotensin-converting enzyme inhibitor,66,67 indicating that this treatment strategy is beneficial and preserves left ventricular systolic function. Adrenergic receptor blockers and angiotensin-converting enzyme inhibitors were compared head-to-head in patients with congestive heart failure in clinical trials such as ELITE I and ELITE II.66,67 There have been some clinical trials of an angiotensin-converting enzyme inhibitor combined with digoxin, diuretics (SOLD-T), and a β1-adrenoceptor blocker (CIBIS-2).63,64,66,67 One-year mortality- related improvement in survival in patients with congestive heart failure was found to be better in patients who received combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor therapy, eg, in the CHARM-Added trial (diuretic + digoxin + angiotensin-converting enzyme inhibitor + β1-adrenoceptor blocker + angiotensin receptor blocker) if compared with SOLD-T (diuretic + digoxin + angiotensin- converting enzyme inhibitor) and CIBIS-2 (diuretic + digoxin + angiotensin-converting enzyme inhibitor + β1-adrenoceptor blocker).65 Overall, the clinical trials demonstrated that combination of an adrenergic receptor blocker + angiotensin-converting enzyme inhibitor produced better outcomes for patients with congestive heart failure, in terms of cardiovascular mortality risk and hospital admissions.
These treatment regimes can restore sympathovagal balance and can reverse remodeling in the failing heart, as evidenced by a significant reduction in left ventricular volume and an improved contractile response.63–65,67 The β1-adrenoceptor blockers failed to meet the necessary safety profile in metabolic syndrome and diabetes because they can affect glycogenolysis, interfere with insulin release, further impair glucose tolerance, and increase serum triglycerides.68 For these reasons, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors or combinations of these agents fit better in the treatment regime.66,67 In fact, these algorithms could be used in clinical trials of metabolic syndrome to explore the most beneficial pharmaceutical strategies.
JTV519 (K201) is a promising, newly developed 1, 4-benzothiazepine drug and a nonspecific blocker of Na+, K+, and Ca2+ channels, with antiarrhythmic and cardioprotective properties.69 Like diltiazem, JTV519 blocks the L-type Ca2+ current, but is not classified as a Ca2+ channel blocker.69,70 Rather than acting as a β-adrenoceptor blocker, it blocks α1-receptors and intracellular Ca2+ pathways.69,70 It is a relatively nonselective blocker of cation currents, including the Na+ current (INA) in a voltage-dependent and frequency-dependent manner.71,72 The time course of Na+ current blockade with JTV519 is slower than with lidocaine, similar to quinidine, and is believed to have intermediate rather than fast kinetics.70 It also blocks the inward rectifying K+ current (IK1) and rapidly activating component of the delayed rectified K+ current (IKr), but not the slowly activating component (IKs).73 JTV519 blocks the Ca2+ current (ICa) and muscarinic acetylcholine receptor-operated K+ current (IKAch).70,73
JTV519 stabilizes the closed state of RyR2 and increases the binding affinity of FKBP12.6 for RyR2 (see Figure 1), thereby reducing and preventing Ca2+ leak, and protecting against ventricular arrhythmia, contractile dysfunction, and Ca2+ overload.2,16,26,69,74 This beneficial combination of activity dramatically ameliorates the progression of heart failure as a result of myocardial damage resulting from Ca2+ overload. The most recent evidence shows that JTV519 protects against Ca2+ leak from the sarcoplasmic reticulum independent of the interaction between FKBP12.6 and RyR2.75 Spontaneous sarcoplasmic reticulum Ca2+ release (leak) also occurs when the Ca2+ content in the sarcoplasmic reticulum reaches a threshold level, ie, overload of Ca2+ stores in the sarcoplasmic reticulum,75 and is known as store overload-induced Ca2+ release (SOICR), which is independent of FKBP12.6 binding to RyR2.70,75 SOICR causes delayed after depolarizations and, in turn, arrhythmias, such as those seen in catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular dysplasia type 2.70,75
Since 2000, JTV519 has been investigated in Phase II trials for its ability to protect against acute myocardial infarction, and is suggested to reduce reperfusion injury following percutaneous transluminal coronary angioplasty and has been shown to be protective in models of ischemia-reperfusion injury and heart failure.76 Stabilization of RyR2 reduces detrimental intracellular Ca2+ leak and improves both diastolic and systolic contractile function in the human heart with or without an FKBP12.6-RyR2 binding interaction. 75 It has also been shown that inhibition of SOICR by JTV519 could be a sophisticated treatment strategy for catecholamine- induced or inherited forms of cardiac arrhythmia (catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular dysplasia type 2).77 JTV519 prevents myocardial infarction and sudden cardiac death.69,70 It provides more effective myocardial protection than calcium channel blockers and β1-adrenoceptor blockers. JTV519 also has fewer negative inotropic and chronotropic effects,69,70 and, in particular, prevents myocardial injury caused by ischemia and catecholamines to a greater extent than do nicorandil, prazosin, propranolol, verapamil, and diltiazem.69,70
Metabolic syndrome is associated with a hyperdynamic circulatory state characterized by increased blood pressure, total blood volume, cardiac output, and metabolic tissue demand.78,79 Many laboratories have demonstrated that basal catecholamine levels are elevated in metabolic syndrome.3,4,9,10,12,16,25,49 We have also previously shown that induction of metabolic syndrome by chronic high-fat feeding increases circulatory plasma epinephrine levels by 55% and norepinephrine levels by 31%.3 The hyperadrenergic state seen in metabolic syndrome could trigger lethal arrhythmias and myocardial ischemia, especially during exercise.3–5
JTV519 is a promising new drug for possible use in the treatment of the cardiovascular disturbances associated with metabolic syndrome. It is a cardioprotective agent against ischemia and hyperadrenergic states for two important reasons; first of all, it can not only protect against but also reverse myocardial damage, and second, it has fewer inotropic and chronotropic effects than the calcium channel blockers and cardioselective β-adrenoceptor blockers.70 JTV519 stabilizes the closed state of RyR2, is thought to increase the binding affinity of FKBP12.6 for RyR2, and protects cardiomyocytes against SOICR, which is independent of the FKBP12.6-RyR2 interaction.70,75
JTV519 blocks cardiac INA, IK1, IKr, ICa and IKAch 70 and inhibits IKAch and IKr in atrial muscle cells, which are potential channels for atrial fibrillation.73,80 It also inhibits IKAch in the atrial appendage which generates atrial fibrillation, mostly in the sinus node.73,80 JTV519 also inhibits ventricular tachycardia and fibrillation,70 and causes prolongation of the QT intervals. It suppresses torsades de pointes in a dose-dependent manner by blocking α1-adrenoreceptors and inhibiting abnormal Ca2+ release from the sarcoplasmic reticulum. 70 SOICR may also cause delayed after depolarizations and contribute to development of ventricular arrhythmias.75 Therefore, JTV519 appears to be a very good candidate as an antiarrhythmic agent in the treatment of atrial and ventricular arrhythmias in patients with metabolic syndrome, especially during exercise.
Another clinical application of JTV519 could be in diastolic heart failure. To date, the exact etiology of diastolic heart failure is unknown and there are no drugs available to treat it. JTV519 could be a good choice because it normalizes left ventricular end-diastolic pressure, protects against the effects of catecholamines, and restores aortic valve opening in the diastolic phase.69,70 Some treatment approaches available for systolic heart failure include digitalis, cyclic nucleotide phosphodiesterase inhibitors (milrinone, inamrinone), angiotensin antagonists (angiotensin-converting inhibitors and receptor blockers), β-adrenoceptor agonists, and α-adrenoreceptor antagonists.69,70
One of the main complications seen in metabolic syndrome is cardiovascular disturbance, ie, hypertension, arrhythmias, coronary artery disease, and heart failure. Metabolic syndrome is associated with a hyperdynamic circulatory state characterized by hypertension and increased cardiac output, with an enhanced cardiometabolic risk profile.4,5,16 JTV519 stabilizes the closed state of RyR2 and increases the binding affinity of FKBP12.6 to RyR2.70 It also protects cardiomyocytes against SOICR, which is independent of the FKBP12.6-RyR2 interaction,75 protects against stress-induced cardiomyopathy,69,70 and prevents and restores myocardial injury more effectively than do the β-adrenoceptor blockers, Ca2+ channel blockers, α-adrenoreceptor antagonists, and vasodilator agents.70 JTV519 appears to be the most effective in not only preventing but also reversing myocardial alterations caused by ischemia and catecholamines, protecting against lethal arrhythmias, and improving diastolic heart failure.70 It could be a future therapeutic strategy to target the arrhythmias, myocardial ischemia, myocardial infarction, and diastolic heart failure seen in metabolic syndrome.
Abbreviations
- MetS
Metabolic syndrome
- CMR
cardio metabolic risk
- GLP-I
glucagon like peptide
- DPP-4
dipeptidyl peptidase-4 inhibitor
- CVS
cardiovascular system
- CAD
coronary arterial disease
- PAD
peripheral arterial disease
- MI
myocardial infarction
- CHF
congestive heart failure
- DM
diabetes mellitus
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HbA1c
glycated hemoglobin A1C
- FAs
fatty acids
- TGs
triglycerides
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoproteins
- RyRs
ryanodine receptors
- RyR1; RyR2 and RyR3
ryanodine receptor type 1; 2 and 3
- SR
sarcoplasmic reticulum
- SNS
sympathetic nervous system
- RAS
renin angiotensin system
- β1-AR
β1-Adrenoreceptor
- β2-AR
β2-Adrenoreceptor
- α1-AR
α1- Adrenoreceptor
- Gsα
stimulatory protein G alpha
- Giα
inhibitory protein G alpha
- AC
adenylyl cyclase
- K 201
JTV 519
- PDE4
phosphodiesterase 4; KBP 12.6; calstabine 2, phosphodiesterase 4D3 binding protein 12.6
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- mKAP
kinase anchoring protein
- EC
excitation contraction
- CaMKII
calcium/calmodulin dependent kinase II
- Ser2808
serin 2808
- Ser2814
serine 2814
- PDE4D3
phosphodiesterase 4D3
- CaM
calmodulin
- RAS
renin angiotensin system
- PP1 and PP2 respectively
protein phosphatases 1 and 2A
- ROS
reactive oxygen species
- PARP
Poly ADP-ribose, polymerase
- AGEs
advanced glycation end products
- SERCA
sarco endo, plasmic reticulum Ca++- ATPase
- K-ATP
potassium adenosine three phosphate
- IGF-1
insulin like growth factor – 1
- ERK
extracellular signal regulated kinase
- PI3K
phospho inositol three kinase
- PKB
protein kinase B
- CNS
central nervous system
- T2DM
type 2 diabetes
- SOICR
store overload induced Ca++ release
- Ang II
angiotensin II
- ACE
angiotensin converting enzyme
- ACE-I
angiotensin converting enzyme inhibitors
- ARB
Angiotensin II receptor blocker
- SOICR
store overload induced Ca++ release
- DAD
delayed after depolarization
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- ARVD2
arrhythmogenic right ventricular dysplasia type 2
Footnotes
Disclosure
Much of the data discussed in this review was presented by the author at the IBC’s Sixth Annual Meeting on Targeting Metabolic Disorders held on March 17–19, 2008, Chapel Hill, NC.
References
- 1.Gallagher EJ, Leroith D, Karnieli E. The metabolic syndrome from insulin resistance to obesity and diabetes. Endocrinol Metab Clin North Am. 2008;37(3):559–579. doi: 10.1016/j.ecl.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Dincer UD. Future therapeutic concepts. Journal of Metabolic Syndrome. 2011;1(1):102. [Google Scholar]
- 3.Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006;13(7):587–595. doi: 10.1080/10739680600885228. [DOI] [PubMed] [Google Scholar]
- 4.Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol. 2006;41(1):108–114. doi: 10.1016/j.yjmcc.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Dincer UD. Cardiac beta-adrenoceptor expression is markedly depressed in Ossabaw swine model of cardiometabolic risk. Int J Gen Med. 2011;4:493–499. doi: 10.2147/IJGM.S18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudson JD, Dincer UD, Dick GM, et al. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(3):H1038–H1046. doi: 10.1152/ajpheart.00244.2005. [DOI] [PubMed] [Google Scholar]
- 7.Knudson JD, Dincer UD, Zhang C, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(1):H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 8.Knudson JD, Rogers PA, Dincer UD, et al. Coronary vasomotor reactivity to endothelin-1 in the prediabetic metabolic syndrome. Microcirculation. 2006;13(3):209–218. doi: 10.1080/10739680600556894. [DOI] [PubMed] [Google Scholar]
- 9.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14(4–5):317–338. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 10.Peters AL. Patient and treatment perspectives: revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk. Cleve Clin J Med. 2009;76(Suppl 5):S20–S27. doi: 10.3949/ccjm.76.s5.04. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S194–S198. doi: 10.2337/dc09-S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9(3):193–199. doi: 10.1007/s11892-009-0032-7. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Banerji M, Bray GA, et al. Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr Disord. 2009;9:17. doi: 10.1186/1472-6823-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Banerji MA, Bray GA, et al. Determinants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW) study. Diabetologia. 2010;53(3):435–445. doi: 10.1007/s00125-009-1614-2. [DOI] [PubMed] [Google Scholar]
- 16.Dincer UD. Cardiac dysfunction in prediabetic metabolic syndrome; Is JTV 519 (K201) future therapy?. IBC’s Drug Discovery Series 6’th Annual Meeting, Targeting Metabolic Disorders; Advancing the Efficacy, Safety and Selectivity of Metabolic Disease Therapeutics; March 17–19, 2008; Chapel Hill, NC. [Google Scholar]
- 17.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95(5):919–937. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1–248. doi: 10.3310/hta14360. [DOI] [PubMed] [Google Scholar]
- 19.Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;10:CD006423. doi: 10.1002/14651858.CD006423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratley RE, Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007;23(4):919–931. doi: 10.1185/030079906x162746. [DOI] [PubMed] [Google Scholar]
- 21.Goodman E, Daniels SR, Dolan LM. Definition of metabolic syndrome. J Pediatr. 2007;150(4):e36–e37. doi: 10.1016/j.jpeds.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, Brands MW, Zappe DH, Onso-Galicia M. Cardiovascular actions of insulin: are they important in long-term blood pressure regulation? Clin Exp Pharmacol Physiol. 1995;22(10):689–700. doi: 10.1111/j.1440-1681.1995.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE, Brands MW, Zappe DH, Alonso GM. Insulin resistance, hyperinsulinemia, and hypertension: causes, consequences, or merely correlations? Proc Soc Exp Biol Med. 1995;208(4):317–329. doi: 10.3181/00379727-208-43862b. [DOI] [PubMed] [Google Scholar]
- 25.Alpert MA. Management of obesity cardiomyopathy. Am J Med Sci. 2001;321(4):237–241. doi: 10.1097/00000441-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Elliott EB, Hasumi H, Otani N, et al. K201 (JTV-519) alters the spatiotemporal properties of diastolic Ca(2+) release and the associated diastolic contraction during beta-adrenergic stimulation in rat ventricular cardiomyocytes. Basic Res Cardiol. 2011;106(6):1009–1022. doi: 10.1007/s00395-011-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guner S, Arioglu E, Tay A, et al. Diabetes decreases mRNA levels of calcium-release channels in human atrial appendage. Mol Cell Biochem. 2004;263(1–2):143–150. doi: 10.1023/B:MCBI.0000041856.92497.0c. [DOI] [PubMed] [Google Scholar]
- 28.Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol. 2010;59:1–30. doi: 10.1016/S1054-3589(10)59001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidasee KR, Dincer UD, Besch HR., Jr Ryanodine receptor dysfunction in hearts of streptozotocin-induced diabetic rats. Mol Pharmacol. 2001;60(6):1356–1364. doi: 10.1124/mol.60.6.1356. [DOI] [PubMed] [Google Scholar]
- 30.Bidasee KR, Nallani K, Henry B, Dincer UD, Besch HR., Jr Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem. 2003;249(1–2):113–123. [PubMed] [Google Scholar]
- 31.Bidasee KR, Nallani K, Besch HR, Jr, Dincer UD. Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2) J Pharmacol Exp Ther. 2003;305(3):989–998. doi: 10.1124/jpet.102.046201. [DOI] [PubMed] [Google Scholar]
- 32.Bidasee KR, Zhang Y, Shao CH, et al. Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53(2):463–473. doi: 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- 33.Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EA. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaburjakova M, Gaburjakova J, Reiken S, et al. FKBP12 binding modulates ryanodine receptor channel gating. J Biol Chem. 2001;276(20):16931–16935. doi: 10.1074/jbc.M100856200. [DOI] [PubMed] [Google Scholar]
- 35.Lehnart SE, Terrenoire C, Reiken S, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103(20):7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehrens XH, Lehnart SE, Huang F, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113(7):829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101(14):1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 38.Lehnart SE, Wehrens XH, Reiken S, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123(1):25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94(6):e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 40.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103(3):511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aracena P, Tang W, Hamilton SL, Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal. 2005;7(7–8):870–881. doi: 10.1089/ars.2005.7.870. [DOI] [PubMed] [Google Scholar]
- 42.Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282(10):6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca(2+) release channel (ryanodine receptor) J Biol Chem. 2003;278(26):23480–23486. doi: 10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi N, Xu L, Evans KE, Pasek DA, Meissner G. Different regions in skeletal and cardiac muscle ryanodine receptors are involved in transducing the functional effects of calmodulin. J Biol Chem. 2004;279(35):36433–36439. doi: 10.1074/jbc.M405834200. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2007;117(5):1344–1353. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lokuta AJ, Meyers MB, Sander PR, Fishman GI, Valdivia HH. Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem. 1997;272(40):25333–25338. doi: 10.1074/jbc.272.40.25333. [DOI] [PubMed] [Google Scholar]
- 47.Farrell EF, Antaramian A, Rueda A, Gomez AM, Valdivia HH. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J Biol Chem. 2003;278(36):34660–34666. doi: 10.1074/jbc.M305931200. [DOI] [PubMed] [Google Scholar]
- 48.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75(6):2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321(4):225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Taur Y, Frishman WH. The cardiac ryanodine receptor (RyR2) and its role in heart disease. Cardiol Rev. 2005;13(3):142–146. doi: 10.1097/01.crd.0000128709.84812.86. [DOI] [PubMed] [Google Scholar]
- 51.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKII delta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107(22):10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wehrens XH, Lehnart SE, Reiken SR, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin 2. Science. 2004;304(5668):292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 53.Xiao B, Tian X, Xie W, et al. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem. 2007;282(41):30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 54.Marks AR, Reiken S, Marx SO. Progression of heart failure: is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation. 2002;105(3):272–275. [PubMed] [Google Scholar]
- 55.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 56.Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–159. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- 57.Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech. 2010;7(2):e135–e143. doi: 10.1016/j.ddmec.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98(5):596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 59.Bidasee KR, Nallani K, Yu Y, et al. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes. 2003;52(7):1825–1836. doi: 10.2337/diabetes.52.7.1825. [DOI] [PubMed] [Google Scholar]
- 60.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88(3):233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 61.Liu GX, Hanley PJ, Ray J, Daut J. Long-chain acyl-coenzyme A esters and fatty acids directly link metabolism to K(ATP) channels in the heart. Circ Res. 2001;88(9):918–924. doi: 10.1161/hh0901.089881. [DOI] [PubMed] [Google Scholar]
- 62.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 63.Dzau VJ, Bernstein K, Celermajer D, et al. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88(9A):1L–20L. doi: 10.1016/s0002-9149(01)01878-1. [DOI] [PubMed] [Google Scholar]
- 64.Dzau VJ, Bernstein K, Celermajer D, et al. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc Drugs Ther. 2002;16(2):149–160. doi: 10.1023/a:1015709617405. [DOI] [PubMed] [Google Scholar]
- 65.McMurray JJ, Pfeffer MA, Swedberg K, Dzau VJ. Which inhibitor of the renin-angiotensin system should be used in chronic heart failure and acute myocardial infarction? Circulation. 2004;110(20):3281–3288. doi: 10.1161/01.CIR.0000147274.83071.68. [DOI] [PubMed] [Google Scholar]
- 66.Erhardt LR. A review of the current evidence for the use of angiotensin- receptor blockers in chronic heart failure. Int J Clin Pract. 2005;59(5):571–578. doi: 10.1111/j.1368-5031.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 67.Eisenberg MJ, Gioia LC. Angiotensin II receptor blockers in congestive heart failure. Cardiol Rev. 2006;14(1):26–34. doi: 10.1097/01.crd.0000182409.91935.85. [DOI] [PubMed] [Google Scholar]
- 68.Taylor AA, Bakris GL. The role of vasodilating beta-blockers in patients with hypertension and the cardiometabolic syndrome. Am J Med. 2010;123(7 Suppl 1):S21–S26. doi: 10.1016/j.amjmed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Toischer K, Lehnart SE, Tenderich G, et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol. 2010;105(2):279–287. doi: 10.1007/s00395-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaneko N, Matsuda R, Hata Y, Shimamoto K. Pharmacological characteristics and clinical applications of K201. Curr Clin Pharmacol. 2009;4(2):126–131. doi: 10.2174/157488409788184972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79(3):275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- 72.Kiriyama K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of JTV-519, a novel anti-ischaemic drug, on the delayed rectifier K+ current in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(6):646–653. doi: 10.1007/s002100000230. [DOI] [PubMed] [Google Scholar]
- 73.Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemura H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br J Pharmacol. 2000;131(7):1363–1372. doi: 10.1038/sj.bjp.0703713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasumi H, Matsuda R, Shimamoto K, Hata Y, Kaneko N. K201, a multi-channel blocker, inhibits clofilium-induced torsades de pointes and attenuates an increase in repolarization. Eur J Pharmacol. 2007;555(1):54–60. doi: 10.1016/j.ejphar.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Hunt DJ, Jones PP, Wang R, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404(3):431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.James AF. Inhibition of SR Ca2+ uptake: a novel action of the RyR2-FKBP12.6 antagonist K201. Cardiovasc Res. 2007;76(2):199–201. doi: 10.1016/j.cardiores.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 77.Jones PP, Jiang D, Bolstad J, et al. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload- induced Ca2+ release. Biochem J. 2008;412(1):171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- 78.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52(4):912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borbouse L, Dick GM, Asano S, et al. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297(5):H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003;14(8):880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]