Abstract
Developmental lung biology is a field that has the potential for significant human impact: lung disease at the extremes of age continues to cause major morbidity and mortality worldwide. Understanding how the lung develops holds the promise that investigators can use this knowledge to aid lung repair and regeneration. In the decade since the “molecular embryology” of the lung was first comprehensively reviewed, new challenges have emerged—and it is on these that we focus the current review. Firstly, there is a critical need to understand the progenitor cell biology of the lung in order to exploit the potential of stem cells for the treatment of lung disease. Secondly, the current familiar descriptions of lung morphogenesis governed by growth and transcription factors need to be elaborated upon with the reinclusion and reconsideration of other factors, such as mechanics, in lung growth. Thirdly, efforts to parse the finer detail of lung bud signaling may need to be combined with broader consideration of overarching mechanisms that may be therapeutically easier to target: in this arena, we advance the proposal that looking at the lung in general (and branching in particular) in terms of clocks may yield unexpected benefits.
1. Introduction
The concept that lung organogenesis is instructed by coordinated mesenchymal-to-epithelial crosstalk originates in the classical recombination experiments of Alescio and Cassini (1962), in which replacing tracheal mesenchyme with mesenchyme from the lung periphery induced ectopic branching of tracheal epithelium in murine embryonic lung organ culture. This idea was extended in an early review by Warburton and Olver (1997) to include the coordination of genetic, epigenetic, and environmental factors in lung development, injury, and repair. Thereafter, a molecular basis of lung morphogenesis was attempted by Warburton et al. (2000). Over the last decade, significant progress has been made in this field as reviewed by Cardoso and Lu (2006), Maeda et al. (2007), and others. Nevertheless, the ultimate goal remains as stated by Warburton and Olver (1997), “to devise new rational and gene therapeutic approaches to ameliorate lung injury and augment lung repair … the ideal agent or agents would therefore mimic the instructive role of lung mesenchyme and would correctly induce the temporospatial pattern of lung-specific gene expression necessary to instruct lung regeneration.” To this overall strategy, we can now add (i) the modulation of lung mechanobiology to favor appropriate lung regeneration and (ii) the stimulation of endogenous stem/progenitor cells or supply of exogenous ones for lung regeneration. Therefore, the current review draws together three important strands of information on lung organogenesis as of April 2010: (i) molecular embryology of the lung, (ii) mechanobiology of the developing lung, and (iii) pulmonary stem/progenitor cell biology. Applying advances in these complementary areas of research to lung regeneration and correction of lung diseases remains the therapeutic goal of this field. With the recent human transplanation of a stem/progenitor cell-derived tissue-engineered major airway (Macchiarini et al., 2008), we can clearly see the potential of this field, while recognizing the many problems yet to be solved.
Before concentrating on the molecular biology, mechanobiology, and stem cell biology of the lung, a first step in regenerative strategies is to consider the developmental anatomy of the lung. From this, we can at least see what type of structures we need to generate.
2. Developmental Anatomy of the Lung
2.1. The bauplan: key steps in lung morphogenesis
A diagrammatic overview of lung morphogenesis is given in Fig. 3.1. Three lobes form on the right side and two lobes on the left side in human lung; in mice four lobes form on the right (cranial, medial, and caudal lobes, plus the accessory lobe) and one on the left. In contrast to humans, in the mouse, there are only 12 airway generations and alveolarization occurs entirely postnatally.
Figure 3.1.
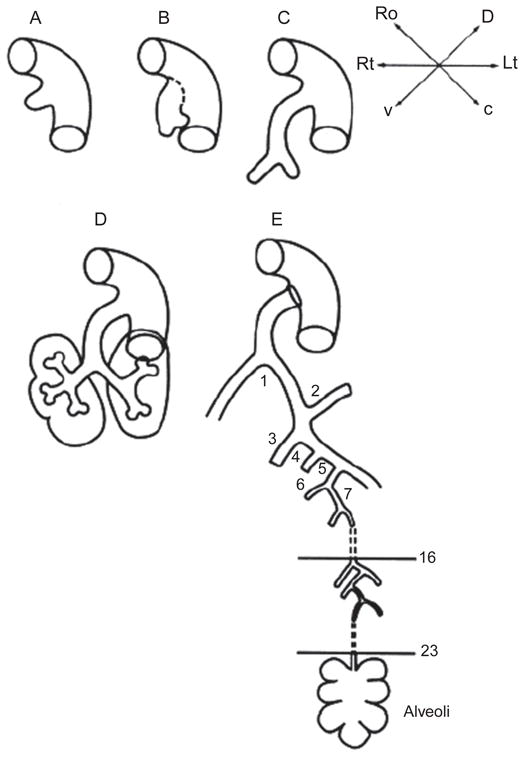
(A) The primitive lung anlage emerges as the laryngotracheal groove from the ventral surface of the primitive foregut at 5 weeks’ gestation in the human. (B) The primitive trachea separates dorsoventrally from the primitive esophagus as the two primary bronchial branches arise from the lateral aspects of the laryngotracheal groove at 5 or 6 weeks’ gestation in the human. (C) The embryonic larynx and trachea with the two primary bronchial branches are separated dorsoventrally from the embryonic esophagus at 6 weeks in the human. (D) The primitive lobar bronchi branching from the primary bronchi at 7 weeks in the human. (E) A schematic rendering of the airway at term in the human. The stereotypical first 16 airway generations are complete by 16 weeks in humans; between 16 and 24 weeks, further branching is nonstereotyped. Alveolarization begins about 20 weeks in humans and is complete by 7 years of age at the earliest. (After West, Burri, Warburton, and others).
2.2. The histological stages of lung development
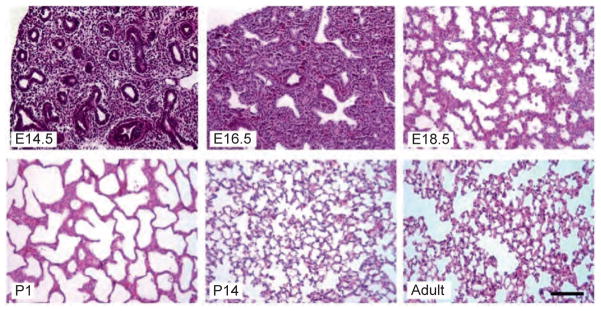
Histologically, lung development and maturation has been divided into four stages: pseudoglandular, canalicular, terminal saccular, and alveolar (Fig. 3.2).
Figure 3.2.
Histology of mouse lung at characteristic stages of development. Embryonic mouse lung develops from pseudoglandular stage (E14.5) to canalicular stage (E16.5) and further terminal sac stage (E18.5 and P1). Neonatal mouse lungs undergo alveolarization, resulting in the formation of many septa (P14). Finally, a mature honeycomb-like structure with alveoli surrounding alveolar ducts conferring normal respiratory structure and function is formed, as observed in the adult. Scale bar: 100 μm.
The pseudoglandular stage (5–17 weeks of human pregnancy, E9.5–16.6 days in mouse embryo)
During this, the earliest developmental stage, epithelial tubes lined with cuboidal epithelial cells undergo branching morphogenesis and resemble an exocrine gland (hence the nomenclature). However, this fluid-filled primitive respiratory tree structure is too immature to support efficient gas exchange.
The canalicular stage (16–25 weeks of human pregnancy, E16.6–17.4 days in mouse embryo)
The cranial part of the lung develops faster than the caudal part, resulting in partial overlap between this stage and the previous stage. During the canalicular stage, the respiratory tree is further expanded in diameter and length, accompanied by vascularization and angiogenesis along the airway. A massive increase in the number of capillaries occurs. The terminal bronchioles are then divided into respiratory bronchioles and alveolar ducts, and the airway epithelial cells are differentiated into peripheral squamous cells and proximal cuboidal cells.
The terminal saccular stage (24 weeks to late fetal period in human, E17.4 to postnatal day 5 (P5) in mouse)
There is substantial thinning of the interstitium during the terminal saccular stage. This results from apoptosis as well as ongoing differentiation of mesenchymal cells (Hashimoto et al., 2002; Lu et al., 2002). Additionally, at this stage, the alveolar epithelial cells (AECs) are more clearly differentiated into mature squamous type I pneumocytes and secretory rounded type II pneumocytes bearing lamellar bodies that contain surfactant. The capillaries also grow rapidly in the mesenchyme surrounding the saccules to form a complex network. In addition, the lymphatic network in lung tissue becomes well developed during this stage. The thick wall of these saccules, also called primary septae, comprises lining epithelial cells on both sides of a connective tissue core, within which there is a double parallel network of capillaries. Toward the end of this stage, the fetal lung can support air exchange in prematurely born human neonates. Maturation of surfactant synthesis and secretion is a key factor in determining whether the newborn lung can sustain gas exchange without collapsing.
The alveolar stage (late fetal period to childhood in human, P5–P30 in mouse)
Alveolarization is the last step of lung development. The majority of the gas exchange surface is formed during this stage.
Genome-wide expression profiling has measured developing human lung transcriptomes in pregnancies terminated between 7 and 22 weeks post conception (Kho et al., 2009). Within the 3,223 gene developing lung-characteristic subtranscriptome, transitions in gene expression correlated with some histological stages, as well as suggesting novel substages exist. For example, induction of surfactant gene expression identifies a “molecular transition” in the pseudoglandular phase.
Hence, the histological account of lung development is complimented by the molecular embryology that we consider in the next main section of the review.
2.3. Focus on branching morphogenesis: simplifying the complexities
Branching morphogenesis is a critical part of overall lung development and a crucial phenomenon in the development of several other organs. Understanding this key hurdle in lung regeneration strategies requires us to appreciate that despite the apparent and beautiful complexity of the lung, there are key simplicities that can help us in our task.
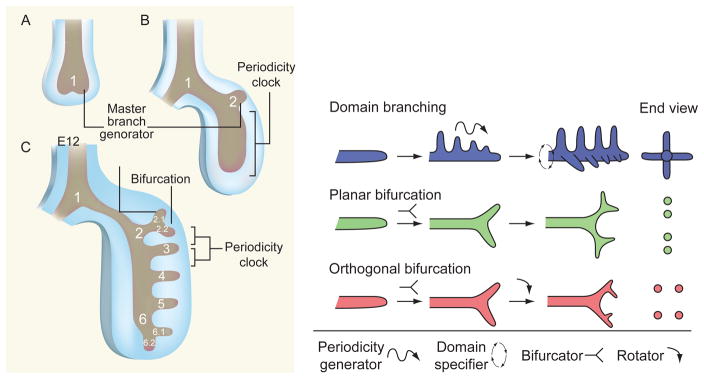
Fractal mathematics has revealed that relatively straightforward algorithms, when applied iteratively, could generate patterning of great complexity (Mandelbrot, 1982). Moreover, when the mathematical parameters were chosen appropriately, the approximation to living branched structures was striking. At first sight, intrapulmonary airway branching (distal to the primary bronchi) appears to become increasingly complicated as it proceeds distally and the number of individual branches increases into the millions. But, once the laryngotracheal complex and left–right laterality are established, distal airway branching is now thought to be driven by a relatively simple set of genetically encoded control routines. Echoing the fractal pattern formation achieved in mathematical models, these include the following: (i) a master branch generator routine, with three slave subroutines instructing a periodicity clock which times the appearance of subsequent branches, (ii) a rotational orientation subroutine which determines the orientation of the branches around the axis of the airway, and (iii) a branch tip division subroutine (Metzger et al., 2008; Warburton, 2008). Thus, branching morphogenesis of the bronchi in early mouse embryo lung can be parsed anatomically into three simple geometric forms, termed domain branching, planar bifurcation, and orthogonal bifurcation (Fig. 3.3). These basic forms are repeated iteratively to form different arrangements of branches. The planar or bottlebrush array describes the sequential proximal to distal emergence of secondary branches along the lateral axis of the primary bronchial airway. The bottlebrush mechanism is then reoriented around the branch axis to form a second row of branches at right angles to the first row. The terms planar array and rosette array describe the patterns formed by sequential bifurcation of the tips of secondary, tertiary, and subsequent buds at right angles to each other. Repetition of these simple branching modules, together with the hierarchical control and coupling of them, may therefore explain how the genome could possibly encode the highly complex yet stereotypic pattern of early bronchial branch formation, using a relatively simple toolbox of genetic modules. In a further illustration of how the mammalian lung uses simple routines and subroutines to construct itself, substantial homology has been identified between the genetic regulation of lung organogenesis and airway morphogenesis in Drosophila (Hacohen et al., 1998; Tefft et al., 1999). Despite the latter’s relative simplicity, it is striking to note not only the genetic homology but also the similar epistatic signaling hierarchy into which these regulators are arranged in the fly.
Figure 3.3.
How the airways can form in a sequential manner by reiterating a few, relatively simple sets of genetic instructions. In the upper panel, which is drawn after Warburton (2008), a master branch generator, a periodicity clock, and a bifurcator program are shown as controlling the layout of the mainstem and lobar branches. At embryonic day (E) 10.5, (A) the primary bronchial branch (1) forms, followed by (B) the development of the left upper-lobe branch (2) by E11, and then (C) the first two segmental branches of the left upper-lobe branch (2.2 and 2.3) form and the subsequent formation of branches 3–6 occurs by E12. The master branch generator is active throughout these events, and the inferred sites of action of the periodicity clock and bifurcator subroutines are shown. Then, in the lower panel, following the views of Metzger et al., (2008), a series of inferred genetic subroutines are shown, all driven by one master branch generator, shown as giving rise to domain or “bottle brush” branching along the lateral proximodistal axis of the main stem bronchi, which can then be rotated at right angles to give rise to a second rank of branches. Then, in subsequent rounds of branching, arising from the tips of the primary and secondary branches, it is shown how the same relatively simple periodicity generator, domain specifier, bifurcator, and rotator subroutines can give rise to apparently more complex patterns of peripheral branching to achieve an ever larger number of space filling terminal branches.
Using real-time microscopic cinematography, individual airway tip branching can be parsed temporally into a branch extension phase, a branch tip arrest phase, and a tip-splitting budding phase, followed once again when the branch budding phase is completed by branch extension until the next round of budding follows once more. A clock mechanism mediated by FGF–FGFR–Sprouty signaling plays a key role in timing the rate of bud extension and hence the inter-branch distance (Unbekandt et al., 2008; Warburton, 2008). Indeed a nested hierarchy of clock routines are likely to be present throughout lung development given the number of oscillating systems intrinsic to the lung (branching, airway peristalsis, calcium oscillations) or visited extrinsically upon it (fetal breathing, circadian rhythms). Branching morphogenesis is accompanied by contractile oscillations (airway peristalsis) that are themselves underpinned by periodic calcium waves (Featherstone et al., 2006; Jesudason et al., 2005). These oscillators appear to be coupled to lung growth, and their precise relation to the timing of branching remains to be determined. However, we postulate that clock routines underlying the linear process of somitogenesis are redeployed three-dimensionally for branching morphogenesis in the lung and other organs (Pourquie, 2003).
2.4. The impact of abnormal lung development
The airway is developed sequentially by early epithelial tube branching and later septation of terminal air sacs. Pulmonary vasculature develops within lung mesenchyme in close conjunction with epithelial morphogenesis. Airway and vascular smooth muscle also develop during early morphogenesis. Perturbation of these developmental processes results in abnormal lung structure, deficiency of gas exchange, and neonatal respiratory failure. Clinical examples of such disruption of normal lung growth include cystic adenomatoid malformation of the lung, bronchopulmonary dysplasia (BPD) (a sequel of premature human delivery), and hypoplasia of the lung (seen in congenital diaphragmatic hernia (CDH), a birth defect as common as cystic fibrosis). More subtle lung dysplasias that are not lethal neonatally may emerge later in life with asthmatic wheezing and perhaps predisposition to early onset of chronic obstructive pulmonary disease (COPD). One of the clearest examples of how early development can affect not only lung organogenesis but also long-term health is the ciliary dyskinesia encountered in Kartagener syndrome and primary ciliary dyskinesia (Storm van’s Gravesande, 2005). Early in embryogenesis, failure of ciliary function leads to randomization of organ situs and hence a supranormal rate of dextrocardia. This is accompanied by randomization of lung asymmetry. Persisting ciliary dysfunction impairs mucociliary clearance in the sinuses as well as the airways and predisposes to chronic lung disease in later life. Crucially one can note that disruption of lung asymmetry does not itself lead to lung malformation: hence the lung “bauplan” is conserved despite the lungs’ left–right asymmetry being the reverse of normal. This observation reiterates to us that the complexities of lung organogenesis may actually be broken down into nested routines and subroutines used to accomplish particular tasks in the overall process.
The implication for lung regeneration is that one need not understand the formation of every last alveolus, but rather that elucidating the iterative routines could suffice to promote pulmonary “self-assembly.”
3. Molecular Embryology of the Lung
This section of the review serves as a comprehensive reference source. For those with no requirement for such detail, the reader is directed to the summary Fig. 3.4.
Figure 3.4.
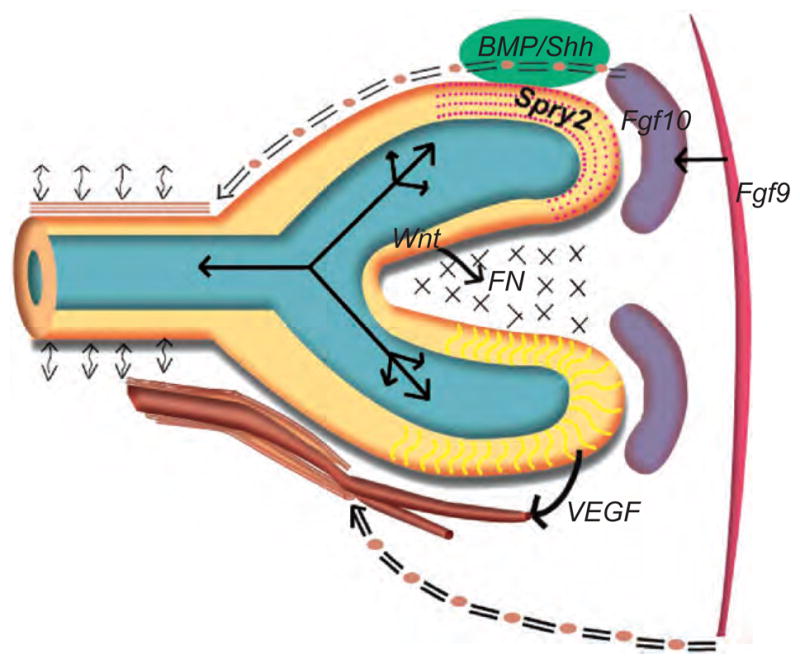
Schematic illustration of the variety of biochemical and biomechanical regulators of lung growth. The epithelium of the end-bud (yellow) encloses a fluid filled lumen (blue-green) in which oscillatory fluid flows are depicted (solid bidirectional arrows) that result from periodic peristaltic contractions (bi-directional curvilinear arrows) of airway smooth muscle (ASM: sample shown in brown running parallel and above the main epithelial trunk). The ASM derives from the FGF10+ precursor pool (seen in purple on the right), and these ASM progenitors are seen becoming more proximal (double-lined “=” at the top of the epithelial outline) relative to the growing epithelium. Examples of key biochemical signaling are given: FGF9 from the mesothelium (far right) regulates the FGF10+ mesenchyme (purple), which in turn interacts locally with epithelial Sprouty (SPRY2), BMP4, and Sonic Hedgehog (SHH) signaling (the latter two epithelial signals are shown in green and, due to space constraint, adjacent to the schematized epithelium). Epithelial Wnt signaling regulates fibronectin (FN) elaboration (shown as “xx”) in the cleft between epithelial branches. Epithelial VEGF signals to developing vasculature shown at the base of the figure. These vessels attract vascular smooth muscle precursors from the mesothelium (shown as “=” at the base of the figure). (See Color Insert.)
We will first use a step-wise “process-driven” description of lung growth followed by a catalogue of the biochemical factors involved: many such factors are involved at multiple stages and do not map neatly on to the “process-driven” account. The biochemical factors are considered as follows: growth and transcription factors in order of first appearance and then other participating factors such as extracellular matrix (ECM) and miRNA.
3.1. Process-driven molecular embryology of the lung
3.1.1. Induction of the early lung anlagen
Early lung induction is regulated by genes that act cooperatively to define the location of laryngotracheal groove and help specify the spatial axes of the developing organ.
Among the earliest endodermal signals essential for gut morphogenesis and gut tube closure are the GATA (zinc-finger proteins that recognize GATA DNA sequence) and hepatocyte nuclear factor (HNF/Fox) transcription factors. Foxa2 is required for gut tube closure, while Gata-6 is required for activation of the lung developmental program within the foregut endoderm. Hnf-3/Foxa2β is a survival factor for the endoderm; its expression is induced by Sonic hedgehog (Shh). Retinoids and their transcriptional factor receptors also play key roles in induction of early lung branching: retinoic acid (RA) deficiency and compound null mutation of retinoid receptors prevent induction of the laryngotracheal groove. Most recently, Wnt2/2b and β-catenin signaling have been shown to be necessary and sufficient to specify lung progenitors in the foregut (Goss et al., 2009; Harris-Johnson et al., 2009). Embryos lacking Wnt2/2b expression exhibit complete lung agenesis and do not express Nkx2.1, the earliest marker of the lung endoderm. This phenotype is recapitulated by an endoderm-restricted deletion of β-catenin. Conversely, conditional expression of an activated form of β-catenin leads to ectopic expansion of the Nkx2.1 expression domain into esophagus and stomach epithelium. Thus, gain or loss of trachea/lung progenitor identity is accompanied, respectively, by contraction or expansion of esophagus/stomach progenitor identity. Taken together, these findings suggest that Wnt2/2b signaling through the canonical Wnt pathway is required to specify lung endoderm progenitors within the foregut. Furthermore, ectopic lung bud formation can be induced in the esophagus by Tbx4 misexpression activating Fgf10 expression (Sakiyama et al., 2003). In addition, left–right asymmetry is controlled by several genes, including nodal, Lefty-1,2, and Pitx-2. For example, single-lobed lungs are found bilaterally in Lefty-1−/− mice, and bilateral isomerism of the lung is found in Pitx2-null mutants.
3.1.2. Tracheoesophageal septation
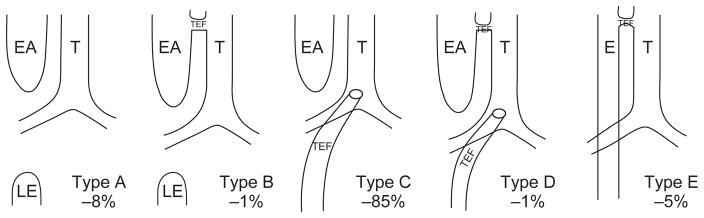
The processes whereby trachea and esophagus form from primitive foregut is of clinical interest due to the common birth defect, tracheoesophageal fistula (TEF) (Fig. 3.5).
Figure 3.5.
Subtypes of esophageal atresia (EA) and/or tracheo(T)–esophageal (E) fistula (TEF) with percentage frequency amongst EA-TEF cases. Type A: ‘Pure’ EA without TEF. Lower esophagus shown (LE); Type B: EA with TEF from proximal esophageal pouch but without any distal TEF; Type C: EA with distal TEF only (the most common variant); Type D: EA with both proximal TEF and distal TEF; Type E: TEF in the absence of EA. Laryngotracheal clefts (not shown here) are still rarer anomalies in which trachea and esophagus form a single lumen for a variable length. In severe variants, a combined tracheo-esophagus connects to the stomach whilst also giving rise to the main bronchi.
Usually encountered in conjunction with esophageal atresia (EA), the combined sequence is sometimes found together with other anomalies of heart, vertebrae, anorectum, and limbs. Genetic defects identified in patients with EA-TEF have recently been comprehensively reviewed (Felix et al., 2009). Transgenic murine mutants with deletions in RA receptors or Gli2/Gli3 feature a form of EA-TEF. Moreover, the transcriptomic changes associated with budding of the lung from the foregut have recently been enumerated. Alongside identifying the known regulators described above, further candidates will need experimental evaluation (Millien et al., 2008). Illustrating that environmental factors may play a role, a EA-TEF phenotype can be generated by exposure of murine embryos to adriamycin (Diez-Pardo et al., 1996). Interestingly, despite the major anomaly of foregut development, lung formation in EA-TEF patients is usually grossly normal. Their respiratory tract morbidity tends to derive from tracheomalacia and, more chronically, reactive airways disease. Whilst the latter is traditionally attributed to gastroesophageal reflux and pulmonary aspiration, it remains possible that some of this pulmonary morbidity stems from subtly abnormal early lung development.
3.1.3. Tracheal cartilage formation
Children with EA-TEF may also suffer from tracheal weakness (tracheomalacia) in which inadequate formation of the tracheal cartilages results in potentially life-threatening airway closure during expiration.
Dorsoventral patterning of the trachea during embryonic development is associated with formation of C-shaped cartilage rings ventrally and trachealis muscle dorsally. Ventral mesenchyme segregates into successive cartilaginous and noncartilaginous domains, providing a compromise between flexibility and rigidity.
Tracheomalacia describes weakness of the walls of the trachea and it may result in life-threatening episodes and/or recurrent hospitalizations for lower airway infections (Austin and Ali, 2003; Boogaard et al., 2005; Carden et al., 2005; McNamara and Crabbe, 2004). It can be an isolated idiopathic anomaly or associated with EA-TEF, primary defects of cartilage synthesis (e.g., dyschondroplasia), cartilage degeneration due to trauma (e.g., long-term tracheal intubation), or extrinsic compressive lesions such as vascular rings or tumors (Berdon, 2000). Tracheal stenosis is narrowing of the trachea: it can follow prolonged intubation or accompany a cartilaginous sleeve malformation of the trachea or may again be associated with extrinsic compressive lesions. Tracheal cartilaginous sleeve comprises fusion of the ventral cartilage rings. It is a rare malformation associated with craniosynostosis syndromes like Crouzon syndrome, Pfeiffer syndrome, Goldenhar syndrome or Apert syndrome (Lin et al., 1995).
Tracheal maldevelopment can be modeled: nitrofen administration to pregnant dams results in tracheal malformations as well as CDH in offspring (Diez-Pardo et al., 1996; Xia et al., 1999). Although genetic control of the regulation of tracheal cartilage versus smooth muscle cell (SMC) formation remains unclear, relevant transgenic murine phenotypes have been observed. Miller et al. (2004) showed that partial Shh inactivation causes tracheobronchial cartilage abnormalities indicative of tracheomalacia. Park et al. (2009) demonstrated Shh augments Sox9 expression: Sox9 induces type II collagen (Col2a1) expression and promotes the chondrocyte lineage amongst mesenchymal cells. Bone morphogenic protein 4 (BMP4) also regulates Sox9 to induce chondroprogenitors amongst mesenchymal cells (Hatakeyama et al., 2004). Impaired BMP signaling induces tracheal cartilage defects with EA-TEF (Que et al., 2006). β-Catenin also interacts with Sox9 but to inhibit differentiation of tracheal chondroprogenitor cells (Akiyama et al., 2004).
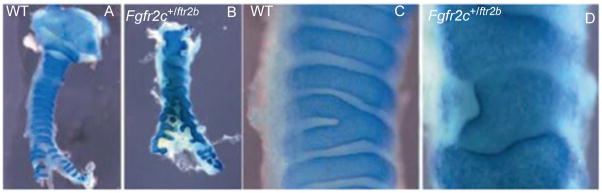
Recently, FGF18 and FGF10 have also been described to play important roles in tracheal cartilage ring formation (Elluru et al., 2009; Tiozzo et al., 2009; Whitsett et al., 2002). Specific targeted inactivation of Fgf18, using the SP-C promoter driving Cre, induced malformation of the cartilage rings (Whitsett et al., 2002). Overexpression of Fgf18 also resulted in malformation of the cartilage rings, possibly via Sox9 upregulation (Elluru et al., 2009). Tiozzo et al. (2009) reported that ectopic fibroblast growth factor receptor (FGFR)2b expression in tracheal mesenchyme renders this hyper-responsive to FGF10, resulting in cartilaginous sleeve formation reminiscent of the Apert syndrome tracheal phenotype (Fig. 3.6). This abnormal cartilage structure arises secondary to increased proliferation of cartilage progenitor cells within tracheal mesenchyme.
Figure 3.6.
Excessive mesenchymal FGF signaling leads to overgrowth of tracheal rings. Wild-type and mutant tracheas are stained with Alcian blue. (A) Wild-type trachea at P0 exhibiting regular cartilage rings separated by noncartilaginous mesenchyme; (B) Fgfr2c+/Fgfr2b trachea at P0 showing excessive growth of the cartilage with absence of noncartilaginous mesenchyme; (C, D) high magnification of A and B, respectively. (See Color Insert.)
Despite incomplete understanding of such genetics, tissue engineered airway (formed using stem cells and cadaveric scaffold) has been successfully transplanted into adult and a pediatric patients to replace damaged bronchus and trachea, respectively (Macchiarini et al., 2008)
3.1.4. Branching morphogenesis of airway and vasculature
A multiplicity of factors are required for normal airway branching morphogenesis. Much of the research in this area is focused on epithelial morphogenesis: this is discussed in detail in subsequent sections dealing with individual signaling pathways. A key insight has been that epithelial morphogenesis proceeds interdependently with vascular development. Indeed tight coupling of endothelial with epithelial development is required for efficient gas transport and fluid clearance at birth. Vascular endothelial growth factor (VEGF) signaling from the epithelium to the developing endothelium is essential for the primitive hemangioblasts to develop into mature capillary networks. Likewise, the endothelium probably signals back to coordinate epithelial morphogenesis. There is a stereotyped anatomical relationship between the developing pulmonary capillaires, arteries, and veins (Fig. 3.7). The arteries run along the superior surface of the developing lobules, while the veins run along the interior surface.
Figure 3.7.
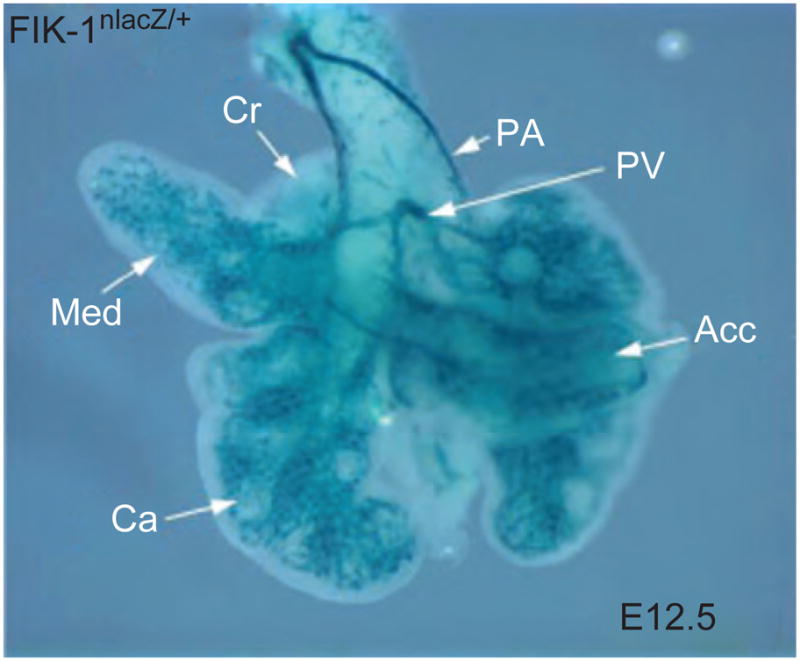
Vascular endothelial development in E12.5 mouse lung is shown in whole mount as a blue signal resulting from transgenic expression of Flk1-β-galactosidase (Flk-1nLacZ/+): pulmonary artery (PA); pulmonary vein (PV); cranial lobe (Cr); medial lobe (Med); caudal lobe (Ca); accessory lobe (Acc). (See Color Insert.)
3.1.5. Alveolar septum formation
Septation of terminal sacs generates alveoli and involves interacting mesenchymal myofibroblasts, epithelial cells, and endothelial cells. Myofibroblasts are smooth muscle precursors with fibroblast morphology that migrate within nascent septa and deposit elastin (particularly at tips) as the first step of secondary septa development (Bostrom et al., 1996; Lindahl et al., 1997). Alveolar myofibroblast differentiaion requires Lunatic fringe and Notch signaling, and their elastin deposition is PGFa- and FGFR3/4−regulated (Xu et al., 2010). Septal thinning and maturation of the alveolar capillary network are also needed. Interstitial thinning proceeds with expansion of septal epithelial, vascular, and airspace compartments but also myofibroblast apoptosis (Awonusonu et al., 1999; Schittny et al., 1998) that affects lipid-filled interstitial fibroblasts (LFIF) rather than non-LFIF (NLFIF). This apoptosis is associated with downregulation of insulin-like growth factor I receptor (Igf-IR) mRNA and cell surface protein expression (Srinivasan et al., 2002).
Finally, the new alveolar septum differentiates into a functional respiratory membrane that consists of type I AECs (AECI), basement membrane, and capillary endothelial cells. The respiratory membrane provides a short distance for diffusion thereby facilitating gas exchange. It is estimated that about 50 million alveoli are present in neonatal lung. However, by age 7–8 years, when alveolarization is largely complete, the number of alveolar units in the lung has increased six-fold to about 300 million. Meanwhile the adut alveolar capillary bed is capable of accommodating the entire adult cardiac output of 5 L/min, rising five-fold to 25 L/min during maximal exercise.
Alveolarization can be adversely affected by premature delivery, hyperoxia, postnatal steroid exposure, and prolonged mechanical ventilation, even with room air (Mokres et al., 2010). Thus, prematurity and hyperoxia plus pressure plus time are well-recognized risk factors for the hypoalveolarization characteristic of BPD in human premature infants. It is postulated that a cascade of events including endotoxin exposure, inflammation, and expression and activation of excessive amounts of transforming growth factor (TGF)-β ligand inhibit aleolarization in BPD and therefore portend an adverse outcome of this disease (reviewed in Shi et al., 2009).
3.2. Cataloguing the biochemical regulators of lung development
Having considered the process of building the lung, we next turn to catalogue the factors required for lung growth and maturation. Transgenic mouse technology has allowed us to evaluate roles in organogenesis by, for example, overexpressing or knocking out a specific genes (Costa et al., 2001). Some murine pulmonary phenotypes resulting from a loss or gain of gene function are listed in Table 3.1, modified from Cardoso and Lu (2006).
Table 3.1.
Examples of mutations in mouse giving a reported lung and/or tracheal phenotype
| Gene symbol | Gene name | Expression pattern | Phenotype | Reference |
|---|---|---|---|---|
| Signaling molecule | ||||
| Egfr | Epidermal growth factor receptor | Epithelium and mesenchyme | Impaired branching and deficient alveolization | Miettinen et al. (1997) |
| Fgf18 | Fibroblast growth factor 18 | Mesenchyme | Deficient alveolization | Usui et al. (2004) |
| Fgf9 | Fibroblast growth factor 9 | Epithelium and pleura | Impaired branching, reduced mesenchyme | Colvin et al. (2001) |
| Greml | Gremlin 1 | Epithelium and mesenchyme | Deficient alveolization | Michos et al. (2004) |
| Hipl | Huntingtin-interacting protein 1 | Mesenchyme | Impaired branching | Chuang et al. (2003) |
| Shh | Sonic hedgehog | Epithelium | Impaired branching, tracheoesophageal fistula | Litingtung et al. (1998) |
| Tgfb3 | Transforming growth factor, β3 | Epithelium and pleura | Impaired branching | Kaartinen et al. (1995) |
| Wnt7b | Wingless-related MMTV integration site 7B | Epithelium | Vascular defect, reduced mesenchyme | Shu et al. (2002) |
| Catnnb1 | β-Catenin | Epithelium | Impaired branching, proximal/distal specification | Mucenski et al. (2003) |
| Ltbp4 | Latent transforming growth factor β binding protein 4 | Not reported | Pulmonary emphysema | Sterner-Kock et al. (2002) |
| Wnt5a | Wingless-related MMTV integration site 5A | Mesenchyme and epithelium | Increased branching, tracheal defect | Li et al. (2002) |
| Fgfl0 | Fibroblast growth factor 10 | Mesenchyme | Lung agenesis | Sekine et al. (1999) |
| Fgfr2b | Fibroblast growth factor receptor 2b | Epithelium | Lung agenesis | De Moerlooze et al. (2000) |
| Fgf8 | Fibroblast growth factor 8 | Not reported | Right pulmonary isomerism | Fischer et al. (2002) |
| TAB1 | TGF-B activated kinase-1 binding protein-1 | Epithelium | Lung dysmorphogenesis | Komatsu et al. (2002) |
| Acvr2b | Activin receptor IIB | Not reported | Right pulmonary isomerism | Oh and Li (1997) |
| Nodal | Nodal | Not reported | Right pulmonary isomerism | Lowe et al. (2001) |
| Lefty1 | Left right determination factor 1 | Not reported | Left pulmonary isomerism | Meno et al. (1998) |
| Traf4 | Tnf receptor associated factor 4 | Not reported | Tracheal defect | Shiels et al. (2000) |
| Fgfr3/Fgfr4 | Fibroblast growth factor receptor 3/4 | Epithelium and mesenchyme | Defective elastin production, alveolarization defect | Weinstein et al. (1998) |
| Nog | Noggin | Mesenchyme | Lobation defect | Weaver et al. (2003) |
| Pitx-2 | Paired-like homeodomain transcription factor 2 | Not reported | Bilateral isomerism | Kitamura et al. (1999) |
| Dermo1 | Twist homolog 2 | Mesenchyme | Impaired branching | De Langhe et al. (2008) |
| BMP4 | Bone morphogenic protein 4 | Epithelium and mesenchyme | Abnormal lung morphogenesis with cystic terminal sacs | Bellusci et al. (1996) |
| Igf1r | Insulin-like growth factor 1 receptor | Not reported | Impaired development | Liu et al. (1993). |
| Notch2/3 | Notch gene homolog 2/3 | Epithelium | Defective myofibroblast differentiation, alveolarization defect | Xu et al. (2009) |
| PDGFa | Platelet derived growth factor a | Epithelium | Defective myofibroblast elastin production, alveolarization defect | Bostrom et al. (2002) |
| Timp3 | Tissue inhibitor of metalloproteinase 3 | Mesenchyme | Reduced number of bronchioles and attenuated alveogenesis | Gill et al. (2003) |
| Transcription factor | ||||
| Cebpa | CCAAT/enhancer binding protein (C/ EBP), α | Epithelium | Hyperproliferation of type II cells | Sugahara et al. (2001) |
| Foxa1/Foxa2 | Forkhead box A1/A2 | Epithelium | Impaired branching, reduced smooth muscle | Wan et al. (2005) |
| Foxf1a | Forkhead box F1a | Mesenchyme | Impaired branching, lobation defect | Lim et al. (2002) |
| Hoxa5 | Homeobox A5 | Mesenchyme | Impaired branching, tracheal defect | Aubin et al. (1997) |
| Klf2 | Kruppel-like factor 2 (lung) | Not reported | Impaired sacculation | Wani et al. (1999) |
| Mycn | Neuroblastoma myc- related oncogene 1 | Epithelium | Impaired branching | Moens et al. (1992) |
| Trp63 | Transformation- related protein 63 | Epithelium | Tracheobronchial defect | Daniely et al. (2004) |
| Titf1 | Thyroid transcription factor 1 | Epithelium | Loss of distal lung fate, impaired branching, tracheoesophageal fistula | Kimura et al. (1996) |
| Nfib | Nuclear factor I/B | Epithelium and mesenchyme | Sacculation defect | Steele-Perkins et al. (2005) |
| Sox11 | SRY-box-containing gene 11 | Epithelium | Hypoplastic lung | Sock et al. (2004) |
| Tcf21 | Transcription factor 21 (Pod1) | Mesenchyme | Impaired branching | Quaggin et al. (1999) |
| Rarb/Rara | Retinoic acid receptor α/β | Epithelium and mesenchyme | Left lung agenesis and right lung hypoplasia | Mendelsohn et al. (1994) |
| Pitx2 | Paired-like homeodomain transcription factor 2 | Mesenchyme | Right pulmonary isomerism | Lin et al. (1999) |
| Foxj1 | Forkhead box J1 | Epithelium | Left– right asymmetry, loss of ciliated cells | Brody et al. (2000) |
| Gata6 | GATA-binding protein 6 | Epithelium | Impaired sacculation | Yang et al. (2002) |
| Gli2/Gli3 | GLI-Kruppel family member GLI2/ GLI3 | Mesenchyme | Lung agenesis | Motoyama et al. (1998) |
| Ascl1 | Achaete-scute complex homolog- like 1 | Neuroendocrine cells | Loss of neuroendocrine cells | Ito et al. (2000) |
| Erm | Ets variant gene 5 | Epithelium | Impaired type I cell formation | Liu and Hogan (2002), Liu et al. (2003) |
| Wnt2/2b | Wingless-related MMTV integration site 2/2b | Mesenchyme | Complete lung agenesis |
Goss et al. (2009) Harris- Johson et al. (2009) |
| Alk3 | Aurora-like kinase | Epithelium | Retardation of lung branching, reduced cell proliferation and differentiation | Sun et al. (2008) |
| Others | ||||
| Eln | Elastin | Mesenchyme | Deficient alveolization | Wendel et al. (2000) |
| Lmnb1 | Lamin B1 | Epithelium and mesenchyme | Deficient alveolization | Vergnes et al. (2004) |
| Lama5 | Lamininα 5 | Epithelium and pleura | Defective lobation | Nguyen et al. (2002) |
| Pcaf | p300/CBP-associated factor | Epithelium and mesenchyme | Defective proximal and distal epithelial cell differentiation | Shikama et al. (2003) |
| Adam17 | A disintegrin and metallopeptidase domain 17 | Epithelium | Impaired epithelial differentiation, impaired branching | Zhao et al. (2001), Peschon et al. (1998) |
| Crh | Corticotropin- releasing hormone | Epithelium | Defective epithelial and mesenchymal maturation | Muglia et al. (1999) |
| Pthlh | Parathyroid hormone- like peptide | Epithelium | Deficient alveolization | Rubin et al. (2004) |
| Itga3 | Integrin α3 | Epithelium | Impaired branching | Kreidberg et al. (1996) |
| Cutl1 | Cut-like 1 | Epithelium | Impaired epithelial differentiation | Ellis et al. (2001) |
| RXRa | Retinoic X receptor alpha | Epithelium and mesenchyme | Decrease in alveolar surface area and alveolar number | McGowan et al. (2000) |
| Tmem16a | Transmembrane protein 16a | Epithelium | Abnormal tracheal cartilages resulting in tracheomegaly | Rock et al. (2008) |
| TACE | Tumor necrosis factor- α converting enzyme | Not reported | Failure to form saccular structures | Zhao et al. (2001) |
| PDGFa | Platelet derived growth factor a | Epithelium | Defective myofibroblast elastin production, alveolarization defect | Bostrom et al. (2002) |
| Na/K ATPase | Sodium/ Potassium ATPase | Epithelium | Failure to absorbfetal lung liquid, causes significant respiratory distress and neonatal lethality | Hummel et al. |
| Lfng | Lunatic Fringe | Epithelium | Impaired myofibroblast differentiation and alveogenesis | Xu et al. (2009) |
3.2.1. Transcription factors
At least four groups of transcription factors, forkhead box, Nkx homeodomain, RA receptors, and Gli family play important roles in lung development.
Forkhead box transcription factor family
Members of the forkhead box family transcription factors, such as Foxa1, Foxa2, HFH8, and HFH4, share homology in the winged-helix DNA-binding domain and regulate pulmonary cellular proliferation and differentiation.
HNF-3α (Foxa1) and HNF-3β (Foxa2) share 93% homology in amino acid sequences and were first identified as factors in hepatocyte differentiation (Qian and Costa, 1995). However, Hnf-3β is expressed in developing lung, with higher levels in proximal epithelial cells and lower levels in distal type II epithelial cells (Zhou et al., 1996b). Overexpression of Hnf-3β under control of epithelial specific SP-C promoter inhibits lung branching morphogenesis and vasculogenesis in vivo (Zhou et al., 1997). HNF-3α and HNF-3β also regulate expression of CCSP and surfactant proteins in bronchiolar and type II epithelial cells (Bingle et al., 1995; Bohinski et al., 1994; He et al., 2000). Hnf-3β is inducible by interferon, and regulates in turn the expression of the Nkx homeodomain transcription factor Nkx2.1 (also termed Ttf-1 and CebpI), which in turn regulates transcription of the surfactant protein genes in peripheral lung epithelium (Ikeda et al., 1996; Samadani et al., 1995).
HFH8 is restricted to splanchnic mesoderm contacting embryonic gut and presumptive lung at E9.5 suggesting that Hfh-8 may participate in lung induction. HFH-8 expression continues in lateral mesoderm-derived tissue during development. By E18.5, Hfh-8 expression is restricted to distal lung mesenchyme and bronchial muscle (Peterson et al., 1997). The level of Hfh-8 expression is important for normal development: alveolar hemorrhage is observed in Hfh8(+/−) mice, while Hfh8(−/−) mice die in utero. Reduced Hfh-8 expression in Hfh-8+/− mutants is accompanied by decreased expression of VEGF and its receptor 2 (Flk-1), bone morphogenetic protein 4 (BMP-4), and the transcription factors of the Brachyury T-Box family (Tbx2–Tbx5) and Lung Kruppel-like factor (Kalinichenko et al., 2001). HFH8 regulates mesebchymal Pdgf receptor (Bostrom et al., 1996; Shinbrot et al., 1994; Souza et al., 1996). HFH8 binding sites are also found in the promoter region of genes, such as Bmp4, Hgf, and Hoxa5, that are critical regulators of lung morphogenesis (Ohmichi et al., 1998; Weaver et al., 1999).
Hfh4 (Foxj1) regulates ciliated epithelial cell differentiation. It is expressed in E15.5 airway epithelium just before ciliated cells appear (Hackett et al., 1995) and Hfh4−/−-null mutant mice feature defective ciliogenesis in airway epithelial cells and randomized left–right asymmetry (mimicking human Kartagener syndrome). The latter can result in perinatal lethality, but in low penetrance it gives rise to situs inversus, sinusitis, bronchiectasis, and sterility, all caused by defects in ciliary beat (Brody et al., 2000; Chen et al., 1998). Interestingly, HFH4 and other proximal lung markers such as CCSP are upregulated by BMP antagonist Noggin in mesenchyme-free airway epithelial culture (Hyatt et al., 2002).
Foxp1, Foxp2, and Foxp4 are highly expressed in mouse lung and gut. Foxp1 and Foxp4 are expressed in both proximal and distal airway epithelium while Foxp2 is expressed primarily in distal epithelium. Foxp1 protein expression is also observed in the mesenchyme and vascular endothelial cells of the lung (Lu et al., 2002).
Nkx and Hox homeodomain transcription factors
One of the most prominent homeodomain transcription factors in lung development is NKX2.1, also called TTF-1 (thyroid-specific transcription factor) or CEBP-1. Nkx2.1 is expressed in epithelial cells derived from foregut endoderm in lungs, thyroid, and pituitary, as well as restricted regions of fetal brain (Guazzi et al., 1990; Lazzaro et al., 1991). Hence, human Nkx2.1 mutants may feature benign hereditary chorea, congenital hypothyroidism, and neonatal respiratory distress at term (sometimes retrieved by the transactivating activity of Pax8) (Carre et al., 2009). Nkx2.1−/− mice exhibit impaired tracheoesophageal separation and early arrest of lung development featuring two main bronchi but no distal branches (Kimura et al., 1996; Minoo et al., 1999). In developing mouse airway epithelium, Nkx2.1 is initially expressed proximally and distally becoming restricted at later stages to distal AECs (Zhou et al., 1996b). Overexpression of Nkx2.1 causes dose-dependent morphological alterations in postnatal lung: modest overexpression raises type II pneumocyte proliferation and SP-B levels; greater overexpression disrupts alveolar septation with emphysema due to alveolar hypoplasia. The highest overexpression of Nkx2.1 in transgenic mice causes severe pulmonary inflammation, fibrosis, and respiratory failure, associated with eosinophil infiltration and increased eotaxin and IL-6 expression (Wert et al., 2002). Nkx2.1 signaling is critical for surfactant protein, T1a, and CC10 gene expression (Boggaram, 2003; Bruno et al., 1995; Guazzi et al., 1990; Ramirez et al., 1997; Whitsett and Glasser, 1998; Yan et al., 1995; Zhang et al., 1997). Nkx2.1-deficient pulmonary epithelial cells fail to express nonciliated marker genes, including differentiated Sp-B, Sp-C, and CC10. Bmp4 expression in these cells is also reduced. In addition to modulating expression of other lung-related genes, it is clear that NKX2.1 phosphorylation plays a crucial role in its signaling: mice with point mutation of seven serine phosphorylation sites of NKX2.1 died immediately following birth with malformation of acinar tubules, pulmonary hypoplasia, and reduced expression of surfactant proteins, CC10/secretoglobulin 1A, and Vegf (DeFelice et al., 2003). Whilst regulating expression of numerous genes, Nkx2.1 expression can itself be activated by transcription factors HNF-3β (Ikeda et al., 1996) and GATA-6 (Shaw-White et al., 1999) during lung morphogenesis.
Hox family transcription factors
Hox transcription factors are expressed with proximodistal polarity in developing lung: Hoxa5, Hoxb2, and Hoxb5 are restricted to distal lung mesenchyme, whilst Hoxb3 and Hoxb4 are expressed in proximal and distal mesenchyme (Aubin et al., 1997; Bogue et al., 1996; Volpe et al., 1997). Illustrating their functional role, Hoxa5−/−-null mutant mice have tracheal defects and occlusions, impaired lung branching morphogenesis, diminished surfactant protein expression, and alveolar wall thickening (Aubin et al., 1997).
GLI family zinc-finger transcription factors
GLI 1, 2, 3 are zinc-finger transcription factors and activated by SHH. All are mesodermally expressed, particularly in the distal lung (Grindley et al., 1997). Combined Gli2−/− and Gli3−/− mutant mice feature lung agenesis. Gli3−/− mice are viable but have small dysmorphic lungs (Grindley et al., 1997). Gli2 regulates normal lung asymmetry: Gli2−/− mice have a fused right and left lung (a small single lobe with defective primary branching in the right lung) and hypoplastic trachea and esophagus that are nevertheless distinct and retain normal proximal–distal differentiation (Motoyama et al., 1998).
3.2.2. Peptide growth factors
Embryonic lung mesenchymal and epithelial cells communicate through autocrine and paracrine factors, as demonstrated by effects of added growth factors on cultured embryonic lung growth (Jaskoll et al., 1988; Warburton et al., 1992).
FGF family
FGF family members are found throughout the vertebrates and invertebrates. Their functions in respiratory organogenesis are conserved from Drosophila to mammals (Glazer and Shilo, 1991; Sutherland et al., 1996). Based on protein sequence homology, FGFs have been divided into 23 subgroups. Similarly, their cognate transmembrane protein tyrosine kinase receptors (FGFRs) are classified into four types, contributing to the specificity of FGF ligand binding (Ornitz and Itoh, 2001). Heparan sulfate proteoglycan, an ECM glycoprotein, has been reported to be essential for FGF ligand–receptor binding and activation (Izvolsky et al., 2003a,b; Lin et al., 1999). FGFs play critical roles in cell proliferation, migration, and differentiation during development. Early inhibition of murine FGFR signaling shows it is required for early lung branching morphogenesis. Later FGFR inhibition in E14.5 lung decreases prenatal airway tubule formation and is associated with severe emphysema at maturity. At E16.5, FGFR inhibition causes mild focal emphysema. Murine mutants lacking FGFR3 and FGFR4 fail to undergo normal alveolarization, with poorly organized myofibroblasts and excessive amounts of poorly organized elastin. However, inhibition of FGFR signaling after birth did not appear to alter postnatal alveolarization (Hokuto et al., 2003).
FGF10 is one of the most-studied family members during lung development. Fgf10-null mice lack distal lung despite formation of larynx and trachea (Min et al., 1998). Fgf10 is expressed focally in E11–12 mouse peripheral lung mesenchyme and signals through adjacent distal epithelial FGFR2IIIb (whose loss also disrupts lung development) (De Moerlooze et al., 2000). These sites of expression change dynamically, compatible with the idea that FGF10 appears at sites of bud formation (Bellusci et al., 1997b). FGF10 has a chemotactic effect on nearby epithelium in culture: epithelial tips will proliferate and migrate toward FGF10 in mesenchyme or on beads (Park et al., 1998; Weaver et al., 2000). FGF10 controls epithelial differentiation, inducing Sp-C expression and downregulating Bmp4 expression (Hyatt et al., 2002). FGF10 dosage and signal transduction level is critical: mice with 20% of normal FGF10 expression (due to an enhancer trap bearing LacZ inserted 100Kb upstream in the FGF10 promoter) feature lung hypoplasia (Ramasamy et al., 2007); similarly, downstream signaling inhibition by misexpression of Sprouty2 under control of the Sftpc promoter induces lung hypoplasia (Mailleux et al., 2001). Several key regulatory molecules such as SHH, BMPs, and TGF-βs crosstalk with FGF10 during embryonic lung morphogenesis: their interactions will be discussed later.
FGF7 (KGF) is found in developing lung mesenchyme at late stages (Post et al., 1996). In early cultured mouse embryonic lung, addition of FGF7 promotes epithelial growth and formation of cyst-like structures with extensive cell proliferation. FGF7 can also contribute to distal airway epithelial cell differentiation (Cardoso et al., 1997; Deterding et al., 1996). Erm and Pea3 are ETS domain transcription factors known to be downstream of FGF signaling. FGF7 can induce Erm/Pea3 expression more effectively than FGF10. Erm is transcribed exclusively in the epithelium, while Pea3 is expressed in both epithelium and mesenchyme. When examined at E18.5, transgenic expression of a repressor form of Erm specifically in the embryonic lung epithelium shows that the distal epithelium of Sp-C-Erm transgenic lungs is composed predominantly of immature type II cells, while no mature type I cells are observed. By contrast, the differentiation of proximal epithelial cells, including ciliated cells and Clara cells, appears to be unaffected (Liu and Hogan, 2002; Liu et al., 2003). FGF7 does not seem to protect against hyperoxic inhibition of normal postnatal alveoli formation and early pulmonary fibrosis, but FGF7 consistently had a significant protective/preventive effect against the development of pulmonary hypertension during hyperoxia (Frank, 2003). However, Fgf7−/− mutant mice have no gross lung abnormalities (Guo et al., 1996), suggesting a FGF7 redundancy during lung development.
FGF9, which signals through FGFR2IIIc, also regulates branching morphogenesis. In E10.5 lung, Fgf9 is expressed in visceral pleura outlining the lung bud and in bronchial epithelium, while Fgfr2IIIc is predominantly expressed in lung mesenchyme. At E12.5 and E14.5, Fgf9 expression persists in visceral pleura but is no longer detected in epithelium (Colvin et al., 1999). Fgf9-null mice exhibit reduced mesenchyme and decreased airway branching but show significant distal airspace formation and pneumocyte differentiation. The reduction in the amount of mesenchyme in Fgf9−/− lungs limits expression of mesenchymal Fgf10 (Colvin et al., 2001). By contrast, addition of recombinant FGF9 protein inhibits the differentiation response of the mesenchyme to N-SHH, but does not affect proliferation (Weaver et al., 2003).
The signaling cascade activated by FGF10 and FGF9 involves FGFR2b and 2c, respectively, as well as Shp2, Raf, MAP ERK kinase (MEK), and extracellular-regulated kinases (ERK) 1 and 2 as signal transducers. MEK inhibition has been shown to reduce lung branching and epithelial cell proliferation, but increase mesenchyme cell apoptosis in fetal lung explants (Papadakis et al., 1997). FGF signaling is regulated at several levels. One of the key inducible negative regulators is the Sprouty family. There are four sprouty (Spry) genes in mouse (mSpry1–4) and human (hSpry1–4). Murine Spry2 is expressed in the distal tip of embryonic lung epithelial branches, but is downregulated between the sites of new bud formation. Murine Spry4 is predominantly expressed in the distal mesenchyme of the embryonic lung (Mailleux et al., 2001), and may play roles in branching morphogenesis. Sprouties (SPRY1, 2, 4) act as suppressors of Ras–MAP kinase signaling (Hacohen et al., 1998; Kramer et al., 1999; Reich et al., 1999). Overexpression of mSpry2 or mSpry4 can inhibit lung branching morphogenesis through reducing epithelium cell proliferation (Hadari et al., 1998; Perl et al., 2003; Tefft et al., 2002). SPRED-1 and SPRED-2 are two sprouty related proteins, which contain Enabled/VAsodilator-Stimulated Phosphoprotein (VASP) Homology-1 (EVH-1) domains. Spreds are predominantly expressed in mesenchymal cells. Expression of Spreds is especially strong in the peripheral mesenchyme and epithelium of new bud formation. After birth, Spreds expression decreases, while the expression of Sprouties expression remains high. Both Sprouties and spreds play important roles in mesenchyme– epithelium interaction during lung development (Hashimoto et al., 2002).
TGF-β/BMP family
The TGF-β superfamily comprises numerous structurally related polypeptide growth factors including TGF-β, BMP, and activin subfamilies. TGF-β ligands bind to cognate cell surface receptors, and activate Smad proteins, which translocate to the nucleus and modulate target gene expression (Massague, 1998; Shi and Massague, 2003).
TGF-β subfamily
The TGF-β ligand subfamily comprises three isoforms, TGF-β1, 2, and 3. TGF-β1 is expressed in early embryonic lung mesenchyme, particularly underlying distal epithelial branch points; TGF-β2 is localized mainly in distal epithelium; TGF-β3 is mainly expressed in proximal mesenchyme and mesothelium (Bragg et al., 2001; Millan et al., 1991; Pelton et al., 1991a,b; Schmidt et al., 1991). Each TGF-β isoform has nonredundant roles revealed by isoform-specific knockouts. Mice lacking TGF-β1 develop apparently normally, but die within 2 months of life from aggressive pulmonary or gut inflammation, as a result of failure to negatively modulate the immune system (McLennan et al., 2000). TGF-β2−/− mutation results in embryonic lethality around E14.5 in mice featuring complex cardiac anomalies and lung dysplasia amongst others (Bartram et al., 2001). TGF-β3−/− mutant mice display cleft palate, retarded lung development, and neonatal lethality with difficulty swallowing and breathing (Kaartinen et al., 1995; Shi et al., 1999). Furthermore, blockade of TGF-β signaling by null mutation of TGF-β activated kinase-1 binding protein-1 (TAB1) results in lethal cardiovascular and lung dysmorphogenesis (Komatsu et al., 2002).
As with the FGFs, the timing and dosage of TGF-β signaling are critical during lung development. Optimal physiological levels of TGF-β-Smad3 signaling appear essential for secondary alveolar septa formation: abrogation of TGF-β type II receptor in lung epithelial cells reduces alveolar septation and allows emergence of AECI (Chen et al., 2008). However, TGF-β1 overexpression in early mouse embryonic lung epithelium inhibits branching morphogenesis (Zhao et al., 1999), whereas misexpression of Sp-C promoter-controlled TGF-β1 in embryonic lung epithelium arrests embryonic lung growth and epithelial cell differentiation whilst inhibiting pulmonary vasculogenesis (Zhou et al., 1996a, 2001). Suggesting a crucial role for optimal TGF-β1 levels in human lung maturation, excessive activated TGF-β1 has been reported in tracheal aspirates of human premature infants who develop more severe BPD (Lecart et al., 2000; Toti et al., 1997). Furthermore, misexpression of TGF-β1 in neonatal rat lung using recombinant adenoviral vectors resulted in neonatal alveolar hypoplasia and interstitial fibrosis; this histological picture closely phenocopies human BPD (Gauldie et al., 2003). By contrast, misexpression of TGF-β1 in adult rats results in chronic progressive interstitial pulmonary fibrosis with increased proliferation and matrix secretion by the mesenchyme (Sime et al., 1997; Zhao et al., 2002). In addition, TGF-β1 may be centrally involved in pulmonary fibrotic responses to bleomycin, or endotoxin and infection (Bonniaud et al., 2005). Blockade of TGF-β signaling via Smad3-null mutation strongly attenuates bleomycin-induced pulmonary fibrosis (Zhao et al., 2002).
The activity of TGF-β signaling is multiply regulated: β6-integrin, Latent transforming growth factor-beta binding proteins (LTBPs), and thrombospondin regulate TGF-β release, whilst β-glycan, endoglin, or decorin modulate TGF-β receptor binding affinity. As expected, mutation of the above genes causes some phenotypes similar to those of TGF-β mutants: loss-of-function mutation in human and mouse endoglin (whose protein binds TGF−β and Alk1, its type I receptor) causes hereditary hemorrhagic telangiectasia (Li et al., 1999; Massague, 2000; McAllister et al., 1994; Urness et al., 2000). Null mutation of LTBP-3 or LTBP-4 causes profound defects in elastin fiber structure and lung alveolarization similar to Smad3 knockout mouse lung (Sterner-Kock et al., 2002; Colarossi et al., 2005; Chen et al., 2005).
In addition, TGF-β signaling blockade has distinct impacts on lung branching morphogenesis and alveolarization depending on whether epithelial or mesenchymal cells are targeted. Mesenchymal TGF-β signaling blockade driven by Dermo1 retards branching after mid-gestation; by contrast, epithelial TGF-β signaling abrogation lacks prenatal impact and only disrupts postnatal lung alveolarization (Chen et al., 2008). Meanwhile, human TGF-β pathway mutations in, for example, TGF-β type II receptor (Alk5) or TGF-β binding proteins such as fibrillin underlie dysplastic matrix elastin defects that predispose to aortic dissection or sudden alveolar rupture in Marfan’s syndrome patients (Kaartinen and Warburton, 2003). Thus, TGF-β signaling has to be regulated “just right” with both deficiency and excess deleterious to normal alveolarization (a concept we term the Goldilocks hypothesis).
BMP subfamily
BMPs, with more than 20 ligand family members, regulate many processes, including lung development (Hogan, 1996). Expression of Bmp3, 4, 5, and 7 is detected in embryonic lung (Bellusci et al., 1996; King et al., 1994; Takahashi and Ikeda, 1996). BMP4 plays a central role in normal lung development (Hogan, 1996). Addition of BMP4 to whole embryonic lung explants stimulates branching (Bragg et al., 2001; Shi et al., 2001). However, BMP4 inhibited FGF10-induced growth of isolated E11.5 mouse lung endoderm cultured in Matrigel (Weaver et al., 2000). Transgenic overexpression of BMP4 in distal fetal lung endoderm, driven by a 3.7 kb human surfactant protein C (SP-C) promoter, causes abnormal morphogenesis with cystic terminal air sacs (Bellusci et al., 1996). Conversely, Sftp-C promoter-driven overexpression of BMP antagonists Noggin or Gremlin severely reduces distal epithelial cell phenotype whilst increasing proximal cell types (Lu et al., 2001; Weaver et al., 1999). Interestingly, blockade of endogenous BMP4 in embryonic mouse lung epithelial cells using a conditional gene knockout approach results in abnormal lung development with similar dilated terminal sacs as seen in BMP4 transgenic mouse lung (Eblaghie et al., 2006). This suggests optimal BMP4 levels are essential for normal lung development. As extracellular growth factors, BMPs bind heteromeric complexes of BMP serine/threonine kinase type I and type II receptors to activate intracellular signal pathway (Massague, 1998; Shi and Massague, 2003). Three cognate BMP type I receptors (Alk2, Alk3, and Alk6) have been identified. Among them, Alk3 is expressed predominantly in distal airway epithelial cells during mouse lung development. Alk3 abrogation in mouse lung epithelia either from early lung organogenesis or from late gestation resulted in similar neonatal respiratory distress phenotypes, accompanied with collapsed lungs (Sun et al., 2008). Early induction of Alk3 knockout in lung epithelial cells causes retardation of early lung branching morphogenesis and reduces cell proliferation and differentiation. But late gestation induction of Alk3 knockout also causes significant epithelial apoptosis accompanied by lack of surfactant secretion (Sun et al., 2008). Furthermore, canonical Wnt signaling was perturbed, possibly through reduced WIF-1 expression in Alk3 knockout lungs (Sun et al., 2008). Therefore, deficiency of appropriate BMP signaling in lung epithelial cells results in prenatal lung malformation, neonatal atelectasis, and respiratory failure.
In addition, BMP signaling is also important in lung vasculogenesis and angiogenesis. Mutations of BMP type II receptor (BMPRII) and change in expression of BMP antagonist Gremlin are associated with primary pulmonary hypertension (PPH) (Lane et al., 2000; Costello et al., 2008). Moreover, upregulation of Gremlin is also associated with pulmonary fibrosis and the severity of the fibrotic pathology (Koli et al., 2006; Myllarniemi et al., 2008)
Sonic hedgehog (Shh) pathway
Sonic hedgehog is a vertebrate homolog of Hedgehog (Hh) that patterns the segment, leg, wing, eye, and brain in Drosophila. Hh binds to patched (Ptc), a transmembrane protein, and releases its inhibitory effect on downstream smoothened (Smo), which is a G protein-coupled transmembrane spanning receptor. This leads to the activation of cubitus interruptus (Ci), a 155-kDa transcription factor that is cleaved to form a 75-kDa transcription inhibitor in cytosol. Elements of the Drosophila Hh signaling pathway and their general functions in the pathway are highly conserved in vertebrates, albeit with increased levels of complexity. Gli1, 2, and 3 are the three vertebrate Ci gene orthologs (van Tuyl and Post, 2000).
The SHH signal transduction pathway plays important roles in mesenchyme–epithelium interaction. In developing mouse lung, Shh is detected in the tracheal diverticulum, the esophagus, and later in the trachea and lung endoderm. Shh is expressed at low levels throughout the epithelium, whilst at higher level in the growing distal buds (Bellusci et al., 1997a; Urase et al., 1996). Null mutation of Shh produces profound lung hypoplasia and failed trachea–esophageal septation. Mesenchymal Ptc, Gli1, and Gli3 expression are all downregulated in Shh knockout lung. Nevertheless, proximodistal differentiation of airway epithelium is preserved (Litingtung et al., 1998; Pepicelli et al., 1998). Also, Fgf10 expression is dysregulated in Shh-null mutant lung compared to the precisely restricted expression seen normally. Lung-specific Shh overexpression results in severe alveolar hypoplasia and significant increase in interstitial tissue caused by increased epithelial and mesenchymal proliferation (Bellusci et al., 1997a). Defective hedgehog signaling may lead to EA and TEF (Spilde et al., 2003).
The membrane-bound Hedgehog interacting protein 1 (HIP1) directly binds mammalian Hedgehog (HH) proteins and attenuates their signaling (Chuang and McMahon, 1999). Hip1 is transcriptionally activated in response to HH signaling, overlapping the expression domains of Ptc1 (Chuang and McMahon, 1999; Goodrich et al., 1996). Targeted disruption of Hip1 results in upregulated Hedgehog signaling and lethal neonatal respiratory failure: left– right asymmetry persists but initial branching from the two primary buds is absent; Fgf10 expression is slightly downregulated at the tips of the primary buds in Hip1−/− lungs at E10.5 but completely absent from the mesenchyme where secondary branching normally initiates (Goodrich et al., 1996). Attenuated PTC1 activity in a Hip1−/− mutant lungs leads to an accelerated lethality. Hip1 and Ptch1 have redundant roles in lung branching control (Goodrich et al., 1996). Both of them can attenuate SHH signal in lung development and pancreas development (Goodrich et al., 1996; Kawahira et al., 2003).
Wnt/β-catenin pathway
Wnt signals are transduced via seven transmembrane Wnt receptors encoded by Frizzled (Fzd) genes to activate the β-catenin T Cell transcription Factor (TCF) pathway, the c-Jun N-terminal kinases (JNK) pathway, or the intracellular Ca2+-releasing pathway. The Wnt/β-catenin pathway plays a critical role in many developmental and tumorigenesis processes. Following Wnt binding to the receptor, β-catenin is dephosphorylated and translocates to the nucleus to activate downstream gene expression (Wodarz and Nusse, 1998).
TOPGAL and BATGAL reporter transgenes have been used to analyze patterns of β-catenin stabilization in developing lung. Within the respiratory precursor region, the TOPGAL reporter is expressed in the undivided proximal endodermal tube and then the lung buds as early as E9.5 (Okubo and Hogan, 2004). This pattern is maintained as the trachea and esophagus separate and the lung buds grow out between E10 and E11.5 (Dean et al., 2005; De Langhe et al., 2005; Okubo and Hogan, 2004; Shu et al., 2005). Between E12.5 and E18.5, analysis of TOPGAL and BATGAL transgene activity suggests a dynamic pattern of TCF/β-catenin-dependent gene expression. Reporter gene activity is found in the tracheal epithelium and cartilaginous condensations at E12.5 but is restricted to the bronchial mesenchyme at E13.5 (De Langhe et al., 2005; Shu et al., 2005). The distal lung epithelium expresses both reporters by E9.5. The pattern of TCF/β-catenin-dependent gene activity in the distal lung at later time points is somewhat variable and dependent on the reporter transgene analyzed. In general, transgene activity clears from the central airways between E13.5 and postnatal day 14 (Okubo and Hogan, 2004; Shu et al., 2005). At E14.5, expression in the distal tip epithelium is either extinguished (TOPGAL) (De Langhe et al., 2005) or restricted to a subset of early alveolar type 2 cells (BATGAL) (Shu et al., 2005). In the adult lung, the TOPGAL transgene is highly expressed in the distal trachea and in clusters of airway secretory and ciliated cells but rarely in the alveolar region (De Langhe, unpublished data).
β-catenin deletion in proximal airway epithelium during development resulted in no obvious alteration to lung structure (Mucenski et al., 2003). By contrast, embryonic deletion of β-catenin in the distal lung epithelium resulted in profound perturbation of normal epithelial, mesenchymal, and vascular development. The latter mice feature proximalization of lung epithelium with decreased expression of alveolar type 2 cell marker Sftpc, vascular endothelial marker PECAM, and α-smooth muscle actin; upper airway epithelial markers (Scgb1a1, FoxJ1, and β-tubulin) were unaltered.
Stabilization of β-catenin in proximal epithelium using the CatnbfloxedExon3 allele raised epithelial β-catenin levels, resulting in squamous, cuboidal, and goblet cell dysplasia in intrapulmonary conducting airways and the appearance of alveolar type 2-like cells in the bronchioles (Mucenski et al., 2005). Epithelial levels of Scgb1a1 immunopositive cells were low whilst SPC expression increased, indicating an increase in Scgb1a1/Sftpc double-positive cells. Similar expansion of Scgb1a1/Sftpc double-positive bronchioalveolar stem cells (BASCs) in response to increased canonical Wnt signaling has been shown in the lung epithelium upon Gata6 loss (Zhang et al., 2008). These authors also showed that canonical Wnt signaling is activated within the niche containing BASCs during lung epithelial regeneration, while forced Wnt activation greatly increases BASC numbers.
Li et al., (2009) stabilized β-catenin in the entire developing lung epithelium using Nkx2.1-cre and Catnb[+/lox(ex3)] mice: in trachea and main bronchi, polyp-like structures formed featuring intracellular β-catenin accumulation suggesting blocked differentiation of spatially-appropriate airway epithelial cell types, Clara cells, ciliated cells, and basal cells (BCs), while activating UCHL1, a marker for pulmonary neuroendocrine cells.
Alternatively, the method of using a Spc promoter-regulated Lef1-dN89β-catenin to stabilize β-catenin from about E10.5 was employed by Okubo and Hogan (2004) to generate mice with widened primary bronchial tubes opening directly into saccules (lined with simple cuboidal or columnar epithelium), decreased progenitor differentiation into secretory and ciliated cells, and absence of alveolar type 2 and type 1 cells. Thus, constitutive β-catenin signaling in developing foregut endoderm partially inhibited branching morphogenesis and blocked expression of lung-specific differentiation genes.
Using a hypomorphic Fgf10 allele, Ramasamy et al. (2007) showed that FGF10 signaling via FGFR2b controls the proliferation of the pulmonary epithelial progenitors in part by autoregulation of β-catenin signaling in the epithelium. This correlation of a reduction in epithelial FGF signaling and epithelial TOPGAL activity has also been demonstrated in lungs of a mouse Apert disease model (De Langhe et al., 2006). Intriguingly, the regulation of epithelial β-catenin signaling by FGF10 and concomitant upregulation of Fgfr2b receptor expression result in potentiating this signaling cascade locally, thus maintaining the distal epithelial progenitor state.
By contrast, the lack of significant activity of well-established Wnt reporters in mesenchyme (including TOPGAL and BATGAL mice) does not support an important role for mesenchymal Wnt signaling during organogenesis. However, expression of several mesenchymal Wnt receptors in the lung has been reported (De Langhe et al., 2005). Furthermore, Wnt5a overexpression either directly or indirectly regulates mesenchymal Fgf10 expression (Li et al., 2005), while Wnt7b acts on lung vascular SMCs via Frizzled 1 and LRP5 (Wang et al., 2005). Besides Lef1/TCF-mediated β-catenin signaling, β-catenin also acts via PITX family transcription factors (Kioussi et al., 2002), which are abundantly expressed in developing mesenchyme (Kitamura et al., 1999). Using Dermo1Cre/+-mediated conditional inactivation (CKO) of β-catenin, De Langhe et al. (2008) showed Dermo1-cre/β-catenin CKO embryos have multiple defects reminiscent of double knockout of Pitx1 and Pitx2 genes (Marcil et al., 2003). Combining fate analysis and global gene expression studies, mesenchymal β-catenin signaling was shown to have dual, lineage-dependant functions: it regulates formation and amplification but not differentiation of Fgf10-expressing parabronchial smooth muscle progenitors (in part via regulation of Fgfr2c expression) but is required for normal endothelial cell differentiation (De Langhe et al., 2008). Cohen et al. (2009) confirmed the role of Wnt in parabronchial smooth muscle development and showed Wnt pathway upregulation in experimental asthma.
Epidermal growth factor (EGF) family growth factors
EGF, TGF-α, and amphiregulin are EGF receptor (EGFR) ligands. Loss- or gain-of-function experiments in mouse, rat, or other animal models prove that EGF ligands positively modulate early mouse embryonic lung branching morphogenesis and cytodifferentiation through EGFR (Schuger et al., 1996a; Seth et al., 1993; Warburton et al., 1992). EGF is expressed in mature AECs and regulates type 2 cell proliferation via autocrine mechanism in culture and in vivo (Raaberg et al., 1992). However, epithelial TGF-α overexpression under Sp-C promoter control induces postnatal lung fibrosis (Korfhagen et al., 1994). TGF-α overexpression caused severe pulmonary vascular disease mediated via EGFR in distal epithelium, but reductions in VEGF may also contribute (Le Cras et al., 2003).
EGFR is a tyrosine kinase receptor whose deletion (Egfr−/−) causes abnormal branching, poor alveolarization, and aberrant matrix metalloprotease protein (MMP) expression (Kheradmand et al., 2002). EGFR phosphorylation in response to stretch induces, at least in part, fetal epithelial cell differentiation via ERK pathway activation. Specific EGFR or ERK pathway blockade reduces stretch-inducible Sp-C mRNA expression. Thus, EGFR may represent a mechanical signal sensor during lung development (Sanchez-Esteban et al., 2003).
Tumor necrosis factor-α (TNFα)-converting enzyme (TACE) is a transmembrane metalloprotease disintegrin that functions as a membrane sheddase to release the ectodomain portions of many transmembrane proteins, including the precursors of TNFα and several other cytokines, as well as the receptors for TNFα, and neuregulin (ErbB4) (Shi et al., 2003). Neonatal TACE-deficient mice had visible respiratory distress and their lungs failed to form normal saccular structures. Mouse embryonic lung explant cultures show that TGF-α and EGF can rescue the inhibition of TACE activity (Zhao et al., 2001).
Platelet-derived growth factors (PDGFs)
PDGF-A and PDGF-B form homodimers (AA or BB) or heterodimers (AB). Two PDGF receptor types, α and β, occur in embryonic mouse lung and are differentially regulated in fetal rat lung epithelium and fibroblasts (Buch et al., 1994). PDGF-A regulates DNA synthesis and branching in cultured embryonic murine lung epithelium (Souza et al., 1995b). Pdgf-a−/− or Pdgf-α−/− mutants die perinatally with loss of alveolar myofibroblasts and SMCs and reduced parenchymal elastin fiber deposition; furthermore, failed alveolar septation is associated with emphysema (Bostrom et al., 1996, 2002). Antisense oligodeoxynucleotide abrogation of PDGF-B reduces the epithelial component of embryonic mouse lung explants but not branch number (Souza et al., 1994). PDGF-B and its receptor are crucial for vascular development during the alveolar phase (Lindahl et al., 1997). PDGF-C and D also dimerize and bind PDGF α or β receptor (LaRochelle et al., 2001; Li et al., 2000). PDGF-C mRNA expression shows a significant increase in bleomycin-induced lung fibrosis (Zhuo et al., 2003).
Insulin-like growth factors (IGFs)
IGFs and their receptors are expressed in rodent and human fetal lung (Batchelor et al., 1995; Lallemand et al., 1995; Maitre et al., 1995; Retsch-Bogart et al., 1996; Schuller et al., 1995). Null mutant mice for the cognate type 1 IGF receptor (Igf1r) gene die at birth with respiratory failure and growth deficiency (45% of normal birth weight). Dwarfism is further exacerbated (70% of size reduction) in either Igf1 or Igf2 double null mutants or in Igf1r and Igf2 double null mutants. There does not appear to be a gross defect in branching morphogenesis per se; the lungs merely appear hypoplastic (Liu et al., 1993). IGF signaling may play a role in facilitating other peptide growth factor pathways during lung morphogenesis, e.g., IGF1R signaling is required for both mitogenic and transforming activities of EGFR (Coppola et al., 1994). Igf1-deficient mice have reduced airspaces, which are exacerbated in mice with additional leukemia inhibitory factor (Lif) deletion (featuring abnormal epithelium and decreased Sp3, Nkx2.1, and Sp-B expression) (Pichel et al., 2003). IGF1 is also trophic for fetal lung endothelial cells: in human fetal lung explants, IGF-IR inactivation results in endothelial cell loss, attenuates time-dependent increase in budding of distal airway, and increases mesenchymal cell apoptosis (Han et al., 2003).
Vascular endothelial growth factor (VEGF) isoforms and cognate receptors
Effective pulmonary gas exchange requires alignment between alveoli and pulmonary capillaries. VEGFs regulate pulmonary vascular development (reviewed extensively in Pauling and Vu, 2004 and in Warburton et al., 2000 and in Warburton et al., 2003; Shi et al., 2007) and signal through cognate receptors Flk-1 (fetal liver kinase-1, VEGFR2) and Flt-1 (fetal liver tyrosinase-1, VEGFR1) (Larrivee and Karsan, 2000). VEGF is regulated by hypoxia-inducible transcription factor-2α (Compernolle et al., 2002) and expression is controlled transcriptionally by hypoxia inducible factor-1 (HIF1) (see Dunwoodie, 2009 for an excellent review). Isoforms VEGF 120, 164, and 188 are expressed in E12.5 murine lung epithelial and mesenchymal cells and regulate endothelial proliferation and microvascular structure (Greenberg et al., 2002; Ng et al., 2001). As epithelial branching progresses, Vegf-A expression becomes restricted to distal lung (Healy et al., 2000), which may be partly due to the high affinity of VEGF-A for matrix components concentrated around branching tips (Acosta et al., 2001; Ng et al., 2001). Epithelial and vascular branching morphogenesis of cultured mouse embryonic lung features epithelial, mesenchymal, and endothelial crosstalk mediated in part by VEGF-A signaling via Flk-1 (Del Moral et al., 2006a). Inhibited VEGF signaling disrupts pulmonary endothelial survival and reduces postnatal alveolarization (Kasahara et al., 2000). Neonatal lungs treated with antibodies to Flt-1 are small with simplified alveoli (Gerber et al., 1999). VEGR3−/− mice have lymphatic hypoplasia and lethally delayed removal of lung liquid at birth. Excessive VEGF signaling also disrupts vascular and epithelial lungmorphogenesis: Vegf misexpression under SP-C promotercontrol yields decreased acinar tubules and mesenchyme (Zeng et al., 1998).
ROBO/SLIT
Roundabout (ROBO) is a receptor involved in repellent signaling and controlling axonal extension and with its ligand SLIT regulates non-neuronal cell migration (Wu et al., 2001). Suggesting interaction during perinatal lung development, Slit-2 is expressed in saccular mesenchyme whilst Robo is expressed in adjacent apical epithelium (Anselmo et al., 2003). A Robo knockout mouse loses alvelolar septation with thickened mesenchyme (Xian et al., 2001).
3.2.3. Other biochemical factors
Small noncoding microRNAs (miRNAs) and lung development
A class of noncoding RNAs called microRNAs (miRNAs) has been recognized for their abundance and conservation between species (Ambros, 2001; Lau et al., 2001; Lagos-Quintana et al., 2001). An intranuclear primary transcript (pri-miRNA) is cleaved by Drosha RNase III endonuclease to liberate the pre-miRNA, a 60–70 nt stem loop intermediate (Lee et al., 2002). Pre-miRNA is transported to the cytoplasm by Exportin-5 (Yi et al., 2003) where Dicer, another RNase III endonuclease, generates the mature miRNA. Mature miRNAs are incorporated as single-stranded RNAs into a ribonucleoprotein complex, the RNA-induced silencing complex (RISC) that downregulates gene expression by mRNA cleavage or translational repression. Mice lacking Dicer die before gastrulation indicating a fundamental developmental role of miRNAs. In lung, conditional epithelial Dicer inactivation arrests branching and disrupts Fgf10 expression (Harris et al., 2006).
Our studies (Carraro et al., 2009) and others’ (Lu et al., 2007) highlighted specific miRNAs’ importance in lung epithelial cell development. Ubiquitous developing epithelial overexpression of the miR-17–92 cluster augments multipotent Sox9-expressing epithelial cell number at the expense of delayed epithelial differentiation (Lu et al., 2007). In addition, miR-17 and its paralogs miR-20a and miR-106b target Stat3 and Mapk14 and thereby regulate E-Cadherin expression by modulating FGF10–FGFR2b downstream signaling. Thus, mir17 paralogs control a key pathway modulating the FGF10–FGFR2b–Sprouty-driven bud morphogenesis periodicity clock (Carraro et al., 2009; Warburton et al., 2008).
Extracellular matrix and lung development
Differentially expressed protein components of extracellular basement membrane, laminins (LNs), entactin/nidogen, type IV collagen, perlecan, SPARC, and fibromodulin mediate cell–cell and cell–ECM interaction during lung morphogenesis. ECM components provide tissue support, may modulate cell proliferation and differentiation (Lwebuga-Mukasa, 1991), and serve as barrier and reservoir for growth factors. Preventing epithelial interaction with basement membrane disrupts lung development (Hilfer, 1996; Minoo and King, 1994).
LNs are glycoproteins involved in cell adhesion, migration, proliferation, and differentiation during tissue development and remodeling. LNs are composed of three chains, one central (α) and two lateral (β and γ), linked by disulfide bonds to form a cross-shaped molecule (Burgeson et al., 1994). To date five α, three β, and three γ chain isoforms have been identified, which suggests their combination can lead to approximately 30 LN variants (Bernier et al., 1995; Ehrig et al., 1990; Galliano et al., 1995; Iivanainen et al., 1995a,b, 1997a,b, 1999; Koch et al., 1999; Pierce et al., 1998; Vuolteenaho et al., 1994). The α1 chain is principally localized in basement membrane at the epithelial–mesenchymal interface with a predilection for specific zones. LN α1 chain also surrounds some mesenchymal cells. A domain in the cross-region of the α1 chain influences lung epithelial cell proliferation (Schuger et al., 1992). The α4 chain in LN8 and LN9 variants is highly expressed developing murine lung and heart (Frieser et al., 1997; Iivanainen et al., 1995a,b, 1997). LN α4 chain is localized around vessels in fetal lung and may assist organization of lung mesenchyme (Miner et al., 1997; Pierce et al., 1998). The α5 chain in LN10 and LN11 is abundantly expressed during lung morphogenesis (Miner et al., 1995, 1998; Pierce et al., 1998): mutated LN α5 chains impair lobe septation and bronchiolar branching in mutant murine lung.
With roles in cell adhesion, β1 and γ1 are constantly expressed during fetal lung development (Durham and Snyder, 1995): globular domains near their N-termini help regulate cell polarization (Schuger et al., 1995, 1996b). LN β2 chain isoform localizes to the basement membrane of prealveolar ducts, airways, SMCs of airways, and arterial blood vessels, as well as type II pneumocytes.
Nidogen (150 kDa) in basement membrane binds γ1 and γ3 chains helping link LN to collagen IV (Dziadek, 1995; Koch et al., 1999; Reinhardt et al., 1993). Nidogen is mesenchymally synthesized and appears to help basement membrane organization during lung morphogenesis (Senior et al., 1996). Blocking Nidogen’s interaction with LN disrupts lung development (Dziadek, 1995; Ekblom et al., 1994; Senior et al., 1996). Nidogen to degradation by matrix metalloproteinases (MMPs) may facilitate remodeling via basement membrane degradation (Mayer et al., 1993).
Proteoglycans comprise a protein core with sulfated carbohydrate side chains. They form flexible structures and function as a reservoir for growth factors, water, and ions. Inhibiting proteoglycan sulfation disrupts branching of E13 mouse lung explants and inhibits epithelial migration toward lung mesenchyme or FGF10-soaked beads (Shannon et al., 2003). Perlecan is a predominant basement membrane proteoglycan, composed of an approximately 450 kDa core protein with three heparan sulfate chains. Pulmonary perlecan synthesis rises sharply with increased fetal SMC proliferation (Belknap et al., 1999).
Fibronectin (FN) is essential for clefting during initial epithelial branching in salivary gland and is accumulated at sites of epithelial constriction and indentation in lung and kidney (Sakai et al., 2003), supporting roles for FN in lung branching morphogenesis (Roman, 1997). Indeed, FN is a Wnt target required in the control mechanism of lung bud tip splitting (De Langhe et al., 2005). Treating lung rudiments with anti-FN antibody or siRNA inhibited branching morphogenesis, while FN supplementation promoted branching (Sakai et al., 2003). Located in epithelium and mesenchyme, the EIIIA segment is one of the alternatively spliced FN segments, modulating FN’s cell proliferative effect: expression decreases from pseudoglandular to saccular stages, increases into the alveolar stage, and parallels changes in Proliferating Cell Nuclear Antigen (PCNA)-positive distal pulmonary cell number (Kikuchi et al., 2003).
MMPs are ECM-degrading enzymes that are inhibited by tissue inhibitors of metalloproteinases (TIMPs). MMPs may alter cell fate and behavior by ECM modulation and modulate signaling of bioactive molecules by their cleavage, by their release from bound stores, or by altering activity of their inhibitors (Vu and Werb, 2000). Timp3-null mutant mice have attenuated airway branching and alveologenesis (Gill et al., 2003) and develop progressive emphysema-like changes (Leco et al., 2001). MMP2, MMP9, and their tissue inhibitors (TIMP1 and TIMP2) are influenced by early postnatal dexamethasone exposure: Timp2 expression falls and Mmp9 expression increases. Such changes may contribute to steroids’ effects on neonatal lung structure (Valencia et al., 2003). MT1-MMP, a potent MMP2 activator, is a major downstream EGFR target. Egfr−/− mice had low MT1-Mmp expression with 10-fold reduction in active MMP2. Abnormal lung alveolarization in Mmp2−/− mice is similar to but less severe than that in neonatal Egfr−/− mice (Kheradmand et al., 2002).
Retinoic acid signaling
Retinoids (all-trans, 9-cis, and 13-cis) are fundamental for development and homeostasis of numerous systems including lung, and there is a well-described mammalian RA synthesis and degradation system (Chambon, 1996). Retinaldehyde dehydrogenase-2 (RALDH-2) plays a prominent role in generating RA during organogenesis (Niederreither et al., 1997, 1999; Ulven et al., 2000). RA signaling is mediated by its nuclear receptors of the steroid hormone receptor superfamily: RAR (α, β, γ) and retinoid RXR (α, β, γ) (Chambon, 1996). RAR/RXR heterodimers also transduce RA signaling in vivo (Kastner et al., 1997). Within E13.5 lung, Rar-β isoform transcripts are localized to proximal airway epithelium and adjacent mesenchyme, whereas Rarα1, Rarα2, and Rarγ2 isoforms are ubiquitously expressed (Chazaud et al., 2003).
RA signaling is required for lung bud initiation. Acute vitamin A deprivation in pregnant rats at the onset of lung development results in blind-ending tracheae and lung agenesis in some embryos, which is similar to the case of Fgf10−/− mutant mice (Dickman et al., 1997; Sekine et al., 1999). Disruption of RA signaling in Rarα/β2 knockout mice causes left lung agenesis and right lung hypoplasia (Mendelsohn et al., 1994). Lung branching morphogenesis is characterized by dramatic downregulation of RA signaling. Preventing this by treating embryonic lung explants with high RA concentrations (10−6–10−5 M) disrupts distal budding with formation of proximal-like immature airways (Cardoso et al., 1995; Malpel et al., 2000). Continued RA activation by overexpression of constitutively activated Rara chimeric receptors meant lungs did not form saccules or identifiable type I cells: raised epithelial Sp-C, Nkx2.1, and Gata6 levels (but not Sp-A or Sp-B) at birth suggested differentiation was arrested early in these lungs. Downregulation of RA signaling is required to allow differentiation to form mature type I and II cells (Wongtrakool et al., 2003). RA inhibits expression and alters Fgf10 and Bmp4 distribution (Cardoso et al., 1995; Malpel et al., 2000). Pan-RAR antagonism alters Tgf-β3, Hnf-3β, and Cftr expression in proximal tubules and Bmp4, Fgf10, and Shh expression in distal buds (Chazaud et al., 2003).
In early E11–12.5 murine lung, Raldh-2 is concentrated in trachea (mesenchyme) and proximal lung (mesothelium) at sites of limited branching: this lack of overlap with Fgf10 suggests RA may restrict Fgf10 expression thereby defining the proximal–distal lung axis. However, during postnatal lung development, RA increases alveolar number, partially rescuing dexamethasone’s effects. In adult rats, RA reverses features of elastase-induced emphysema (Maden and Hind, 2003; Massaro and Massaro, 1996, 2000). RAR-γ-null mice have defective alveolar septation consistent with abnormal elastin deposition (Chytil, 1996). Additional deletion of a retinoid X receptor (RXRα) allele decreases alveolar surface area and number (McGowan et al., 2000). RA is a vitamin A metabolite. Vitamin A deficiency injures lung and impairs rat type II pneumocyte function (McGowan et al., 2000). This combined evidence suggests RA may have a complex role in alveolar development.
4. Mechanobiology of the Developing Lung
Mechanical stimuli to lung development have been long appreciated. Recent advances in molecular and stem cell biology allow these fields to be integrated with modern mechanobiology (Ingber, 2003). For example, ECM polymers, in addition to binding and presenting growth factors, provide resistance to deformation, fluid flow, and diffusion, and transmit force over surprisingly long distances. Adhesion molecules regulate cell motility and tissue structure, and these in turn interact through forces and deformations. Cytoskeletal components generate and transmit forces and conversely provide resistance to deformation. If we do not understand the relevant physics, we miss major factors in development, physiology, and regulation.
In an inflated rubber glove, raising internal pressure decreases curvature with simplification of form. By contrast, in lung increased internal pressure is associated with increased curvature and more complex morphology. Why this difference? Part of the answer is that the rubber glove is a thin elastic shell whilst embryonic tissue better resembles a viscoelastic fluid (Forgacs et al., 1998; Jakab et al., 2008). Over the timescale of growth, the elastic component of tissue viscoelasticity may be neglected; the tissue therefore behaves mechanically as a fluid, such that a simple mechanobiological model (Lubkin and Murray, 1995) predicts pulmonary pressure–morphology relationships (Unbekandt et al., 2008).
Mechanics also influence differentiation: in vitro mesenchymal stem cells differentiate toward neurons at 1 kPa, muscle at 10 kPa, and cartilage at 30 kPa (Engler et al., 2006). Hypothesizing that lung seeks to equilibrate tangential epithelial stress, a mechanobiological model of pseudoglandular lung (Lubkin and Murray, 1995) treated the epithelium as a viscous fluid with surface tension (Foty et al., 1994) to predict that branch size will be inversely related to pressure difference between external medium (and native mesenchyme) and lumen. Indeed embryonic lung epithelium appears to regulate tangential stress by modulating cytoskeletal tension via the Rho– ROCK system (Moore et al., 2005).
4.1. Lessons on mechanobiology from human and in vivo studies
Human birth defects and in utero experiments have demonstrated lung development is subject to mechanics. For example, CDH (Smith et al., 2005) comprises a diaphragmatic defect, intrathoracic herniation of abdominal viscera and lung hypoplasia: affected newborns retain a high mortality rate due to inadequate lung growth. Traditionally, lung hypoplasia was attributed to lung compression by herniated abdominal viscera. Indeed lung growth is impaired when fetal CDH is created surgically (Starrett and de Lorimier, 1975). Similarly, human fetuses with renal agenesis or profound renal failure exhibit Potter’s syndrome, in which an underfilled amniotic cavity is thought to cause lung hypoplasia due to excessive lung fluid loss and/or fetal thorax compression. Certainly, bilateral fetal nephrectomy impairs ovine lung growth (Wilson et al., 1993). Alternatively, lung hypoplasia may result from developmental insults to the lung that precede or coincide with the origins of CDH and renal agenesis, respectively. For example, in the nitrofen-induced CDH model, early lung malformation precedes CDH (Jesudason et al., 2000). Similarly, lung hypoplasia emerges before fetal urine output normally contributes to amniotic fluid in a transgenic murine model of renal dysgenesis (Smith et al., 2006). Synthesizing these positions argues for an early developmental insult to the lung that is then compounded by unfavorable mechanical influences (Keijzer et al., 2000). In addition to extrinsic forces acting on fetal lung, a distending pressure is generated by lung liquid production. Draining this fluid by fetal tracheostomy is associated with lung hypoplasia (Fewell et al., 1983). Likewise, retention of this fluid in congenital laryngeal atresia is associated with lung overgrowth and distension (Harding and Hooper, 1996). This led to development of fetal tracheal occlusion to rescue hypoplastic lung growth in human CDH (Harrison et al., 2003; Hedrick et al., 1994). The normal fetal larynx appears to open only during diaphragmatic contraction (fetal breathing movements: FBMs), which restricts lung liquid efflux (Fewell and Johnson, 1983). Hence, failure of FBM in CDH may also contribute to lung hypoplasia. Experimental FBM abolition by phrenic nerve section is associated with lung hypoplasia (Miller et al., 1993). Loss of skeletal muscle formation also causes lung hypoplasia: thinned diaphragms in MyoD−/− mice cannot support FBM and the lungs are hypoplastic with reduced cell proliferation at E18.5 (Inanlou and Kablar, 2003). Neonatally, mechanical ventilation conspires with factors such as inflammation to generate BPD in premature newborns (Warburton et al., 2001). Mechanical factors appear influential beyond this period: compensatory lung growth follows lung resection (Thurlbeck, 1983) comprising lung distension and parenchymal growth. This postpneumonectomy effect suggests the lung responds to altered mechanics and that the organism to reduced alveolar surface area. At a smaller scale, airway smooth muscle (ASM) hypertrophy and hyperreactivity in asthma are associated with air trapping and acute lung distension; however, with time, this is associated with airway remodeling and chronic lung hyperexpansion. ASM-led airway occlusions in asthma may therefore have analogous effects to fetal tracheal occlusion (which distends and remodels prenatal lung) (Jesudason, 2007). Moreover, transient endogenous ASM-led airway occlusions occur in fetal lung (known as airway peristalsis), and this contractility may be an important regulator of lung growth (discussed below) (Jesudason, 2006a). With this in mind, we next focus on three areas of interest in lung mechanobiology: (i) lung liquid, (ii) airway contractility, and (iii) calcium signaling in this secretory, contractile environment.
4.2. The impact of hydraulic pressure on lung organogenesis
Prenatal lung liquid is neither plasma ultrafiltrate nor “inhaled” amniotic fluid (Adamson et al., 1969). Lung liquid is produced throughout prenatal lung development by incompletely understood mechanisms that involve active Cl− transport from blood/interstitium into lumen (Olver and Strang, 1974). Intracellular Cl− accumulation is energized by the basolateral Na+/K+-ATPase (Bland and Boyd, 1986) and accomplished via Na+-linked cellular Cl− uptake through the Na+/K+/2Cl− co-transporter (Thom and Perks, 1990); indeed Cl− secretion rate depends on NKCC1 expression (Gillie et al., 2001). Movement of accumulated Cl− down its concentration gradient via apical Cl− channels results in accompanying Na+and water flux to generate fetal lung fluid (see Olver et al., 2004 for comprehensive review). Whilst active Cl− and fluid secretion are crucial to lung growth (Alcorn et al., 1977), they may not contribute to branching per se (Souza et al., 1995a).
The identity of the apical Cl− channel remains unclear. Numerous channels are demonstrated in fetal alveolar type II cells, including a G protein-regulated maxiCl channel (Kemp et al., 1994), cystic fibrosis transmembrane conductance regulator (CFTR) (McCray et al., 1993), at least one member of the Chloride Channel (CLC) channel family (Blaisdell et al., 2004; Murray et al., 1995), and a Ca2+-activated Cl− channel, TMEM16a (Rock et al., 2008). CFTR−/− mice have normal prenatal lungs (Wallace et al., 2008), suggesting CFTR plays no role in producing lung liquid or there is functional redundancy. Although a definitive link between CLC channels and lung liquid production remains to be established in vivo, there is evidence that CLC-2 contributes to fluid secretion and cyst expansion in vitro (Blaisdell et al., 2004). Interestingly, murine TMEM16a−/− mutants die of respiratory failure at an interval after birth with characteristic tracheomegaly and disruption of trachealis formation (Rock et al., 2008).
The rate of liquid production and the laryngeal valve function help determine hydraulic pressure in the lung. Obstructing the prenatal trachea increases intraluminal pressure two- to three-fold and airway branching three-fold; the rate of bud extension increases about two-fold whilst inter-bud distance is halved. These effects depend on FGF10–FGFR2b–Sprouty signaling (Unbekandt et al., 2008). Several studies have used tracheal obstruction to try to improve lung growth in human CDH (Harrison et al., 2003; Jani et al., 2005). However, clinical evidence of benefit of this potentially hazardous intervention remains limited. An alternative being explored is to exploit spontaneous airway occlusions that may be important for lung growth and perhaps avoid invasive fetal interventions (Jesudason, 2009).
4.3. The impact of embryonic airway peristalsis in lung organogenesis
Early mammalian airway exhibits spontaneous transient airway occlusions due to airway peristalsis. This is mediated by spontaneous ASM contractions that occur in birds and humans and which increase in frequency from embryonic stages to birth (Schittny et al., 2000). Peristaltic contractions and airway occlusions direct waves of fluid toward the lung’s tips. This results in rhythmic stretch and relaxation of growing buds (Fig. 3.8). Hence airway peristalsis and occlusions are well placed to regulate both pressure and stretch in the tips of developing lung (Jesudason, 2009). These ASM waves emanate from pacemaker areas in proximal airway before transmission distally (Jesudason et al., 2005). This pacemaker-driven airway contractility may even be important postnatally in asthma (Jesudason et al., 2006b). Thus, putative pulmonary pacemakers might be targeted for ablation by bronchial thermoplasty for asthma (Jesudason, 2009). Studying frequency of peristalsis in embryonic lung culture revealed that it is amenable to acceleration by cholingergic agents as well as growth factors (FGF10). These accelerated rates accompany enhanced in vitro lung growth. Similarly, in vitro inhibition of peristalsis is associated with reduced lung growth (Jesudason et al., 2005). This apparent coupling raised interest in mechanisms linking morphogenesis and peristalsis-led airway occlusions. In particular, Ca2+-imaging studies revealed that prenatal lung features spontaneous regenerative intercellular ASM calcium waves that propagate along main airways immediately prior to the wave of peristaltic contractility (Featherstone et al., 2005). Using pharmacological inhibitors, we showed that ASM calcium waves depend on extra- and intracellular calcium as well as gap junction integrity. Moreover, these calcium waves are abnormal in experimental lung hypoplasia (Featherstone et al., 2006). Thus, if peristaltic airway contractions do regulate lung growth, it means that underlying calcium oscillations govern lung development.
Figure 3.8.
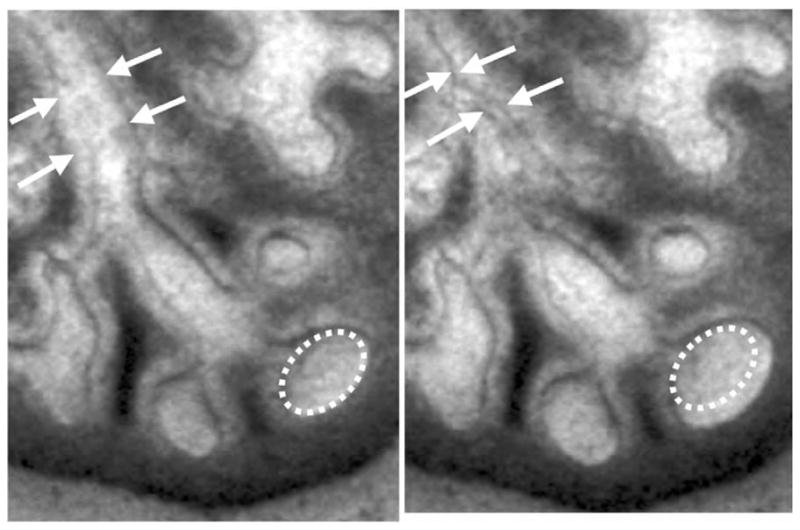
Peristalsis occludes airway proximally whilst distending it distally. Sequential photomicrographs of cultured embryonic lung: in both panels the arrows outline proximal airway (open in the top picture but occluded by contraction in the lower one). The peristaltic airway occlusion’s distal effect is shown in a terminal bud, which rapidly rhythmically increases in size (the same ovoid outline is applied to both pictures). This distension and relaxation recurs with each wave.
4.4. Lung stretch transduction and parathyroid hormone-related protein (PTHrP)
Airway peristalsis is coupled to lung growth, responsible for phasic lung stretch and underpinned by calcium oscillations. Transduction of such mechanical activity involves key modulators and sensors of serum Ca2+. For example, stretching alveolar type II cells produces parathyroid hormone-related protein (PTHrP) (Torday et al., 2002), which binds in paracrine fashion to its cognate receptor on adjacent adepithelial fibroblasts (Torday and Rehan, 2002); the latter are induced to differentiate into a lipofibroblast lineage (Schultz et al., 2002). The lipofibroblasts, discovered by Vaccaro and Brody (1978), protect against oxidant injury (Torday et al., 2001) and produce leptin, which stimulates synthesis of surfactant phospholipids and proteins, by ligating its receptor on alveolar type II cells (Torday et al., 2002a,b). Alveolar stretch releases PTHrP, which dilates alveolar capillaries (Gao and Raj, 2005) and coordinates surfactant production with alveolar perfusion. The Torday group showed that fluid distension permits cultured lung to differentiate like lung in vivo, agreeing with the concept discussed elsewhere that rhythmic mechanical distension serves as a clock or “zeitgeber” mechanism for organogenesis (Gross et al., 1978). Conversely, inhibiting lung fluid in explants inhibits the putative “zeitgeber,” while PTHrP treatment rescues the temporal molecular development of the lung. Fluid distension also stimulates endodermal Shh expression, which in turn stimulates the mesodermal Wnt pathway. Wnt increases endodermal PTHrP expression, which feeds back negatively on the Wnt pathway by stimulating protein kinase A. Wnt/β-catenin signaling has recently been linked to both PTHrP and the CFTR (Cohen et al., 2008).
4.5. Lung development and the Ca2+-sensing receptor (CaSR)
Stretch-induced PTHrP regulates Ca2+ and lung growth, and stretch-inducing airway peristalsis is underpinned by Ca2+ signaling. However, the developing fetus is actually hypercalcemic compared to the adult, with free extracellular Ca2+ concentration ([Ca2+]o) of around 1.7 mM, while maternal [Ca2+]o is 1.1.–1.3 mM. Studies in mouse lung explant culture demonstrated fetal [Ca2+]o suppresses branching and cellular proliferation while enhancing Cl−-dependent fluid secretion (Finney et al., 2008). Since effects of fetal [Ca2+]o are mimicked by pharmacological activation of Ca2+-sensing receptor (CaSR), the G protein-coupled extracellular CaSR and fetal Ca2+ via CaSR may balance branching morphogenesis and lumen distension. CaSR expression has been documented in mouse (Finney et al., 2008) and humans (Finney, Wilkinson, Kemp, and Riccardi, unpublished observations), throughout the pseudoglandular stage. At the start of this phase, CaSR is restricted to the epithelium where its expression is maintained for at least 48 h in the organ culture. From E14.5 in mouse (and week 9 in humans) mesenchymal CaSR expression appears. CaSR expression wanes during the canalicular phase and is absent from adult mouse, rat, and bovine lung (Brown et al., 1993; Finney et al., 2008; Riccardi et al., 1995). CaSR-dependent regulation of branching and fluid secretion occurs via phospholipase C-dependent rises in intracellular Ca2+ concentration and phosphoinositide 3 (PI3) kinase activation (Fig. 3.9). Whether CaSR-dependent rises in Ca2+ affects lung development through peristaltic waves (discussed above) and/or controls growth by altering epithelial integrity via activation of PI3 kinase-dependent β-catenin signaling remains unclear. CaSR activation could also affect clock mechanisms of airway branching by interfering with FGF–FGFR–Sprouty signaling events: lungs cultured in 1.7 mM Ca2+ delay both bud extension and tip-splitting, when compared to lungs cultured in lower, adult levels of [Ca2+]o (1.2 mM) (Finney et al., 2008). Finally, patients with inactivating CaSR mutations have increased risk of interstitial lung disease (Auwerx et al., 1985a,b). Given that CaSR expression appears confined to the pseudoglandular phase, we need to test if this phenotype originates prenatally.
Figure 3.9.
CaSR-evoked inhibition of branching and its dependence on Pulmonary lymphangitiic carcinomatosis (PLC) and phosphoinositide 3 (PI3) kinase signaling. Effect on branching of 1.05 mM (A, upper panels) or 1.7 mM (A, lower panels) Ca2+o in the absence (0.1% DMSO vehicle control; A left panels) or presence (A, right panels) of 5 μM of the PLC inhibitor, U73122. Quantification of branching at 48 h in the four conditions is shown in (B). Inhibition of PLC rescues suppression of branching evoked by 1.7 mM Ca2+o. Bars = 700 μm. Effect on branching of 1.05 mM Ca2+o (C, upper panels), 1.05 mM Ca2+o plus 30 nM R-568 (C, middle panels), and 1.7 mM Ca2+o (C, lower panels) in the absence (0.05% DMSO vehicle control) or presence of 12.5 μM of the PI3K inhibitor, LY294002. Bars = 750 μm. Quantification of branching at 48 h in the six conditions is shown in (D). The calcimimetic R-568 mimics the suppressive effect of high Ca2+o on branching, further implicating CaSR in the process. Note PI3 kinase inhibition rescues suppression of branching, whether evoked by high Ca2+o or calcimimetic. Adapted from Finney et al. (2008).
5. Stem/Progenitor Cell Biology of the Lung
A stem cell describes a self-renewing, primitive, undifferentiated, multipotent source of multiple cell lineages. Such cells are critical for development and growth; pools of adult stem cells are hypothetical sources for tissue regeneration and repair as well as cancers. In contrast to embryonic stem cells and tumor cells, adult stem cells reduce telomere length with age (Warburton et al., 2008).
In the lung, there is limited knowledge about existence of self-renewing cells, whether such cells conform to classic or nonclassic stem cell hierarchies and whether a single stem/progenitor cell suffices to generate the more than 40 distinct cell types required in mature lung. At least five putative epithelial stem/progenitor cell niches are reported in adult mouse airway (Liu and Engelhardt, 2008), as well as endothelial stem cells in the pulmonary vasculature and ASM stem cells. Additionally, circulating stem/progenitors may lodge in the lung. Unlike skin and gastrointestinal tract, postnatal lung turns over slowly, which hampers putative progenitor identification. Specific markers and clonality assays also limit attempts at isolation. Herein we review new information regarding the development and function of lung stem/progenitor cells in organogenesis.
5.1. Endogenous epithelial progenitor cells
Failing regeneration and repair with age has been suggested to be due to stem cell failure. In pseudoglandular lung, tips of the branching tubules appear to contain undifferentiated multipotent epithelial progenitors. In adult lung, putative endogenous epithelial progenitors have been located in the basal layer of the upper airways, within or near pulmonary neuroendocrine cell rests as well as at the bronchoalveolar junction and in the alveolar epithelium (Engelhardt, 2001; Giangreco et al., 2002, 2004; Kim et al., 2005; Rawlins and Hogan 2006; Reddy et al., 2004; Reynolds et al., 2000, 2004). The distal-most epithelial cells were shown to be a multi-potent progenitor cell population during branching morphogenesis of the lung (Rawlins et al., 2009a). A recent model suggests, as the lung branches, descendents of distal tip progenitors are left to differentiate in the stalks, whereas self-renewing progenitors remain in the epithelial tips: distal epithelial cells have a unique gene expressionpattern, including high levels of Sox 9, Id2 (inhibitor of differentiation 2), N-myc, and Etv5/ERM (ets variant gene 5). In addition, they are exposed to high levels of FGF, Wnt, Shh, and BMP signaling (Bellusci et al., 1996; Liu et al., 2002; Shu et al., 2005). Many of these pathways including sox9, etv5, and FGFR signaling are associated with stem/progenitor cells in other endodermally derived organs (Seymour et al., 2007; Zhou et al., 2007). Distal epithelial cells also have different cell cycle kinetics compared with the rest of the epithelium; a higher proportion of them incorporate the thymidine analog bromodeoxyuridine (BrdU) during a short pulse (Okubo et al., 2005).
5.1.1. Tracheal and bronchial epithelial progenitors
Candidate endogenous stem or progenitor cells have been identified in trachea and lung, using lung injury/repair models. For example, CC10/Scgb1a1+ Clara cells self-renew and proliferate after tracheal injury, but seem not the main source for tracheal epithelial regeneration (Rawlins et al., 2009b). However, subsets of Keratin-14 (K-14)-expressing BCs in the trachea (Hong et al., 2004a) can restore differentiated epithelium after injury and are distinct from bronchial BCs (Hong et al., 2004b). Lineage tracing in the adult mouse lung and trachea showed K-14-positive cells act as progenitors and ciliated cells cannot (Hong, et al., 2004a; Rawlins, et al., 2007). Human SpC and rat CC10 promoters have also been used for lineage tracing in lung (Perl et al., 2002, 2005a).
Since pseudostratified epithelium of mouse trachea and human airways contains a BC population expressing cytokeratin 5 (Krt5), a recent study using Krt5-CreERT2 transgenic mouse line for lineage tracing showed BCs of mouse trachea function as progenitors during postnatal growth, in adult homeostasis and also in epithelial repair of experimentally-induced SO2 damage (Rock et al., 2009). A clonality assay also found BCs of mouse and human airways self-renew and differentiate into mucus and ciliated lineages in the absence of stroma or columnar epithelial cells.
There is also a rare mixed population of pluripotent cells in lower respiratory tract characterized as a Hoechst dye effluxing side population (SP) cells. They express molecular markers of airway and mesenchymal origin (Giangreco et al., 2004). CD45− SP cells isolated from human tracheobronchial epithelium have proliferative potential. Increased numbers of these cells in asthmatic airways suggest that dysregulation of pluripotent cells may play a role in this chronic disorder (Hackett et al., 2008). In developing lung, some SP cells, which are CD45+ and CD45− have endothelial progenitor cell (EPC) potential in response to hyperoxia (Irwin et al., 2007).
The submucosal gland ducts in proximal airway are likewise suspected to contain stem cells (Liu and Engelhardt, 2008). However, relatively little is known about glandular stem/progenitor cells and their niche(s). Studies have suggested regenerating tracheal epithelium after naphthalene injury arises from cells migrating from gland ducts (Borthwick et al., 2001).
Rawlins et al. (2009b) used lineage tracing to circumvent obstacles that hampered earlier studies of lung stem/progenitor cells. Using the restricted expression of CC10/Scgb1a1, they generated a “knocking” transgenic mouse with a tamoxifen (TM) inducible Cre-recombinase (ScgB1a1-CreER™) that lineage-tags Clara cells of the airway. By varying dose and timing of TM administration, they discovered that epithelial reconstitution in the bronchioles involves Clara cell self-renewal and differentiation into ciliated cells. These data argue that bronchiolar ScgB1a1-expressing cells, largely mature Clara cells, are a self-renewing progenitor pool. The observation that lineage tags are chased into ciliated cells over time is consistent with early findings of Evans and colleagues (1978) that Clara cells are progenitors for ciliated cell renewal. On the other hand, Rawlins et al. (2009b) showed that lineage tags introduced into ScgB1a1-expressing cells of tracheobronchial airways were depleted within the ScgB1a1-expressing population over time. Collectively, these data suggest that ScgB1a1-expressing cells of proximal airways behave like transit amplifying (TA) cells, like those of intestinal epithelium, whereas ScgB1a1 cells of bronchiolar airways behave like self-renewing progenitors present in the interfollicular epidermis (reviewed by Chen et al., 2009).
A different approach by Giangreco and colleagues (2009) to investigate long-term behavior of airway progenitors in normal and injured airways showed in concordance with Rawlins et al. (2009b) that during homeostasis an abundant progenitor cell pool maintains the airway epithelium (rather than rare tissue stem cells). However, clonal patches of labeled cells emanate from tissue-specific stem cells located at airway branch points or bronchioalveolar duct junctions, after Clara cell depletion resulting from naphthalene exposure. In this naphthalene injury case, repairing bronchiolar airways more closely resemble the renewing epidermis after wounding, wherein stem cells are recruited from the hair follicle bulge to replace the depleted BC pool of the interfollicular epidermis (Zemke et al., 2009).
Varying dose and timing of TM administration, Rawlins et al. (2009a) discovered that reconstitution of bronchiolar epithelium involves Clara cell self-renewal and differentiation into ciliated cells and that Clara cells contribute to tracheal repair. Using lineage tracing, this study showed that a special population of BASCs which coexpress CC10 and SP-C, which have been proposed to contribute to both bronchioles and alveoli, has no apparent function during postnatal growth, adult homeostasis, or alveolar repair. Thus, they propose that trachea, bronchioles, and alveoli are maintained by distinct progenitor populations (Rawlins et al., 2009a). Currently, the significance that some Scgb1a1+ bronchiolar Clara cells express SftpC and some alveolar type 2 cells express Scgb1a1 is not understood. There is accumulating evidence the (Scgb1a1+, SftpC+) coexpressing cell population increases in number in murine lung cancer models (Ventura et al., 2007; Yang et al., 2008). However, it is unclear if this is due to preferential proliferation of preexisting (Scgb1a1+, SftpC+) cells or oncogenic upregulation of SftpC or Scgb1a1.
In a recent study (Tompkins et al., 2009), selective Sox2 deletion in Clara cells with Scgb1a1-Cre showed that Clara cell Sox2 is required for differentiation and/or maintenance of ciliated, Clara, and goblet cells in bronchiolar epithelium after birth and caused progressive loss of ciliated, Clara, and goblet cells and an inability to produce goblet cells in response to allergen. The findings indicate Clara cells can serve as common progenitors of ciliated, Clara, and goblet cells in a process requiring Sox2.
5.1.2. Alveolar epithelial progenitors
Epithelial progenitors of the alveoli have yet to be identified. An interesting model is that the alveolar progenitors are located in distal epithelial tips during the canalicular stage. However, there is no published evidence to support, or refute, this hypothesis. Presumably, because of its vast area a large number of AECs must function as a “ready reserve” to repair damaged alveolar surface. For instance, the expression of telomerase, a stem/progenitor cell marker, after acute oxygen injury is widely upregulated in AECs during recovery (Driscoll et al., 2000). This suggests that either AECs contain a progenitor cell subpopulation or that the majority of AECs undergo reactivation progenitor-like states after injury (Driscoll et al., 2000). In addition, without telomerase, resistance to injury and repopulation of damaged alveoli are compromised, indicating this pathway is likely critical for alveolar progenitor cell activity (Driscoll et al., personal communication). Moreover, Kim and colleagues (2005) described BASCs, which possess stem cell characteristics, are resistant to naphthaline injury and proliferate after airway or alveolar injury. Such BASCs reside near bronchioalveolar junctions and coexpress both alveolar (SP-C) and airway (CC10) epithelial cell markers, as well as coexpressing Sca-1. They are capable of self-renewal and differentiation into Clara cells and alveolar cells, and are also multipotent in clonal assays. Moreover, studies by Hong and coworkers (2001) identified variant Clara cells as endogenous lung stem cells, which infrequently proliferate during steady state but are held responsible for repopulating distal airway epithelium after injury. Variant Clara cells express Clara cell secretory protein, but survive naphthalene injury. As the lung continues to grow postnatally, Clara cells both self-renew and act as progenitors for ciliated cells, based on kinetics of cell labeling after a pulse of tritiated [3H]-thymidine (McDowell et al., 1985; Plopper, et al., 1992). This is supported by recent lineage labeling (Perl et al., 2005a). Whether all Clara cells have this capacity requires investigation. Moreover, type II cells proliferate and give rise to type I cells after adult alveolar injury, and this probably also occurs during postnatal growth (Evans et al., 1975). Several putative endogenous alveolar stem cell populations thus provide targets for directed regenerative therapies. Taking acute oxygen injury as an example, AECs undergo DNA and other forms of damage such as mitochondrial failure, glutathione depletion, and apoptosis (Buckley et al., 1998; Lee et al., 2006; Roper et al., 2004).
5.2. Endogenous mesenchymal progenitors
Several studies have shown that signals from lung mesenchyme are essential for branching morphogenesis. Mesothelium-derived FGF9 activates and controls FGF10 signaling from peripheral mesenchyme via FGFR2b, SHP2, Grb2, Sos, Ras, and Sprouty in epithelium (Del Moral et al., 2006b; Bellusci et al., 1997b; Tefft et al., 2002, 2005).
5.2.1. Smooth muscle progenitors
Distal Fgf10-expressing mesenchymal cells serve as progenitors for peripheral ASM (De Langhe et al., 2006; Mailleux et al., 2005; Ramasamy et al., 2007). Fgf10-lacz lineage tracing reveals ASM progenitors begin as Fgf10-expressing cells that, as the airway elongates, become distributed along peripheral airway. Transdifferentiation to express alpha–smooth muscle actin occurs under the control of SHH and BMP4, which are expressed proximal to the airway tip. Thus, increase in population size and localization of peripheral ASM progenitors occur early in development. Another population of ASM progenitors arise in proximal mesenchyme and advance peripherally (Shan et al., 2008).
5.2.2. Vascular progenitors
Lung microcirculation is rich in progenitors, but our understanding of these is limited. Mesothelium overlying the lung contains progenitors that give rise to pulmonary vascular (but not airway) SMCs during embryonic development (Que et al. 2008). Endothelial progenitors arise from endogenous vascular wall or from circulating progenitors. Similar to lung epithelial cells, heterogenous pulmonary endothelial cells may require a site-specific niche (Clark et al., 2008); alternatively, putative resident endothelial progenitors may constitute a universal pool of progenitors that lack segmental specification (Blaisdell et al., 2009).
Distal airspace and vascular growth are coordinated so injury can affect both (Jakkula et al., 2000). Balasubramaniam et al. (2007) examined endothelial progenitors in BPD to show that hyperoxia disrupts alveolar and vascular growth, limiting surface area for gas exchange. In the lung, nitric oxide, VEGF, and erythropoietin contribute to mobilization and homing of EPCs. Several related developmental changes occur after hyperoxia in neonatal mice: expression of endothelial nitric oxide synthase, VEGF, and erythropoietin receptor and the number of EPCs in the blood, bone marrow, and lung were all reduced (Balasubramaniam et al., 2007).
Primitive capillaries surround the laryngotracheal groove as the lung buds from foregut and can be visualized by β-galactosidase expression under control of Flk1 promoter. This promoter is active and the earliest known marker of hemangioblasts. Under stimulation of epithelial VEGF, these hemangioblasts differentiate into a capillary network that surrounds bronchial, lobar, and segmental airways (Del Moral et al., 2006a; Ramasamy et al., 2007). Organization of this plexus appears essential for correct branching and perfusion. Thus, mesothelial–mesenchymal–epithelial–endothelial crosstalk matches epithelial and vascular progenitor function and will likely be essential for lung regeneration to succeed. Further studies are needed to define phenotypes of the pulmonary endothelial cell but also SMCs within the vasculature (Stevens et al., 2008).
5.3. Control of lung progenitor cell proliferation
Embryonic progenitors undergo symmetric and asymmetric divisions. To distinguish these, one can look at differences in spindle orientation or differential inheritance of cytoplasmic or membrane-bound proteins such as cell fate determinant Numb and atypical protein kinase C (PKC) (Huttner and Kosodo, 2005; Morrison and Kimble, 2006; Wang et al., 2009; El-Hashash and Warburton, unpublished data). Cells divide asymmetrically in response to extrinsic or intrinsic fate determinants: extrinsically, daughter cells placed in different microenvironments adopt different fates; intrinsically, cytoplasmic cell fate determinants (e.g., Numb) are asymmetrically localized within a cell and segregate differentially into daughters that adopt different fates (reviewed by Yamashita, 2009). Comparing progenitor numbers in mutant and sibling control lungs, we infer that certain molecules promote progenitor self-renewal or differentiation (Rawlins, 2008).
Several transcription factors and signaling molecules control lung growth and therefore probably affect progenitor cell proliferation. Thyroid transcription factor 1 (Ttf-1/Nkx2.1) expression marks lung lineage commitment in the early embryo and is critical for distal lung progenitor development (Kimura et al., 1999). Ttf1−/−-null mice have insufficiently differentiated lungs for survival (Kimura, et al., 1996). HMG box transcription factor, Sox9 is intensively expressed in distal epithelial progenitors from E11.5 to E16.5 (Liu and Hogan, 2002). However, lung-specific conditional deletion has no effect on progenitor cell behavior (Perl et al., 2005b). Sox9 may therefore act redundantly with other, as yet unknown, regulators: N-myc is also essential for maintaining a distal population of undifferentiated, proliferating progenitor cells, and may promote their self-renewal (Okubo et al., 2005).
In addition, several forkhead/winged helix (fox) family transcription factors have mutant knockout phenotypes and may promote lung epithelial progenitor proliferation. For example, conditional deletion of both foxa1 and foxa2 genes in lung results in small lungs with decreased cell division rates (Wan et al., 2005). A similar phenotype was reported after conditional deletion of both foxp1 and foxp2, which are enriched in the distal epithelial progenitors. In foxp22/2; foxp11/2 double mutants, the lungs are smaller than normal, with inhibited proliferation, but normal proximal–distal patterning (Shu et al., 2007). This suggests an essential role of fox transcription factors in the maintenance of the progenitor cell population and their self-renewing divisions.
Similarly, five key signaling molecules regulate many processes in embryonic development: Wnt, Notch, Hedgehog, FGF, and TGF-β family. Embryos lacking Wnt2/2b exhibit lung agenesis and do not express Nkx2.1, the earliest marker of lung endoderm. Endoderm-restricted deletion of β-catenin replicates this, suggesting canonical Wnt2/2b signaling is required to specify lung endoderm progenitors in the foregut (Goss et al., 2009, Harris-Johnson, 2009). FGF signaling plays an essential role in specification of distal lung lineages (De Langhe et al., 2008; Ramasamy et al., 2007) and others (Serls et al., 2005). FGF10 is expressed by lung mesenchyme and is a chemotaxin during morphogenesis. FGF10 overexpression maintains epithelial progenitor cell proliferation and leads to goblet cell metaplasia (Nyeng et al., 2008). In addition, FGF10 coordinates alveolar SMC formation and vascular development (Ramasamy et al., 2007). RA signaling is also essential for expansion of lung progenitors and formation of primary lung buds, by affecting Fgf10 expression through TGF-β signaling (Chen et al., 2007). Similarly, Shh in distal epithelium controls proliferation and branching and is believed to promote progenitor proliferation (Pepicelli et al., 1998). Autocrine Bmp signaling is likewise important for proliferation of the distal epithelial progenitor cell compartment. Wnt5a is also highly expressed around distal epithelial tips. Wnt5a−/− lungs have increased cell proliferation and an additional airway branch (Li et al., 2002), but it is unknown if this phenotype relates to defective progenitors. The details of how these signaling pathways regulate distal epithelial progenitor cells remain to be determined.
5.4. Embryonic lung progenitors and proximal–distal patterning
Recent studies suggest that Wnt and Bmp signaling controls proximal–distal lung patterning, but there is currently no evidence to confirm that this is mediated through progenitors. Shu et al. (2005) demonstrated that proximal–distal lung patterning depends on Wnt/β-catenin signaling and is mediated, in part, through regulation of N-myc, Bmp-4, and FGF signaling. Potentiation of β-catenin signaling in proximal airway results in arrested differentiation of immature bronchiolar stem cells, but β-catenin is unnecessary for adult bronchiolar stem cell maintenance (Zemke et al., 2009). Fortunately, reporters of Wnt pathway activity are highly active in distal lung epithelial cells. Recent studies suggested that Wnt signaling regulates proximal–distal patterning and progenitor proliferation independently, and that Wnt promotes distal airway fate at the expense of the proximal. (Mucenski et al., 2003; Shu et al., 2005). Shu and coworkers overexpressed Dickkopf-1 to inhibit Wnt pathway activity throughout developing epithelium: this expands proximal (conducting) airways at the expense of the distal, without effects on total levels of cell proliferation (Shu et al., 2005). Similarly, Mucenski et al. (2003) showed that lung-specific deletion of β-catenin abrogates distal epithelial differentiation. Notch signaling favors progenitor identity at the expense of differentiated phenotypes in different organs (Jadhav et al., 2006; Mizutani et al., 2007) and is also required for lung epithelial progenitors. Notch1 is highly expressed in distal epithelial progenitors during the pseudoglandular stage (Post et al., 2000). Notch controls cell fates in developing airways (Tsao et al., 2009), and arrests normal differentiation of distal lung progenitors before they initiate an alveolar program (Guseh et al., 2009). Notch misexpression in the distal lung prevented the differentiation of alveolar cell types (Guseh et al., 2009); expression of a constitutively active form of Notch3 throughout the developing lung epithelium prevents cell differentiation (Dang et al., 2003).
Furthermore, BMP signaling is also required for lung epithelium development, probably by promoting distal and repressing proximal cell fate. Inactivation of Bmp signaling by overexpression of a dominant-negative BMP receptor, or BMP antagonists Gremlin or Noggin, results in proximalization of lung epithelium (Weaver et al., 1999; Lu et al., 2001). Thus, reduction of BMP or Wnt signaling causes lung proximalization phenotypes (Eblaghie et al., 2006; Li et al., 2002).
5.5. Emergence of specific cell types during lung organogenesis
At least 40 differentiated cell types emerge during lung organogenesis. Early trachea and esophagus are both lined with ciliated epithelium; following their septation, esophageal epithelium becomes squamous, while tracheal epithelium retains cilia. Primitive airway epithelium expresses several marker proteins including cGRP, Clara cell protein, and SP-A: its differentiation starts around E16 in mouse with emergence of pulmonary neurendocrine (PNE) cell rests, surrounded shortly after by Clara cells. In the periphery, AEC2 differentiation in E18 mouse is denoted by glycogen granules’ disappearance and emergence of surfactant-containing lamellar bodies with increased SP-C expression.
In mature lung, epithelial lineages are arranged proximodistally along the airways. Cartilage lies outside the submucosa and decreases in amount as bronchial caliber decreases; it is absent from bronchioles. The two major epithelial cell types in proximal bronchi are pseudostratified ciliated columnar cells and mucous (goblet) cells. Both arise from BCs, but ciliated cells predominate. Goblet cells begin to mature around 13 weeks’ gestation in humans (mature ciliated columnar cells are already present), express mucin markers (MUC5B, 5A, 5C), and release mucus granules into the airway which reduces drying and, through ciliary-driven cephalad mucus flow, cleanses the airway. In cystic fibrosis, mutation of the cystic fibrosis transmembrane conductance regulator (Cftr) gene disrupts expression of the encoded transmembrane Na+ ion transporter protein leading to thick mucus that overwhelms ciliary clearance and increases susceptibility to infection. In chronic airway injury, goblet cell hyperplasia may follow repair or experimental epithelial IL-9 exposure; the latter increases epithelial lysozyme and mucus production (Vermeer et al., 2003). IL-4, IL-13, and allergens enhance TGF-α release, which is a ligand for the EGFR that also stimulates goblet cell differentiation (Lordan et al., 2002).
There are three types of cells in bronchial submucosal glands. Myoepithelial cells surround the gland, while mucous cells (pale cytoplasm) and serous cells (basophilic cytoplasm) produce mucins. These secreted mucins mix with lysozyme and IgA on airway surface.
Kulchitsky cells are also found next to bronchial glands, but their function is unclear. It is believed they are pulmonary neuroendocrine cells (PNECs) producing peptides such as serotonin and calcitonin. Their cytoplasmic extensions usually reach the airway lumen. Kulchitsky cells expressing gastrin-releasing peptide (GRP), calcitonin gene-related peptide (CGRP), and chromogranin may be related to small cell carcinoma and carcinoid tumors. However, PNEC differentiate earlier by 10 weeks’ human gestation and are the first fully differentiated murine airway epithelial cells.
Clara cells reside in distal bronchiolar airway epithelium (normally lacking mucous cells) and produce mucus-poor, watery secretion. They emerge during the 19th week in humans and appear to assist with clearance, detoxification, and surface tension reduction in small airways. Clara cell-specific protein (CC10, CCSP, or uteroglobin) and cytochrome P450 reductase CC10 can be used as Clara cell markers. Whilst normal mice feature few mucin-positive cells in the airway, mucus metaplasia is associated with numerous Clara cell-derived mucous cells with excess mucin production or reduced secretion (Evans et al., 2004).
Most of the alveolar surface is covered by flat type I epithelial cells that are believed to be terminal differentiated and express several markers, such as T1a and aquaporin 5. T1a is developmentally regulated and encodes an apical membrane protein of unknown function. Absence of T1a protein blocks type I cell differentiation. Homozygous T1a null mice die at birth of respiratory failure with lungs that will not inflate normally (Ramirez et al., 2003). Aquaporin 5 is a water-channel in type I epithelial cells. Recently, a knock in of Cre-ERT2 into the Aqp5 locus has been reported (Borok, personal communication). These mice will be useful, not only to target gene deletion in type I cells but also for lineage tracing of the type I cells under development, injury, and repair.
Whilst type I epithelial cells cover more than 95% of the alveolar surface area, they account for only 40% of total AECs: the other 60% are rounded type II pneumocytes. These plump or cuboidal cells can regenerate and replace type I cells post injury and have finely stippled cytoplasm and surface microvilli. They manufacture surfactant phospholipids and proteins that modulate alveolar surface tension, such that despite varied size, alveoli remain open and end-expiratory atelectasis is reduced. Surfactant protein C (SftpC) is a commonly used type II cell marker. The four surfactant proteins, SP-A, B, C, and D play critical roles: SP-A and SP-D participate in airway host defense; SP-B and SP-C contribute to surfactant’s surface tension reduction (Whitsett et al., 2002).
Macrophages, although a small percentage of alveolar cells, are a major sentinel of host defense and derived primarily from blood monocytes; once in the lung, their turnover is extremely slow.
5.6. Stem and progenitor cells in the postnatal respiratory system
Stem and progenitor cells presumably help repair damaged lung, but identification of such cells remains problematic. The large surface area, numerous branches, and folded topography suggest lung harbors several stem or progenitor cell types. In trachea and bronchi, certain BCs and mucous-gland duct cells are believed to be stem/progenitor cells. Clara-like cells and type II pneumocytes are also thought to function as stem/progenitor cells in bronchioles and alveoli, respectively. Another population of stem/progenitor cells apparently lies at the bronchoalveolar duct junction (Kim et al., 2005). They have been proposed to function as bipotential precursors of both the SP-C- and CC10-expressing cell lineages.
It has recently been reported that bone marrow-derived mesenchymal stem cells can differentiate into airway epithelial cells and alveolar type I pneumocytes, particularly post-injury. By contrast, in vitro cell culture indicates Syrian hamster fetal lung epithelial M3E3/C3 cells differentiate into Clara cells and type II pneumocytes under different culture conditions. Whether CCSP-expressing cells with pre-Clara cell phenotypes are stem cells for the entire respiratory tract remains to be determined. In addition, the concept of a pluripotent stem cell for the whole lung needs to be further investigated due to the great differences between identified stem cell or progenitor candidates in proximal bronchi and distal alveoli. Recently, we discovered lung contains cells with stem or progenitor characteristics that can be FACS sorted from adult rat and mouse lung and which are relatively apoptosis-resistant and perhaps responsible for post-injury alveolar repopulation. Another such population sorted as “side cells” on FACS may repopulate several tissues including bone marrow.
ASM derives from at least two progenitor populations: one comes from the lung periphery and arises from Fgf10-expressing cells in subepithelial mesenchyme. By virtue of FGF10 expression, these cells first help mediate epithelial branching; however, as the airway elongates into peripheral mesenchyme, these progenitors remain to lie along the more proximal stalk of the distal bud. Here they differentiate into ASM, most probably under paracrine induction of BMP4 and Sonic hedgehog from the adjacent epithelium (DeLanghe et al., 2006). The second ASM progenitor population arises around the proximal large airways (Shan et al., 2008) and appears to meet with the distally-derived counterparts beyond major lobar and segmental branches. It is speculated that whilst size of such ASM progenitor populations are determined during embryonic airway branching, they may nevertheless determine susceptibility to later BPD and asthma. Moreover, maternal smoking may dysregulate ASM progenitors and their progeny via the cholinergic-agonist, nicotine.
5.7. Potential strategies to protect lung progenitors
Both FGF7 and inosine treatment ameliorate DNA damage in AECs, as well as enhancing mitochondrial protection and the ability of AEC to migrate and repair in an in vitro scratch assay (Buckley et al., 1997). FGF7 has also been evaluated by others in vivo as a treatment to enhance resistance to alveolar injury in animal models (Plantier et al., 2007; Ray et al., 2003). Also, FGF10 has a protective effect against lung injury and fibrosis (Gupte et al., 2009). We have also shown that inosine has protective properties against oxygen injury, including glutathione repletion, mitochondrial protection, decreased apoptosis, and increased VEGF expression (Buckley et al., 2005). Thus, it appears that protection or enhancement of alveolar progenitor cell function may be a viable therapeutic option that could possibly be evaluated in clinical trials of lung progenitor cell protection using small molecules such as inosine or FGF7 or FGF10.
6. Postnatal and Adult Lung
6.1. The transition to air breathing
Maturation of the surfactant system is one of two key steps to prepare fetal lung for air breathing. During the last 8 weeks of human gestation, fetal lung glycogen is converted into surfactant phospolipids, the most important of which is disaturated phosphatidylcholine (DSPC). This maturation is under the control of, and can be stimulated by, corticosteroids since it is blocked in mice with null mutations of glucocorticoid receptors or corticotrophin-releasing hormone. Human mutations have been found, such as surfactant protein B, that adversely affect stability of surfactant and hence the ability to maintain lung inflation.
The transition to air breathing occurs rapidly in mature neonatal lung. Immediately following severance of the umbilical circulation, a spike in catecholamine levels switches off chloride secretion and stimulates sodium/potassium ATPase (Brown et al., 1983; Olver and Strang, 1974; Olver et al., 1986). This replaces tracheal fluid production with its rapid absorption into lung interestiitum (and thence to lymphatic and capillary circulations). Null mutation of Na/K ATPase in mice leads to failure to absorb fetal lung liquid, which causes significant respiratory distress and even neonatal lethality (Hummler et al., 1996). In humans delayed lung liquid absorption manifests as transient tachypnea of the newborn.
6.2. Lung aging and involution
From middle age in normal humans, an inexorable decline in lung function supervenes (illustrated by FEV1). By 120 years, FEV1 resembles that end-stage COPD in a younger person; hence, degenerating lung function appears currently to limit human life expectancy (Fletcher and Peto, 1977; Shi, W. and Warburton, D. 2010). Whilst some genetic mutations and/or environmental exposures fundamentally disrupt lung development and result in pre- or perinatal death, less critical leions may only be manifest as lung disease in infancy, childhood, or beyond. For example, minor genetic changes such as DNA polymorphisms may have very subtle impacts on lung organogenesis with apparently normal neonatal phenotype. Nevertheless, such lungs may have abnormal responses to subsequent environmental injury (e.g., cigarette smoke or vehicular pollution) that degrade lung anatomy and physiology faster than normal and predispose to, for example, COPD (Figure 3.10). Therefore, by understanding, protecting, and re-entraining developmental processes, amelioration or reversal of lung degeneration may permit enhanced duration and quality of life.
Figure 3.10.
Smoking and genetics synergize to degrade lung function with age (modified after Fletcher and Peto, 1977; Shi, W. and Warburton, D. 2010). Wild-type lungs “grown in room air” achieve greatest capacity and remain healthy despite age-related degradation. Smoking exacerbates degradation of lung function even in the healthy. Genetic alterations decrease the potential to develop maximal lung capacity compared to wild type and smoking exacerbates lung degradation still further. A 0.5% loss of lung function per year is assumed for normal lungs and double that for smokers. Lungs with a genetic defect were assumed to have 95% of the growth rate of a normal lung. Growth rate is considered as a process of doublings, but the rate of doublings declines from maturity exponentially with age. This is expressed in a simple differential equation for modeling lung function; , where mat is the maturation coefficient, r is the doubling coefficient, and a is the loss-of-function coefficient.
7. Conclusions
Appreciating that distal lung mesenchyme could trigger epithelial airway development has stimulated the search for controls of lung development. Given the mortality and morbidity of lung disease at all stages of life, lung regeneration is a global therapeutic priority. To achieve such goals, clinicians and scientists need to decipher how the lung is formed. Whilst this understanding began with histological analyses, advances in biology have allowed the “molecular embryology” of the lung to be elucidated. In parallel with this progress, lessons from human lung maldevelopment illustrate the importance of mechanical forces to normal lung growth. Such forces encompass both extrinsic factors (thoracic size, FBMs) and intrinsic ones (lung fluid, airway peristalsis, endogenous airway occlusions). Attempting to weave these diverse influences to facilitate regenerative lung growth appears a daunting task. Nevertheless, there are reasons for optimism: first, following Alan Turing’s insight, complex (lung) morphogenesis may arise via simple iterative biochemical signaling; secondly, Benoit Mandelbrot illustrated that simple mathematics can be applied to generate apparently complex form; thirdly, D’Arcy Thompson made clear that the set of genetically possible forms are vastly constrained by fundamental physical constraints; fourth, despite huge uncertainties about the regulation of lung development, regenerative medicine has already allowed transplantation of autologous tissue-engineered airway to aid patients. Hence, despite the structural complexity of the lung, its organogenesis is governed by simpler routines more readily susceptible to discovery and therapeutic exploitation. In pursuing the latter, we may similarly be reassured that physical constraints limit the possible structures we may engineer. Finally, despite all that we do not know, clinically important aspects of pulmonary regeneration can already be achieved. The challenge for the future will be the generation of more complex and vascularized structures that can ultimately support and/or replace impaired lung function.
Acknowledgments
We apologize to those colleagues whose important work in this field we have failed to cite.
Funding sources: National Heart, Lung and Blood Institute, National Institutes of Health, USA, National Science Foundation, USA, California Institute for Regenerative Medicine, Medical Research Council UK, Biotechnology and Biological Sciences Research Council, UK, Foreign and Commonwealth Office UK/USA stem cell collaboration grant, American Heart Association, American Lung Association, and Pasadena Guild of Childrens Hospital Los Angeles.
Editorial assistance: Zoe Ly and Theresa Webster.
Facilitation of US/UK collaborations on this review: UK Science & Innovation Network, British Consulate-General Los Angeles.
References
- Acosta JM, Thebaud B, Castillo C, Mailleux A, Tefft D, Wuenschell C, Anderson KD, Bourbon J, Thiery JP, Bellusci S, Warburton D. Novel mechanisms in murine nitrofen-induced pulmonary hypoplasia: FGF-10 rescue in culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L250–257. doi: 10.1152/ajplung.2001.281.1.L250. [DOI] [PubMed] [Google Scholar]
- Adamson TM, Boyd RD, Platt HS, Strang LB. Composition of alveolar liquid in the foetal lamb. J Physiol. 1969;204:159–168. doi: 10.1113/jphysiol.1969.sp008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649–660. [PMC free article] [PubMed] [Google Scholar]
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Anselmo MA, Dalvin S, Prodhan P, Komatsuzaki K, Aidlen JT, Schnitzer JJ, Wu JY, Bernard KT. Slit and robo: Expression patterns in lung development. Gene Expr Patterns. 2003;3:13–19. doi: 10.1016/s1567-133x(02)00095-9. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieux M, Tremblay M, Berard J, Jeannotte L. Early postnatal lethality in hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Austin J, Ali T. Tracheomalacia and bronchomalacia in children: Pathophysiology, assessment, treatment and anaesthesia management. Pediatr Anaesth. 2003;13:3–11. doi: 10.1046/j.1460-9592.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Demedts M, Bouillon R, Desmet J. Coexistence of hypocalciuric hypercalcaemia and interstitial lung disease in a family: A cross-sectional study. Eur J Clin Invest. 1985a;15(1):6–14. doi: 10.1111/j.1365-2362.1985.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Boogaerts M, Ceuppens JL, Demedts M. Defective host defence mechanisms in a family with hypocalciuric hypercalcaemia and coexisting interstitial lung disease. Clin Exp Immunol. 1985b;62:57–64. [PMC free article] [PubMed] [Google Scholar]
- Awonusonu F, Srinivasan S, Strange J, Al Jumaily W, Bruce MC. Developmental shift in the relative percentages of lung fibroblast subsets: Role of apoptosis postseptation. Am J Physiol. 1999;277:L848–L859. doi: 10.1152/ajplung.1999.277.4.L848. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: Implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Batchelor DC, Hutchins AM, Klempt M, Skinner SJ. Developmental changes in the expression patterns of IGFs, type 1 IGF receptor and IGF-binding proteins-2 and -4 in perinatal rat lung. J Muscoskel Pain. 1995;15:105–115. doi: 10.1677/jme.0.0150105. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Weiser-Evans MC, Grieshaber SS, Majack RA, Stenmark KR. Relationship between perlecan and tropoelastin gene expression and cell replication in the developing rat pulmonary vasculature. Am J Respir Cell Mol Biol. 1999;20:24–34. doi: 10.1165/ajrcmb.20.1.3321. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997a;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997b;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Berdon WE. Rings, slings, and other things: Vascular compression of the infant trachea updated from the midcentury to the millennium—the legacy of Robert E. Gross, M.D., and Edward B. D. Neuhauser, M.D. Radiology. 2000;216:624–632. doi: 10.1148/radiology.216.3.r00se40624. [DOI] [PubMed] [Google Scholar]
- Bernier SM, Utani A, Sugiyama S, Doi T, Polistina C, Yamada Y. Cloning and expression of laminin alpha 2 chain (M-chain) in the mouse. Matrix Biol. 1995;14:447–455. doi: 10.1016/0945-053x(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Bingle CD, Hackett BP, Moxley M, Longmore W, Gitlin JD. Role of hepatocyte nuclear factor-3 alpha and hepatocyte nuclear factor-3 beta in Clara cell secretory protein gene expression in the bronchiolar epithelium. Biochem J. 1995;308(Pt 1):197–202. doi: 10.1042/bj3080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell CJ, Gail DB, Nabel EG. National heart, lung, and blood institute perspective: Lung progenitor and stem cells–gaps in knowledge and future opportunities. Stem Cells. 2009;27(9):2263–2270. doi: 10.1002/stem.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell CJ, Morales MM, Andrade AC, Bamford P, Wasicko M, Welling P. Inhibition of CLC-2 chloride channel expression interrupts expansion of fetal lung cysts. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L420–L426. doi: 10.1152/ajplung.00113.2003. [DOI] [PubMed] [Google Scholar]
- Bland RD, Boyd CA. Cation transport in lung epithelial cells derived from fetal, newborn, and adult rabbits. J Appl Physiol. 1986;61(2):507–515. doi: 10.1152/jappl.1986.61.2.507. [DOI] [PubMed] [Google Scholar]
- Boggaram V. Regulation of lung surfactant protein gene expression. Front Biosci. 2003;8:d751–d764. doi: 10.2741/1062. [DOI] [PubMed] [Google Scholar]
- Bogue CW, Lou LJ, Vasavada H, Wilson CM, Jacobs HC. Expression of Hoxb genes in the developing mouse foregut and lung. Am J Respir Cell Mol Biol. 1996;15:163–171. doi: 10.1165/ajrcmb.15.2.8703472. [DOI] [PubMed] [Google Scholar]
- Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390–5395. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: Incidence and patient characteristics. Chest. 2005;128:3391–3397. doi: 10.1378/chest.128.5.3391. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Bostrom H, Gritli-Linde A, Betsholtz C. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn. 2002;223:155–162. doi: 10.1002/dvdy.1225. [DOI] [PubMed] [Google Scholar]
- Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Tornell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Bragg AD, Moses HL, Serra R. Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. Mech Dev. 2001;109:13–26. doi: 10.1016/s0925-4773(01)00508-1. [DOI] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Brown L, Erdmann E, Thomas R. Digitalis structure-activit relationship analyses. Conclusions from indirect binding studies with cardiac (Na+ +K+)-ATPase. Biochem Pharmacol. 1983;32:2767–2774. doi: 10.1016/0006-2952(83)90090-4. [DOI] [PubMed] [Google Scholar]
- Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- Buch S, Jassal D, Cannigia I, Edelson J, Han R, Liu J, Tanswell K, Post M. Ontogeny and regulation of platelet-derived growth factor gene expression in distal fetal rat lung epithelial cells. Am J Respir Cell Mol Biol. 1994;11:251–261. doi: 10.1165/ajrcmb.11.3.8086163. [DOI] [PubMed] [Google Scholar]
- Buckley S, Barsky L, Weinberg K, Warburton D. In vivo inosine protects alveolar epithelial type 2 cells against hyperoxia-induced DNA damage through MAP kinase signaling. Am J Physiol Lung Cell Mol Physiol. 2005;288:L569–L575. doi: 10.1152/ajplung.00278.2004. [DOI] [PubMed] [Google Scholar]
- Buckley S, Driscol B, Anderson KD, Warburton D. Apoptosis and DNA damage in AEC2 cultured from hyperoxic rats. Am J Physio. 1998;274(5 Pt 1):L714–L720. doi: 10.1152/ajplung.1998.274.5.L714. [DOI] [PubMed] [Google Scholar]
- Buckley S, Driscoll B, Anderson KD, Warburton D. Cell cycle in alveolar epithelial type II cells: Integration of matrigel and KGF. Am J Physiol. 1997;273:L572–L580. doi: 10.1152/ajplung.1997.273.3.L572. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: An in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: Auestions, facts and controversies. Development. 2006;133(9):1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Itoh A, Nogawa H, Mason I, Brody JS. FGF-1 and FGF−7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev Dyn. 1997;208:398–405. doi: 10.1002/(SICI)1097-0177(199703)208:3<398::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Williams MC, Mitsialis SA, Joyce-Brady M, Rishi AK, Brody JS. Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitro. Am J Respir Cell Mol Biol. 1995;12:464–476. doi: 10.1165/ajrcmb.12.5.7742011. [DOI] [PubMed] [Google Scholar]
- Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, Warburton D. MiR−17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-cadherin distribution. Dev Biol. 2009;333(2):238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre A, Szinnai G, Castanet M, Sura-Trueba S, Tron E, Broutin-L’Hermite I, Bara P, Goizet C, Lacombe D, Moutard ML, Raybaud C, Raynaud-Ravni C, Romana S, Ythier H, Leger J, Polka M. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chazaud C, Dolle P, Rossant J, Mollard R. Retinoic acid signaling regulates murine bronchial tubule formation. Mech Dev. 2003;120:691–700. doi: 10.1016/s0925-4773(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of TGF b signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Chen H, Sun J, Buckley S, Chen C, Warburton D, Wang XF, Shi W. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol. 2005;288:L683–L691. doi: 10.1152/ajplung.00298.2004. [DOI] [PubMed] [Google Scholar]
- Chen J, Lecuona E, Briva A, Welch LC, Sznajder JI. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. Am J Repir Cell Mol Biol. 2008;38:32–37. doi: 10.1165/rcmb.2007-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Przyboroski M, Berthaume F. Stem cells for skin tissue engineering and would healing. Crit Rev Biomed Eng. 2009;37:399–421. doi: 10.1615/critrevbiomedeng.v37.i4-5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian hedgehog signaling by the hedgehog-binding protein, hip1, modulates fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- Chytil F. Retinoids in lung development. FASEB J. 1996;10:986–992. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- Clark J, Alvarez DF, Alexeyev M, King JA, Huang L, Yoder MC, Stevens T. Regulatory role for nucleosomeassembly protein-1 in the proliferative and vasculogenic phenotypeof pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L431–L439. doi: 10.1152/ajplung.00316.2007. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119(9):2538–2549. doi: 10.1172/JCI38079. 38079 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Larson JE, Killeen E, Love D, Takemaru K. CFTR and Wnt/beta-catenin signaling in lung development. BMC Dev Biol. 2008;6(8):70. doi: 10.1186/1471-213X-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol. 2005;167:419–428. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev Dyn. 1999;216:72–88. doi: 10.1002/(SICI)1097-0177(199909)216:1<72::AID-DVDY9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Coppola D, Ferber A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. Am J Physiol Lung Cell Mol Physiol. 2001;280:L823–L838. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- Costello CM, Howell K, Cahill E, McBryan J, Konigshoff M, Eickelberg O, Gaine S, Martin F, McLoughlin P. Lung-selective gene responses to alveolar hypoxia: potential role for the bone morphogenetic antagonist gremlin in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L272–L284. doi: 10.1152/ajplung.00358.2007. [DOI] [PubMed] [Google Scholar]
- Dang TP, Eichenberger S, Gonzalez A, Olson S, Carbone DP. Constitutive activation of notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene. 2003;22:1988–1997. doi: 10.1038/sj.onc.1206230. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- De Felice M, Silberschmidt D, DiLauro R, Xu Y, Wert SE, Weaver TE, Bachurski CJ, Clark JC, Whitsett JA. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J Biol Chem. 2003;278:35574–35583. doi: 10.1074/jbc.M304885200. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS ONE. 2008;3(1):e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol. 2006;299(1):52–62. doi: 10.1016/j.ydbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277(2):316. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006a;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol. 2006b;293:77–89. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286(1):270–286. doi: 10.1016/j.ydbio.2005.07.034. S0012-1606(05)00517-8 [pii] [DOI] [PubMed] [Google Scholar]
- Deterding RR, Jacoby CR, Shannon JM. Acidic fibroblast growth factor and keratinocyte growth factor stimulate fetal rat pulmonary epithelial growth. Am J Physiol. 1996;271:L495–L505. doi: 10.1152/ajplung.1996.271.4.L495. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- Diez-Pardo JA, Qi BQ, Navarro C, Tovar JA. A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: Preliminary report. J Pediatr Surg. 1996;31(4):498–502. doi: 10.1016/s0022-3468(96)90482-0. [DOI] [PubMed] [Google Scholar]
- Driscoll B, Buckley S, Bui KC, Anderson KD, Warburton D. Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1191–L1198. doi: 10.1152/ajplung.2000.279.6.L1191. [DOI] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Durham PL, Snyder JM. Characterization of alpha 1, beta 1, and gamma 1 laminin subunits during rabbit fetal lung development. Dev Dyn. 1995;203:408–421. doi: 10.1002/aja.1002030404. [DOI] [PubMed] [Google Scholar]
- Dziadek M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51:901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- Ellis T, Gambardella L, Horcher M, Tschanz S, Capol J, Bertram P, Jochum W, Barrandon Y, Busslinger M. The transcriptional repressor CDP (cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes Dev. 2001;15:2307–2319. doi: 10.1101/gad.200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elluru RG, Thompson F, Reece A. Fibroblast growth factor 18 gives growth and directional cues to airway cartilage. Laryngoscope. 2009;119:1153–1165. doi: 10.1002/lary.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol. 2001;24:649–652. doi: 10.1165/ajrcmb.24.6.f206. [DOI] [PubMed] [Google Scholar]
- Esposito G, Fogolari F, Damante G, Formisano S, Tell G, Leonardi A, Di Lauro R, Viglino P. Analysis of the solution structure of the homeodomain of rat thyroid transcription factor 1 by 1h-NMR spectroscopy and restrained molecular mechanics. Eur J Biochem. 1996;241:101–113. doi: 10.1111/j.1432-1033.1996.0101t.x. [DOI] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, Ngam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Featherstone NC, Connell MG, Fernig DG, Wray S, Burdyga TV, Losty PD, Jesudason EC. Airway smooth muscle dysfunction precedes teratogenic congenital diaphragmatic hernia and May contribute to hypoplastic lung morphogenesis. Am J Respir Cell Mol Biol. 2006;35(5):571–578. doi: 10.1165/rcmb.2006-0079OC. [DOI] [PubMed] [Google Scholar]
- Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol. 2005;33(2):153–160. doi: 10.1165/rcmb.2005-0137OC. [DOI] [PubMed] [Google Scholar]
- Felix JF, de Jong EM, Torfs CP, de Klein A, Rottier RJ, Tibboel D. Genetic and environemental factors in the etiology of esophageal atresia and/or tracheoesophageal fistula: an overview of the current concepts. Birth Defects Re A Clin Mol Teratol. 2009;85:747–754. doi: 10.1002/bdra.20592. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Johnson P. Upper airway dynamics during breathing and during apnoea in fetal lambs. J Physiol. 1983;339:495–505. doi: 10.1113/jphysiol.1983.sp014729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Hislop AA, Kitterman JA, Johnson P. Effect of tracheostomy on lung development in fetal lambs. J Appl Physiol. 2003;55:1103–1108. doi: 10.1152/jappl.1983.55.4.1103. [DOI] [PubMed] [Google Scholar]
- Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol. 2008;15(586 Pt 24):6007–6019. doi: 10.1113/jphysiol.2008.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Viebahn C, Blum M. FGF8 acts as a right determinant during establishment of the left-right axis in the rabbit. Curr Biol. 2002;12:1807–1816. doi: 10.1016/s0960-9822(02)01222-8. [DOI] [PubMed] [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: A quantitative study. Biophys J. 1998;74(5):2227–2234. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Forgacs G, Pfleger CM, Steinberg MS. Liquid properties of embryonic tissues: Measurement of interfacial tensions. Phys Rev Lett. 1994;72:2298–2301. doi: 10.1103/PhysRevLett.72.2298. [DOI] [PubMed] [Google Scholar]
- Frank L. Protective effect of keratinocyte growth factor against lung abnormalities associated with hyperoxia in prematurely born rats. Biol Neonate. 2003;83:263–272. doi: 10.1159/000069480. [DOI] [PubMed] [Google Scholar]
- Frieser M, Nockel H, Pausch F, Roder C, Hahn A, Deutzmann R, Sorokin LM. Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium. Eur J Biochem. 1997;246:727–735. doi: 10.1111/j.1432-1033.1997.t01-1-00727.x. [DOI] [PubMed] [Google Scholar]
- Galliano MF, Aberdam D, Aguzzi A, Ortonne JP, Meneguzzi G. Cloning and complete primary structure of the mouse laminin alpha 3 chain. Distinct expression pattern of the laminin alpha 3a and alpha 3b chain isoforms. J Biol Chem. 1995;270:21820–21826. doi: 10.1074/jbc.270.37.21820. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raj JU. Parathyroid hormone-related protein-mediated responses in pulmonary arteries and veins of newborn lambs. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L60–L66. doi: 10.1152/ajplung.00411.2004. [DOI] [PubMed] [Google Scholar]
- Gauldie J, Galt Tl, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of tranforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol. 2003;163:2575–2584. doi: 10.1016/s0002-9440(10)63612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Strip BR. Terminal bronchiales harbor a unique airway stem cell population that localized to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Pape MC, Khokha R, Watson AJ, Leco KJ. A null mutation for tissue inhibitor of metalloproteinases-3 (timp-3) impairs murine bronchiole branching morphogenesis. Dev Biol. 2003;261:313–323. doi: 10.1016/s0012-1606(03)00318-x. [DOI] [PubMed] [Google Scholar]
- Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2-chloride transporter. Am J Respir Cell Mol Biol. 2001;25(1):14–20. doi: 10.1165/ajrcmb.25.1.4500. [DOI] [PubMed] [Google Scholar]
- Glazer L, Shilo BZ. The drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev. 1991;5:697–705. doi: 10.1101/gad.5.4.697. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JM, Thompson FY, Brooks SK, Shannon JM, McCormick-Shannon K, Cameron JE, Mallory BP, Akeson AL. Mesenchymal expression of vascular endothelial growth factors D and A defines vascular pattering in developing lung. Dev Dyn. 2002;224:144–153. doi: 10.1002/dvdy.10095. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol. 1997;188:337–348. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- Gross I, Smith GJ, Maniscalco WM, Czajka MR, Wilson CM, Rooney SA. An organ culture model for study of biochemical development of fetal rat lung. J Appl Physiol. 1978;45(3):355–362. doi: 10.1152/jappl.1978.45.3.355. [DOI] [PubMed] [Google Scholar]
- Guazzi S, Price M, De Felice M, Damante G, Mattei MG, Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, Guenther A, Warburton D, Driscoll B, Minoo P, Bellusci S. Over-expression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Repir Crit Care Med. 2009;180:424–436. doi: 10.1164/rccm.200811-1794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136(10):1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett BP, Brody SL, Liang M, Zeitz ID, Bruns LA, Gitlin JD. Primary structure of hepatocyte nuclear factor/forkhead homologue 4 and characterization of gene expression in the developing respiratory and reproductive epithelium. Proc Natl Acad Sci USA. 1995;92:4249–4253. doi: 10.1073/pnas.92.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RN, Post M, Tanswell AK, Lye SJ. Insulin-like growth factor-I receptor-mediated vasculogenesis/angiogenesis in human lung development. Am J Respir Cell Mol Biol. 2003;28:159–169. doi: 10.1165/rcmb.4764. [DOI] [PubMed] [Google Scholar]
- Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol. 1996;81(1):209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. Beta-catenin promotes respiratory progenitor identify in mouse foregut. Pro Natl Acad Sci USA. 2009;106 (38):16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, Lee H, Filly RA, Farrell JA, Albanese CT. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349(20):1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Nakano H, Singh G, Katyal S. Expression of spred and sprouty in developing rat lung. Mech Dev. 2002;119(Suppl 1):S303–S309. doi: 10.1016/s0925-4773(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91:1204–1217. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- He Y, Crouch EC, Rust K, Spaite E, Brody SL. Proximal promoter of the surfactant protein D gene: Regulatory roles of AP-1, forkhead box, and GT box binding proteins. J Biol Chem. 2000;275:31051–31060. doi: 10.1074/jbc.M003499200. [DOI] [PubMed] [Google Scholar]
- Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev Dyn. 2000;219:341–352. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hedrick MH, Estes JM, Sullivan KM, Bealer JF, Kitterman JA, Flake AW, Adzick NS, Harrison MR. Plug the lung until it grows (PLUG): A new method to treat congenital diaphragmatic hernia in utero. J Pediatr Surg. 1994;29(5):612–617. doi: 10.1016/0022-3468(94)90724-2. [DOI] [PubMed] [Google Scholar]
- Hilfer SR. Morphogenesis of the lung: Control of embryonic and fetal branching. Annu Rev Physiol. 1996;58:93–113. doi: 10.1146/annurev.ph.58.030196.000521. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hokuto I, Perl AK, Whitsett JA. Prenatal, but not postnatal, inhibition of fibroblast growth factor receptor signaling causes emphysema. J Biol Chem. 2003;278:415–421. doi: 10.1074/jbc.M208328200. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein- expressing cells of the airway neuroepithelial body micro−environment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004a;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004b;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha ENaC-deficient mice. Nat Genet. 1996;12(3):325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17(6):648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. BMP4 modulates fibroblast growth factor-mediated induction of proximal and distal lung differentiation in mouse embryonic tracheal epithelium in mesenchyme-free culture. Dev Dyn. 2002;225:153–165. doi: 10.1002/dvdy.10145. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Morita T, Tryggvason K. Molecular cloning and tissue-specific expression of a novel murine laminin gamma3 chain. J Biol Chem. 1999;274:14107–14111. doi: 10.1074/jbc.274.20.14107. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Bergmann U, Thesleff I, Tryggvason K. Primary structure, developmental expression, and immunolocalization of the murine laminin alpha4 chain. J Biol Chem. 1997;272:27862–27868. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Sainio K, Sariola H, Tryggvason K. Primary structure and expression of a novel human laminin alpha 4 chain. FEBS Lett. 1995a;365:183–188. doi: 10.1016/0014-5793(95)00462-i. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Vuolteenaho R, Sainio K, Eddy R, Shows TB, Sariola H, Tryggvason K. The human laminin beta 2 chain (S-laminin): Structure, expression in fetal tissues and chromosomal assignment of the LAMB2 gene. Matrix Biol. 1995b;14:489–497. doi: 10.1016/0945-053x(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol Cell Biol. 1996;16:3626–3636. doi: 10.1128/mcb.16.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanlou MR, Kablar B. Abnormal development of the diaphragm in mdx: MyoD−/−(9th) embryos leads to pulmonary hypoplasia. Int J Dev Biol. 2003;47:363–371. [PubMed] [Google Scholar]
- Ingber D. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Irwin D, Helm K, Campbell N, Imamura M, Faggan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, Dempsey EC, West J, Majka S. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia−induced lung simplification. Am J Physiol Lung Cell Mol Physiol. 2007;293:L941–L951. doi: 10.1152/ajplung.00054.2007. [DOI] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcriptionfactors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol. 2003a;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, Cardoso WV. Heparan sulfates expressed in the distal lung are required for fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol. 2003b;285:L838– L846. doi: 10.1152/ajplung.00081.2003. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci USA. 2006;103:18998–19003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab K, Damon B, Marga F, Doaga O, Mironov V, Kosztin I, Markwald R, Forgacs G. Relating cell and tissue mechanics: Implications and applications. Dev Dyn. 2008;237:2438–2449. doi: 10.1002/dvdy.21684. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Jani J, Gratacos E, Greenough A, Piero JL, Benachi A, Harrison M, Nicolaides J, Deprest J. Percutaneous fetal endoscopic tracheal occlusion (FETO) for severe left-sided congenital diaphragmatic hernia. Clin Obstet Gynecol. 2005;48(4):910–922. doi: 10.1097/01.grf.0000184774.02793.0c. [DOI] [PubMed] [Google Scholar]
- Jaskoll TF, Don-Wheeler G, Johnson R, Slavkin HC. Embryonic mouse lung morphogenesis and type II cytodifferentiation in serumless, chemically defined medium using prolonged in vitro cultures. Cell Differ. 1988;24:105–117. doi: 10.1016/0045-6039(88)90062-0. [DOI] [PubMed] [Google Scholar]
- Jesudason EC. Airway smooth muscle: An architect of the lung? Thorax. 2009;64(6):541–545. doi: 10.1136/thx.2008.107094. [DOI] [PubMed] [Google Scholar]
- Jesudason EC. Exploiting mechanical stimuli to rescue growth of the hypoplastic lung. Pediatr Surg Int. 2007;23:827–836. doi: 10.1007/s00383-007-1956-0. [DOI] [PubMed] [Google Scholar]
- Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am J Physiol Lung Cell Mol Physiol. 2006a;291(4):L559–L565. doi: 10.1152/ajplung.00498.2005. [DOI] [PubMed] [Google Scholar]
- Jesudason EC. Small lungs and suspect smooth muscle: Congenital diaphragmatic hernia and the smooth muscle hypothesis. J Pediatr Surg. 2006b;41(2):431–435. doi: 10.1016/j.jpedsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD. Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am J Respir Cell Mol Biol. 2005;32(2):118–127. doi: 10.1165/rcmb.2004-0304OC. [DOI] [PubMed] [Google Scholar]
- Jesudason EC, Connell MG, Fernig DG, Lloyd DA, Losty PD. Early lung malformations in congenital diaphragmatic hernia. J Pediatr Surg. 2000;35(1):124–127. doi: 10.1016/s0022-3468(00)80028-7. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Warburton D. Fibrillin controls TGF-beta activation. Nat Genet. 2003;33:331–332. doi: 10.1038/ng0303-331. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC, Costa RH. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by hetero-dimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- Kawahira H, Ma NH, Tzanakakis ES, McMahon AP, Chuang PT, Hebrok M. Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development. 2003;130:4871–4879. doi: 10.1242/dev.00653. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156(4):1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PJ, Roberts GC, Boyd CA. Identification and properties of pathways for K+ transport in guinea-pig and rat alveolar epithelial type II cells. J Physiol. 1994;476:79–88. [PMC free article] [PubMed] [Google Scholar]
- Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–848. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho AT, Bhattacharya S, Mecham BH, Hong J, Kohane IS, Mariani TJ. Expression profiles of the mouse lung identify a molecular signature of time-to-birth. Am J Repir Cell Mol Biol. 2009;40:47–57. doi: 10.1165/rcmb.2008-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi W, Arai H, Ishida A, Takahashi Y, Takada G. Distal pulmonary cell proliferation is associated with the expression of eiiia+ fibronectin in thedeveloping rat lung. Exp Lung Res. 2003;29:135–147. doi: 10.1080/01902140303774. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ward JM, Minoo P. Thyroid-specific enhancer-binding protein/thyroid transcription factor 1 is not required for the initial specificationof the thyroid and lung primordia. Biochimie. 1999;81:321–327. doi: 10.1016/s0300-9084(99)80077-7. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol. 1994;166:112–122. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin –> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111(5):673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suerhiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra-and periocular mesoderm and right pulmonary isomerism. Development. 1999;126(24):5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin gamma3 chain: A novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koli K, Myllarniemi M, Vuorinen K, Salmenkivi K, Ryynanen MJ, Kinnula VL, Keski-Oja J. Bone morphogenetic protein-4 inhibitor gremlin is over-expressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169:61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Shibuya H, Takeda N, Ninomiya-Tsuji J, Yasui T, Miyado K, Sekimoto T, Ueno N, Matsumoto K, Yamada G. Targeted disruption of the tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev. 2002;119:239–249. doi: 10.1016/s0925-4773(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Swantz RJ, Wert SE, McCarty JM, Kerlakian CB, Glasser SW, Whitsett JA. Respiratory epithelial cell expression of human transforming growth factor-alpha induces lung fibrosis in transgenic mice. J Clin Invest. 1994;93:1691–1699. doi: 10.1172/JCI117152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: A common antagonist of FGF and EGF signaling pathways in drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial rolein kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Idenfication of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lallemand AV, Ruocco SM, Joly PM, Gaillard DA. In vivo localization of the insulin-like growth factors I and II (IGF I and IGF II) gene expression during human lung development. Int J Dev Biol. 1995;39:529–537. [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JAIII, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- Larrivee B, Karsan A. Signaling pathways induced by vascular endothelial growth factor. Int J Mol Med. 2000;5:447–456. doi: 10.3892/ijmm.5.5.447. [DOI] [PubMed] [Google Scholar]
- LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS. PDGF-D, a new protease-activated growth factor. Nat Cell Biol. 2001;3:517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probably regulatory role in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- Lazzaro D, Price M, De Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, Hardie WD, Fagan K, Whitsett JA, Korfhagen TR. Disrupted pulmonary vascular development and pulmonary hypertension in transgenic mice overexpressing transforming growth factor-a. Am J Physiol Lung Cell Mol Physiol. 2003;258:L1046–L1054. doi: 10.1152/ajplung.00045.2003. [DOI] [PubMed] [Google Scholar]
- Lecart C, Cayabyab R, Buckley S, Morrison J, Kwong KY, Warburton D, Ramanathan R, Jones CA, Minoo P. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol Neonate. 2000;77:217–223. doi: 10.1159/000014219. [DOI] [PubMed] [Google Scholar]
- Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Reddy R, Barsky L, Weinberg K, Driscoll B. Contribution of proliferation and DNA damage repair to alveolar epithelial type 2 cell recovery from hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L685–L694. doi: 10.1152/ajplung.00020.2005. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li A, Li M, Xing Y, Chen H, Hu L, Tiozzo C, Anderson S, Taketo MM, Minoo P. Stabilized beta-catenin in lung epithelial cells changes cell fate and leads to tracheal and bronchial polyposis. Dev Biol. 2009;334:97–108. doi: 10.1016/j.ydbio.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, DeLanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287(1):86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Lim L, Kalinichenko VV, Whitsett JA, Costa RH. Fusion of lung lobes and vessels in mouse embryos heterozygous for the forkhead box f1 targeted allele. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1012–L1022. doi: 10.1152/ajplung.00371.2001. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Lin SY, Chen JC, Hotaling AJ, Holinger LD. Congenital tracheal cartilaginous sleeve. Laryngoscope. 1995;105:1213–1219. doi: 10.1288/00005537-199511000-00014. [DOI] [PubMed] [Google Scholar]
- Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277(4519–4525):22. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc. 2008;5:682–688. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hogan BL. Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns. 2002;2:229–233. doi: 10.1016/s1567-133x(02)00057-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Lordan JL, Bucchieri F, Richter A, Konstantinidis A, Holloway JW, Thornber M, Puddicombe SM, Buchanan D, Wilson SJ, Djukanovic R, Holgate ST, Davies DE. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol. 2002;169:407–414. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodalfunction in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Lu MM, Li S, Yang H, Morrisey EE. Foxp4: A novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Mech Dev. 2002;119(Suppl 1):S197–S202. doi: 10.1016/s0925-4773(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Lu MM, Yang H, Zhang L, Shu W, Blair DG, Morrisey EE. The bone morphogenic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev Dyn. 2001;222:667–680. doi: 10.1002/dvdy.1231. [DOI] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microrna mir-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkin SR, Murray JD. A mechanism for early branching in lung morphogenesis. J Med Entomol. 1995;34(1):77–94. doi: 10.1007/BF00180137. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa JS. Matrix-driven pneumocyte differentiation. Am Rev Respir Dis. 1991;144(2):452–457. doi: 10.1164/ajrccm/144.2.452. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Maitre B, Clement A, Williams MC, Brody JS. Expression of insulin-like growth factor receptors 1 and 2 in the developing lung and their relation to epithelial cell differentiation. Am J Respir Cell Mol Biol. 1995;13:262–270. doi: 10.1165/ajrcmb.13.3.7654382. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Mandelbrot B. The Fractal Geometry of Nature. Freeman; San Francisco: 1982. [Google Scholar]
- Maquet E, Costagliola S, Parma J, Christophe-Hobertus C, Oligny LL, Fournet JC, Robitaille Y, Vuissoz JM, Payot A, Laberge S, Vassart G, Van Vliet G, Deladoey J. Lethal respiratory failure and mild primary hypothyroidism in a term girl with a de novo heterozygous mutation in the TITF1/NKX2.1 gene. J Clin Endocrinol Metab. 2009;94:197–203. doi: 10.1210/jc.2008-1402. [DOI] [PubMed] [Google Scholar]
- Marcil A, Dumontier E, Chamberland M, Camper SA, Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130(1):45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- Mayer U, Mann K, Timpl R, Murphy G. Sites of nidogen cleavage by proteases involved in tissue homeostasis and remodelling. Eur J Biochem. 1993;217:877–884. doi: 10.1111/j.1432-1033.1993.tb18316.x. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- McCray PB, Jr, Bettencourt JD, Bastacky J, Denning GM, Welsh MJ. Expression of CFTR and a cAMP-stimulated chloride secretory current in cultured human fetal alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993;9(6):578–585. doi: 10.1165/ajrcmb/9.6.578. [DOI] [PubMed] [Google Scholar]
- McDowell EM, Newkirk C, Coleman B. Development of hamster tracheal epithelium: II. Cell proliferation in the fetus. Anat Rec. 1985;213:448–456. doi: 10.1002/ar.1092130310. [DOI] [PubMed] [Google Scholar]
- McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Poussart Y, Koishi K. Development of skeletal muscles in transforming growth factor-beta 1 (TGF-beta1) null-mutant mice. Dev Dyn. 2000;217:250–256. doi: 10.1002/(SICI)1097-0177(200003)217:3<250::AID-DVDY3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McNamara VM, Crabbe DC. Tracheomalacia. Paediatr Respir Rev. 2004;5:147–154. doi: 10.1016/j.prrv.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. Lefty-1 is required for left-rightdetermination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453(7196):745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymalfeedback signaling controlling metanephric. Development. 2004;131:3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Warburton D, Bu D, Zhao JS, Berger JE, Minoo P, Koivisto T, Allen L, Dobbs L, Werb Z, Derynck R. Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev Biol. 1997;186:224–236. doi: 10.1006/dbio.1997.8593. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Miller AA, Hooper SB, Harding R. Role of fetal breathing movements in control of fetal lung distension. J Appl Physiol. 1993;75(6):2711–2717. doi: 10.1152/jappl.1993.75.6.2711. [DOI] [PubMed] [Google Scholar]
- Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- Millien G, Beane J, Lenburg M, Tsao PN, Lu JN, Spira A, Ramirez MI. Characterization of the mid-foregut transcriptome identifies genes regulated during lung bud induction. Gene Expr Patterns. 2008;8(2):124–139. doi: 10.1016/j.modgep.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: Expression, developmental transitions, and chromosomal locations of alpha1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–28526. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Minoo P, King RJ. Epithelial-mesenchymal interactions in lung development. Annu Rev Physiol. 1994;56:13–45. doi: 10.1146/annurev.ph.56.030194.000305. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Moens CB, Auerbach AB, Conlon RA, Joyner AL, Rossant J. A targeted mutation reveals a role for N-myc in branchingmorphogenesis in the embryonic mouse lung. Genes Dev. 1992;6:691–704. doi: 10.1101/gad.6.5.691. [DOI] [PubMed] [Google Scholar]
- Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270–1277. doi: 10.1172/JCI114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induced apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L23–L35. doi: 10.1152/ajplung.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232(2):268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L971–L979. doi: 10.1152/ajplung.00172.2005. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278(41):40231– 40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA. Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol. 1999;20:181–188. doi: 10.1165/ajrcmb.20.2.3381. [DOI] [PubMed] [Google Scholar]
- Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: A developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol. 1995;12(6):597–604. doi: 10.1165/ajrcmb.12.6.7766424. [DOI] [PubMed] [Google Scholar]
- Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-medicated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol. 2002;246:231–244. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, McCaffery P, Drager UC, Chambon P, Dolle P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. Fgf10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol. 2008;8:2. doi: 10.1186/1471-213X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activinreceptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development. 1998;125:1315–1324. doi: 10.1242/dev.125.7.1315. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132(6):1363–1374. doi: 10.1242/dev.01678. dev.01678 [pii] [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3(3):11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Walters DV, MWS Developmental regulation of lung liquid transport. Annu Rev Physiol. 2004;66:77–101. doi: 10.1146/annurev.physiol.66.071702.145229. [DOI] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. J Physiol. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974;241(2):327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis K, Luks FI, De Paepe ME, Piasecki GJ, Wesselhoeft CW., Jr Fetal lung growth after tracheal ligation is not solely a pressure phenomenon. J Pediatr Surg. 1997;32:347–351. doi: 10.1016/s0022-3468(97)90208-6. [DOI] [PubMed] [Google Scholar]
- Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2009 doi: 10.1002/dvdy.22192. dvdy.22192 [pii] [DOI] [PubMed] [Google Scholar]
- Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol. 1998;201:125–134. doi: 10.1006/dbio.1998.8994. [DOI] [PubMed] [Google Scholar]
- Pauling MH, Vu TH. Mechanisms and regulation of lung vascular development. Curr Top Dev Biol. 2004;64:73–99. doi: 10.1016/S0070-2153(04)64005-1. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Johnson MD, Perkett EA, Gold LI, Moses HL. Expression of transforming growth factor-beta 1, -beta 2, and -beta 3 mRNA and protein in the murine lung. Am J Respir Cell Mol Biol. 1991a;5:522–530. doi: 10.1165/ajrcmb/5.6.522. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: Expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991b;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol. 2005a;33:455–462. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after sox9 inactivation in the respiratory epithelium. Genesis. 2005b;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Perl AK, Hokuto I, Impagnatiello MA, Christofori G, Whitsett JA. Temporal effects of Sprouty on lung morphogenesis. Dev Biol. 2003;258:154–168. doi: 10.1016/s0012-1606(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lim L, Ye H, Zhou H, Overdier DG, Costa RH. The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech Dev. 1997;69:53–69. doi: 10.1016/s0925-4773(97)00153-6. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Fernandez-Moreno C, Vicario-Abejon C, Testillano PS, Patterson PH, de Pablo F. Developmental cooperation of leukemia inhibitory factor and insulin-like growth factor I in mice is tissue-specific and essential for lung maturation involving the transcription factors Sp3 and TTF-1. Mech Dev. 2003;120:349–361. doi: 10.1016/s0925-4773(02)00449-5. [DOI] [PubMed] [Google Scholar]
- Pierce RA, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Sanes JR, Miner JH, Senior RM. Expression of laminin alpha3, alpha4, and alpha5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol. 1998;19:237–244. doi: 10.1165/ajrcmb.19.2.3087. [DOI] [PubMed] [Google Scholar]
- Plantier L, Marchand-Adam S, Antico VG, Boyer L, De Coster C, Marchal J, Bachoual R, Mailleux A, Boczkowski J, Crestani B. Keratinocyte growth factor protects against elastase-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1230–L1239. doi: 10.1152/ajplung.00460.2006. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Nishio SJ, Alley JL, Kass P, Hyde DM. The role of the nonciliated bronchiolar epithelial (Clara) cell as the progenitor cell during bronchiolar epithelial differentiation in the perinatal rabbit lung. Am J Respir Cell Mol Biol. 1992;7:606–613. doi: 10.1165/ajrcmb/7.6.606. [DOI] [PubMed] [Google Scholar]
- Post LC, Ternet M, Hogan BL. Notch/delta expression in the developing mouse lung. Mech Dev. 2000;98:95–98. doi: 10.1016/s0925-4773(00)00432-9. [DOI] [PubMed] [Google Scholar]
- Post M, Souza P, Liu J, Tseu I, Wang J, Kuliszewski M, Tanswell AK. Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development. 1996;122:3107–3115. doi: 10.1242/dev.122.10.3107. [DOI] [PubMed] [Google Scholar]
- Pourquie O. The segmentation clock: Converting embryonic time into spatial pattern. Science. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Qian X, Costa RH. Analysis of hepatocyte nuclear factor-3 beta protein domains required for transcriptional activation and nuclear targeting. Nucleic Acids Res. 1995;23:1184–1191. doi: 10.1093/nar/23.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci USA. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: Current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Raaberg L, Nexo E, Buckley S, Luo W, Snead ML, Warburton D. Epidermal growth factor transcription, translation, and signal transduction by rat type II pneumocytes in culture. Am J Respir Cell Mol Biol. 1992;6:44–49. doi: 10.1165/ajrcmb/6.1.44. [DOI] [PubMed] [Google Scholar]
- Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, DeLanghe S, Parsa S, Kelly LK. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307(2):237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Rishi AK, Cao YX, Williams MC. TGT3, thyroid transcription factor I, and Sp1 elements regulate transcriptional activity of the 1.3-kilo-base pair promoter of T1alpha, a lung alveolar type I cell gene. J Biol Chem. 1997;272:26285–26294. doi: 10.1074/jbc.272.42.26285. [DOI] [PubMed] [Google Scholar]
- Rawlins EL. Lung Epithelial Progenitor Cells Lessons from development. Proc Am Thorac Soc. 2008;5:675–681. doi: 10.1513/pats.200801-006AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009a;136(22):3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cellsin the long-term maintenance and repair of lung airway, but not alveolar epithelium. Cell Stem Cell. 2009b;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: Contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci USA. 2003;100:6098–6103. doi: 10.1073/pnas.1031851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L658–L667. doi: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mann K, Nischt R, Fox JW, Chu ML, Krieg T, Timpl R. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J Biol Chem. 1993;268:10881–10887. [PubMed] [Google Scholar]
- Retsch-Bogart GZ, Moats-Staats BM, Howard K, D’Ercole AJ, Stiles AD. Cellular localization of messenger RNAs for insulin-like growth factors (IGFs), their receptors and binding proteins during fetal rat lung development. Am J Respir Cell Mol Biol. 1996;14:61–69. doi: 10.1165/ajrcmb.14.1.8534487. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: Selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1256–L1265. doi: 10.1152/ajplung.00203.2004. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA. 1995;92(1):131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321(1):141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Roman J. Fibronectin and fibronectin receptors in lung development. Exp Lung Res. 1997;23:147–159. doi: 10.3109/01902149709074027. [DOI] [PubMed] [Google Scholar]
- Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC, O’Reilly MA. In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1045–L1054. doi: 10.1152/ajplung.00376.2003. [DOI] [PubMed] [Google Scholar]
- Rubin LP, Kovacs CS, De Paepe ME, Tsai SW, Torday JS, Kronenberg HM. Arrested pulmonary alveolar cytodifferentiation and defective surfactant synthesis in mice missing the gene for parathyroid hormone-related protein. Dev Dyn. 2004;230:278–289. doi: 10.1002/dvdy.20058. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sakiyama J, Yamagishi A, Kuroiwa A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development. 2003;130:1225–1234. doi: 10.1242/dev.00345. [DOI] [PubMed] [Google Scholar]
- Samadani U, Porcella A, Pani L, Johnson PF, Burch JB, Pine R, Costa RH. Cytokine regulation of the liver transcription factor hepatocyte nuclear factor-3 beta is mediated by the C/EBP family and interferon regulatory factor 1. Cell Growth Differ. 1995;6:879–890. [PubMed] [Google Scholar]
- Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP. Mechanical stretch induces fetal type II cell differentiation via an EGFR-ERK signaling pathway. Am J Respir Cell Mol Biol. 2003;30(1):76–83. doi: 10.1165/rcmb.2003-0121OC. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittny JC, Miseroccandi G, Sparrow MP. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol. 2000;23(1):11–18. doi: 10.1165/ajrcmb.23.1.3926. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Djonov V, Fine A, Burri PH. Programmed cell death contributes to postnatal lung development. Am J Respir Cell Mol Biol. 1998;18:786–793. doi: 10.1165/ajrcmb.18.6.3031. [DOI] [PubMed] [Google Scholar]
- Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. Differential expression of TGF beta 1, beta 2 and beta 3 genes during mouse embryogenesis. Development. 1991;111:117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- Schuger L, Johnson GR, Gilbride K, Plowman GD, Mandel R. Amphiregulin in lung branching morphogenesis: Interaction with heparan sulfate proteoglycan modulates cell proliferation. Development. 1996a;122:1759–1767. doi: 10.1242/dev.122.6.1759. [DOI] [PubMed] [Google Scholar]
- Schuger L, Skubitz AP, Gilbride K, Mandel R, He L. Laminin and heparan sulfate proteoglycan mediate epithelial cell polarization in organotypic cultures of embryonic lung cells: Evidence implicating involvement of the inner globular region of laminin beta 1 chain and the heparan sulfate groups of heparan sulfate proteoglycan. Dev Biol. 1996b;179:264–273. doi: 10.1006/dbio.1996.0256. [DOI] [PubMed] [Google Scholar]
- Schuger L, Skubitz AP, de las MA, Gilbride K. Two separate domains of laminin promote lung organogenesis by different mechanisms of action. Dev Biol. 1995;169:520–532. doi: 10.1006/dbio.1995.1166. [DOI] [PubMed] [Google Scholar]
- Schuger L, Varani J, Killen PD, Skubitz AP, Gilbride K. Laminin expression in the mouse lung increases with development and stimulates spontaneous organotypic rearrangement of mixed lung cells. Dev Dyn. 1992;195:43–54. doi: 10.1002/aja.1001950105. [DOI] [PubMed] [Google Scholar]
- Schuller AG, van Neck JW, Beukenholdt RW, Zwarthoff EC, Drop SL. IGF, type I IGF receptor and IGF-binding protein mRNA expression in the developing mouse lung. J Muscoskel Pain. 1995;14:349–355. doi: 10.1677/jme.0.0140349. [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L288–L296. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Pierce RA. Entactin expression by rat lung and rat alveolar epithelial cells. Am J Resp Cell Mol Biol. 1996;14(3):239–247. doi: 10.1165/ajrcmb.14.3.8845174. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132(1):35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Seth R, Shum L, Wu F, Wuenschell C, Hall FL, Slavkin HC, Warburton D. Role of epidermal growth factor expression in early mouse embryo lung branching morphogenesis in culture: Antisense oligodeoxynucleotide inhibitory strategy. Dev Biol. 1993;158(2):555–559. doi: 10.1006/dbio.1993.1213. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. Sox9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104(6):1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Subramaniam M, Emanuel RL, Degan S, Johnston P, Tefft D, Warburton D, Sunday ME. Centrifugal migration of mesenchymal cells in embryonic lung. Dev Dyn. 2008;237:750–757. doi: 10.1002/dvdy.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JM, McCormick-Shannon K, Burhans MS, Shangguan X, Srivastava K, Hyatt BA. Chondroitin sulfate proteoglycans are required for lung growth and morphogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2003;285(6):L1323–L1336. doi: 10.1152/ajplung.00226.2003. [DOI] [PubMed] [Google Scholar]
- Shaw-White JR, Bruno MD, Whitsett JA. GATA-6 activates transcription of thyroid transcription factor-1. J Biol Chem. 1999;274:2658–2664. doi: 10.1074/jbc.274.5.2658. [DOI] [PubMed] [Google Scholar]
- Shi W, Warburton D. Is COPD in adulthood really so far removed from early development? Eur Respir J. 2010;35:12–13. doi: 10.1183/09031936.00145809. [DOI] [PubMed] [Google Scholar]
- Shi W, Xu J, Warburton D. Development, repair, and fibrosis: what is common and why it matters. Respirology. 2009;14:656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;2:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen H, Sun J, Buckley S, Zhao J, Anderson KD, Williams RG, Warburton D. TACE is required for fetal murine cardiac development and modeling. Dev Biol. 2003;261:371–380. doi: 10.1016/s0012-1606(03)00315-4. [DOI] [PubMed] [Google Scholar]
- Shi W, Zhao J, Anderson KD, Warburton D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1030–L1039. doi: 10.1152/ajplung.2001.280.5.L1030. [DOI] [PubMed] [Google Scholar]
- Shi W, Heisterkamp N, Groffen J, Zhao J, Warburton D, Kaartinen V. TGF-beta3-null mutation does not abrogate fetal lung maturation in vivo by glucocorticoids. Am J Physiol. 1999;277(6 Pt 1):L1205–L1213. doi: 10.1152/ajplung.1999.277.6.L1205. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shiels H, Li X, Schumacker PT, Maltepe E, Padrid PA, Sperling A, Thompson CB, Lindsten T. TRAF4 deficiency leads to tracheal malformation with resulting alterations in air 3ow to the lungs. Am J Pathol. 2000;157:679–688. doi: 10.1016/S0002-9440(10)64578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama N, Lutz W, Kretzschmar R, Sauter N, Roth JF, Marino S, Wittwer J, Scheidweiler A, Eckner R. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J. 2003;22:5175–5185. doi: 10.1093/emboj/cdg502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbrot E, Peters KG, Williams LT. Expression of the platelet-derived growth factor beta receptor during organogenesis and tissue differentiation in the mouse embryo. Dev Dyn. 1994;199:169–175. doi: 10.1002/aja.1001990302. [DOI] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283(1):226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector−mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NP, Jesudason EC, Featherstone NC, Corbett HJ, Losty PD. Recent advances in congenital diaphragmatic hernia. Arch Dis Child. 2005;90(4):426–428. doi: 10.1136/adc.2003.045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NP, Losty PD, Connell MG, Mayer U, Jesudason EC. Abnormal lung development precedes oligohydramnios in a transgenic murine model of renal dysgenesis. J Urol. 2006;175(2):783–786. doi: 10.1016/S0022-5347(05)00169-2. [DOI] [PubMed] [Google Scholar]
- Sock E, Rettig SD, Enderich J, Bosl MR, Tamm ER, Wegner M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol. 2004;24:6635–6644. doi: 10.1128/MCB.24.15.6635-6644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza P, Tanswell AK, Post M. Different roles for PDGF-alpha and -beta receptors in embryonic lung development. Am J Respir Cell Mol Biol. 1996;15:551–562. doi: 10.1165/ajrcmb.15.4.8879189. [DOI] [PubMed] [Google Scholar]
- Souza P, O’Brodovich H, Post M. Lung fluid restriction affects growth but not airway branching of embryonic rat lung. Int J Dev Biol. 1995a;39(4):629–637. [PubMed] [Google Scholar]
- Souza P, Kuliszewski M, Wang J, Tseu I, Tanswell AK, Post M. PDGF-AA and its receptor influence early lung branching via an epithelial-mesenchymal interaction. Development. 1995b;121:2559–2567. doi: 10.1242/dev.121.8.2559. [DOI] [PubMed] [Google Scholar]
- Souza P, Sedlackova L, Kuliszewski M, Wang J, Liu J, Tseu I, Liu M, Tanswell AK, Post M. Antisense oligodeoxynucleotides targeting PDGF-B mRNA inhibit cell proliferation during embryonic rat lung development. Development. 1994;120:2163–2173. doi: 10.1242/dev.120.8.2163. [DOI] [PubMed] [Google Scholar]
- Spilde TL, Bhatia AM, Mehta S, Ostlie DJ, Hembree MJ, Preuett BL, Prasadan K, Li Z, Snyder CL, Gittes GK. Defective sonic hedgehog signaling in esophageal atresia with tracheoesophageal fistula. Surgery. 2003;134:345–350. doi: 10.1067/msy.2003.243. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Strange J, Awonusonu F, Bruce MC. Insulin-like growth factor I receptor is downregulated after alveolarization in an apoptotic fibroblast subset. Am J Physiol Lung Cell Mol Physiol. 2002;282:L457–L467. doi: 10.1152/ajplung.00050.2001. [DOI] [PubMed] [Google Scholar]
- Starrett RW, de Lorimier AA. Congenital diaphragmatic hernia in lambs: Hemodynamic and ventilatory changes with breathing. J Pediatr Surg. 1975;10(5):575–582. doi: 10.1016/0022-3468(75)90359-0. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM. The transcription factor gene N3b is essential for both lung maturation and brain development. Mol Cell Biol. 2005;25:685–698. doi: 10.1128/MCB.25.2.685-698.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: Endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm van’s Gravesande K, Omran H. Primary ciliary dykskinesia: clinical presentation, diagnosis and genetics. Ann Med. 2005;37:439–449. doi: 10.1080/07853890510011985. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Iyama KI, Kimura T, Sano K, Darlington GJ, Akiba T, Takiguchi M. Mice lacking CCAAt/enhancer-binding protein-alphashow hyperproliferation of alveolar type II cells and increased surfactant protein mRNAs. Cell Tissue Res. 2001;306:57–63. doi: 10.1007/s004410100420. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172(3):571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. Branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ikeda T. Transcripts for two members of the transforming growth factor-beta superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev Dyn. 1996;207:439–449. doi: 10.1002/(SICI)1097-0177(199612)207:4<439::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tefft D, De Langhe SP, Del Moral PM, Sala F, Shi W, Bellusci S, Warburton D. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev Biol. 2005;282:422–431. doi: 10.1016/j.ydbio.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Tefft D, Lee M, Smith S, Crowe DL, Bellusci S, Warburton D. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am J Physiol Lung Cell Mol Physiol. 2002;283:L700–L706. doi: 10.1152/ajplung.00372.2001. [DOI] [PubMed] [Google Scholar]
- Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- Thom J, Perks AM. The effects of furosemide and bumetanide on lung liquid production by in vitro lungs from fetal guinea pigs. Can J Physiol Pharmacol. 1990;68(8):1131–1135. doi: 10.1139/y90-169. [DOI] [PubMed] [Google Scholar]
- Thurlbeck W. Postpneumonectomy compensatory lung growth. Am Rev Respir Dis. 1983;128(6):965–967. doi: 10.1164/arrd.1983.128.6.965. [DOI] [PubMed] [Google Scholar]
- Tiozzo C, De Langhe S, Carraro G, Alam DA, Nagy A, Wigfall C, Hajihosseini MK, Warburton D, Minoo P, Bellusci S. Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of apert syndrome. Pediatr Res. 2009;66:386–390. doi: 10.1203/PDR.0b013e3181b45580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, Ciliated, and Goblet cells. PLoS One. 2009;4(12):e1932. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L405–L410. doi: 10.1152/ajplung.2002.282.3.L405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L130–L135. doi: 10.1152/ajplung.00380.2001. [DOI] [PubMed] [Google Scholar]
- Torday JS, Torday DP, Gutnick J, Qin J, Rehan V. Biologic role of fetal lung fibroblast triglycerides as antioxidants. Pediatr Res. 2001;49(6):843–849. doi: 10.1203/00006450-200106000-00021. [DOI] [PubMed] [Google Scholar]
- Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: An immunohistochemical study. Pediatr Pulmonol. 1997;24:22–28. doi: 10.1002/(sici)1099-0496(199707)24:1<22::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136(13):2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unbekandt M, del Moral PM, Sala FG, Bellusci S, Warburton D, Fleury V. Tracheal occlusion increases the rate of epithelial branching of embryonic mouse lunch via the FGF10-FGFR2b-Sprouty2 pathway. Mech Dev. 2008;120(3–4):314–324. doi: 10.1016/j.mod.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven SM, Gundersen TE, Weedon MS, Landaas VO, Sakhi AK, Fromm SH, Geronimo BA, Moskaug JO, Blomhoff R. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: Important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev Biol. 2000;220:379–391. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- Urase K, Mukasa T, Igarashi H, Ishii Y, Yasugi S, Momoi MY, Momoi T. Spatial expression of Sonic hedgehog in the lung epithelium during branching morphogenesis. Biochem Biophys Res Commun. 1996;225:161–166. doi: 10.1006/bbrc.1996.1147. [DOI] [PubMed] [Google Scholar]
- Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- Usui H, Shibayama M, Ohbayashi N, Konishi M, Takada S, Itoh N. Fgf18 is required for embryonic lung alveolar development. Biochem Biophys Res Commun. 2004;322:887–892. doi: 10.1016/j.bbrc.2004.07.198. [DOI] [PubMed] [Google Scholar]
- Vaccaro C, Brody JS. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec. 1978;192(4):467–479. doi: 10.1002/ar.1091920402. [DOI] [PubMed] [Google Scholar]
- van Tuyl M, Post M. From fruitflies to mammals: Mechanisms of signalling via the Sonic hedgehog pathway in lung development. Respir Res. 2000;1:30–35. doi: 10.1186/rr9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia AM, Beharry KD, Ang JG, Devarajan K, Van Woerkom R, Abrantes M, Nishihara K, Chang E, Waltzman J, Modanlou HD. Early postnatal dexamethasone influences matrix metalloproteinase-2 and -9, and heir tissue inhibitors in the developing rat lung. Pediatr Pulmonol. 2003;35:456–462. doi: 10.1002/ppul.10293. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Tenbaum S, Perdiguero E, Hth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–295. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Martin A, Vosatka RJ, Mazzoni CL, Nielsen HC. Hoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fate. Histochem Cell Biol. 1997;108:495–504. doi: 10.1007/s004180050190. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho R, Nissinen M, Sainio K, Byers M, Eddy R, Hirvonen H, Shows TB, Sariola H, Engvall E, Tryggvason K. Human laminin M chain (merosin): Complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124:381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H, Connell M, Losty P, Jesudason E, Southern KW. Embryonic lung growth is normal in a cftr-knockout mouse model. Exp Lung Res. 2008;34(10):717–727. doi: 10.1080/01902140802389719. [DOI] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- Wang C, Chang KC, Somers G, Virshup D, Ang BT, Tang C, Yu F, Wang H. Protein phosphatase 2A regulates self-renewal of Drosophila neural stem cells. Development. 2009;136(13):2287–2296. doi: 10.1242/dev.035758. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25(12):5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani MA, Wert SE, Lingrel JB. Lung Kruppel-like factor, a zinc 3nger transcription factor, is essential for normal lung development. J Biol Chem. 1999;274:21180– 21185. doi: 10.1074/jbc.274.30.21180. [DOI] [PubMed] [Google Scholar]
- Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5(6):703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92(1):55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Warburton D, Tefft D, Mailleux A, Bellusci S, Thiery JP, Zhao J, Buckley S, Shi W, Driscoll B. Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med. 2001;164(10 Pt 2):S59–S62. doi: 10.1164/ajrccm.164.supplement_2.2106064. [DOI] [PubMed] [Google Scholar]
- Warburton D, Olver BE. Coordination of genetic, epigenetic, and environmental factors in lung development, injury, and repair. Chest. 1997;111(6S):119S–122S. doi: 10.1378/chest.111.6_supplement.119s. [DOI] [PubMed] [Google Scholar]
- Warburton D, Seth R, Shum L, Horcher PG, Hall FL, Werb Z, Slavkin HC. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev Biol. 1992;149:123–133. doi: 10.1016/0012-1606(92)90269-m. [DOI] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Wert SE, Dey CR, Blair PA, Kimura S, Whitsett JA. Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Dev Biol. 2002;242:75–87. doi: 10.1006/dbio.2001.0540. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Clark JC, Picard L, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT. Fibroblast growth factor 18 influences proximal programming during lung morphogenesis. J Biol Chem. 2002;277:22743–22749. doi: 10.1074/jbc.M202253200. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Glasser SW. Regulation of surfactant protein gene transcription. Biochem Biophys Acta. 1998;1408:303–311. doi: 10.1016/s0925-4439(98)00076-3. [DOI] [PubMed] [Google Scholar]
- Wilson J, DiFiore J, Peters C. Experimental fetal tracheal ligation prevents the pulmonary hypoplasia associated with fetal nephrectomy: Possible application for congenital diaphragmatic hernia. J Pediatr Surg. 1993;28(11):1433–1439. doi: 10.1016/0022-3468(93)90426-l. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wongtrakool C, Malpel S, Gorenstein J, Sedita J, Ramirez MI, Underhill TM, Cardoso WV. Downregulation of retinoic acid receptor alpha signaling is required for sacculation and type 1 cell formation in the developing lung. J Biol Chem. 2003;278(47):46911–46918. doi: 10.1074/jbc.M307977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z, Zhang X, Rao Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Migliazza L, Diez-Pardo JA, Tovar JA. The tracheobronchial tree is abnormal in experimental congenital diaphragmatic hernia. Pediatr Surg Int. 1999;15(3–4):184–187. doi: 10.1007/s003830050550. [DOI] [PubMed] [Google Scholar]
- Xian J, Clark KJ, Fordham R, Pannell R, Rabbitts TH, Rabbitts PH. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt1/Robo1 gene. Proc Natl Acad Sci USA. 2001;98:15062–15066. doi: 10.1073/pnas.251407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Nieuwenhuis E, Cohen BL, Wang W, Canty AJ, Danska JS, Coultas L, Rossant J, Wu MY, Piscione TD, Nagy A, Gossler A, Hicks GG, Hui CC, Henkelman RM, Yu LX, Sled JG, Gridley T, Egan SE. Lunatic Fringe-mediated Notch signaling is required for lung alveogenesis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L45–L56. doi: 10.1152/ajplung.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y. Asymmetric stem cell division and pathology: insights from Drosophila stem cell systems. J Pathol. 2009;217:181–185. doi: 10.1002/path.2470. [DOI] [PubMed] [Google Scholar]
- Yan C, Sever Z, Whitsett JA. Upstream enhancer activity in the human surfactant protein B gene is mediated by thyroid transcription factor 1. J Biol Chem. 1995;270:24852–24857. doi: 10.1074/jbc.270.42.24852. [DOI] [PubMed] [Google Scholar]
- Yang Y, Iwanaga K, Raso MG, Wislez M, Hanna AE, Wieder ED, Molidrem JJ, Wistuba II, Powis G, Demayo FJ, Kim CF, Kurie JM. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS One. 2008;3:e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. b-Catenin is not necessary or maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol. 2009;41(5):535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn. 2001;221:289–301. doi: 10.1002/dvdy.1140. [DOI] [PubMed] [Google Scholar]
- Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Zhang L, Whitsett JA, Stripp BR. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim Biophys Acta. 1997;1350:359–367. doi: 10.1016/s0167-4781(96)00180-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40(7):862–870. doi: 10.1038/ng.157. ng.157 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin−induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585– L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Chen H, Peschon JJ, Shi W, Zhang Y, Frank SJ, Warburton D. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev Biol. 2001;232:204–218. doi: 10.1006/dbio.2001.0176. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sime PJ, Bringas P, Jr, Tefft JD, Buckley S, Bu D, Gauldie J, Warburton D. Spatial-specific TGF-beta1 adenoviral expression determines morphogenetic phenotypes in embryonic mouse lung. Eur J Cell Biol. 1999;78:715–725. doi: 10.1016/s0171-9335(99)80040-5. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dey CR, Wert SE, Yan C, Costa RH, Whitsett JA. Hepatocyte nuclear factor-3beta limits cellular diversity in the developing respiratory epithelium and alters lung morphogenesis in vivo. Dev Dyn. 1997;210:305–314. doi: 10.1002/(SICI)1097-0177(199711)210:3<305::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev Biol. 1996a;175:227–238. doi: 10.1006/dbio.1996.0110. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem. 1996b;44:1183–1193. doi: 10.1177/44.10.8813084. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Zhang J, Laboy M, Lasky JA. Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;286(1):L182–L188. doi: 10.1152/ajplung.00083.2003. [DOI] [PubMed] [Google Scholar]