Background: RIPK1 is a central kinase in TNFR1 signaling participating in NF-κB activation, necroptosis, and apoptosis.
Results: Ectopic expression of a RIPK1 mutant lacking the intermediate domain induces a shift from TNF-induced necroptosis to RIPK1 kinase-dependent apoptosis.
Conclusion: The intermediate domain of RIPK1 harbors an anti-apoptotic function.
Significance: We developed a cellular model to identify RIPK1 targets during RIPK1-dependent apoptosis.
Keywords: Apoptosis, Cell Death, Necrosis (Necrotic Death), Signal Transduction, Tumor Necrosis Factor (TNF)
Abstract
Receptor-interacting protein kinase 1 (RIPK1) is an important component of the tumor necrosis factor receptor 1 (TNFR1) signaling pathway. Depending on the cell type and conditions, RIPK1 mediates MAPK and NF-κB activation as well as cell death. Using a mutant form of RIPK1 (RIPK1ΔID) lacking the intermediate domain (ID), we confirm the requirement of this domain for activation of these signaling events. Moreover, expression of RIPK1ΔID resulted in enhanced recruitment of caspase-8 to the TNFR1 complex II component Fas-associated death domain (FADD), which allowed a shift from TNF-induced necroptosis to apoptosis in L929 cells. Addition of the RIPK1 kinase inhibitor necrostatin-1 strongly reduced recruitment of RIPK1 and caspase-8 to FADD and subsequent apoptosis, indicating a role for RIPK1 kinase activity in apoptotic complex formation. Our study shows that RIPK1 has an anti-apoptotic function residing in its ID and demonstrates a cellular system as an elegant genetic model for RIPK1 kinase-dependent apoptosis that, in contrast to the Smac mimetic model, does not rely on depletion of cellular inhibitor of apoptosis protein 1 and 2 (cIAP1/2).
Introduction
TNF is a pleiotropic cytokine involved in diverse cellular responses, such as proliferation, differentiation, cell survival, and death. It is a key player in a variety of physiological and pathological processes. TNF signals through two cell surface receptors, TNFR16 and TNFR2, but most of its biological activities are initiated by TNFR1 (1). Ligand binding triggers the sequential formation of different protein complexes (2, 3), and the identity of the complex that is formed determines the subsequent intracellular signaling and the cellular response. Upon TNFR1 stimulation, a prosurvival complex I is formed by recruitment of, among others, TNF receptor-associated death domain (TRADD), receptor-interacting protein kinase 1 (RIPK1), TRAF2 (TNF receptor-associated factor 2), cellular inhibitor of apoptosis protein 1 and 2 (cIAP1 and cIAP2) and the linear ubiquitin chain assembly complex (1, 2, 4). In this complex, RIPK1 is conjugated with non-degradative polyubiquitin chains on Lys-377 (5, 6) by the E3 ubiquitin ligases cIAP1 and cIAP2 (7–10). Addition of those chains to RIPK1 has two major consequences. First, they serve as substrates for the ubiquitin-binding domain of the TAK1-associated binding (TAB) proteins and potentially of NEMO. This allows recruitment and activation of the TAB/TAK and the IκB kinase complexes, which leads to activation of NF-κB and MAPK (4). Second, they prevent RIPK1 from integrating into death-inducing complexes (7, 11–13). However, the need for RIPK1 in TNF-mediated NF-κB activation was reported to be cell-specific. Indeed, NF-κB activation is normal in some RIPK1-depleted cells, indicating that other proteins in complex I can activate the IκB kinase complex in the absence of RIPK1 (14, 15).
Although RIPK1 overexpression can induce apoptotic cell death (16–18), many reports conclude that RIPK1 is redundant for death receptor-induced apoptosis (12, 19, 20). However, in the presence of Smac mimetics, which induce proteasomal degradation of cIAP1/2 and consequently inhibit RIPK1 ubiquitylation in complex I, cells switch to a RIPK1-dependent apoptotic pathway in response to TNF (7, 9, 19, 21–23). In these conditions, kinase-active RIPK1 was crucial for the formation of a caspase-8 activating complex, whereas in the absence of Smac mimetics, RIPK1 had no role in apoptosis induction upon TNF stimulation (23). Interestingly, a death complex with similar core composition has been reported to assemble spontaneously upon cIAP depletion, indicating that cIAPs negatively regulate formation of both RIPK-containing death complexes (24, 25). This last complex has been referred to as the ripoptosome (24, 25).
Besides its function as a central mediator of cell survival and its role in apoptosis induced by Smac mimetics, RIPK1 is important in death-receptor-induced necroptosis (19, 26). This cell death pathway is initiated by the interplay between RIPK1 and RIPK3 in a complex known as the necrosome, in which RIPK1 and RIPK3 interact through a RIP homotypic interaction motif (RHIM) present in both kinases (27–30). Necroptosis is initiated after TNFR1 stimulation alone in certain cell types or when caspase activation is simultaneously blocked (19, 31–33). Although the RIPK1 kinase activity is dispensable for survival signaling (19, 34), the kinase activity of both RIP kinases is crucial for initiating a signaling cascade that leads to necroptotic cell death (27, 29, 30).
RIPK1 is thus a central molecule in the diverse TNF-induced signaling pathways: it functions as a platform mediating survival signals and as an active kinase during necroptosis and apoptosis in the presence of Smac mimetics. In this study, by exploring the structure-function relationship of RIPK1 in necroptosis, we developed a genetic model for TNF-induced RIPK1-dependent apoptosis in L929sA cells. Our model resembles the model of RIPK1-dependent apoptosis induced by TNF plus Smac mimetics but has the advantage that no additional factors are required so that this cellular model is very useful to study the downstream targets of RIPK1 kinase leading to apoptosis.
EXPERIMENTAL PROCEDURES
Cell Lines
L929sA, a TNF-sensitive derivative of the murine fibrosarcoma cell line L929, was transfected with cDNA encoding the poxviral caspase-8 inhibitor CrmA or the human Fas receptor. The two resultant strains were L929sACrmA and L929sAhFas cells, respectively (32), and the latter is referred to as L929sA for simplicity. The amphotropic packaging cell line Phoenix-Ampho was provided kindly by the Nolan Laboratory (56); the cells were transfected with a control vector (pdLZRS-IRES-eGFP, constructed by Dr. C. Stove and compatible with Gateway system, Invitrogen) or with a vector containing the RIPK1ΔID sequence (pdLZRS-mRIPK1ΔID-IRES-eGFP). After 30 h, the viral supernatant was harvested and used to infect L929sA cells. GFP-positive cells were selected by flow cytometry-based cell sorting. All cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin (100 IU/ml), streptomycin (0.1 mg/ml), l-glutamine (0.03%), and in the case of Phoenix-Ampho, sodium pyruvate (0.4 mm).
Antibodies, Cytokines, and Reagents
Recombinant human TNF was produced in Escherichia coli and purified to at least 99% homogeneity in our laboratories. The specific biological activity was 3 × 107 IU/ml as determined in a standardized cytotoxicity assay on L929sA cells. The caspase peptide inhibitor Z-VAD-fmk (Bachem, Bubendorf, Switzerland) was used at 10 μm. 5-Diphenyltetrazolium bromide (Sigma Aldrich) was used at 500 mg/ml. Nec-1 (Calbiochem, San Diego, CA) was used at 10 μm. Propidium iodide (Sigma Aldrich) was used at 3 μm. The following antibodies were used for L929sA cells: anti-cIAP1 (RIAP1 antibody (35), a kind gift from Dr. R. G. Korneluk, University of Ottawa, Ottawa, Canada); anti-β-tubulin (HRP) (Abcam, Cambridge, UK); anti-murine caspase-3 (rabbit polyclonal antibody made in-house); anti-cleaved caspase-3 (Asp-175). The antibodies used from Cell Signaling Technology (Beverly, MA) were as follows: anti-phospho-IκBα (Ser-32/36) (5A5); anti-p38 MAPK; anti-phospho-p38 MAPK (Thr-180/Tyr-182); anti-JNK/SAPK. We also used the following antibodies: anti-phospho-JNK/SAPK (pTpY183/185) (Invitrogen); anti-caspase-8 (1G-12) (Alexis Biochemicals, San Diego, CA); anti-IκBα (C21) and anti-TRADD (H-278), (Santa Cruz Biotechnology, Santa Cruz, CA); anti-RIPK1 (610459) (BD Biosciences); anti-RIPK3 (Sigma Aldrich); and anti-FADD (12E7, obtained from Dr. Strasser, WEHI, Melbourne, Australia; M19, sc-6036, Santa Cruz Biotechnology).
Analysis of Cell Survival and Cell Death
Cells were seeded at a density of 7500 cells per well in 96-well BD-imaging plates. After ∼20 h, cells were treated with hTNF (10000 IU/ml) in the presence of Hoechst 33342 (1 μg/ml; Invitrogen) and propidium iodide (PI, 1 μg/ml; Sigma). Images were acquired using a BDPathwayTM 855 instrument (BD Biosciences) equipped with an environmental control unit to ensure a constant temperature of 37 °C and 5% CO2 during image acquisition. Images were taken using a 10× objective (Olympus) in a montage of 4 × 4, including ∼2000 cells per image and treatment condition. Hoechst 33342 labeling was used to segment the nuclei and to extract Hoechst and PI intensity values of each nucleus, with BD Attovision analysis software (BD Biosciences). The percentage of PI-positive nuclei per image was calculated as the percentage of nuclei with PI intensities above the threshold of healthy, untreated nuclei. In other experiments, cell death and DNA fragmentation were analyzed flow cytometrically by measuring PI-emitted fluorescence on an LSR-II with 96-well HTS and FACSDiva software (BD Biosciences) after stimulation with hTNF (10,000 IU/ml) and in situ PI staining (1 μg/ml). Cell death or loss of plasma membrane integrity was measured on freshly harvested cells. DNA fragmentation or hypoploidy was measured after freezing cells at −70 °C and thawing them. To measure cell survival, cells were treated with a concentration gradient of hTNF and survival was determined by a 5-diphenyltetrazolium bromide assay following a standard protocol.
Fluorogenic Substrate Assay for Caspase Activity
The fluorogenic substrate assay was carried out as described (31). Cells were lysed in caspase lysis buffer, and cell debris was removed by centrifugation. Caspase activity was measured by incubating 15 μg of protein with 50 μm Ac-DEVD-MCA (3171-V, peptide, Scientific Marketing Associate) in 150 μl of cell-free system buffer containing 10 mm Hepes, pH 7.4, 220 mm mannitol, 68 mm sucrose, 2 mm NaCl, 2.5 mm KH2PO4, 0.5 mm EGTA, 2 mm MgCl2, 0.5 mm sodium pyruvate, 0.5 mm l-glutamine, and 10 mm dithiothreitol. The release of fluorescent aminomethylcoumarin was measured for 1 h at 2-min intervals by fluorometry (excitation at 360 nm and emission at 480 nm) (Cytofluor; PerSeptiveBiosystems, Cambridge, MA); the maximal rate of increase in fluorescence was calculated (ΔF/min).
RNAi-mediated Knockdown
L929sA cells were transfected in six-well plates according to the manufacturer's protocol using siRNA targeting caspase-8, TRADD, or RIPK3. As a negative control, we used siCONTROL non-targeting siRNA (ON-TARGETplus SMARTpool siRNA; Dharmacon, Thermo Fisher Scientific, Waltham, MA). INTERFERin (Polyplus-transfection SA, Illkirch, France) was used as a transfection reagent. After 72 h, L929sA cells were stimulated with hTNF, and cell survival or death was determined as described above. Knockdown efficiency was checked by Western blot.
Immunoprecipitation
For immunoprecipitation of FADD, L929sA cells were seeded at 3.5 × 106 cells per 60-cm2 Petri dish. The next day, cells were lysed and scraped in 1 ml of Nonidet P-40 buffer (150 mm NaCl, 1% Nonidet P-40, 10% glycerol, 10 mm Tris, pH 8) containing complete, EDTA-free protease inhibitor mixture tablets (11873580001) and phosphatase inhibitor mixture tablets (PhosSTOP, 04906837001) (both from Roche Diagnostics). Beads were incubated in 5% BSA for 8 h at room temperature before binding of anti-FADD antibody (M19, sc-6036, Santa Cruz Biotechnology) to the beads overnight at 4 °C. The next day, FADD was immunoprecipitated at 4 °C overnight. Beads were washed three times in Nonidet P-40 buffer and boiled in sample buffer before Western blot analysis. For immunoprecipitation of processed caspase-8, L929sA cells were lysed in RIPA buffer and incubated for 3 h at 4 °C with streptavidin-agarose beads (Invitrogen, S951), which had previously been incubated with biotin-IETD-fmk (MBL, JM-1121-20C).
High Resolution Live Cell Imaging
L929sA cells were seeded in an eight-well chambered coverglass (NalgeNunc Intl., Rochester, NY) and stimulated with hTNF (10,000 IU/ml) in the presence of Hoechst 33342 (0.5 μg/ml, Invitrogen) and propidium iodide (1 μg/ml). Time-lapse microscopy was done using an Application Solution multidimensional work station (Leica Microsystems, Wetzlar, Germany), including a DM IRE2 microscope, HCX PL APO 63×/1.3 glycerin-corrected 37 °C objective, 75-W Xenon burner (with monochromator) and a 12-bit Coolsnap HQ Camera. During acquisition, cells were kept at 37 °C in a 5% CO2 environment. Cell morphology was observed using differential interference contrast. Hoechst 33342 and PI were excited at 380 and 540 nm, respectively, and emission was detected using a BP340/80/FT400/LP425 filter cube and a BP515–560/FT580/LP590 filter cube, respectively. Z-stack images (1-μm interval) were acquired every 15 min. Maximum intensity projections (for Hoechst 33342 and PI) and autofocus images (for differential interference contrast) were made for each time point using an in-house script for Fiji public domain imaging software.
RESULTS
Ectopic Expression of RIPK1ΔID Induces Shift from Necroptosis to Apoptosis in Response to TNF
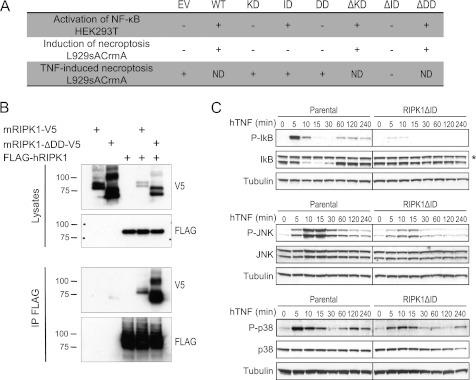
RIPK1 contains an N-terminal kinase domain (KD) followed by an ID and a C-terminal death domain (DD) (supplemental Fig. S1A). To study the role of the different RIPK1 domains in TNF-induced necroptosis, we performed a structure-function analysis. We overexpressed the three single-domain mutants (KD, ID, and DD) and the three domain-deletion mutants (ΔKD, ΔID, and ΔDD) of RIPK1 (supplemental Fig. S1A) in L929sA CrmA cells and analyzed the ability of each mutant to induce necrotic cell death. Mouse fibrosarcoma L929sA cells die by necroptosis in response to TNF, even in the absence of caspase inhibitors and transcription or translation inhibitors (31). Nevertheless, these initial studies were performed in the presence of the caspase-8 inhibitor CrmA to ensure necrotic conditions. A viability assay showed that ectopic expression of wild-type RIPK1 (WT), RIPK1ΔKD, or RIPK1ΔDD induced cytotoxicity even in absence of any trigger (Fig. 1A and supplemental Fig. S1B). These observations suggest that ectopically expressed RIPK1 can oligomerize in the absence of DD, which was confirmed in HEK293T cells by demonstrating interaction of overexpressed RIPK1ΔDD with full-length RIPK1 (Fig. 1B). Our results further indicate that ID is involved in induction of necrotic cell death, as ectopic expression of RIPK1ΔID did not induce cell death. Even more, stimulation with TNF resulted in substantially lower induction of necrotic cell death in these cells as compared with L929sA CrmA cells expressing the other RIPK1 mutants (Fig. 1A and supplemental Fig. S1C), further demonstrating the involvement of ID in cell death induction.
FIGURE 1.
Absence of the RIPK1 intermediate domain inhibits TNF-induced necroptosis and prosurvival signaling. A, overview of the results of the structure-function analysis of RIPK1 in TNF signaling. A series of RIPK1 mutants was overexpressed in HEK293T or L929sACrmA cells as indicated, and their effect on activation of NF-κB, induction of necroptosis, and TNF-induced necroptosis was analyzed. −, no induction of NF-κB or necroptosis; +, induction of NF-κB or necroptosis; ND, not done due to toxic effect of overexpression. B, V5-tagged mRIPK1ΔDD and mRIPK1 were overexpressed together with FLAG-tagged hRIPK1 in HEK293T cells, and their interaction was analyzed by FLAG-mediated immunoprecipitation. C, parental and RIPK1ΔID expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) for the indicated durations. Lysates were made and analyzed for phospho-IκB and total IκB (upper panel), phospho-JNK, and total JNK (middle panel), and phospho-p38 and total p38 (lower panel). An asterisk indicates a nonspecific band.
As RIPK1 is important for activation of NF-κB in several signaling pathways (36), we also analyzed the ability of each RIPK1 mutant to induce an NF-κB-dependent luciferase reporter. Because overexpression of several RIPK1 mutants in L929sA CrmA cells rapidly resulted in necrotic death, this assay was performed in HEK293T cells, which are devoid of the necrotic mediator RIPK3 (29). Overexpression of RIPK1 ID was sufficient to induce activation of NF-κB, whereas overexpression of RIPK1ΔID failed to do so (Fig. 1A and supplemental Fig. S1D), confirming the crucial role of ID in this pathway, which is known to contain several regions responsible for interaction with proteins involved in the NF-κB activation pathway.
We next analyzed whether ectopically expressed RIPK1ΔID acted as dominant negative on TNF-induced NF-κB and MAPK signaling in L929sA cells. In cells expressing RIPK1ΔID and stimulated with TNF, both early and late waves of NF-κB activation were abrogated in comparison with parental cells (Fig. 1C). This abrogation was likely caused by the absence of Lys-377 in RIPK1ΔID, which has been reported to act as acceptor site for the polyubiquitin chains that allow recruitment of the TAB-TAK and IκB kinase complexes (5, 6). Similarly, ectopic expression of RIPK1ΔID strongly reduced both early and late phosphorylation of p38 and JNK in response to TNF (Fig. 1C). We therefore decided to focus our study on RIPK1ΔID because this mutant acted as a dominant negative, inhibiting both TNF-induced NF-κB activation in L929sA and necroptosis in L929sA CrmA cells.
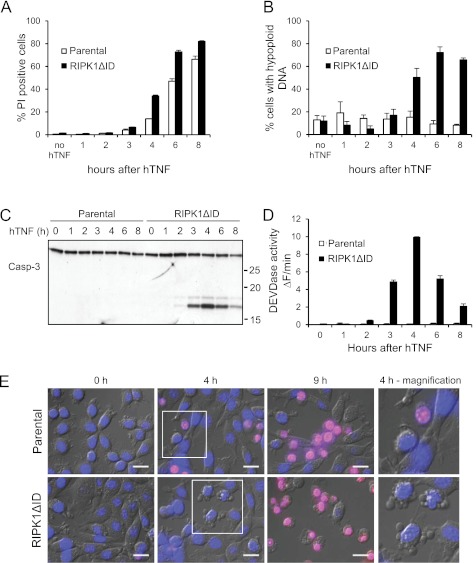
Necroptosis depends on the kinases RIPK1 and RIPK3, which interact through their RHIM domains to form the necrosome (28, 37). Because the RHIM domain of RIPK1 is situated in its ID, the dominant negative effect of RIPK1ΔID on TNF-induced necroptosis can be explained by defects in necrosome formation. Intriguingly, in the absence of caspase inhibition, L929sA cells expressing RIPK1ΔID showed more cell death in response to TNF than parental cells (Fig. 2A). Analysis of several biochemical parameters, such as DNA hypoploidy (Fig. 2B), caspase-3 cleavage (Fig. 2C), and caspase activity (Fig. 2D), revealed that cells expressing RIPK1ΔID underwent apoptosis, whereas parental L929sA cells underwent necroptosis. The biochemical assessment of the RIPK1ΔID-induced shift from necroptosis to apoptosis was confirmed by analysis of cell morphology, which showed typical apoptotic features such as blebbing and DNA condensation (Fig. 2E, bottom panels), whereas parental L929sA cells had a typical necrotic morphology (Fig. 2E, upper panels). Together, these results demonstrate that absence of the ID of RIPK1 causes L929sA cells to respond to TNF by apoptosis rather than by necroptosis. This finding indicates that apoptosis in parental L929sA cells is inhibited by a RIPK1-dependent mechanism that relies on RIPK1 ID.
FIGURE 2.
Ectopic expression of RIPK1ΔID induces a shift from necroptosis to apoptosis in response to TNF. A, parental and RIPK1ΔID-expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) and cell death (% PI-positive cells) was analyzed by flow cytometry. B, cells from A were freeze-thawed, and their content of hypoploid DNA was analyzed by flow cytometry. C and D, parental and RIPK1ΔID-expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) for the indicated time points. Lysates were collected and analyzed for caspase-3 cleavage (Casp-3; C) and caspase activity (D). Error bars represent standard deviation of biological triplicates. E, parental and RIPK1ΔID-expressing L929sA cells were stained with Hoechst 33242 (blue) and PI (red) for live-cell imaging and monitored for 12 h after stimulation with hTNF (10,000 IU/ml). Representative overlay snapshots of the fluorescent and transmitted light images at the indicated time points after hTNF stimulation are shown. Scale bar, 20 μm.
Ectopic Expression of RIPK1ΔID Promotes Formation of Caspase-8-activating Complex Independently of TRADD
Internalization of TNFR1 induces the transition from a prosurvival complex I to a cytosolic death-inducing complex II that contains FADD, caspase-8, RIPK1, and RIPK3 (2, 27). Depending on the stimulus and cell line, other signaling molecules, such as TRADD, might also be present in this complex (2, 23).
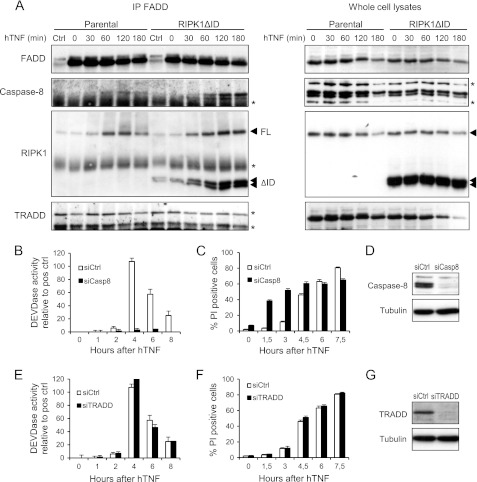
Our results indicated that distinct functional complexes are formed during necroptotic and RIPK1ΔID-dependent apoptotic cell death in response to TNF. We therefore analyzed the composition of TNFR1 complex II by immunoprecipitating FADD in parental and RIPK1ΔID-expressing L929sA cells after different durations of TNF stimulation. We found similar time-dependent increases in recruitment of endogenous RIPK1 to FADD, with a peak between 60 and 120 min in both cell types (Fig. 3A). The recruitment of RIPK1ΔID to FADD in cells expressing RIPK1ΔID followed similar kinetics (Fig. 3A). In contrast, recruitment of caspase-8 to FADD 2 h after TNF treatment was enhanced only in cells expressing RIPK1ΔID (Fig. 3A), which is in line with the shift of these cells toward apoptosis. Furthermore, caspase-8 activation in RIPK1ΔID-expressing cells was confirmed by immunoprecipitating processed caspase-8 p45 fragments using biotin-IETD-fmk as an activity-based probe (supplemental Fig. S2). Together, those results suggest that absence of RIPK1 ID allows apoptotic signaling by facilitating the formation of the caspase-8 activating complex. In contrast to caspase-8, no substantial levels of TRADD could be detected in the necroptotic or apoptotic cell death-inducing complex (Fig. 3A), illustrating that the presence of TRADD in TNFR1 complex II might vary with cell type and stimulus (2, 23).
FIGURE 3.
Ectopic expression of RIPK1ΔID promotes the formation of a caspase-8 activating complex. A, parental and RIPK1ΔID-expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) after pretreatment with Z-VAD-fmk (20 μm). Lysates were made at the indicated time points, and FADD was immunoprecipitated. Levels of FADD, caspase-8, RIPK1, RIPK1ΔID, and TRADD in the immunocomplex were measured by Western blot. An asterisk indicates nonspecific bands. B and C, L929sA cells expressing RIPK1ΔID were transfected with siRNA targeting caspase-8 before stimulation with hTNF (10,000 IU/ml). Caspase activity (B) and cell death (C) (% PI-positive cells measured using a BDPathwayTM 855 instrument) were analyzed at the indicated time points. D, Western blot showing efficiency of caspase-8 knockdown. E and F, L929sA cells expressing RIPK1ΔID were transfected with siRNA targeting TRADD before stimulation with hTNF (10,000 IU/ml). Caspase activity (E) and cell death (F) (% PI-positive cells measured using a BDPathwayTM 855 instrument) were analyzed at the indicated time points. G, Western blot showing efficiency of TRADD knockdown. Error bars represent S.D. of biological triplicates. Ctrl, control.
To confirm the contribution of caspase-8 in TNF-induced apoptosis in cells expressing RIPK1ΔID, and to further investigate the role of TRADD therein, cells deficient in caspase-8 or TRADD were generated by an RNAi technique (Fig. 3, D and G). Knockdown of caspase-8 abrogated TNF-induced caspase activity (Fig. 3B). However, this did not rescue the cells from TNF-induced cell death (Fig. 3C). On the contrary, cell death was accelerated, even when caspase activity was absent, indicating that cells underwent a shift back to caspase-independent necroptosis. In these conditions, Nec-1 or knockdown of RIPK3 strongly diminished TNF-induced cell death (supplemental Fig. S3, A and B). Addition of the pan-caspase inhibitor Z-VAD-fmk to L929sA cells expressing RIPK1ΔID induced a similar shift to necroptosis in response to TNF (supplemental Fig. S3C). These results illustrate that caspase-8 deficiency or inhibition favors necroptosis, as demonstrated by others in cell lines (19, 31, 38) and recently also in vivo (39–41). On the other hand, knockdown of TRADD affected neither caspase activity nor apoptotic cell death (Fig. 3, E–G), which is in agreement with the observation that TRADD is not present in TNFR1 complex II when RIPK1ΔID is expressed. This resembles the apoptotic pathway activated when TNF and Smac mimetics are used together (23).
In Absence of ID of RIPK1, TNF-induced Formation of Caspase-8 Activating Complex II Requires RIPK1 Kinase Activity
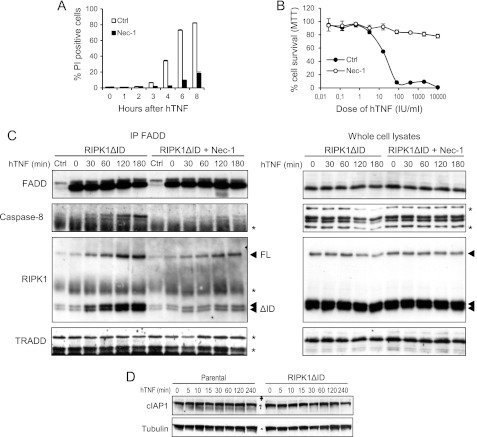
RIPK1 kinase activity is crucial not only for TNF-induced necroptosis but also for apoptosis under specific conditions, e.g. after TNF stimulation in the presence of Smac mimetics (23, 42, 43). Therefore, we investigated whether RIPK1 kinase activity contributes to TNF-induced apoptosis in cells expressing RIPK1ΔID. Inhibition of RIPK1 kinase activity by Nec-1 almost completely inhibited the apoptotic response to TNF (Fig. 4A), and this inhibition persisted for 18 h after stimulation (Fig. 4B). These results clearly indicate that activation of RIPK1 kinase activity is causally linked to TNF-induced apoptosis in this cellular system.
FIGURE 4.
TNF-induced formation of the caspase-8 activating complex II and consequent apoptosis are dependent on RIPK1 kinase activity. A and B, L929sA cells expressing RIPK1ΔID were pretreated with Nec-1 (10 μm) for 1 h and stimulated with hTNF (10,000 IU/ml). Cell death (% PI-positive cells) was analyzed by flow cytometry (A) or cell survival was measured 20 h after hTNF treatment by 5-diphenyltetrazolium bromide (MTT) B, error bars represent S.D. of biological triplicates. C, parental and RIPK1ΔID-expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) after treatment with Z-VAD-fmk (20 μm) and Nec-1 (10 μm). Lysates were made at indicated time points, and FADD was immunoprecipitated. Levels of FADD, caspase-8, RIPK1, RIPK1ΔID, and TRADD in the immunocomplex were measured by Western blot. An asterisk indicates nonspecific bands. D, parental and RIPK1ΔID-expressing L929sA cells were stimulated with hTNF (10,000 IU/ml) for the indicated durations. Lysates were made and analyzed for cIAP1 levels by Western blot.
Next, we investigated whether Nec-1 affects the formation of TNFR1 complex II. In line with the observation that Nec-1 rescued cells expressing RIPK1ΔID from TNF-induced apoptosis, Nec-1 pretreatment almost completely abrogated the interaction of caspase-8 with FADD (Fig. 4C). In addition, recruitment of endogenous RIPK1 and ectopic RIPK1ΔID in the FADD complex was significantly decreased. Collectively, these results indicate that RIPK1ΔID promotes the formation of a caspase-8-activating complex by a mechanism that requires RIPK1 kinase activity.
Remarkably, our cellular model of RIPK1 kinase-dependent apoptosis is very similar to the TNF plus Smac mimetic model. Smac mimetics induce autodegradation of cIAPs, preventing TAK1 recruitment and activation of the MAPK and NF-κB pathways and preventing RIPK1 from integrating into and activating death complexes (7, 11–13). Depending on the cell line, this enhances either necrotic or apoptotic cell death. Because of the close resemblance of our model to the Smac mimetic model, we checked whether ectopic expression of RIPK1ΔID affected cIAP1 levels after TNF stimulation. cIAP1 levels remained unchanged during RIPK1-dependent apoptosis in cells expressing RIPK1ΔID (Fig. 4D).
In conclusion, our cellular system provides an elegant genetic model for studying RIPK1 kinase-dependent apoptosis. In contrast to the TNF plus Smac mimetic model, in our model, RIPK1 kinase-dependent apoptosis does not rely on cIAP depletion, making it particularly interesting for identifying RIPK1 targets in apoptotic signaling.
Reconstitution of RIPK1-deficient Cells with RIPK1ΔID Leads to TNF-mediated RIPK1 Kinase-dependent Apoptosis
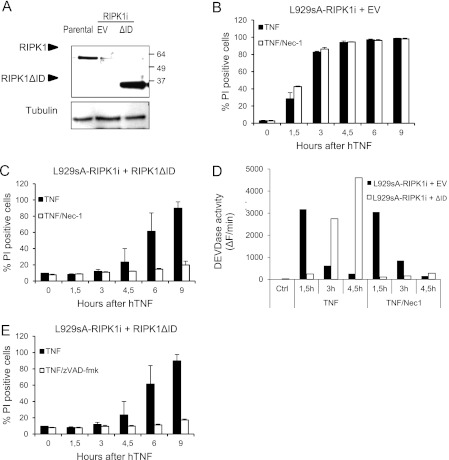
To evaluate the contribution of endogenous RIPK1 to our observations, L929sA cells deficient in RIPK1 were generated by stable expression of a miRNA directed against the 3′ UTR of the RIPK1 mRNA (Fig. 5A). As previously reported, L929sA cells deficient in RIPK1 switch from a necrotic to an apoptotic pathway in response to TNF, which occurs independently of RIPK1 kinase activity (Fig. 5, B and D) (44). Interestingly, expression of RIPK1ΔID in RIPK1-depleted cells delayed caspase activation and shifted the cell death response to RIPK1 kinase-dependent apoptosis, as Nec-1 prevented against caspase activation and consequent cell death (Fig. 5, C and D). These results further suggest that endogenous RIPK1 is not involved in apoptosis induction and that RIPK1 ID prevents RIPK1 kinase activity from initiating apoptotic signaling. Interestingly, whereas addition of zVAD-fmk to wild-type L929sA cells expressing RIPK1ΔID shifted the response to necrosis (supplemental Fig. S3C), Z-VAD-fmk protected the RIPK1-depleted cells reconstituted with RIPK1ΔID (Fig. 5E). These results indicate that endogenous RIPK1 is required for the switch back to the necrotic signaling cascade in the wild-type L929sA cells expressing RIPK1ΔID.
FIGURE 5.
Ectopic expression of RIPK1ΔID in RIPK1-depleted cells shifts the cell death response to apoptosis inhibited by Nec-1. L929sA cells were transduced with a miRNA directed against the 3′ UTR of the RIPK1 mRNA (RIPK1i) and reconstituted with an empty vector (EV) or RIPK1ΔID. A, expression levels of endogenous RIPK1 and RIPK1ΔID in parental L929sA cells and L929sA-RIPK1i + EV or RIPK1ΔID were analyzed by Western blot. B and C, RIPK1-depleted L929sA cells reconstituted with empty vector (B) or RIPK1ΔID (C) were stimulated with hTNF (10,000 IU/ml) after treatment with Nec-1 (10 μm), and cell death levels were analyzed via PI uptake. D, lysates were made from cells in B and C, and caspase activity was analyzed via DEVDase activity. E, RIPK1-depleted L929sA cells reconstituted with RIPK1ΔID were stimulated with hTNF (10,000 IU/ml) after treatment with Z-VAD-fmk (20 μm), and cell death levels were analyzed via PI uptake. Error bars represent S.D. of biological duplicates. Ctrl, control.
DISCUSSION
The last decade of research revealed an important role for RIPK1 in TNFR1 signaling pathways, involving different RIPK1 domains and posttranslational modifications (28, 45). Most cell lines respond to TNF by activating prosurvival or proinflammatory pathways and require the addition of transcriptional or translational inhibitors for cell death to occur. Alternatively, addition of Smac mimetics has been shown to greatly sensitize cells to death, which in most cell lines is executed via an apoptotic program. Although RIPK1 is dispensable for the classical apoptotic pathway induced by TNF, in the presence of Smac mimetics, RIPK1 is converted into a prodeath molecule that is required for the formation of a caspase-8 activating complex (7, 9, 19, 21–23). In this study, by performing a structure-function analysis of RIPK1 in TNF signaling, we observed that ectopic expression of a RIPK1 mutant lacking the intermediate domain (RIPK1ΔID) in L929sA cells converted the cellular response to TNF from RIPK1 kinase-dependent necroptosis to RIPK1 kinase-dependent apoptosis. Thus, our experimental setup represents a genetic cellular model for RIPK1 kinase-dependent apoptosis, which resembles the apoptotic pathway in response to TNF plus Smac mimetics in several aspects (23). First, because in both cellular systems RIPK1 cannot be conjugated with non-degradative polyubiquitin chains, TNFR1 complex I signaling is abrogated. Second, formation of the caspase-8-activating complex is enhanced in both systems, and the complex is devoid of TRADD. This indicates that RIPK1 kinase-dependent apoptosis is different from classical apoptosis, in which TRADD serves as an initial platform for recruitment of FADD and caspase-8 (2, 44, 46, 47). Third, both pathways require kinase-active RIPK1 for formation of the caspase-8-activating complex and subsequent cell death, which were abrogated in the presence of kinase-inactive RIPK1.
The observed shift from TNF-induced necrosis to apoptosis upon deletion of the ID domain suggests that the ID domain harbors an anti-apoptotic function. Indeed, RIPK1ΔID lacks both the Lys-377 ubiquitylation site and the RHIM domain, the absence of which eliminates both survival signaling and necroptotic signaling, respectively. Therefore, it is conceivable that the simultaneous deletion of both functions is the cause of the observed shift in cell death mode from necroptosis to apoptosis. However, blockade of survival signaling and necroptosis in parental L929sA cells by depletion of both cIAPs and RIPK3 does not cause a shift to apoptosis but instead cells survive (11), indicating that an additional anti-apoptotic function resides in RIPK1 ID.
Depleting caspase-8 in RIPK1ΔID-expressing cells shifted the TNF response back to RIPK-dependent necroptosis. Thus, our data illustrate that TNF-induced cell death in L929sA cells is regulated at several levels by RIPK1 ID and caspase-8. Parental cells by default activate RIPK1/RIPK3 kinase-dependent necroptosis. However, deleting the RIPK1 ID apparently removes an anti-apoptotic signal emanating from the ID, and the result is RIPK1 kinase-dependent apoptosis. In turn, depletion of caspase-8 in these conditions redirects the cell death response to RIPK1/RIPK3 kinase-dependent necroptosis. These results are in line with the observation that the proapoptotic proteins caspase-8 and FADD negatively regulate RIPK1/RIPK3-dependent necroptosis not only on the cellular level (19, 31, 38), but also in vivo (39–41). Deletion of RIPK1 and RIPK3 rescued embryonic lethality of FADD and caspase-8 deficiency in mice, respectively, indicating that embryonic lethality in these mice is caused by massive necrosis (39–41). As blockade of caspase activation in RIPK1-depleted L929sA cells reconstituted with RIPK1ΔID did not result in a shift to necrosis, we think that the necrotic signaling in L929sA cells expressing both endogenous RIPK1 and RIPK1ΔID is due to recruitment of RIPK3 via RHIM-mediated interaction by endogenous RIPK1.
Our genetic cellular model may have some advantages over the Smac mimetic model for studying RIPK1 kinase-dependent apoptotic pathways. Smac mimetics induce autodegradation of cIAP1 and cIAP2 and affect XIAP activity, thereby abrogating all their functions, which extend beyond mere inhibition of RIPK1 polyubiquitylation. For example, XIAP effectively binds and inhibits caspase-3, -7, and -9, whereas cIAP1/2 might target caspase-3 and -7 for proteasome-mediated degradation (48, 49). Hence, Smac mimetics not only sensitize for cell death by their action on RIPK1 but also by relieving caspase inhibition. Since expression of RIPK1ΔID specifically targets RIPK1 signaling in TNFR1 complex I without affecting the function of XIAP and other functions of cIAP1/2, as is the case when using Smac mimetics, the L929sA cell line expressing RIPK1ΔID can be considered an elegant genetic model of TNF-induced apoptosis that is RIPK1 kinase-dependent. This cellular model of RIPK1 kinase-dependent apoptosis has several potential applications. It is of particular interest for investigating the underlying mechanisms that drive cells to either RIPK1/RIPK3 kinase-dependent necroptosis in parental L929sA cells or to apoptosis in RIPK1ΔID expressing L929sA cells in response to the same death stimulus. To date, downstream substrates of RIPK1 during necroptotic or apoptotic signaling are unknown. Use of this cellular system in phosphoproteomic studies will allow identification of the differential RIPK1-mediated phosphoproteome in both signaling pathways.
Finally, our results shed new light on the use of necrostatin-1 in vivo. Nec-1 currently is used as an inhibitor of necroptosis and has been proven successful in the treatment of several pathological conditions such as neurodegenerative diseases, ischemia-reperfusion injury, myocardial infarction, and head trauma (50–53). However, one should interpret these results with caution as RIPK1 kinase activity is not linked exclusively to TNF-induced necroptosis but in some conditions it is also critical for activating caspase-8 in TNFR1 complex II. RIPK1 kinase-dependent apoptosis has been demonstrated in the presence of Smac mimetics (23) as well as after stimulation with TWEAK, which induces cIAP degradation upon binding to its cognate receptor FN14 and thus represents a more physiological context in which RIPK1 kinase-dependent apoptosis may occur (54, 55). Hence, protection by Nec-1 points to involvement of the RIPK1 kinase activity, which does not necessarily mean that necroptosis is implicated. In these studies, precise analysis of cell death mode and/or validation of the results in RIPK3-deficient conditions is therefore appropriate.
Supplementary Material
Acknowledgments
We thank Sigrid Cornelis, Saskia Lippens, and Franky Van Herreweghe for scientific discussion; Isabel Vanoverberghe, Wilma Burm, and Ann Meeuws for expert technical assistance; and Amin Bredan for excellent editing.
This work was supported by European grants (FP6 ApopTrain, MRTN-CT-035624; FP7 European Commission for Research and Technological Development Integrated Project, Apo-Sys, FP7–200767; Euregional PACT II), Belgian grants (Interuniversity Attraction Poles, IAP 6/18), Flemish grants (Research Foundation Flanders, FWO G.0875.11 and FWO G.0973.11), Ghent University grants (Multidisciplinary Research Partnerships, GROUP-ID consortium), and grants from Flanders Institute for Biotechnology (VIB).

This article contains supplemental Figs. S1–S3.
- TNFR1
- tumor necrosis factor receptor 1
- RIPK1
- receptor-interacting protein kinase 1
- FADD
- Fas-associated death domain
- TRADD
- TNF receptor-associated death domain
- RHIM
- RIP homotypic interaction motif
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- PI
- propidium iodide
- KD
- kinase domain
- DD
- death domain
- eGFP
- enhanced GFP
- hTNF
- human TNF.
REFERENCES
- 1. Wilson N. S., Dixit V., Ashkenazi A. (2009) Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 10, 348–355 [DOI] [PubMed] [Google Scholar]
- 2. Micheau O., Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 3. Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., Mentlein R., Kabelitz D., Schütze S. (2004) Compartmentalization of TNF receptor 1 signaling: Internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 [DOI] [PubMed] [Google Scholar]
- 4. Wajant H., Scheurich P. (2011) TNFR1-induced activation of the classical NF-κB pathway. FEBS. J. 278, 862–876 [DOI] [PubMed] [Google Scholar]
- 5. Ea C. K., Deng L., Xia Z. P., Pineda G., Chen Z. J. (2006) Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 [DOI] [PubMed] [Google Scholar]
- 6. Li H., Kobayashi M., Blonska M., You Y., Lin X. (2006) Ubiquitination of RIP is required for tumor necrosis factor α-induced NF-κB activation. J. Biol. Chem. 281, 13636–13643 [DOI] [PubMed] [Google Scholar]
- 7. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 8. Mahoney D. J., Cheung H. H., Mrad R. L., Plenchette S., Simard C., Enwere E., Arora V., Mak T. W., Lacasse E. C., Waring J., Korneluk R. G. (2008) Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl. Acad. Sci. U.S.A. 105, 11778–11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varfolomeev E., Goncharov T., Fedorova A. V., Dynek J. N., Zobel K., Deshayes K., Fairbrother W. J., Vucic D. (2008) c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vince J. E., Pantaki D., Feltham R., Mace P. D., Cordier S. M., Schmukle A. C., Davidson A. J., Callus B. A., Wong W. W., Gentle I. E., Carter H., Lee E. F., Walczak H., Day C. L., Vaux D. L., Silke J. (2009) TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-κB and to prevent TNF-induced apoptosis. J. Biol. Chem. 284, 35906–35915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vanlangenakker N., Vanden Berghe T., Bogaert P., Laukens B., Zobel K., Deshayes K., Vucic D., Fulda S., Vandenabeele P., Bertrand M. J. (2011) cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Donnell M. A., Legarda-Addison D., Skountzos P., Yeh W. C., Ting A. T. (2007) Ubiquitination of RIP1 regulates an NF-κB-independent cell death switch in TNF signaling. Curr. Biol. 17, 418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Legarda-Addison D., Hase H., O'Donnell M. A., Ting A. T. (2009) NEMO/IKKγ regulates an early NF-κB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 16, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong W. W., Gentle I. E., Nachbur U., Anderton H., Vaux D. L., Silke J. (2010) RIPK1 is not essential for TNFR1-induced activation of NF-κB. Cell Death Differ. 17, 482–487 [DOI] [PubMed] [Google Scholar]
- 15. Bertrand M. J., Vandenabeele P. (2010) RIP1's function in NF-κB activation: From master actor to onlooker. Cell Death Differ. 17, 379–380 [DOI] [PubMed] [Google Scholar]
- 16. Grimm S., Stanger B. Z., Leder P. (1996) RIP and FADD: Two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc. Natl. Acad. Sci. U.S.A. 93, 10923–10927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanger B. Z., Leder P., Lee T. H., Kim E., Seed B. (1995) RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81, 513–523 [DOI] [PubMed] [Google Scholar]
- 18. Hsu H., Huang J., Shu H. B., Baichwal V., Goeddel D. V. (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 [DOI] [PubMed] [Google Scholar]
- 19. Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J. L., Schneider P., Seed B., Tschopp J. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 [DOI] [PubMed] [Google Scholar]
- 20. Kelliher M. A., Grimm S., Ishida Y., Kuo F., Stanger B. Z., Leder P. (1998) The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 [DOI] [PubMed] [Google Scholar]
- 21. Petersen S. L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. (2007) Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vince J. E., Wong W. W., Khan N., Feltham R., Chau D., Ahmed A. U., Benetatos C. A., Chunduru S. K., Condon S. M., McKinlay M., Brink R., Leverkus M., Tergaonkar V., Schneider P., Callus B. A., Koentgen F., Vaux D. L., Silke J. (2007) IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 [DOI] [PubMed] [Google Scholar]
- 23. Wang L., Du F., Wang X. (2008) TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 [DOI] [PubMed] [Google Scholar]
- 24. Feoktistova M., Geserick P., Kellert B., Dimitrova D. P., Langlais C., Hupe M., Cain K., MacFarlane M., Häcker G., Leverkus M. (2011) cIAPs block Ripoptosome formation, an RIP1/caspase-8-containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., Meier P. (2011) The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 [DOI] [PubMed] [Google Scholar]
- 26. Berghe T. V., Vanlangenakker N., Parthoens E., Deckers W., Devos M., Festjens N., Guerin C. J., Brunk U. T., Declercq W., Vandenabeele P. (2010) Necroptosis, necrosis, and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 [DOI] [PubMed] [Google Scholar]
- 27. Cho Y. S., Challa S., Moquin D., Genga R., Ray T. D., Guildford M., Chan F. K. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Declercq W., Vanden Berghe T., Vandenabeele P. (2009) RIP kinases at the cross-roads of cell death and survival. Cell 138, 229–232 [DOI] [PubMed] [Google Scholar]
- 29. He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 [DOI] [PubMed] [Google Scholar]
- 30. Zhang D. W., Shao J., Lin J., Zhang N., Lu B. J., Lin S. C., Dong M. Q., Han J. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 [DOI] [PubMed] [Google Scholar]
- 31. Vercammen D., Beyaert R., Denecker G., Goossens V., Van Loo G., Declercq W., Grooten J., Fiers W., Vandenabeele P. (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vercammen D., Brouckaert G., Denecker G., Van de Craen M., Declercq W., Fiers W., Vandenabeele P. (1998) Dual signaling of the Fas receptor: Initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin Y., Choksi S., Shen H. M., Yang Q. F., Hur G. M., Kim Y. S., Tran J. H., Nedospasov S. A., Liu Z. G. (2004) Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 279, 10822–10828 [DOI] [PubMed] [Google Scholar]
- 34. Lee T. H., Shank J., Cusson N., Kelliher M. A. (2004) The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 279, 33185–33191 [DOI] [PubMed] [Google Scholar]
- 35. Holcik M., Lefebvre C. A., Hicks K., Korneluk R. G. (2002) Cloning and characterization of the rat homologues of the inhibitor of apoptosis protein 1, 2, and 3 genes. BMC Genomics 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Festjens N., Vanden Berghe T., Cornelis S., Vandenabeele P. (2007) RIP1, a kinase on the cross-roads of a cell's decision to live or die. Cell Death Differ. 14, 400–410 [DOI] [PubMed] [Google Scholar]
- 37. Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. (2010) Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 [DOI] [PubMed] [Google Scholar]
- 38. Bell B. D., Leverrier S., Weist B. M., Newton R. H., Arechiga A. F., Luhrs K. A., Morrissette N. S., Walsh C. M. (2008) FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc. Natl. Acad. Sci. U.S.A. 105, 16677–16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaiser W. J., Upton J. W., Long A. B., Livingston-Rosanoff D., Daley-Bauer L. P., Hakem R., Caspary T., Mocarski E. S. (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oberst A., Dillon C. P., Weinlich R., McCormick L. L., Fitzgerald P., Pop C., Hakem R., Salvesen G. S., Green D. R. (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang H., Zhou X., McQuade T., Li J., Chan F. K., Zhang J. (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Degterev A., Hitomi J., Germscheid M., Ch'en I. L., Korkina O., Teng X., Abbott D., Cuny G. D., Yuan C., Wagner G., Hedrick S. M., Gerber S. A., Lugovskoy A., Yuan J. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knox P. G., Davies C. C., Ioannou M., Eliopoulos A. G. (2011) The death domain kinase RIP1 links the immunoregulatory CD40 receptor to apoptotic signaling in carcinomas. J. Cell Biol. 192, 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vanlangenakker N., Bertrand M. J., Bogaert P., Vandenabeele P., Berghe T. V. (2011) TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Donnell M. A., Ting A. T. (2011) RIP1 comes back to life as a cell death regulator in TNFR1 signaling. FEBS J. 278, 877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ermolaeva M. A., Michallet M. C., Papadopoulou N., Utermöhlen O., Kranidioti K., Kollias G., Tschopp J., Pasparakis M. (2008) Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat. Immunol. 9, 1037–1046 [DOI] [PubMed] [Google Scholar]
- 47. Pobezinskaya Y. L., Kim Y. S., Choksi S., Morgan M. J., Li T., Liu C., Liu Z. (2008) The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat. Immunol. 9, 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gyrd-Hansen M., Meier P. (2010) IAPs: From caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 [DOI] [PubMed] [Google Scholar]
- 49. Choi Y. E., Butterworth M., Malladi S., Duckett C. S., Cohen G. M., Bratton S. B. (2009) The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J. Biol. Chem. 284, 12772–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G. D., Mitchison T. J., Moskowitz M. A., Yuan J. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 [DOI] [PubMed] [Google Scholar]
- 51. Lim S. Y., Davidson S. M., Mocanu M. M., Yellon D. M., Smith C. C. (2007) The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 21, 467–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. You Z., Savitz S. I., Yang J., Degterev A., Yuan J., Cuny G. D., Moskowitz M. A., Whalen M. J. (2008) Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 1564–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yuan J., Lipinski M., Degterev A. (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413 [DOI] [PubMed] [Google Scholar]
- 54. Ikner A., Ashkenazi A. (2011) TWEAK induces apoptosis through a death-signaling complex comprising receptor-interacting protein 1 (RIP1), Fas-associated death domain (FADD), and caspase-8. J. Biol. Chem. 286, 21546–21554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vince J. E., Chau D., Callus B., Wong W. W., Hawkins C. J., Schneider P., McKinlay M., Benetatos C. A., Condon S. M., Chunduru S. K., Yeoh G., Brink R., Vaux D. L., Silke J. (2008) TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFα. J. Cell Biol. 182, 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kinsella T. M., Nolan G. P. (1996) Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7, 1405–1413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.