Background: N-Myristoylated GCAP1 controls Ca2+ sensitivity of cGMP synthesis in photoreceptors.
Results: Hydrophobic residues replacing the myristoyl group in GCAP1 restore activation of its target enzyme but not Ca2+ binding.
Conclusion: The fatty chain regulates sensitivity of EF-hand located on the opposite side via intramolecular tug.
Significance: Mechanisms of Ca2+ binding by GCAP1 are critical for normal vision and visual disorders.
Keywords: Calcium-binding Proteins, Neurobiology, Photoreceptors, Phototransduction, Protein-Protein Interactions, Vision
Abstract
Guanylyl cyclase-activating protein 1 (GCAP1), a myristoylated Ca2+ sensor in vision, regulates retinal guanylyl cyclase (RetGC). We show that protein-myristoyl group interactions control Ca2+ sensitivity, apparent affinity for RetGC, and maximal level of cyclase activation. Mutating residues near the myristoyl moiety affected the affinity of Ca2+ binding to EF-hand 4. Inserting Phe residues in the cavity around the myristoyl group increased both the affinity of GCAP1 for RetGC and maximal activation of the cyclase. NMR spectra show that the myristoyl group in the L80F/L176F/V180F mutant remained sequestered inside GCAP1 in both Ca2+-bound and Mg2+-bound states. This mutant displayed much higher affinity for the cyclase but reduced Ca2+ sensitivity of the cyclase regulation. The L176F substitution improved affinity of myristoylated and non-acylated GCAP1 for the cyclase but simultaneously reduced the affinity of Ca2+ binding to EF-hand 4 and Ca2+ sensitivity of the cyclase regulation by acylated GCAP1. The replacement of amino acids near both ends of the myristoyl moiety (Leu80 and Val180) minimally affected regulatory properties of GCAP1. N-Lauryl- and N-myristoyl-GCAP1 activated RetGC in a similar fashion. Thus, protein interactions with the central region of the fatty acyl chain optimize GCAP1 binding to RetGC and maximize activation of the cyclase. We propose a dynamic connection (or “tug”) between the fatty acyl group and EF-hand 4 via the C-terminal helix that attenuates the efficiency of RetGC activation in exchange for optimal Ca2+ sensitivity.
Introduction
Neuronal calcium sensor (NCS)2 proteins form a distinct group in the EF-hand superfamily of calcium-binding proteins (1, 2). A typical NCS molecule is formed by two pairs of EF-hand calcium binding domains (conventionally numbered as EF-1 through EF-4 from the N to C terminus) creating two semiglobular structures connected by a flexible hinge region between EF-hands 2 and 3 (2–5) (see Fig. 1). Proteins of this group include S-modulin/recoverin, neurocalcin (6, 7), guanylyl cyclase-activating proteins (GCAPs) (8–11), and a variety of other calcium-binding proteins of which some are highly conserved in eukaryotes, whereas others are predominantly expressed only in neural tissues of higher organisms (1, 2). Proteins of the NCS family typically lack some of the Ca2+-coordinating side chains in the loop of EF-hand 1. In GCAPs, this EF-hand apparently evolved by trading the ability to bind Ca2+ for the ability to recognize a target enzyme (12–14). Most of the NCS proteins undergo fatty acylation at the N terminus (15, 16) widely known as myristoylation. The functional role of the fatty acyl group remains unclear because in different NCS proteins it acts differently, both structurally and functionally. In some (recoverin, neurocalcin, and NCS-1), it contributes to membrane binding (17, 18) via a “calcium-myristoyl switch” (19–21). In response to Ca2+ binding in recoverin, its fatty acylated N terminus is extruded from a hydrophobic pocket formed by EF-hands 1 and 2 (17, 19–21). In contrast, there is equally compelling NMR (22, 23) and x-ray crystallography (24) evidence that the Ca2+-myristoyl switch does not occur in GCAPs.
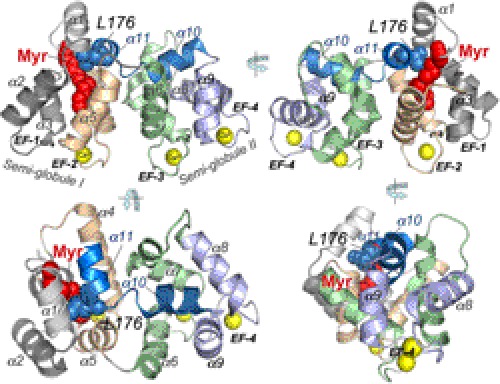
FIGURE 1.
Ca2+GCAP1 model adopted from Stephen et al. (24). The crystal structure depicts the two semiglobular portions of the molecule created by two pairs of EF-hands (EF-1 through EF-4). The α-helical regions are marked as α1 through α11. The yellow spheres represent Ca2+ ions bound in EF-hands. The Myr moiety is shown in red spheres. The cavity embedding the Myr fatty acyl chain is located in semiglobule I formed by the N-terminal EF-hands (EF-1 and EF-2). The main chain exiting as an α-helix (α9) from EF-hand 4 in semiglobule II extends back to semiglobule I via two short α-helical stretches, α10 and α11 (blue ribbons), of which the C-terminal helix, α11 (Asp175–Leu183; numeration by bovine GCAP1 sequence), is in direct contact with the Myr group via Leu176 (blue spheres).
GCAPs 1 and 2 (8, 9) play an essential physiological role in photoreceptor recovery and light adaptation by accelerating the synthesis of cGMP by retinal guanylyl cyclase in response to illumination (25–29). Light-induced activation of the phototransduction cascade in rods and cones depletes cGMP in the outer segment of photoreceptor and shuts down the influx of Na+ and Ca2+ through the cGMP-gated channels (for reviews, see Refs. 30–32). This results in decreased levels of intracellular Ca2+ (33–35), but not Mg2+ (36), that in turns converts GCAPs from their Ca2+-bound state to a Mg2+-bound state (37). The Mg2+-bound form activates RetGC and stimulates cGMP production (for a review, see Ref. 38), which reopens the cGMP-gated channel and enables rods and cones to recover from excitation in a timely manner (29, 39). In the dark, the cGMP hydrolysis is stopped, and the restored influx of Ca2+ through reopened cGMP-gated channels converts GCAPs back to their inhibitory Ca2+-bound state in which they decelerate production of cGMP (40, 41). Different isoforms of GCAPs have variable sensitivity for Ca2+ (41–45) and therefore contribute to the acceleration of cGMP synthesis at different phases of photoresponse, adjusting the shape of the photoresponse to the proper recovery kinetics (44). When Ca2+ sensitivity of GCAP1 is decreased as a result of human disease-related mutations affecting Ca2+ binding in EF-hands 3 and 4 (46, 47), the concentrations of cGMP and free Ca2+ in photoreceptors rise above the normal levels (48, 49) and trigger retinal degeneration in transgenic animal models (48–50).
The myristoyl group in a crystal structure of Ca2+-bound GCAP1 (24) remains constrained inside a hydrophobic cavity formed by the first pair of EF-hands (designated as a “semiglobule I” in Fig. 1). It was documented previously (51, 52) that myristoylation is required for GCAP1 to efficiently activate RetGC despite that the overall thermal stability of a metal-liganded non-acylated GCAP1 does not appear critically compromised (53). It cannot be ruled out that fatty acylation improves stability of at least some regions in GCAP1 by the embedded fatty chain (24), but we found in the present study that the role of myristoylation is much more than possible general stabilization of protein structure. We present evidence that interaction of the myristoyl group with surrounding amino acid side chains adjusts both the Ca2+ binding affinity and the extent of cyclase activation. We reason that the N-myristoyl group functionally connects the two semiglobular parts of GCAP1. This bridging by the myristoyl moiety creates a dynamic tug that provides a trade-off between the maximal achievable activation of RetGC and Ca2+ sensitivity of cyclase regulation in photoreceptors.
MATERIALS AND METHODS
Protein Expression for Biochemical Studies
Myristoylated bovine D6S GCAP1 was produced in BL21(DE3) or BLR(DE3) Escherichia coli strains harboring yeast N-myristoyltransferase and purified to a final purity of ∼95% after Ca2+ precipitation, hydrophobic chromatography on butyl-Sepharose, and gel filtration as described previously (37) except that a high resolution Superose 12 10/30 HR FPLC column (GE Healthcare) was used for the gel filtration step (37). Non-myristoylated GCAP1 was expressed and purified using the same method except for the absence of the N-myristoyltransferase-coding plasmid in the expression system. The protein samples were concentrated to ∼6–10 mg/ml in 10 mm Tris-HCl (pH 7.5) and 30 μm EDTA, aliquoted, and kept frozen at −70 °C before using them in assays. To verify the presence or absence of fatty acylation, the protein samples were desalted in 0.1% trifluoroacetic acid using C4 TopTip minicolumns (Glygen); eluted in 80% acetonitrile, 0.05% trifluoroacetic acid; and analyzed by electrospray ionization/time-of-flight mass spectrometry. The molecular masses of the acylated and non-acylated GCAP1 and its mutants matched within 1-mass unit accuracy the respective calculated average molecular masses. Recombinant RetGC1 was expressed in HEK293 cells transfected using a calcium/phosphate precipitation technique with the pRCCMV plasmid harboring human RetGC1 cDNA, and the membrane fraction containing recombinant cyclase was isolated as described previously (54).
Ca2+/EGTA Buffers
Ca2+/EGTA mixtures were prepared according to the protocol of Tsien and Pozzan (55) and verified with Ca2+ fluorescent indicator dyes as described previously (37). The free metal concentrations in assays containing 2 mm Ca2+/EGTA buffer were calculated using Bound and Determined and MaxChelator software correcting for pH, salt and nucleotide concentrations, and temperature.
Guanylyl Cyclase Assays
RetGC activity was assayed as described previously (43, 54). Briefly, the assay mixture (25 μl), which was incubated at 30 °C, contained 30 mm MOPS/KOH (pH 7.2), 60 mm KCl, 4 mm NaCl, 1 mm DTT, 2 mm Ca2+/EGTA buffer, 1 mm free Mg2+, 0.3 mm ATP, 4 mm cGMP, 10 mm creatine phosphate, 0.5 unit of creatine phosphokinase, 1 mm GTP, 1 μCi of [α-32P]GTP, 0.1 μCi of [8-3H]cGMP (PerkinElmer Life Sciences), and the PDE6 inhibitors zaprinast and dipyridamole. The resultant [32P]cGMP product and the [3H]cGMP internal standard were analyzed by TLC using fluorescently backed polyethyleneimine cellulose plates (Merck) developed in 0.2 m LiCl as described previously (56).
Mutagenesis
Mutations were introduced in bovine GCAP1 cDNA by the “splicing by overlap extension” technique using PCRs catalyzed by high fidelity Phusion Flash polymerase (Finnzymes). The resultant products were ligated into the NcoI/BamHI sites of pET11d (Novagen) vector, sequenced, and transformed into expressing cell lines as described previously in detail (37).
Metal-sensitive Trp Fluorescence
The intrinsic Trp fluorescence of GCAP1 and its mutants was recorded as described previously in detail (37). Briefly, fluorescence emission at 332 nm (excitation at 290 nm) was recorded at 23 °C using ∼4 μm GCAP1 in 0.6 ml of 100 mm MOPS/KOH (pH 7.2), 40 mm KCl, 1 mm EGTA, and the specified concentrations of MgCl2. Small aliquots of concentrated CaCl2 solution were added to obtain the desired free Ca2+ concentrations. The free Ca2+ and Mg2+ concentrations in the solution were calculated according to the method of Brooks and Storey (57) utilizing the algorithm of Marks and Maxfield (58).
Ca2+ Binding Stoichiometry
The stoichiometry of Ca2+ binding to myristoylated and non-acylated GCAP1 and its mutants was determined using a fluorescent Ca2+ indicator dye method previously described in detail (37) with minor modifications. Briefly, each protein sample containing a known amount of GCAP1 was incubated at a known total concentration of CaCl2, and the free Ca2+ concentration ([Ca2+]free) in the sample was then determined using Ca2+ fluorescent indicator dyes (BAPTA-2, BAPTA-6F, and Fluo-4FF, all from Invitrogen/Molecular Probes) to calculate the concentration of Ca2+ bound to the protein. The readings of the fluorescence intensity were corrected for dilution caused by addition of CaCl2. Free Ca2+ in the reaction mixture was calculated using the formula: [Ca2+]free = Kd × (F − Fmin)/(100 − F) where F is the fluorescence intensity of the Ca2+ indicator in the assay mixture expressed as a percentage of the fluorescence of the Ca2+-saturated indicator (recorded at the end of each experiment in 1 mm [Ca2+]free), Fmin is the fluorescence intensity of the Ca2+ indicator in the absence of Ca2+ and also expressed as a percentage of the fluorescence of the Ca2+-saturated indicator, and Kd is a corrected constant of the indicator dye for Ca2+ (37). GCAP1 was diluted from 300–350 μm stock solution to 20–40 μm final concentration in 0.6 ml of 100 mm MOPS/KOH (pH 7.2), 40 mm KCl, 1 mm dithiothreitol, 0 or 1 mm MgCl2, and 0.5 μm appropriate fluorescent Ca2+ indicator dye (BAPTA-2, Fluo-4FF, or BAPTA-6F). The mixture was assembled in a plastic cuvette and titrated at 23 °C with 3-μl aliquots of CaCl2 solution. Because the direct Ca2+ binding isotherm for GCAP1 is not cooperative (37), the fluorescence data were fitted using a simplified saturating hyperbolic function: ([Ca2+]bound/[GCAP1]) = Bmax × [Ca2+]free/([Ca2+]free + Kd) where [Ca2+]bound is the concentration of Ca2+ bound to GCAP1 calculated as [Ca2+]bound = [Ca2+]total − [Ca2+]free, Bmax is the mol of Ca2+ ions bound/mol of GCAP1 at saturation, and Kd is the apparent affinity of GCAP1 for Ca2+. The trace amounts of EDTA introduced into the assay from the stock solutions of the proteins were negligible compared with the protein concentration in the assay. The data shown are representative from independent experiments producing virtually identical results.
NMR Spectroscopy
NMR samples of GCAP1-FFF with a covalently attached 13C-labeled myristoyl group were prepared as described previously (23) in 5 mm Tris-d11-HCl (pH 7.4) buffer containing 5 mm dithiothreitol-d10 and 5 mm MgCl2 (Mg2+-bound) or 5 mm CaCl2 (Ca2+-bound) or 8 m urea (denatured state) in 99.9% D2O. The protein sample concentration was typically 0.3 mm. All NMR spectra were acquired using a Bruker Advance III 800-MHz spectrometer equipped with a triple resonance cryoprobe and z gradient. Spectra were obtained at 310 K with 64 scans per increment. Two-dimensional 1H-13C heteronuclear multiple quantum correlation spectra were recorded with 2048 × 256 points (20, 23). Three-dimensional 13C-edited (F1) and 13C-filtered (F3) heteronuclear single quantum correlation-NOESY spectra (120-ms mixing time) were obtained with 1024 (F3: 1H) × 128 (F2: 13C) × 32 (F1: 1H) points (23, 59, 60).
RESULTS
Stoichiometry of Ca2+ Binding by GCAP1 Does Not Depend on Its N-Fatty Acylation
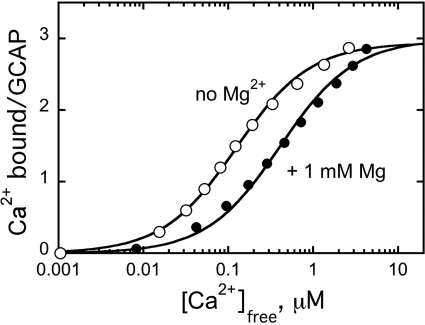
The stoichiometry of Ca2+ binding tested by fluorescent indicator dye BAPTA-2 titration (37) was the same for the non-acylated wild-type GCAP1 as for the myristoylated GCAP1 (37): three Ca2+ ions bound to the protein (Fig. 2). Independent trials using two other Ca2+ indicator dyes, Fluo-4FF, and BAPTA-6F, yielded the same binding stoichiometry (not shown). Just like in myristoylated GCAP1 (37, 54), Ca2+ binding in non-acylated protein also remained sensitive to Mg2+ (Fig. 2). This argues that in the conditions of our experiments the lack of fatty acylation did not cause the protein to undergo nonspecific unfolding.
FIGURE 2.
Ca2+ binding by non-myristoylated recombinant GCAP1. Ca2+ binding was measured in the absence (○) or in the presence (●) of 1 mm free Mg2+. The concentrations of free Ca2+ were calculated using a Ca2+ fluorescent indicator dye, BAPTA-2, as described (37). The data were produced and analyzed as described under “Materials and Methods.”
Myristoyl Moiety Affects Three Major Regulatory Characteristics of GCAP1
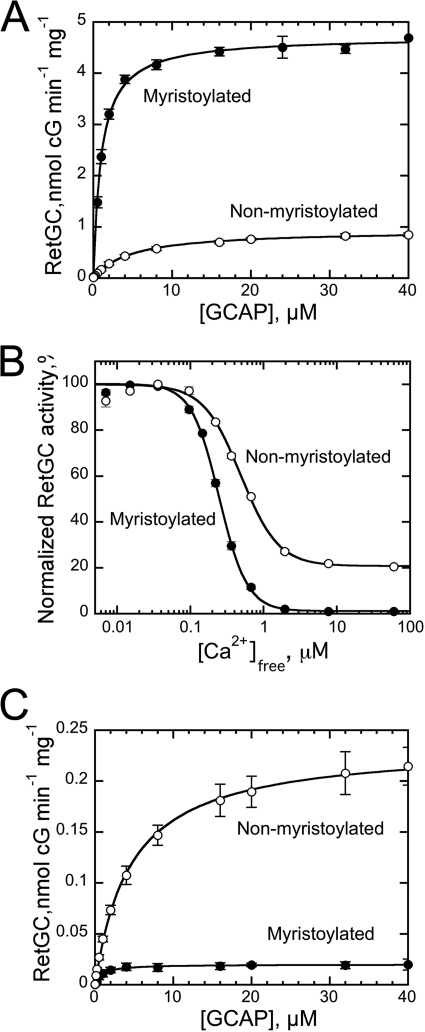
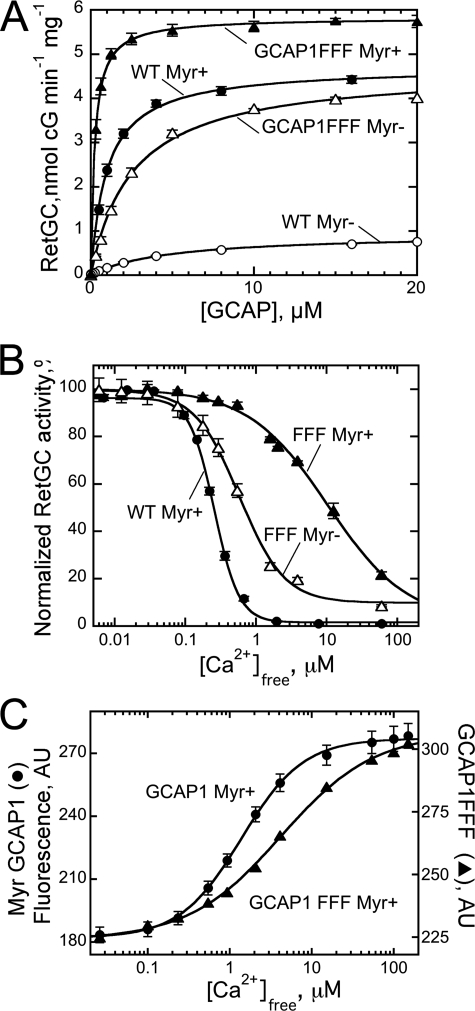
Consistent with previous observations (51–53), the lack of myristoylation in GCAP1 damages its ability to regulate RetGC. The results in Fig. 3 show that the N-fatty acylation affects three major parameters of cyclase regulation by GCAP1. Myristoylation increased 5-fold the apparent affinity of RetGC binding to the activator form, Mg2+GCAP1 (K½GCAP1, 1.1 ± 0.1 μm acylated versus 4.9 ± 0.4 μm non-acylated, mean ± S.E.) (Fig. 3A). The affinity of GCAP binding to RetGC cannot be measured directly in binding experiments because detergents required for extraction of RetGC from the membrane disrupt the cyclase interaction with GCAPs in solution (61). Therefore, only the apparent affinity can be evaluated using a RetGC activation assay (45, 54). Myristoylation of GCAP1 also elevated the maximal activation of RetGC at saturation with GCAP1 nearly 5-fold (Fig. 3A). Lastly, the fatty acylation sensitized Ca2+-dependent inhibition of the cyclase ∼2-fold (Ca2+½, 240 ± 7 nm (n = 5) versus 470 ± 24 nm (n = 3) for myristoylated and non-acylated GCAP1, respectively) (Fig. 3B). In the presence of myristoylated Ca2+GCAP1, the activity fell below 1% of that stimulated by the Mg2+GCAP1. For the non-myristoylated Ca2+GCAP1, the activity of the cyclase remained near 20% of the Mg2+GCAP1-stimulated level (Fig. 3B), similar to that reported for RetGC regulation in washed bovine outer segment membranes (52).
FIGURE 3.
A, dose dependence of RetGC1 activation by myristoylated (●) or non-myristoylated (○) wild-type GCAP1 in the presence of 1 mm free Mg2+ and 2 mm EGTA. The data were fitted by the equation a = amax [GCAP1]/(K½GCAP1 + [GCAP1]) where a is the activity of RetGC in the assay, amax is the maximal activity of RetGC, [GCAP1] is the concentration of GCAP1, and K½GCAP1 is the GCAP1 concentration required for half-maximal activation. B, the Ca2+ sensitivity of RetGC1 regulation by myristoylated (●) or non-myristoylated (○) GCAP1 at 1 mm free Mg2+. The data were fitted by the equation a = (amax − amin)/(1 + ([Ca2+]free/Ca2+½)n) + amin where a is the activity of RetGC, amax and amin are the maximal and minimal activity of RetGC in the assay, respectively, Ca2+½ is the free Ca2+ concentration required for half-maximal inhibition of RetGC, and n is the cooperativity coefficient. C, activation of RetGC1 by Ca2+-saturated myristoylated (●) or non-myristoylated (○) GCAP1 in the presence of 1 mm free Mg2+ and 200 μm free Ca2+. The data were fitted as described in A; error bars, S.E.
The Ca2+ sensitivity of the cyclase regulation by GCAP depends on two main factors: the intrinsic affinity of the GCAP1 for Ca2+ and the relative affinities of Mg2+-bound (activator) versus Ca2+-bound (inhibitor) forms of GCAP1 for RetGC (54, 62, 63). Measuring the apparent affinity for RetGC binding to the myristoylated wild-type Ca2+GCAP1 is particularly challenging because the levels of activity become very low. However, because recombinant RetGC1 does not display intrinsic basal activity in the absence of GCAPs, it can be quantified (54). The K½GCAP1 values of 0.9 ± 0.13 (n = 3) and 4.6 ± 0.5 (n = 3) μm, respectively, for myristoylated and non-myristoylated Ca2+GCAP1 (Fig. 3C) were virtually identical to the corresponding values for Mg2+GCAP1 (Fig. 3A). Hence, myristoylation equally affects the relative affinities for cyclase binding to both the activator and inhibitor forms of GCAP1. This indicates that the difference in Ca2+ sensitivity of the cyclase regulation by the acylated versus non-acylated GCAP1 is due to the change in Ca2+ binding properties.
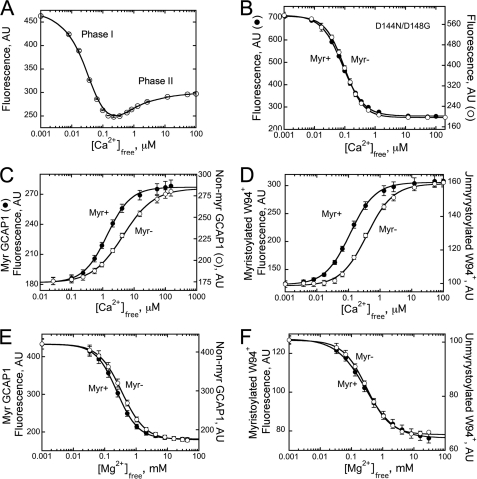
Titration of metal-free GCAP1 changes its intrinsic Trp fluorescence in a biphasic fashion (37, 62, 64, 65). At first, high affinity Ca2+ binding in EF-hands 2 and 3 sharply decreases fluorescence of Trp21 and Trp54 (“phase I”), but a further increase in Ca2+ concentrations elevates the fluorescence of Trp94 located in the entering helix of EF-hand 3 (“phase II”) (37, 62). Both phases characteristic for myristoylated GCAP1 were also well defined in the non-myristoylated GCAP1 (Fig. 4A), which also argues in support of its normal general folding. Although phase I is produced by divalent cation binding in EF-hands 2 and 3, phase II specifically reflects Ca2+ binding in EF-hand 4 (37). Also, prevention of Ca2+ binding in EF-hand 4 of the non-myristoylated D144N/D148G GCAP1 mutant eliminated phase II in the same manner as it does in the myristoylated (37) mutant (Fig. 4B). These results strongly argue that myristoylation does not critically affect Ca2+ binding in EF-hands 2 and 3.
FIGURE 4.
A, Ca2+-dependent changes in Trp fluorescence intensity of non-myristoylated wild-type GCAP1. B, Ca2+-dependent changes in Trp fluorescence intensity of myristoylated (Myr+; ●) or non-myristoylated (Myr−; ○) GCAP1 mutant in which Ca2+ binding to EF-hand 4 was inactivated by D144N/D148G substitutions (37). C, Ca2+-dependent changes in Trp fluorescence intensity of myristoylated (●) or non-myristoylated (○) wild-type GCAP1 in the presence of 10 mm Mg2+. D, Ca2+-dependent changes in Trp fluorescence intensity of myristoylated (●) or non-myristoylated (○) W94+GCAP1 mutant in which Trp21 and Trp54 were replaced with Phe (37). E, Mg2+-dependent changes in Trp fluorescence intensity of myristoylated (●) or non-myristoylated (○) wild-type GCAP1. F, Mg2+-dependent changes in Trp fluorescence intensity of myristoylated (●) or non-myristoylated (○) W94+GCAP1. AU, arbitrary units; error bars, S.E.
In contrast, we found that myristoylation markedly affects the conformational changes resulting from binding of Ca2+ in EF-hand 4 (Fig. 4C). Phase II of Trp fluorescence can be isolated by presaturation of GCAP1 with 10 mm Mg2+, which eliminates the high affinity phase I transition from the metal-free into metal-bound state (37, 62). In the non-myristoylated GCAP1, the remaining phase II required higher Ca2+ concentrations than in fatty acylated GCAP1 (Ca2+½, ∼4.9 versus 1.4 μm). In Fig. 4D, we also directly compared the Ca2+ sensitivity of the fluorescence in myristoylated versus non-myristoylated W94+GCAP1 mutant containing a single Trp94 (37). This Trp residue located in the entering helix of EF-hand 3 responds to the binding of Ca2+ in neighboring EF-hand 4 by an increase of fluorescence-producing phase II. Myristoylation shifts the Ca2+-specific increase in Trp94 fluorescence to the lower physiological free Ca2+ range (Ca2+½, ∼120 nm) compared with the non-acylated GCAP1 (Ca2+½, ∼390 nm). Contrary to that, myristoylation had little effect on Trp fluorescence accompanying transition of the inactive apoGCAP1 (63) into its Mg2+-bound activator state (37, 62, 63) both in wild type (Fig. 4E) and in the W94+GCAP1 (Fig. 4F).
Taken together, the experiments presented in Figs. 2 and 4 argue that in the conditions of our study the non-myristoylated GCAP1 is a generally normally folded protein that binds divalent metal ions in all three EF-hands. The only obvious metal binding function strongly affected by myristoylation was the conformational change caused by Ca2+ binding in EF-hand 4.
Filling Hydrophobic Cavity of Semiglobule I with Phe Can Partially Mimic Presence of Fatty Acyl Moiety
One could anticipate that the N-myristoyl group in semiglobule I would affect GCAP1 affinity for the cyclase (Fig. 2) because the EF-1 domain in GCAPs evidently participates in recognition of the target enzyme (12–14). However, it was rather surprising that the N-terminal fatty acyl group so markedly affected Ca2+ binding in EF-hand 4 located on the opposite side of the molecule. This suggested that the presence of the myristoyl fatty chain somehow bridges the two parts of the protein and creates an intramolecular dynamic tug between the two semiglobules of GCAP1.
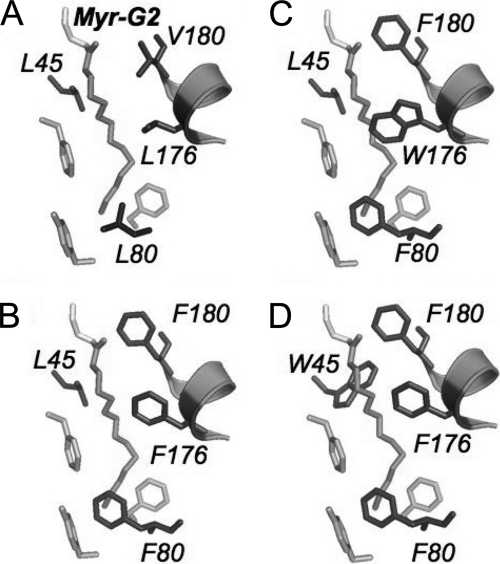
Semiglobule I formed by a non-metal-binding EF-hand 1 and a Ca2+/Mg2+-binding EF-hand 2 includes a portion of α-helix 11 from the C-terminal part of the molecule (24) (Fig. 1). Consequently, the residues facing the N-myristoyl group inside the hydrophobic cavity of semiglobule I presented in Fig. 5 include those that belong to EF-hand 1 (Leu45 and Phe43), EF-hand 2 (Leu80), and the α-helix 11 (Val180 and Leu176). Among those, Leu80 is located near the methyl group of Myr-Gly2, at the “bottom” of the cavity, Val180 is proximal to the N-acyl group of Myr-Gly2 at the “entrance” to the cavity, and the other residues are located along the midportion of the fatty acyl moiety.
FIGURE 5.
Substitutions introduced in hydrophobic pocket of semiglobule I. A, wild-type residues proximal to myristoyl residue include Leu45 of EF-hand 1, Leu80 of EF-hand 2, and Leu176 and Val180 of the C-terminal α-helix α11. B, GCAP1-FFF mutant (L80F/L176F/L180F). C and D, larger Trp residues were incorporated into the GCAP1-FFF mutant: L80F/L176W/L180F (C) and L45W/L80F/L176F/L180F (D).
In the Ca2+GCAP1 crystal structure determined by Stephen et al. (24), the myristoyl group is sequestered inside semiglobule I. On the other hand, NMR spectroscopy of the myristoylated GCAP1 reveals substantial flexibility in the region surrounding the fatty residue in the GCAP1 solution structure (23). Therefore, we mutated amino acid residues proximal to the fatty acyl group to alter the hydrophobic interactions between the myristoyl group and its protein environment. We reasoned that if the myristoyl group creates a dynamic tug affecting GCAP1 conformation then we could expect that introduction of large hydrophobic residues inside the cavity at certain positions would be able to change the properties of GCAP1 in a manner similar to the effect of myristoylation.
We therefore introduced three Phe residues to substitute several smaller hydrophobic side chains at different positions inside the cavity, L80F/L176F/V180F (“GCAP1-FFF mutant”). The triple Phe substitutions resulted in a dramatic change of the biochemical and regulatory properties in both non-myristoylated and myristoylated GCAP1 (Fig. 6). The non-acylated GCAP1-FFF restored activation of RetGC to the level stimulated by myristoylated wild-type GCAP1 (Fig. 6A). The mutation also improved 2-fold the apparent affinity (K½GCAP1) of the non-acylated GCAP1 for the cyclase to 2.6 ± 0.16 from 4.9 ± 0.04 μm (n = 3). Therefore, strengthening the hydrophobic interactions inside the hydrophobic cavity in the non-myristoylated GCAP1 can adjust its conformation to improve interaction with the cyclase. However, unlike the myristoyl moiety, the substitution failed to improve Ca2+ sensitivity of the cyclase regulation for the non-acylated GCAP1-FFF (Ca2+½, 590 ± 30 versus 490 ± 5 nm (n = 3) in the non-acylated wild type) (Fig. 6B).
FIGURE 6.
A, dose dependence of RetGC1 activation by myristoylated (Myr+) GCAP1 (●) and L80F/L176F/V180F (▴) GCAP1 (GCAP1FFF Myr+) or non-myristoylated (Myr−) wild-type GCAP1 (○) and L80F/L176F/V180F GCAP1 (△) (GCAP1FFF Myr−) in the presence of 1 mm free Mg2+ and 2 mm EGTA. Data were fitted as in Fig. 3A. B, the Ca2+ sensitivity of RetGC1 regulation by myristoylated ▴) and non-myristoylated (△) L80F/L176F/V180F GCAP1 (FFF) or myristoylated wild-type GCAP1 (●) at 1 mm free Mg2+. Data were fitted as in Fig. 3B. C, Ca2+ sensitivity of Trp94 fluorescence increases in myristoylated wild-type (●) and L80F/L176F/V180F (▴) GCAP1 preincubated with 10 mm MgCl2. AU, arbitrary units; error bars, S.E.
The addition of the myristoyl (Myr) moiety in the GCAP1-FFF mutant makes its affinity for the cyclase much higher than in myristoylated wild type (K½GCAP1, 0.29 ± 0.02 versus 1.1 ± 0.1 μm, respectively) (Fig. 6A). This sharp increase in the affinity for the cyclase was accompanied by a strong decrease in Ca2+ sensitivity of RetGC regulation: the Ca2+½ rose from 0.24 ± 0.01 μm in WT to 10.8 ± 0.7 μm (n = 3) (Fig. 6B). Consistent with the abnormal sensitivity of the cyclase, Trp fluorescence produced by Ca2+ binding in EF-hand 4 was shifted toward higher free Ca2+ concentrations than in wild type (Fig. 6C). In separate experiments (not shown), we also found that the relative affinities for the cyclase of the Mg2+-bound versus Ca2+-bound myristoylated mutant (K½GCAP1, 0.29 and 0.66 μm, respectively) increased more than 2-fold in favor of the activator form compared with the wild type (Fig. 3C).
Myristoyl Group Remains Sequestered in Hydrophobic Cavity of L80F/L176F/V180F GCAP1
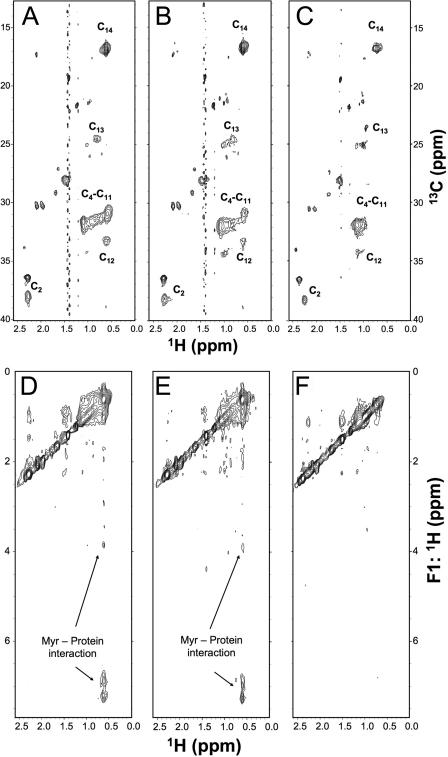
In recoverin, Ca2+ binding causes conformational changes that lead to extrusion of the fatty acyl group (calcium-myristoyl switch) and thus promote stronger association with membranes (17, 19–21). In contrast to recoverin, both Ca2+- and Mg2+-liganded GCAP1 retains the fatty acyl group sequestered inside semiglobule I (23, 24). We wondered whether substitution of a bulky Phe in place of residues near the fatty chain (Leu80, Leu176, and Leu180) might result in expulsion of the myristoyl group. If the filling of the cavity with the three Phe residues causes extrusion of the fatty acyl group, then this could promote stronger association of the triple mutant with membranes containing RetGC. Alternatively, the triple mutant might retain a sequestered myristoyl group, and the observed higher affinity for the cyclase could be explained by internal structural changes caused by the three Phe residues. To distinguish the two possibilities, we compared two-dimensional NMR spectra of L80F/L176F/V180F GCAP1 containing a 13C-labeled myristoyl group and unlabeled protein in both Mg2+- and Ca2+-bound forms (Fig. 7). Two-dimensional 13C-1H heteronuclear multiple quantum correlation NMR spectra of the myristoyl chain attached to Mg2+-bound (Fig. 7A) and Ca2+-bound (Fig. 7B) GCAP1-FFF mutant look similar and indicate that the myristoyl group is sequestered inside the protein in both states. The methylene proton chemical shift (assigned at the H11 and H12 positions) is upfield-shifted in both Mg2+-bound and Ca2+-bound states (0.5 ppm) compared with that in a solvent-exposed environment (1.0 ppm in 8 m urea). The upfield shift is likely caused by a ring current effect from the three Phe residues. To selectively probe atoms of the protein that are less than 5 Å away from the fatty acyl chain, we recorded three-dimensional 13C-edited (F1) and 13C-filtered (F3) NOESY spectra (Fig. 7D-F). The NOESY spectra show cross-peaks representing methylene protons of the C14:0 chain that contact aromatic protons from the three Phe residues (Fig. 7, D and E, peaks near 7.0 ppm). The protein-myristoyl cross-peaks disappear when the myristoyl fatty chain becomes solvent-exposed upon protein denaturation in 8 m urea (Fig. 7F). These NMR data confirm that the myristoyl group remains sequestered inside the hydrophobic cavity of GCAP1-FFF in both Mg2+- and Ca2+-bound states just as in wild type (23, 24). Hence, the effect on the cyclase regulation observed for the triple Phe mutant must be due to internal adjustment of the protein structure caused by the three Phe residues.
FIGURE 7.
NMR spectra of L80F/L176F/V180F GCAP1 containing 13C-labeled myristoyl group. 13C-1H heteronuclear multiple quantum correlation spectra of the 13C-labeled myristoyl group attached to unlabeled L80F/L176F/V180F GCAP1 in the Mg2+-bound activator state (A), Ca2+-bound inhibitory state (B), and denatured state in 8 m urea (C) are shown. Two-dimensional projections of 13C-edited NOESY spectra of the 13C-labeled myristoyl group attached to unlabeled L80F/L176F/V180F GCAP1 in the Mg2+-bound activator state (D), Ca2+-bound inhibitory state (E), and denatured state in 8 m urea (F) are shown. All NMR spectra were recorded at 800-MHz proton frequency. Sample conditions are described under “Materials and Methods.”
Substitutions Facing Different Portions of Myr Group Differentially Affect Regulatory Properties of GCAP1
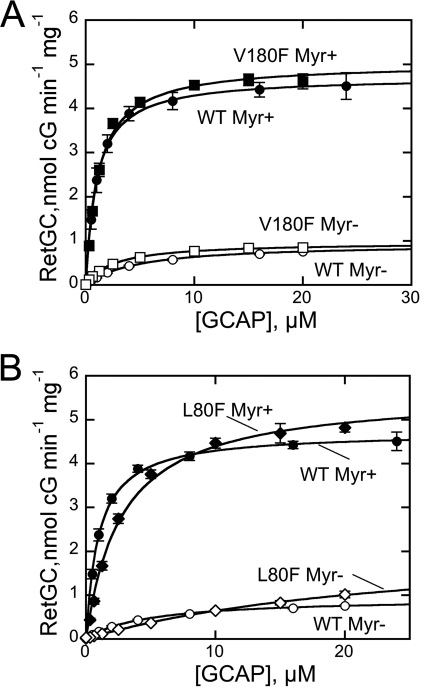
To identify the residues responsible for the effects of the L80F/L176F/V180F GCAP1 mutant, we tested single amino acid substitutions. The Val180 located in the Asp175–Leu183 helix 11 near the acyl group of the Myr residue at the top of the cavity was replaced with Phe without causing major effects on the cyclase regulation. The maximal level of the cyclase activation by the myristoylated V180F GCAP1 was similar to that of the wild type (Fig. 8A). The non-myristoylated mutant showed only a small increase in the RetGC activation compared with the non-myristoylated wild type, and the overall RetGC activity remained 4 times lower than with myristoylated wild type.
FIGURE 8.
A, dose dependence of RetGC1 activation by myristoylated GCAP1 (●), myristoylated V180F GCAP1 (■), non-myristoylated GCAP1 (○), or non-myristoylated V180F GCAP1 (□). B, dose dependence of RetGC1activation by myristoylated wild-type (●), myristoylated L80F (♦), non-myristoylated wild-type (○), or non-myristoylated L80F (♢) GCAP1. The data were fitted as in Fig. 3A; error bars, S.E.
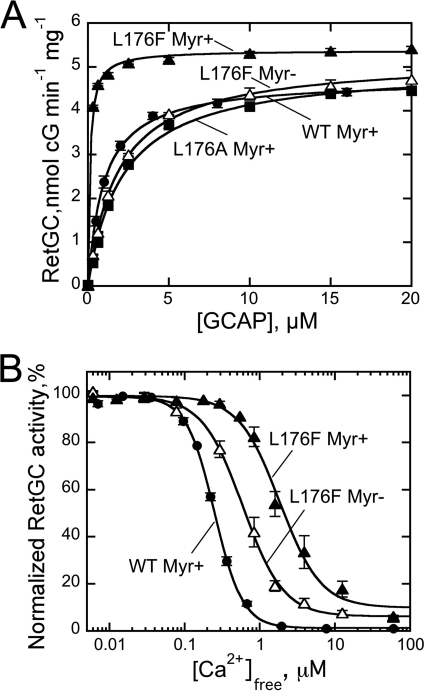
The L80F mutation that brings a large hydrophobic chain closer to the methyl end of the myristoyl group slightly increased the cyclase activation by myristoylated GCAP1 (5.6 ± 0.2 (n = 3) versus 4.8 nmol of cGMP/min/mg in WT; Fig. 8B). At the same time, the presence of a Phe residue at the bottom of the hydrophobic cavity in the non-myristoylated L80F GCAP1 apparently further reduced its apparent affinity for the cyclase (K½GCAP1 > 10 μm; Fig. 8B). This decrease was not caused by a nonspecific denaturation of the unmyristoylated L80F mutant because the stoichiometry of Ca2+ binding remained three Ca2+ ions per protein molecule (not shown). However, the presence of the myristoyl group largely mitigated the loss of the affinity for the cyclase in the L80F GCAP1 (K½GCAP1 = 2.7 ± 0.2 μm, n = 3; Fig. 8A). The Ca2+ sensitivity of both myristoylated and non-myristoylated L80F GCAP1 was similar to that of the non-myristoylated wild type (Ca2+½ ∼ 0.5–0.7 μm). These results suggested that it was mainly the L176F substitution that was responsible for the dramatic increase of the apparent affinity for the cyclase and the concurrent strong decrease of Ca2+ sensitivity of its regulation in the GCAP1-FFF mutant.
“Tug” Effects of Substitutions in Side Chains Proximal to Midportion of Myristoyl Fatty Chain
The substitution by Phe of Leu176 in α-helix 11 facing the midportion of the myristoyl group (24) profoundly altered GCAP1 properties (Fig. 9). The activity of a non-myristoylated L176F GCAP1 rose dramatically (Fig. 9A). Much like the L80F/L176F/V180F GCAP1, this substitution alone made RetGC activation by GCAP1 largely independent of myristoylation. The L176F GCAP1 fully restored RetGC activity to the level achieved by the myristoylated wild type, and the apparent affinity for the cyclase was similar to or exceeded that of the myristoylated wild type in both non-acylated (K½GCAP1 = 1.87 ± 0.2 μm, n = 3) and myristoylated (K½GCAP1 ∼ 0.1 ± 0.01 μm, n = 3) L176F GCAP1. In contrast, substitution of Leu176 with Ala slightly decreased the affinity of the myristoylated GCAP1 for the cyclase from 1.1 to 2.1 μm (Fig. 9A). Similar to the L80F/L176F/V180F GCAP1, the L176F substitution decreased Ca2+ sensitivity of RetGC inhibition for myristoylated GCAP1 (Ca2+½, ∼1.9 ± 0.3 versus 0.24 ± 0.01 μm in wild type) (Fig. 9B) but not in the non-acylated GCAP1 (Ca2+½ = 0.5, 0.6, and 0.6 μm for the wild type, L80F/L176F/V180F, and L176F GCAP1, respectively).
FIGURE 9.
A, dose dependence of RetGC1 activation by myristoylated wild-type (●), L176F (▴), or L176A (■) GCAP1 or non-myristoylated L176F GCAP1 (△) in the presence of 1 mm free Mg2+ and 2 mm EGTA. The data were fitted as in Fig. 3A. B, the Ca2+ sensitivity of RetGC1 regulation by myristoylated (▴) or non-myristoylated (△) L176F GCAP1 compared with myristoylated wild-type GCAP1 (●) at 1 mm free Mg2+. The data were fitted as in Fig. 3B; error bars, S.E.
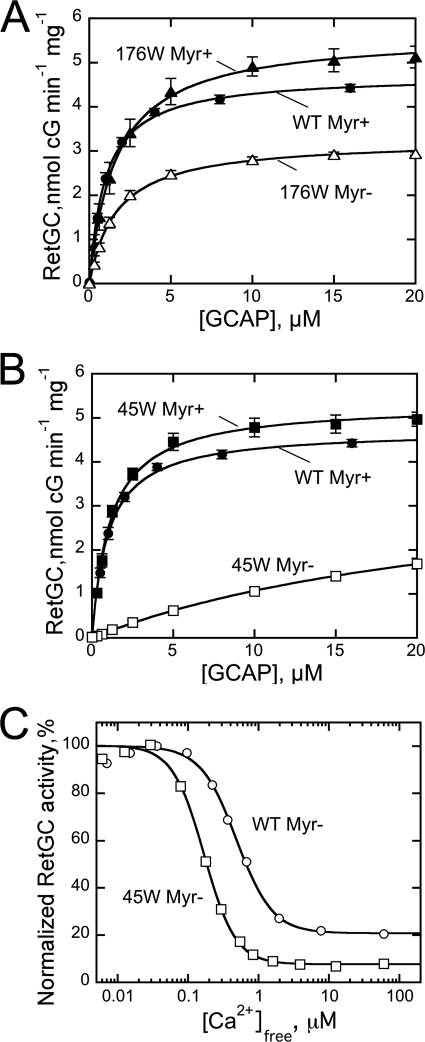
A further increase in size and hydrophobicity of the residue contacting the central region of the myristoyl group was tested by inserting the Trp residues in L80F/L176W/V180F and the L45W/L80F/L176F/V180F mutants (Fig. 10). Much like the L176F or the L80F/L176F/V180F GCAP1, the L80F/L176W/V180F mutant, now containing a larger Trp residue in position 176, displayed fairly high affinity for the cyclase in both myristoylated and non-acylated forms (K½GCAP1, 1.74 ± 0.3 and 1.71 ± 0.2 μm; n = 3). However, compared with the myristoylated GCAP1-FFF or L176F GCAP1, the presence of Trp176 noticeably lowered the GCAP1 affinity for RetGC. Evidently, the effect of the side chain positioned in the C-terminal α-helix 11 near the center of the myristoyl residue was not due to a simple increase in hydrophobicity but would rather be consistent with a molecular tug of a proper length of the link between helix 11 and the myristoyl fatty chain. Hence, although Phe interaction with the Myr group maximized the GCAP1 affinity for the target enzyme, a larger Trp residue in this position partially attenuated it.
FIGURE 10.
A, dose dependence of RetGC1 activation by myristoylated (▴) or non-myristoylated (△) L80F/L176W/V180F GCAP1 (176W). B, dose dependence of RetGC1 activation by myristoylated (■) or non-myristoylated (□) L45W/L80F/L176F/V180F GCAP1 (45W) in the presence of 1 mm free Mg and 2 mm EGTA. C, the Ca2+ sensitivity of RetGC1 regulation by non-myristoylated wild-type GCAP1 (○) or non-myristoylated L45W/L80F/L176F/V180F GCAP1 (45W) (□) at 1 mm free Mg2+; error bars, S.E.
Likewise, when the Trp45 residue was introduced into the L80F/L176F/V180F GCAP1 on the opposite side of the Myr group from the Phe176, the affinity for the cyclase remained fairly high in fatty acylated protein (K½GCAP1 ∼ 1.1 ± 0.1 μm, n = 3) but nonetheless >3-fold lower than in the myristoylated GCAP1-FFF mutant (Fig. 10B). In addition to that, Trp45 very strongly decreased the affinity of the non-myristoylated GCAP1 mutant for RetGC (K½GCAP1 > 20 μm). At the same time, it also markedly increased Ca2+ sensitivity of the cyclase regulation by non-myristoylated mutant (Ca2+½ = 167 ± 3 nm, n = 3) (Fig. 10C), which is the only case when the sensitivity was increased for a non-acylated GCAP1 in our experiments.
Comparison of N-Laurylated and N-Myristoylated GCAP1
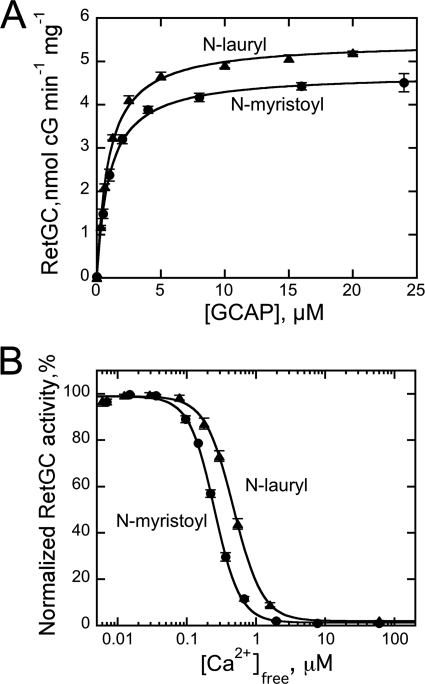
N-Myristoyltransferase in photoreceptors acylates its protein substrates with both C14 and C12 fatty acyl groups (15, 16). Because our mutagenesis study suggested that the contacts between the hydrophobic residues near the central region of the fatty acyl residue are the most critical for adjusting the activator properties of GCAP1, we also tested the regulatory properties of the N-laurylated GCAP1 (Fig. 11). The C12:0 acylated protein activated RetGC essentially to the same level and with the same affinity as the myristoylated GCAP1 (K½GCAP1 = 0.94 ± 0.03 μm, n = 3). However, Ca2+ sensitivity of RetGC inhibition by the laurylated GCAP1 (Ca2+½ = 473 ± 24 nm, n = 3) was lower compared with the C14:0-GCAP1 (240 nm). This implies that interactions at the C14-methyl end of the myristoyl chain do not control the activator conformation of GCAP1 but may contribute to the fine adjustment of the Ca2+ sensitivity.
FIGURE 11.
A, dose dependence of RetGC1 activation by N-myristoylated (●) or N-laurylated (▴) GCAP1 in the presence of 1 mm free Mg2+ and 2 mm EGTA. The data were fitted as in Fig. 3A. B, Ca2+ sensitivity of RetGC1 regulation by N-myristoylated (●) or N-laurylated (▴) GCAP1 at 1 mm free Mg2+. The data were fitted as in Fig. 3B; error bars, S.E.
DISCUSSION
Myristoylation Affects Specific Regulatory Properties of GCAP1 Rather than General Folding of Protein
The lack of myristoylation decreased by ∼5-fold the apparent affinity of GCAP1 for RetGC (Fig. 3). That could suggest that misfolding of a non-myristoylated GCAP1 proportionally decreased the concentration of the folded GCAP1 in the assay. However, the stoichiometry of Ca2+ binding in an unfolded protein would have to become much lower than in myristoylated GCAP1. In contrast, it remained exactly the same: three Ca2+ ions per GCAP1 molecule (Fig. 2). In G2A GCAP1 mutant, which is not a substrate for N-myristoyltransferase (51), the Ca2+ binding isotherm is slightly shifted toward a higher free Ca2+ range compared with the wild type, but the stoichiometry of Ca2+ binding also remains unchanged (66). Good overall preservation of Ca2+ binding by non-myristoylated GCAP1 in our experiments is in accord with the CD spectroscopy evidence that the lack of fatty acylation does not strongly affect the thermal stability of the secondary structure in a metal-liganded GCAP1 especially below 50 °C (53). Likewise, the NMR spectra of non-acylated GCAP1 give no indications that its main chain undergoes unfolding at 37–47 °C (23). Taken together, this group of evidence strongly argues that the structural role of the myristoyl fatty chain inside the protein is to help create a specific conformation required for efficient regulation of the cyclase rather than merely prevent general misfolding of the GCAP1 molecule at physiologically relevant temperatures.
In particular, the myristoyl group is needed to increase the affinity of GCAP1 for the target enzyme and to adjust its conformation to the maximal efficacy of the cyclase regulation. The latter involves two aspects: an increase in RetGC activity by Mg2+GCAP1 and reduction of the “background” (basal) activity of RetGC by Ca2+GCAP1 (Fig. 3). The offset of RetGC suppression by the non-myristoylated GCAP1 at saturating concentrations of Ca2+ observed by Hwang and Koch (52) in reconstituted outer segment membranes remains evident in the case of recombinant RetGC1 (Fig. 3), which indicates that the non-myristoylated Ca2+GCAP1 acquires a “partial activator” conformation. Although stimulation of RetGC by non-myristoylated Ca2+GCAP1 is much lower than it is by Mg2+GCAP1, it is nevertheless measurably higher than in the presence of myristoylated Ca2+GCAP1. Myristoylation also adjusts the Ca2+ sensitivity of GCAP1 to a proper Ca2+ range of the intracellular Ca2+ concentrations (Fig. 3).
Myristoyl Group in GCAP1 as Intramolecular Tug
GCAPs play an essential role in the physiology of photoreceptors by accelerating their recovery from excitation (29, 39, 44, 67–69). The N-myristoylation in GCAP1 is required for the Ca2+ sensitivity of RetGC to be within physiological range (33–35) of the intracellular free Ca2+ concentrations (Figs. 3 and 4). In particular, it has a strong effect on Ca2+ binding in EF-hand 4, which is primarily responsible for the inactivation of RetGC in the dark when Ca2+ levels rise (37, 63). Ca2+ binding to EF-4 is promoted by its binding to EF-3 (63). Therefore, if Ca2+ binding in EF-hand 4 is blocked by naturally occurring mutations that cause human blindness (50), GCAP1 enters a constitutively active state capable of triggering photoreceptor degeneration (48–50).
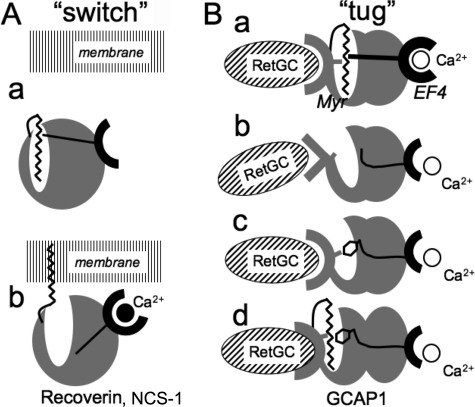
The increase of Trp94 fluorescence occurs when Ca2+ replaces Mg2+ in EF-hand 4 (37). Evidently, conformational changes caused by either Mg2+ binding in all three EF-hands or Ca2+ binding in EF-hands 2 and 3 do not critically depend on myristoylation (Fig. 4). We have also found in a recent separate study that when EF-hands 3 and 4 are both inactivated in GCAP1 the high affinity Ca2+ binding isotherm in the remaining EF-hand 2 (proximal to the myristoyl group in GCAP1 structure; Fig. 1) remains virtually unchanged in the absence of myristoylation (66). In contrast, Trp94 fluorescence affected by Ca2+ binding in EF-hand 4 in the present study clearly depends on the fatty acylation (Fig. 4). Paradoxically, the fatty acyl group and EF-hand 4 are located on opposite sides of the GCAP1 molecule (24) (Fig. 1). The N-terminal myristoyl is retained inside the hydrophobic cavity of the first semiglobular portion of GCAP1 both in Mg2+- and Ca2+-liganded states and does not undergo a calcium-myristoyl switch as a part of its physiological function (23, 24). Hence, the myristoyl fatty acyl chain can only affect the properties of the GCAP1 through its intramolecular interactions (see the schematics in Fig. 12).
FIGURE 12.
Two types of calcium-myristoyl functional links in NCS proteins. A, calcium-myristoyl switch in recoverin and NCS-1 (17, 19, 60). The myristoyl chain in Ca2+-free protein (a) is constrained within the hydrophobic pocket. A conformational change in response to the binding of Ca2+ releases the myristoyl moiety from the constraint (b), and the extruded fatty acyl chain anchors the protein to the membrane (modified from Refs. 17 and 19). B, “calcium-myristoyl tug” hypothesis. The fatty chain interacting with the surrounding hydrophobic amino acid residues adjusts the conformation of the RetGC-binding interface, which evidently includes part of the neighboring non-metal-binding EF-hand 1 (12–14, 66) (a). It also provides intramolecular pull (or push) across the entire protein globule on the remote EF-hand 4 (EF4) presumably transmitted via α-helices 11 and 10. Consequently, the lack of proper hydrophobic interaction in the pocket of the non-acylated GCAP1 (b) simultaneously perturbs the cyclase-binding interface and reduces the intramolecular pull from the myristoyl moiety, thus lowering the affinity for Ca2+ of EF-hand 4. A large hydrophobic side chain (c) on the opposite side of the pocket can provide the hydrophobic interaction needed for adjustment of the cyclase-binding interface, but because of the altered size of the side chain, it also creates hindrance for the proper tug effect needed for high affinity Ca2+ binding in EF-hand 4. In myristoylated L176F mutant (d), the conformation of the cyclase recognition domain becomes optimized even further at the expense of the weaker heave on EF-hand 4. Hence, only the myristoyl fatty chain allows the proper tug action on EF-hand 4 required for the normal Ca2+ sensor function of GCAP1. See “Discussion” for other details.
At least one structural element that can couple the hydrophobic pocket with the opposite side of the molecule is the stretch of α-helices, α10 and α11, that lies across the protein molecule from the exiting helix 9 of EF-hand 4 to the N-terminal semiglobule (24) (highlighted in blue in Fig. 1). The most C-proximal helix, α11, comes into direct contact with the fatty chain inside semiglobule I through Leu176 and Val180 (Figs. 1 and 5). Our data strongly argue that the side chain 176 in the C-terminal helix is essential for the proper functional coupling between the two semiglobules of GCAP1. It creates a proper dynamic tug that in an opposite manner affects Ca2+ binding in EF-hand 4 and the conformation of GCAP1 required for interaction with the cyclase. Large hydrophobic residues at this position in the cavity, Phe176 or Trp176 (Fig. 5), can mimic the effect of the myristoyl fatty chain by increasing the affinity for the cyclase but only at the expense of reduced Ca2+ sensitivity. These effects are strongest when substitutions are introduced in the region surrounding the midregion of the myristoyl moiety (Phe176, Trp176, and Trp45) (Figs. 6, 9, and 10), indicating that it is the central region of the fatty acyl chain that contributes the most to the conformational tuning of GCAP1 as the cyclase regulator. The Myr-Gly2 portion near the entrance to the hydrophobic cavity does not play a critical role in such tuning. Likewise, the contact between the myristoyl group and Leu80 at the bottom of the cavity makes only a modest contribution (Fig. 8). This is consistent with the ability of a shorter N-lauryl group to maintain a nearly normal function of GCAP1 (Fig. 11). However, the C14-methyl end of the myristoyl group can (to at least some extent) contribute to the fine conformational tuning required for proper Ca2+ sensitivity because the Ca2+½ remained slightly elevated in the laurylated GCAP1.
Opposite Effects of Mutation in Hydrophobic Pocket in GCAP1 on Its Affinities for Cyclase and Ca2+
Affinity for Ca2+ is very sensitive to even minor changes in the hydrophobic cavity of semiglobule I. Replacement of the side chains inside the cavity can evidently either “pull” or “push” the dynamic tug just enough to decrease the affinity of EF-hand 4. It should be emphasized that none of the mutations that increase GCAP1 affinity for the cyclase in our experiments improved Ca2+ sensitivity of RetGC regulation. On the contrary, much higher than normal affinity of the myristoylated L176F GCAP1 or GCAP1-FFF for the cyclase strongly suppressed Ca2+ sensitivity of the cyclase regulation (Figs. 6 and 9). The only mutant we encountered (L45W/L80F/L176F/V180F) that increased the affinity of a non-myristoylated GCAP1 for Ca2+ also drastically reduced the affinity for the cyclase (Fig. 10). Therefore, high affinity for the cyclase and high sensitivity for Ca2+-dependent regulation of RetGC by GCAP1 tend to be in a “tug-of-war” relationship in which the role of the tug is likely played by the structural link between the myristoyl moiety and the exiting α-helix 9 of the EF-hand 4. When mutations facing the midportion of the myristoyl group limit its flexibility, GCAP1 conformation becomes more suitable for binding to the cyclase compared with wild type (K½GCAP1 ∼ 0.1–0.2 μm) but adversely affects the proper pull from the “myristoyl tug,” thus interfering with the Ca2+ binding affinity of EF-hand 4. Apparently, the fatty acyl chain in the hydrophobic cavity evolved to balance the affinity for binding to the cyclase versus Ca2+. The presence of the evolutionarily conserved Leu45 and Leu176 in GCAP1 evidently provides just enough flexibility for the myristoyl tug. This allows the myristoyl moiety to maintain sufficiently high affinity for the cyclase (K½GCAP1 ∼ 1 μm) without excessive pull/push on the second semiglobule that would otherwise reduce Ca2+ binding in EF-hand 4.
Conclusion
In contrast to those myristoylated NCS proteins that undergo a calcium-myristoyl switch (17, 19, 60), the myristoyl group in GCAP1 evolved to fine tune the protein structure to bind and regulate the cyclase. We present evidence that the central region of the fatty acyl chain interacts with the C-terminal helix 11 via Leu176 to create an intramolecular tug (Fig. 12) that balances two opposing tendencies in GCAP1 molecules: high affinity for the target enzyme versus high affinity for Ca2+ in EF-hand 4. The fatty acyl chain anchors hydrophobic residues in the pocket to help maintain the proper cyclase-binding interface and simultaneously creates an intramolecular heave adjusting the Ca2+ sensor, EF-hand 4, at the opposite end.
This work was supported, in whole or in part, by National Institutes of Health Grants EY11522 (to A. M. D.) and EY012347 (to J. B. A.) from the NEI. This work was also supported by a formula grant from the Pennsylvania Department of Health.
- NCS
- neuronal calcium sensor
- GCAP
- guanylyl cyclase-activating protein
- RetGC
- retinal guanylyl cyclase
- Myr
- myristoyl.
REFERENCES
- 1. Burgoyne R. D. (2007) Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 8, 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ames J. B., Lim S., Ikura M. (2012) Molecular structure and target recognition of neuronal calcium sensor proteins. Front. Mol. Neurosci. 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Flaherty K. M., Zozulya S., Stryer L., McKay D. B. (1993) Three-dimensional structure of recoverin, a calcium sensor in vision. Cell 75, 709–716 [DOI] [PubMed] [Google Scholar]
- 4. Ames J. B., Dizhoor A. M., Ikura M., Palczewski K., Stryer L. (1999) Three-dimensional structure of guanylyl cyclase activating protein-2, a calcium-sensitive modulator of photoreceptor guanylyl cyclases. J. Biol. Chem. 274, 19329–19337 [DOI] [PubMed] [Google Scholar]
- 5. Stephen R., Palczewski K., Sousa M. C. (2006) The crystal structure of GCAP3 suggests molecular mechanism of GCAP-linked cone dystrophies. J. Mol. Biol. 359, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawamura S., Tachibanaki S. (2002) S-modulin. Adv. Exp. Med. Biol. 514, 61–68 [DOI] [PubMed] [Google Scholar]
- 7. Vijay-Kumar S., Kumar V. D. (1999) Crystal structure of recombinant bovine neurocalcin. Nat. Struct. Biol. 6, 80–88 [DOI] [PubMed] [Google Scholar]
- 8. Palczewski K., Subbaraya I., Gorczyca W. A., Helekar B. S., Ruiz C. C., Ohguro H., Huang J., Zhao X., Crabb J. W., Johnson R. S., Walsh K. A., Gray-Keller M. P., Detwiller P. B., Baehr W. (1994) Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 13, 395–404 [DOI] [PubMed] [Google Scholar]
- 9. Dizhoor A. M., Olshevskaya E. V., Henzel W. J., Wong S. C., Stults J. T., Ankoudinova I., Hurley J. B. (1995) Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J. Biol. Chem. 270, 25200–25206 [DOI] [PubMed] [Google Scholar]
- 10. Imanishi Y., Yang L., Sokal I., Filipek S., Palczewski K., Baehr W. (2004) Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP1–8) in pufferfish (Fugu rubripes). J. Mol. Evol. 59, 204–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takemoto N., Tachibanaki S., Kawamura S. (2009) High cGMP synthetic activity in carp cones. Proc. Natl. Acad. Sci. U.S.A 106, 11788–11793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ermilov A. N., Olshevskaya E. V., Dizhoor A. M. (2001) Instead of binding calcium, one of the EF-hand structures in guanylyl cyclase activating protein-2 is required for targeting photoreceptor guanylyl cyclase. J. Biol. Chem. 276, 48143–48148 [DOI] [PubMed] [Google Scholar]
- 13. Helten A., Koch K. W. (2007) Calcium-dependent conformational changes in guanylate cyclase-activating protein 2 monitored by cysteine accessibility. Biochem. Biophys. Res. Commun. 356, 687–692 [DOI] [PubMed] [Google Scholar]
- 14. Hwang J. Y., Schlesinger R., Koch K. W. (2004) Irregular dimerization of guanylate cyclase-activating protein 1 mutants causes loss of target activation. Eur. J. Biochem. 271, 3785–3793 [DOI] [PubMed] [Google Scholar]
- 15. Dizhoor A. M., Ericsson L. H., Johnson R. S., Kumar S., Olshevskaya E., Zozulya S., Neubert T. A., Stryer L., Hurley J. B., Walsh K. A. (1992) The NH2 terminus of retinal recoverin is acylated by a small family of fatty acids. J. Biol. Chem. 267, 16033–16036 [PubMed] [Google Scholar]
- 16. Johnson R. S., Ohguro H., Palczewski K., Hurley J. B., Walsh K. A., Neubert T. A. (1994) Heterogeneous N-acylation is a tissue- and species-specific posttranslational modification. J. Biol. Chem. 269, 21067–21071 [PubMed] [Google Scholar]
- 17. Dizhoor A. M., Chen C. K., Olshevskaya E., Sinelnikova V. V., Phillipov P., Hurley J. B. (1993) Role of the acylated amino terminus of recoverin in Ca2+-dependent membrane interaction. Science 259, 829–832 [DOI] [PubMed] [Google Scholar]
- 18. Ladant D. (1995) Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J. Biol. Chem. 270, 3179–3185 [PubMed] [Google Scholar]
- 19. Zozulya S., Stryer L. (1992) Calcium-myristoyl protein switch. Proc. Natl. Acad. Sci. U.S.A 89, 11569–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ames J. B., Tanaka T., Ikura M., Stryer L. (1995) Nuclear magnetic resonance evidence for Ca2+-induced extrusion of the myristoyl group of recoverin. J. Biol. Chem. 270, 30909–30913 [DOI] [PubMed] [Google Scholar]
- 21. Ames J. B., Ishima R., Tanaka T., Gordon J. I., Stryer L., Ikura M. (1997) Molecular mechanics of calcium-myristoyl switches. Nature 389, 198–202 [DOI] [PubMed] [Google Scholar]
- 22. Hughes R. E., Brzovic P. S., Dizhoor A. M., Klevit R. E., Hurley J. B. (1998) Ca2+-dependent conformational changes in bovine GCAP-2. Protein Sci. 7, 2675–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim S., Peshenko I., Dizhoor A., Ames J. B. (2009) Effects of Ca2+, Mg2+, and myristoylation on guanylyl cyclase activating protein 1 structure and stability. Biochemistry 48, 850–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stephen R., Bereta G., Golczak M., Palczewski K., Sousa M. C. (2007) Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure 15, 1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koch K. W., Stryer L. (1988) Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature 334, 64–66 [DOI] [PubMed] [Google Scholar]
- 26. Gorczyca W. A., Gray-Keller M. P., Detwiler P. B., Palczewski K. (1994) Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc. Natl. Acad. Sci. U.S.A 91, 4014–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dizhoor A. M., Lowe D. G., Olshevskaya E. V., Laura R. P., Hurley J. B. (1994) The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron 12, 1345–1352 [DOI] [PubMed] [Google Scholar]
- 28. Duda T., Goraczniak R., Surgucheva I., Rudnicka-Nawrot M., Gorczyca W. A., Palczewski K., Sitaramayya A., Baehr W., Sharma R. K. (1996) Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry 35, 8478–8482 [DOI] [PubMed] [Google Scholar]
- 29. Mendez A., Burns M. E., Sokal I., Dizhoor A. M., Baehr W., Palczewski K., Baylor D. A., Chen J. (2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 98, 9948–9953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pugh E. N., Jr., Duda T., Sitaramayya A., Sharma R. K. (1997) Photoreceptor guanylate cyclases: a review. Biosci. Rep. 17, 429–473 [DOI] [PubMed] [Google Scholar]
- 31. Pugh E. N., Jr., Nikonov S., Lamb T. D. (1999) Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr. Opin. Neurobiol. 9, 410–418 [DOI] [PubMed] [Google Scholar]
- 32. Fu Y., Yau K. W. (2007) Phototransduction in mouse rods and cones. Pflugers Arch. 454, 805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gray-Keller M. P., Detwiler P. B. (1994) The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron 13, 849–861 [DOI] [PubMed] [Google Scholar]
- 34. Sampath A. P., Matthews H. R., Cornwall M. C., Bandarchi J., Fain G. L. (1999) Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J. Gen. Physiol. 113, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woodruff M. L., Sampath A. P., Matthews H. R., Krasnoperova N. V., Lem J., Fain G. L. (2002) Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542, 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakatani K., Chen C., Yau K. W., Koutalos Y. (2002) Calcium and phototransduction. Adv. Exp. Med. Biol. 514, 1–20 [DOI] [PubMed] [Google Scholar]
- 37. Peshenko I. V., Dizhoor A. M. (2006) Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 281, 23830–23841 [DOI] [PubMed] [Google Scholar]
- 38. Dizhoor A. M., Olshevskaya E. V., Peshenko I. V. (2010) Mg2+/Ca2+ cation binding cycle of guanylyl cyclase activating proteins (GCAPs): role in regulation of photoreceptor guanylyl cyclase. Mol. Cell. Biochem. 334, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burns M. E., Mendez A., Chen J., Baylor D. A. (2002) Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36, 81–91 [DOI] [PubMed] [Google Scholar]
- 40. Dizhoor A. M., Hurley J. B. (1996) Inactivation of EF-hands makes GCAP-2 (p24) a constitutive activator of photoreceptor guanylyl cyclase by preventing a Ca2+-induced “activator-to-inhibitor” transition. J. Biol. Chem. 271, 19346–19350 [DOI] [PubMed] [Google Scholar]
- 41. Dizhoor A. M., Boikov S. G., Olshevskaya E. V. (1998) Constitutive activation of photoreceptor guanylate cyclase by Y99C mutant of GCAP-1. Possible role in causing human autosomal dominant cone degeneration. J. Biol. Chem. 273, 17311–17314 [DOI] [PubMed] [Google Scholar]
- 42. Hwang J. Y., Lange C., Helten A., Höppner-Heitmann D., Duda T., Sharma R. K., Koch K. W. (2003) Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca2+-sensitivity. Eur. J. Biochem. 270, 3814–3821 [DOI] [PubMed] [Google Scholar]
- 43. Peshenko I. V., Olshevskaya E. V., Savchenko A. B., Karan S., Palczewski K., Baehr W., Dizhoor A. M. (2011) Enzymatic properties and regulation of the native isozymes of retinal membrane guanylyl cyclase (RetGC) from mouse photoreceptors. Biochemistry 50, 5590–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Makino C. L., Peshenko I. V., Wen X. H., Olshevskaya E. V., Barrett R., Dizhoor A. M. (2008) A role for GCAP2 in regulating the photoresponse. Guanylyl cyclase activation and rod electrophysiology in GUCA1B knock-out mice. J. Biol. Chem. 283, 29135–29143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scholten A., Koch K. W. (2011) Differential calcium signaling by cone specific guanylate cyclase-activating proteins from the zebrafish retina. PLoS One 6, e23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Payne A. M., Downes S. M., Bessant D. A., Taylor R., Holder G. E., Warren M. J., Bird A. C., Bhattacharya S. S. (1998) A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum. Mol. Genet. 7, 273–277 [DOI] [PubMed] [Google Scholar]
- 47. Wilkie S. E., Li Y., Deery E. C., Newbold R. J., Garibaldi D., Bateman J. B., Zhang H., Lin W., Zack D. J., Bhattacharya S. S., Warren M. J., Hunt D. M., Zhang K. (2001) Identification and functional consequences of a new mutation (E155G) in the gene for GCAP1 that causes autosomal dominant cone dystrophy. Am. J. Hum. Genet. 69, 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olshevskaya E. V., Calvert P. D., Woodruff M. L., Peshenko I. V., Savchenko A. B., Makino C. L., Ho Y. S., Fain G. L., Dizhoor A. M. (2004) The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. J. Neurosci. 24, 6078–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woodruff M. L., Olshevskaya E. V., Savchenko A. B., Peshenko I. V., Barrett R., Bush R. A., Sieving P. A., Fain G. L., Dizhoor A. M. (2007) Constitutive excitation by Gly90Asp rhodopsin rescues rods from degeneration caused by elevated production of cGMP in the dark. J. Neurosci. 27, 8805–8815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang L., Zhang H., Dizhoor A. M., Boye S. E., Hauswirth W. W., Frederick J. M., Baehr W. (2011) Long-term RNA interference gene therapy in a dominant retinitis pigmentosa mouse model. Proc. Natl. Acad. Sci. U.S.A 108, 18476–18481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Otto-Bruc A., Buczylko J., Surgucheva I., Subbaraya I., Rudnicka-Nawrot M., Crabb J. W., Arendt A., Hargrave P. A., Baehr W., Palczewski K. (1997) Functional reconstitution of photoreceptor guanylate cyclase with native and mutant forms of guanylate cyclase-activating protein 1. Biochemistry 36, 4295–4302 [DOI] [PubMed] [Google Scholar]
- 52. Hwang J. Y., Koch K. W. (2002) Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry 41, 13021–13028 [DOI] [PubMed] [Google Scholar]
- 53. Dell'Orco D., Behnen P., Linse S., Koch K. W. (2010) Calcium binding, structural stability and guanylate cyclase activation in GCAP1 variants associated with human cone dystrophy. Cell. Mol. Life Sci. 67, 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peshenko I. V., Moiseyev G. P., Olshevskaya E. V., Dizhoor A. M. (2004) Factors that determine Ca2+ sensitivity of photoreceptor guanylyl cyclase. Kinetic analysis of the interaction between the Ca2+-bound and the Ca2+-free guanylyl cyclase activating proteins (GCAPs) and recombinant photoreceptor guanylyl cyclase 1 (RetGC-1). Biochemistry 43, 13796–13804 [DOI] [PubMed] [Google Scholar]
- 55. Tsien R., Pozzan T. (1989) Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 172, 230–262 [DOI] [PubMed] [Google Scholar]
- 56. Olshevskaya E. V., Hughes R. E., Hurley J. B., Dizhoor A. M. (1997) Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J. Biol. Chem. 272, 14327–14333 [DOI] [PubMed] [Google Scholar]
- 57. Brooks S. P., Storey K. B. (1992) Bound and determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 201, 119–126 [DOI] [PubMed] [Google Scholar]
- 58. Marks P. W., Maxfield F. R. (1991) Preparation of solutions with free calcium concentration in the nanomolar range using 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. Anal. Biochem. 193, 61–71 [DOI] [PubMed] [Google Scholar]
- 59. Lee W., Revington M. J., Arrowsmith C., Kay L. E. (1994) A pulsed field gradient isotope-filtered 3D 13C HMQC-NOESY experiment for extracting intermolecular NOE contacts in molecular complexes. FEBS Lett. 350, 87–90 [DOI] [PubMed] [Google Scholar]
- 60. Lim S., Strahl T., Thorner J., Ames J. B. (2011) Structure of a Ca2+-myristoyl switch protein that controls activation of a phosphatidylinositol 4-kinase in fission yeast. J. Biol. Chem. 286, 12565–12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koch K. W. (1991) Purification and identification of photoreceptor guanylate cyclase. J. Biol. Chem. 266, 8634–8637 [PubMed] [Google Scholar]
- 62. Peshenko I. V., Dizhoor A. M. (2004) Guanylyl cyclase-activating proteins (GCAPs) are Ca2+/Mg2+ sensors: implications for photoreceptor guanylyl cyclase (RetGC) regulation in mammalian photoreceptors. J. Biol. Chem. 279, 16903–16906 [DOI] [PubMed] [Google Scholar]
- 63. Peshenko I. V., Dizhoor A. M. (2007) Activation and inhibition of photoreceptor guanylyl cyclase by guanylyl cyclase activating protein 1 (GCAP-1): the functional role of Mg2+/Ca2+ exchange in EF-hand domains. J. Biol. Chem. 282, 21645–21652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sokal I., Otto-Bruc A. E., Surgucheva I., Verlinde C. L., Wang C. K., Baehr W., Palczewski K. (1999) Conformational changes in guanylyl cyclase-activating protein 1 (GCAP1) and its tryptophan mutants as a function of calcium concentration. J. Biol. Chem. 274, 19829–19837 [DOI] [PubMed] [Google Scholar]
- 65. Sokal I., Li N., Klug C. S., Filipek S., Hubbell W. L., Baehr W., Palczewski K. (2001) Calcium-sensitive regions of GCAP1 as observed by chemical modifications, fluorescence, and EPR spectroscopies. J. Biol. Chem. 276, 43361–43373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peshenko I. V., Olshevskaya E. V., Dizhoor A. M. (2012) Interaction of GCAP1 with retinal guanylyl cyclase and calcium: sensitivity to fatty acylation. Front. Mol. Neurosci. 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sakurai K., Chen J., Kefalov V. J. (2011) Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pennesi M. E., Howes K. A., Baehr W., Wu S. M. (2003) Guanylate cyclase-activating protein (GCAP) 1 rescues cone recovery kinetics in GCAP1/GCAP2 knockout mice. Proc. Natl. Acad. Sci. U.S.A. 100, 6783–6788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Howes K. A., Pennesi M. E., Sokal I., Church-Kopish J., Schmidt B., Margolis D., Frederick J. M., Rieke F., Palczewski K., Wu S. M., Detwiler P. B., Baehr W. (2002) GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. EMBO J. 21, 1545–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]