Background: Auxiliary β subunits regulate the voltage-gated sodium channels of dorsal root ganglion (DRG) neurons.
Results: β subunits are differentially expressed in subpopulations of DRG neurons and regulate Nav1.7 channels in an isoform-specific manner.
Conclusion: Differential β subunit expression and isoform-specific regulation have important implications for the sodium currents of DRG neurons.
Significance: β subunits are important determinants of sodium channel function and sensory neuron excitability.
Keywords: Molecular Cell Biology, Patch Clamp Electrophysiology, Polymerase Chain Reaction (PCR), RNA Abundance, Sodium Channels, Dorsal Root Ganglion, Nociceptor, Sensory Neuron, Single-cell RT-PCR
Abstract
The small-diameter (<25 μm) and large-diameter (>30 μm) sensory neurons of the dorsal root ganglion (DRG) express distinct combinations of tetrodotoxin sensitive and tetrodotoxin-resistant Na+ channels that underlie the unique electrical properties of these neurons. In vivo, these Na+ channels are formed as complexes of pore-forming α and auxiliary β subunits. The goal of this study was to investigate the expression of β subunits in DRG sensory neurons. Quantitative single-cell RT-PCR revealed that β subunit mRNA is differentially expressed in small (β2 and β3) and large (β1 and β2) DRG neurons. This raises the possibility that β subunit availability and Na+ channel composition and functional regulation may differ in these subpopulations of sensory neurons. To further explore these possibilities, we quantitatively compared the mRNA expression of the β subunit with that of Nav1.7, a TTX-sensitive Na+ channel widely expressed in both small and large DRG neurons. Nav1.7 and β subunit mRNAs were significantly correlated in small (β2 and β3) and large (β1 and β2) DRG neurons, indicating that these subunits are coexpressed in the same populations. Co-immunoprecipitation and immunocytochemistry indicated that Nav1.7 formed stable complexes with the β1–β3 subunits in vivo and that Nav1.7 and β3 co-localized within the plasma membranes of small DRG neurons. Heterologous expression studies showed that β3 induced a hyperpolarizing shift in Nav1.7 activation, whereas β1 produced a depolarizing shift in inactivation and faster recovery. The data indicate that β3 and β1 subunits are preferentially expressed in small and large DRG neurons, respectively, and that these auxiliary subunits differentially regulate the gating properties of Nav1.7 channels.
Introduction
The sensory neurons of the dorsal root ganglia (DRG)2 give rise to nerve fibers that convey information about thermal, mechanical, and chemical stimulation from peripheral tissues to the central nervous system. These neurons express a unique combination of tetrodotoxin-sensitive (TTX-S) and tetrodotoxin-resistant (TTX-R) Na+ currents that produce the rapid rising phase of the action potentials. Much of what is currently known about Na+ channel expression in sensory neurons has been derived from electrophysiological studies of cultured DRG neurons (1–3). The small-diameter neurons (<25 μm) isolated from the DRG represent the cell bodies of unmyelinated nociceptors and preferentially express TTX-R Na+ current, whereas the large-diameter neurons (>30 μm), typically associated with low threshold mechanoreceptors, predominately express TTX-S Na+ current. DRG sensory neurons express at least six distinct Na+ channel isoforms that display properties similar to the endogenous TTX-S (Nav1.1, Nav1.2, Nav1.6, and Nav1.7) and TTX-R (Nav1.8 and Nav1.9) Na+ currents observed in these neurons (4–7).
In vivo, voltage-gated sodium channels form complexes with auxiliary β subunits that regulate the trafficking, gating properties, and kinetics of the endogenous Na+ channels (8–12). β subunits are relatively small proteins (33–36 kDa) composed of a single membrane-spanning α helix, a short intracellular C terminus, and a large extracellular N terminus incorporating an immunoglobulin-like fold similar to that found in adhesion molecules (8, 13). Immunocytochemistry and in situ hybridization indicate that all four isoforms of the β subunit (β1–β4) are expressed in sensory neurons (12, 14, 15).
In this study, we employed a combination of single-cell RT-PCR, immunocytochemistry, immunoprecipitation, and electrophysiology to further investigate β subunit expression in DRG sensory neurons. The data indicate that small and large DRG neurons express different complements of β subunits. The functional consequences of β subunit expression were evaluated by examining their regulation of Nav1.7, a TTX-S Na+ channel widely expressed in sensory neurons and an important contributor to pain sensation (19, 20). The β3 and β1 subunits differentially regulated heterologously expressed Nav1.7 channels. The preferential expression of β subunits in small (β2 and β3) and large (β1 and β2) neurons, coupled with the isoform-specific β subunit regulation of Nav1.7 activation (β3) and inactivation (β1), predicts substantial differences in the TTX-S currents of DRG sensory neurons.
EXPERIMENTAL PROCEDURES
Preparation of DRG Neurons
Postnatal day 7 Sprague-Dawley rats (P7) were anesthetized with isoflurane before decapitation, and the DRG were harvested from all accessible levels. The ganglia were incubated for 30 min at 37 °C in 2 ml of Hanks' balanced salt solution/HEPES containing 1.5 mg/ml collagenase (Sigma-Aldrich), followed by 1 mg/ml trypsin (Sigma-Aldrich) for an additional 30 min. Trypsin was removed, and the ganglia were transferred to Leibovitz's L-15 medium supplemented with 1% fetal bovine serum (Invitrogen), 2 mm glutamine, 2% penicillin/streptomycin (Invitrogen), and 50 ng/ml nerve growth factor (Sigma-Aldrich). The ganglia were disrupted using fire-polished Pasteur pipettes, and dissociated neurons were plated onto polylysine-coated glass coverslips and placed into 35-mm dishes containing supplemented Leibovitz's medium. Neurons were suitable for single-cell harvesting and electrophysiology for up to 8 h after plating. Animal protocols were approved by the Animal Care and Use Committee of Thomas Jefferson University.
Single-cell RT-PCR
Detailed methods for performing single-cell RT-PCR with dissociated DRG neurons were published recently (7). Small-diameter (<25 μm) and large-diameter (>30 μm) DRG neurons were individually harvested by drawing them into a large bore pipette (30–50-μm diameter) containing sterile bath solution. The neurons were osmotically lysed by 10-fold dilution with sterile water and rapidly frozen. The mRNA present in the cell lysates was reverse-transcribed using random hexamer primers (Stratagene) in a standard 25-μl Moloney murine leukemia virus reverse transcription reaction (Fisher). Aliquots of the transcription reaction (1–2 μl) were quantitatively analyzed using a SYBR Green reaction mixture on an Mx30005P real-time PCR machine (Agilent Technologies). β-Actin was quantitatively measured in each sample and used to normalize for differences in cellular mRNA expression. The absolute number of mRNA copies of each transcript was determined by comparing the threshold cycle (Ct) of the single-cell lysates with known cDNA standards assayed in parallel reactions. PCR primers were designed to span exon/intron borders to eliminate the detection of genomic DNA, and concentrations (50–200 nm) were optimized to achieve high amplification efficiency without the formation of primer dimers (Proligo, Sigma-Aldrich). The specificity of the real-time detections was assessed using melting curve analysis, and the identity of the amplified DNA was determined by sequencing.
Nav1.7 Stable Cell Line
Rat Nav1.7 cDNA was subcloned into the pcDNA3 expression vector (Invitrogen) and transfected into HEK293 cells using a standard calcium phosphate precipitation method (Invitrogen). After 2 weeks of selection for neomycin resistance (800 μg/ml), the remaining colonies were isolated and transferred to separate culture plates for expansion. Nav1.7 expression was verified using RT-PCR and electrophysiology to measure Na+ currents. The HEK293 cell line stably expressing Nav1.7 was maintained under standard culture conditions in DMEM supplemented with 10% FBS, 2 mm l-glutamine, 100 units/ml penicillin, 10 mg/ml streptomycin, and 400 μg/ml neomycin (Invitrogen).
Electrophysiology
Macroscopic Na+ currents of HEK293 cells stably expressing the Nav1.7 channel were recorded using the whole-cell patch clamp technique. The pipette solution contained 5 mm NaCl, 135 mm CsF, 10 mm EGTA, and 10 mm HEPES (pH 7.4). The bath solution contained 150 mm NaCl, 2 mm KCl, 1.5 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES (pH 7.4). Patch electrodes were fashioned from Corning 8161 borosilicate glass and coated with Sylgard (Dow Corning Corp.) to minimize pipette capacitance. Recording pipettes had low access resistances (<1 megohm), and the residual series resistance was 80% compensated. A correction for the liquid junction potential between the pipette and the bath solutions (−7 mV) was applied to the holding potential before the formation of gigohm seals. After establishing the whole-cell configuration, the cells were dialyzed for 10 min at room temperature (22 °C) prior to recording Na+ currents. Voltage pulses were generated, and currents were recorded using pCLAMP and an Axopatch 200 amplifier (Molecular Devices). Whole-cell currents were filtered at 5 kHz and digitized at 10 kHz with a Digidata 1440A system (Molecular Devices).
Current-voltage relationships were obtained by plotting the current density (picoamperes/picofarad) versus the test voltage. Normalized Na+ conductance (GNa) was calculated from the peak Na+ current (INa) at each test potential (V): GNa = INa/(V − ENa), where ENa is the measured Na+ ion reversal potential. The steady-state inactivation was determined by normalizing the peak Na+ current (I) measured after conditioning prepulses (−130 to −10 mV for 500 ms) to the maximal Na+ current amplitude (Imax) measured after prepulses to −140 mV and plotted against the conditioning voltage. The activation and steady-state inactivation were fitted to Boltzmann functions: G/Gmax(I/Imax) = 1/(1 + exp(V0.5 − V)/kv), where V0.5 is the midpoint, and kv is the slope factor. The predicted window currents were calculated from the product of the activation and steady-state inactivation curves as described previously (21). Recovery from inactivation was determined using depolarizing prepulses (−30 mV/20 ms) before returning to −100 mV for variable intervals (0–1200 ms). Standard test pulses (−30 mV/20 ms) were used to assess availability. The recovery time course was fitted to the sum of two exponentials, yielding estimates of the fast (τf) and slow (τs) time constants.
Rat β1–β3 subunits were cloned in our laboratory as described previously (22). The β4 subunit was a gift from Dr. Lori Isom (University of Michigan). The Na+ channel β1–β4 subunits (piRES/CD8/β1–4) and CD8 cDNA were subcloned into the piRES vector (Clontech). HEK293 cells stably expressing the Nav1.7 channel were transiently transfected with piRES/CD8/β1–4 cDNA using a calcium phosphate precipitation method (23). Prior to recording, the cells were briefly incubated in PBS containing anti-CD8 antibody-coated beads to identify cells expressing the CD8 antigen (Dynal, Lake Success, NY).
β Subunit Chimeras
The β1/β2 chimeras (β211, β221, β112, and β11Δ) were a gift from Dr. Thomas Zimmer (Friedrich-Schiller Universität, Jena, Germany). The three subscripted numbers refer to the extracellular N-terminal, membrane-spanning, and intracellular C-terminal domains. In this nomenclature, the wild-type β1 and β2 subunits are designated β111 and β222, respectively. β211 contains the extracellular domain of β2 and the membrane-spanning and intracellular domains of β1. β221 incorporates the extracellular and membrane-spanning domains of β2 and the intracellular domain of β1. β112 contains the extracellular and membrane-spanning domains of β1 and the intracellular domain of β2. β11Δ contains the extracellular and membrane-spanning domains of β1 and a deletion of the 41 amino acids from the intracellular C-terminal domain (see Fig. 6A). β211, β221, β112, and β11Δ were transferred to the piRES vector for expression in mammalian cells (piRES/CD8/β211, piRES/CD8/β221, piRES/CD8/β112, and piRES/CD8/β11Δ) and transiently transfected into our Nav1.7 stable cell line.
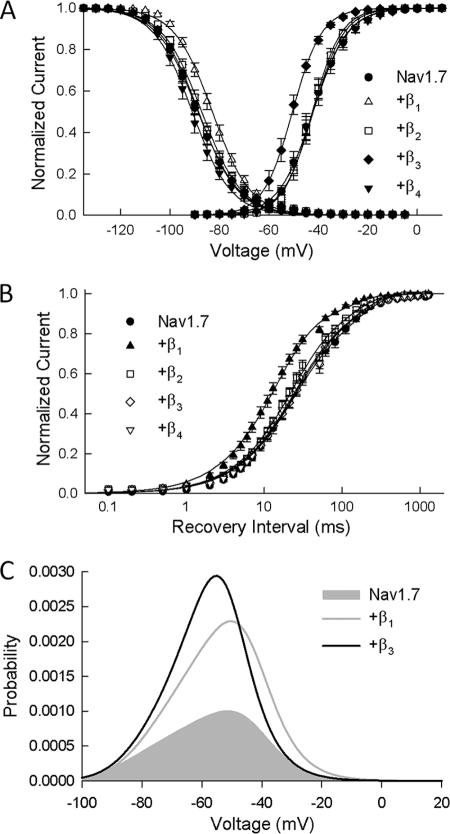
FIGURE 6.
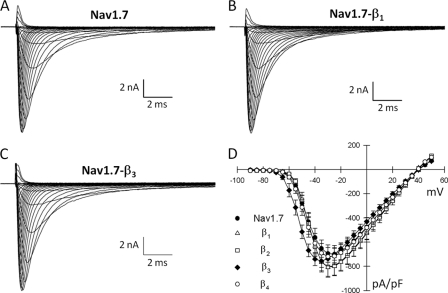
β subunits shift activation and inactivation of Nav1. 7 channels. A, the normalized conductance was determined from the peak Na+ currents and is plotted versus the test potential. Also plotted is the steady-state inactivation obtained using 500-ms prepulses to voltages between −130 and −5 mV. The smooth curves are fits of the activation and inactivation data to Boltzmann functions with the parameters listed in Table 1. Data are the means ± S.E. of 14 (Nav1.7), 26 (β1), 9 (β2), 21 (β3), and 8 (β4) determinations. B, Na+ channels were inactivated by a brief depolarization (−30 mV/20 ms), and the recovery time course (0–1200 ms) was measured at −100 mV. The smooth curves are biexponential curve fits with the fast and slow time constants listed in Table 1. Data are the means ± S.E. of 15 (Nav1.7), 22 (β1), 10 (β2), 17 (β3), and 8 (β4) determinations. C, window current probabilities predicted from the activation and steady-state inactivation of the Nav1.7 channels expressed alone or with either the β1 or β3 subunits.
Immunoprecipitation and Western Analysis
Rat DRG were harvested and immediately placed in ice-cold Hanks' balanced salt solution. The ganglia were washed with ice-cold Hanks' balanced salt solution and pelleted by low speed centrifugation at 4 °C. Hanks' balanced salt solution was replaced with ice-cold lysis buffer (50 mm Tris, 1.0 mm EDTA, 1.0 mm EGTA, 150 mm NaCl, and 1.0% Triton X-100) supplemented with protease inhibitors (Sigma-Aldrich). The samples were homogenized on ice and centrifuged at 15,000 rpm for 20 min at 4 °C. The supernatant was recovered and assayed for protein concentration using the Bradford method (Bio-Rad). Lysates (1 mg) were incubated overnight at 4 °C in 1 ml of lysis buffer containing either 10 μg of control mouse IgG or 10 μg of mouse anti-Nav1.7 monoclonal antibody N68/6 (UC Davis/NIH NeuroMab Facility). Anti-Nav1.7 antibody N68/6 does not cross-react with other Na+ channel isoforms or channel proteins extracted from adult rat brain. Protein G-agarose resin (Thermo Scientific) was added (100 μl), and the lysates were incubated for 6 h at 4 °C before washing with ice-cold lysis buffer. Proteins were eluted from the protein G-agarose by the addition of 50 μl of 0.2 m glycine buffer (pH 2.5). The pH was neutralized by adding 10 μl of 1 m Tris buffer (pH 9.0), mixed with 3× sample buffer, and separated on 12% SDS-polyacrylamide gels. Proteins were transferred to Protran nitrocellulose membranes (Whatman); blocked with 5% BSA; washed with Tris-buffered saline with 0.1% Tween 20 (TBS/Tween); and incubated overnight with rabbit anti-SCN1B (Cell Applications), rabbit anti-SCN2B (Sigma-Aldrich), or rabbit anti-SCN3B (Abcam) polyclonal antibody in TBS/Tween containing 5% BSA. These commercial antibodies (SCN1B, SCN2B, and SCN3B) are highly specific and do not display cross-reactivity with other members of the β subunit family. The membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibody (Thermo Scientific) for 1 h at room temperature, and labeled proteins were detected by chemiluminescence (Thermo Scientific). We routinely failed to observe Nav1.7 or β subunit precipitation from cell lysates preincubated with control IgG, further supporting the specificity of the Nav1.7 immunoprecipitations. The low level expression of the β4 subunits in DRG neurons (see Fig. 1) combined with the poor quality of available anti-β4 antibodies prevented detailed analysis of this protein.
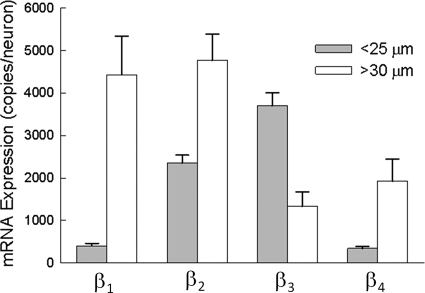
FIGURE 1.
Single-cell analysis of β subunit mRNA. Small-diameter (<25 μm) and large-diameter (>30 μm) DRG neurons were individually harvested, and the mRNA present in the cell lysates was reverse-transcribed and quantitatively measured by real-time PCR. The data are expressed as the number of mRNA copies present in each neuron. The data are the means ± S.E. of 74 small and 21 large neurons.
Immunocytochemistry
Dissociated DRG neurons were plated onto polylysine-coated glass coverslips and fixed in PBS containing 4% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min before several washes with PBS. Nonspecific antibody binding was reduced by incubating the cells with 5% BSA and 5% goat serum in TBS/Tween for 60 min. Permeabilized cells were incubated with mouse anti-Nav1.7 monoclonal antibody or rabbit anti-SCN1B, rabbit anti-SCN2B, or rabbit anti-SCN3B polyclonal antibody (1:500 dilution) for 60 min before adding Alexa Fluor 488-conjugated anti-mouse or Alexa Fluor 594-conjugated anti-rabbit fluorescent secondary antibody (Invitrogen) for 60 min. After several washes with PBS, the coverslips were dried overnight and mounted onto glass slides with Mowiol 4.88 (Calbiochem). The slides were imaged on a Zeiss LSM 510 META confocal microscope equipped with FITC and rhodamine filter sets at the Kimmel Cancer Center at Jefferson Medical College.
RESULTS
The expression of β subunits was investigated in acutely dissociated DRG sensory neurons isolated from 7-day-old neonatal rats. Neurons were individually harvested, and the mRNA present in the cell lysates was quantitatively measured (mRNA copies/neuron) using real-time PCR. Fig. 1 compares the expression of the β subunit transcripts in small-diameter (<25 μm) and large-diameter (>30 μm) DRG neurons. The data indicate that small neurons preferentially expressed the β2 and β3 isoforms (2000–4000 copies/neuron). Although β1 was also detected in these neurons, the mRNA copy number was 5-fold lower (<400 copies/neuron). This contrasts with large-diameter neurons, which highly expressed β1 and β2 mRNAs (≈4500 copies/neuron), whereas β3 was present at lower levels (<2000 copies/neuron). The β4 subunit was expressed at comparatively low levels in both the small (<500 copies/neuron) and large (<2000 copies/neuron) neurons. The data indicate that small (β2 and β3) and large (β1 and β2) DRG neurons express different complements of auxiliary β subunits.
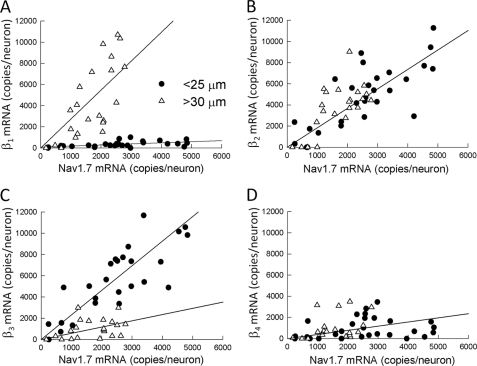
To investigate the relationship between Nav1.7 and β subunits, the mRNAs encoding these subunits were quantitatively measured in small and large DRG neurons. Fig. 2 plots the number of Nav1.7 mRNA copies versus the β subunit mRNA measured from the same neurons. The data were statistically evaluated using Pearson product-moment correlation analysis to determine the strength of mRNA coexpression in these neurons. The Nav1.7-β2 and Nav1.7-β3 mRNAs were found to be significantly correlated, with Pearson coefficients (r) of 0.777 and 0.775, respectively (p < 0.001). Despite the low expression of β1 mRNA (344 copies/neurons), these subunits were significantly correlated with Nav1.7 (r = 0.537, p < 0.01), although the physiological relevance of this association is not clear. The Nav1.7 and β4 mRNAs were not associated in these neurons (r = 0.193). These data indicate that β2 and β3 subunit transcripts are abundantly expressed in small DRG neurons and are significantly correlated with Nav1.7 mRNA.
FIGURE 2.
Correlation of Nav1. 7 and β subunit mRNA expression in small and large DRG neurons. The mRNAs (copies/neuron) of Nav1.7 and β subunits were measured in the same populations of small-diameter (<25 μm) and large-diameter (>30 μm) neurons. Plots are shown of Nav1.7 mRNA versus β1 (A), β2 (B), β3 (C), and β4 (D). The straight lines are simple linear regressions. The data represent the means ± S.E. of mRNA measurements from 29 small and 21 large DRG neurons.
Fig. 2 also shows the correlation of Nav1.7 and β subunit mRNAs in large neurons. Nav1.7 expression was significantly correlated with the β1 (r = 0.732) and β2 (r = 0.680) subunits (p < 0.001). This contrasted with the β3 (r = 0.357, p = 0.112) and β4 (r = 0.342, p = 0.152) subunits, which were not correlated with Nav1.7. The data indicate that the Nav1.7, β1 subunit, and β2 subunit mRNAs are coexpressed in the same population of large-diameter neurons.
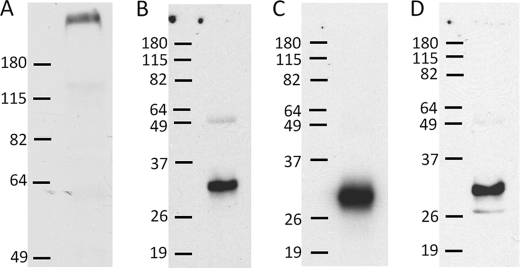
Nav1.7-β interactions were further investigated using co-immunoprecipitation and Western blotting (Fig. 3). Fig. 3A shows a Western blot of DRG homogenates isolated from postnatal day 7 animals probed with the anti-Nav1.7 antibody. The anti-Nav1.7 antibody labeled a single high molecular mass protein (≈270 kDa) that is characteristic of Nav1.7 channels. Immunoprecipitated Nav1.7 complexes were separated on acrylamide gels, transferred to nitrocellulose membranes, and probed with β-specific antibodies. Fig. 3 (B–D) shows that the anti-β subunit antibodies labeled low molecular mass proteins (32–34 kDa) that are slightly smaller than what was previously reported for the β1 (36 kDa) and β2 (33 kDa) subunits of adult rats (24). β subunits are highly glycosylated proteins containing 30–36% carbohydrate by weight (24, 25). Differences in the carbohydrate content of these subunits account for variations in the molecular mass of the β1 subunit expressed in skeletal muscle (25). We speculate that the lower molecular masses observed in P7 animals (≈1–2 kDa) may represent partially glycosylated β subunits (26). The immunoprecipitation data show that the β1–β3 subunits formed stable complexes with Nav1.7 channels isolated from the DRG and are therefore candidates for regulating these channels in vivo. Unfortunately, it is impossible to associate the Nav1.7-β interactions detected using immunoblotting techniques with specific subpopulations of small and large DRG neurons.
FIGURE 3.
Co-immunoprecipitation of Nav1.7 and β subunits. DRG homogenates were separated on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with Nav1.7-specific antibodies (A). Nav1.7 channel complexes were immunoprecipitated from DRG lysates; separated on SDS-polyacrylamide gels; and probed with antibodies specific for β1 (B), β2 (C), and β3 (D). Bars indicate the positions of molecular mass markers (shown in kilodaltons).
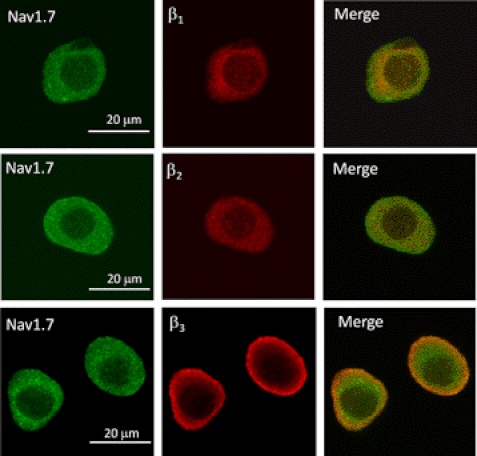
Potential Nav1.7-β subunit interactions were further investigated by immunocytochemistry. Fig. 4 shows the confocal imaging of small neurons labeled with Nav1.7- and β-specific antibodies. The cytoplasm of these neurons displayed diffuse labeling for Nav1.7 and the β1 and β2 subunits. Merged images revealed some overlap of Nav1.7 with the β1 and β2 subunits, predominately within the intracellular compartment. By contrast, the majority of the β3 immunofluorescence was localized along the cell periphery, consistent with the labeling of membrane-bound proteins. The merged images display considerable overlap of Nav1.7 and β3 around the cell periphery, consistent with the co-localization of these proteins near the plasma membrane.
FIGURE 4.
Imaging of Nav1. 7 and β subunits in small DRG neurons. Small-diameter DRG neurons (<25 μm) were immunolabeled with Nav1.7- and β-specific (β1–β3) antibodies and reacted with fluorochrome-conjugated secondary antibodies before confocal imaging. Left panels, Nav1.7 immunostaining; middle panels, β subunit staining; right panels, merged images.
Initial attempts to investigate the β subunit regulation of endogenous Nav1.7 channels in dissociated DRG neurons were complicated by the variable expression of Nav1.7 and β subunits and the presence of multiple overlapping components of TTX-S Na+ current in these neurons. We therefore conducted heterologous expression studies to further investigate the β subunit regulation of Nav1.7 channels. HEK293 cells stably expressing Nav1.7 were transiently transfected with β subunits. Fig. 5 shows examples of whole-cell Na+ currents recorded from cells expressing Nav1.7 alone or with coexpressed β1 or β3 subunits. In the absence of β subunits, the Nav1.7 channels produced rapidly gating Na+ current. Coexpressing β subunits (β1–β4) had no effect on the current kinetics or peak Na+ current amplitudes.
FIGURE 5.
β subunit regulation of heterologously expressed Nav1. 7 channels. Shown are whole-cell Na+ currents of HEK293 cells stably expressing the Nav1.7 channels. Currents were elicited by depolarizing voltage pulses between −90 and +50 mV from a holding potential of −120 mV. A–C, representative Na+ currents of Nav1.7 channels expressed alone (A) or coexpressed with β1 (B) or β3 (C) subunits. D, plot of the peak current density (picoamperes/picofarads) of Nav1.7 channels alone or coexpressed with β subunits (β1–β4). Data are the means ± S.E. of 13 (Nav1.7), 26 (β1), 9 (β2), 18 (β3), and 8 (β4) determinations.
To investigate potential changes in voltage-dependent gating, the Na+ conductance was calculated from the peak currents and plotted versus the test voltage (Fig. 6A). Coexpressing the β3 subunit produced a significant hyperpolarizing shift (−9 mV) in Nav1.7 activation. Steady-state inactivation was determined using 500-ms prepulses to voltages between −130 and −5 mV. β1 induced a depolarizing shift (+5 mV) in the midpoint of Nav1.7 inactivation (Fig. 6A). By contrast, coexpressing the β2 or β4 subunits did not alter the activation or the steady-state inactivation of the channels.
Recovery from inactivation was determined by applying depolarizing prepulses (−20 mV/30 ms) before returning to −100 mV for varying intervals (0–1200 ms). The recovery time course of Nav1.7 channels was biexponential, with τf and τs time constants of 26 and 153 ms, respectively (Fig. 6B). Coexpressing β1 significantly reduced both τf (14 ms) and τs (67 ms), consistent with a more rapid recovery from inactivation (Fig. 6B). The remaining β subunits (β2–β4) had no effect on recovery from inactivation (Table 1).
TABLE 1.
β subunit regulation of Nav1.7 gating
The parameters were obtained from curve fits of Nav1.7 activation, inactivation, and recovery from inactivation (Fig. 6). The data were tested for significant differences by analysis of variance (p < 0.001), followed by Dunnett's post hoc test at a significance level of p < 0.05. For Dunnett's test, the effects of β subunits were compared with values measured for Nav1.7 channels expressed alone. Data are the means ± S.E. of between 8 and 30 experiments.
| Activation |
Inactivation |
Recovery |
|||||
|---|---|---|---|---|---|---|---|
| V0.5 | kv | V0.5 | kv | τf | τs | Af | |
| mV | mV | ms | % | ||||
| Nav1.7 | −42 ± 1 | 6.0 ± 0.3 | −88 ± 2 | 7.2 ± 0.3 | 26 ± 2 | 153 ± 11 | 68 |
| β1 | −43 ± 1 | 5.7 ± 0.3 | −83 ± 1a | 7.2 ± 0.2 | 14 ± 1a | 67 ± 7a | 67 |
| β2 | −43 ± 1 | 5.3 ± 0.3 | −87 ± 1 | 7.2 ± 0.2 | 22 ± 2 | 128 ± 12 | 70 |
| β3 | −51 ± 1a | 5.0 ± 0.3 | −88 ± 1 | 7.1 ± 0.2 | 24 ± 2 | 142 ± 11 | 70 |
| β4 | −41 ± 1 | 6.9 ± 0.2 | −91 ± 2 | 7.6 ± 1.0 | 25 ± 2 | 136 ± 11 | 73 |
| β1/β2 chimeras | |||||||
| β112 | −44 ± 1 | 5.9 ± 0.2 | −83 ± 1a | 6.3 ± 0.1 | 16 ± 1a | 58 ± 6a | 73 |
| β11Δ | −44 ± 1 | 4.9 ± 0.2 | −82 ± 1a | 6.3 ± 0.2 | 16 ± 2a | 60 ± 6a | 71 |
| β211 | −43 ± 1 | 5.4 ± 0.3 | −87 ± 1 | 7.2 ± 0.2 | 22 ± 1 | 126 ± 11 | 69 |
| β221 | −44 ± 1 | 4.6 ± 0.2 | −88 ± 1 | 6.9 ± 0.2 | 22 ± 2 | 133 ± 10 | 67 |
a Values indicate significant differences (p<0.05).
The overlap of activation and steady-state inactivation of Na+ channels defines a range of voltages (i.e. window) where Na+ channels can be partially activated but are not fully inactivated. Na+ channels within this hyperpolarized range of voltages may become persistently activated, resulting in inward Na+ currents that could potentially depolarize the resting membrane potential and increase neuronal excitability. β subunit-induced increases in the overlap of Na+ channel activation and inactivation tend to expand this window and, consequently, the fraction of persistently activated channels. The β1 subunit produced a +5-mV depolarizing shift in steady-state inactivation, whereas β3 produced a −9-mV shift in Nav1.7 activation (Table 1) that could potentially increase the window currents. Fig. 6C shows the predicted window currents of Nav1.7 channels coexpressed with either the β1 or β3 subunits. Despite acting by different mechanisms, the β1 and β3 subunits produced similar 2–3-fold increases in the Nav1.7 window current.
To gain a better understanding of the mechanism of β subunit regulation, chimeras were generated by exchanging the structural domains of the β1 subunit that shifted steady-state inactivation and accelerated recovery from inactivation with the homologous domains of the β2 subunit that had no effect on Nav1.7 gating (Table 1). The extracellular N-terminal, intracellular C-terminal, and membrane-spanning domains of β1 were systematically replaced with those of β2 and transiently expressed in HEK293 cells stably expressing Nav1.7 channels. Chimeras that retained the extracellular N-terminal domain of β1 (β112 and β11Δ) fully recapitulated the hyperpolarizing shift in steady-state inactivation and faster recovery observed with the wild-type β1 subunit (Table 1). Conversely, substitutions that replaced the N terminus of β1 (β211 and β221) completely abolished Nav1.7 regulation. C-terminal deletions of the β1 subunit (β11Δ) retained full activity, indicating that the intracellular domain is not essential. The data indicate that the extracellular N-terminal domain of β1 is critical for the functional regulation of Nav1.7 channels.
DISCUSSION
The goal of this study was to investigate the expression of auxiliary β subunits in DRG neurons and to characterize the β subunit regulation of Nav1.7, a TTX-S Na+ channel widely expressed in sensory neurons. Single-cell analysis demonstrated that β subunit mRNAs were differentially expressed in small (β2 and β3) and large (β1 and β2) DRG neurons (Fig. 1). Comparisons of Nav1.7, β2, and β3 mRNAs measured in individual small neurons showed that the expression of these subunits was significantly correlated (Fig. 2), indicating that these transcripts are coexpressed in the same neurons. By contrast, the Nav1.7 mRNA of large neurons was found to be significantly correlated with the β1 and β2 subunits. These data indicate that the Nav1.7 channels present in small and large DRG neurons are coexpressed with different complements of auxiliary β subunits.
Interactions between Nav1.7 and β subunits were further explored by co-immunoprecipitation of Nav1.7 channels. Nav1.7 coprecipitated with the β1–β3 subunits (Fig. 3), indicating that these subunits form stable complexes in vivo. Despite supporting a direct physical interaction between Nav1.7 and the β1–β3 subunits, it is impossible to ascertain the neurons in which these interactions occurred (i.e. small versus large) using immunoprecipitation techniques. However, immunofluorescence imaging showed that Nav1.7 and β3 co-localized near the periphery of the small DRG neurons (Fig. 4). Although β2 subunits are also highly expressed in small neurons, they failed to display obvious co-localization with Nav1.7 channels. The combination of Nav1.7-β3 mRNA correlation (Fig. 2), co-immunoprecipitation (Fig. 3), and co-localization near the plasma membrane (Fig. 4) supports the idea that β3 subunits partner with Nav1.7 channels. Although these data do not preclude Nav1.7 interaction with other β subunits, they suggest an important contribution of Nav1.7-β3 channels to the TTX-S Na+ currents of small DRG neurons.
Previous studies of β subunit regulation of heterologously expressed Nav1.7 channels have produced conflicting data. Initial studies of Nav1.7 channels expressed in Xenopus oocytes indicated that the β1 and β2 subunits failed to alter the expression or gating properties of Nav1.7, suggesting that these channels may be not regulated by these auxiliary subunits (27, 28). Subsequent work, also in oocytes, found that coexpressing β1 accelerated inactivation and recovery kinetics and produced a hyperpolarizing shift in Nav1.7 activation (29). The regulation of Nav1.7 channels by the β3 and β4 subunits has not been investigated.
In this study, HEK293 cells stably expressing Nav1.7 channels were employed to further investigate the functional consequences of Nav1.7-β interactions. Coexpressing β subunits (β1–β4) did not alter the peak Na+ current densities or Nav1.7 current kinetics. However, β1 produced a depolarizing shift in steady-state inactivation and a faster recovery from inactivation (Table 1). At voltages near the resting membrane potentials of DRG neurons (≈ −60 mV), depolarizing shifts in inactivation would tend to increase the fraction of Nav1.7 channels available to open in response to depolarization. Similar increases in availability along with the associated increase in Na+ current density are well known to reduce the threshold for initiating action potentials (30–32). The rate of Na+ channel recovery from inactivation is an important determinant of the absolute and relative refractory periods of action potentials. The faster recovery of Nav1.7-β1 channels predicts rapid repriming at hyperpolarized voltages that may reduce the duration of the refractory periods, thereby enabling increased firing frequency in large-diameter neurons highly expressing the Nav1.7-β1 combination.
The β3 subunit produced a −9-mV shift in Nav1.7 activation, causing the channels to open at more hyperpolarized voltages (Table 1). Such shifts in activation and the accompanying increase in Na+ current at more hyperpolarized voltages are predicted to increase neuronal excitability and could potentially reduce the threshold for firing action potentials in small-diameter neurons. This mechanism is consistent with studies showing that Na+ channels with low activation thresholds are critical determinants of action potential initiation at the axon initial segment (33, 34).
Nav1.7-β subunit interactions that induce hyperpolarizing shifts in activation (β3) or depolarizing shifts in inactivation (β1) tend to increase the overlap of activation and inactivation gating (Fig. 6C). At voltages within this overlap region, Na+ channels are partially activated but not fully inactivated, increasing the potential of persistent window currents (35). At −50 mV, the peak window current probability predicts that a small percentage (0.1%) of Nav1.7 channels will be persistently activated. Coexpressing the β1 or β3 subunits increased the probability of persistent activation by 2–3-fold. Persistent activation of Nav1.7-β1 and Nav1.7-β3 channels and the resulting inward Na+ current at resting membrane potentials could depolarize the neuron, leading to increased excitability of DRG neurons. Similar mechanisms are believed to underlie the increased excitability of sensory neurons harboring inherited human pain disorder mutations that produce shifts in Nav1.7 activation and inactivation of similar polarity and magnitude as those observed for the Nav1.7-β1 and Nav1.7-β3 channels (36–38).
Previous studies have employed chimeras, deletion analysis, and mutations to define the structural domains of β subunits that are critical for Na+ channel regulation (21, 39–42). The findings indicate that the extracellular N-terminal domain of β1 is essential for the functional regulation of neuronal and skeletal muscle Na+ channels. This contrasts with the β1 regulation of cardiac Na+ channels, where the membrane-spanning domain was found to be critical for the increased expression and accelerated recovery of Nav1.5 channels (43). These data imply that different structural domains and therefore different molecular interactions are responsible for β1 regulation of neuronal and cardiac Na+ channels.
β1 mRNA is highly expressed in large DRG neurons (Fig. 1), where it is significantly correlated with Nav1.7, indicating that these subunits are coexpressed in the same population of large-diameter neurons (Fig. 2). β subunit chimeras were employed to identify the structural domains of β1 required to produce the observed depolarizing shift in steady-state inactivation and the accelerated recovery of Nav1.7 channels (Table 1). Chimeras incorporating the extracellular N-terminal domain of β1 (β112) retained the shift in inactivation and faster recovery, whereas replacing the extracellular domain (β211) completely eliminated these effects. These data indicate that the N-terminal domain of the β1 subunit is required for Nav1.7 regulation. β1 subunits with a truncated C terminus (β11Δ) retained full functional regulation, indicating that the intracellular domain is nonessential. Interactions between the N terminus of β1 and extracellular loops of Nav1.7 may be important for the functional regulation of these channels, similar to what has been described previously for other neuronal Na+ channels (40, 41).
Recent work employed a similar approach to investigate the β1 regulation of Nav1.8, a TTX-R channel that produces the majority of the inward Na+ current in small-diameter DRG neurons (21). Substitution of the extracellular N-terminal domain of β1 had no effect on the expression or gating properties of Nav1.8 channels. Rather, the intracellular C-terminal domain of β1 was found to be the critical determinant of Nav1.8 regulation. These data indicate that the N and C termini of the β1 subunit differentially regulate the gating properties of Nav1.7 and Nav1.8 channels.
Much of what is currently known about β subunit expression in the DRG has been derived from immunocytochemistry and in situ hybridization (4, 12, 15–18, 44). These studies indicate that all four isoforms of β subunits (β1–β4) are present in the DRG and that these subunits are differentially expressed in subpopulations of sensory neurons (12, 15). β3 subunits are prominently expressed in small and medium neurons, whereas β1 and β4 are preferentially expressed in large neurons (4, 16, 17). β2 appears to be widely expressed in the DRG and does not show a clear preference for neuronal size (15, 45). These findings are in good agreement with our single-cell analysis of gene expression and are consistent with the conclusion that β subunits are differentially expressed in subpopulations of DRG neurons. Unfortunately, histological approaches do not provide quantitative assessments of β subunit expression levels or insight into the functional regulation of Na+ channels by β subunits. Our data indicate that the differential expression of β subunits in DRG neurons combined with isoform-specific β subunit regulation of Nav1.7 activation (β3) and inactivation (β1) predicts substantial differences in the predominant TTX-S Na+ currents of small and large sensory neurons.
Previous work investigated the role of the β1 and β2 subunits in sensory neurons using Scn1b and Scn2b null mice (45, 46). Whole-cell recordings from DRG neurons isolated from the β1 knock-outs revealed small changes in the amplitudes and gating properties of TTX-S and TTX-R Na+ currents (46). The relatively subtle effects of the Scn1b knock-out on DRG Na+ currents coupled with the low level expression of β1 subunits in small-diameter sensory neurons suggest that these subunits may not be important regulators of the Na+ channels expressed in nociceptors. Neurons from the Scn2b null mice displayed reductions in TTX-S Na+ current amplitude and Na+ channel mRNA and protein (46). Although the underlying mechanism is unclear, the Scn2b knock-out appears to reduce TTX-S Na+ currents by decreasing Na+ channel mRNA and protein expression. Based on the comparison of Na+ currents recorded from control and Scn2b null mice, the β2 subunits were proposed to increase Na+ channel expression (Nav1.1, Nav1.6, and Nav1.7), produce hyperpolarizing shifts in activation, and accelerate the kinetics of the endogenous TTX-S Na+ currents (46). These effects were not recapitulated in our heterologous expression studies of Nav1.7-β2 channels, where no changes in Na+ current density, voltage dependence, or current kinetics were observed. Rather, our findings are consistent with previous work showing that the β2 subunit has no effect on the expression or gating properties of the Nav1.3, Nav1.6, and Nav1.8 channels (21, 47). The reasons for the apparent discrepancy between in vivo knockdown and heterologous expression studies are not known but may reflect contributions by endogenous regulatory pathways that are specific to the DRG or the compensatory up-regulation of other β subunits in the sensory neurons of Scn2b null mice.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM078244 from NIGMS (to M. E. O'L.). This work was also supported by the Heart and Stroke Foundation of Québec (HSFQ) and Canadian Institutes of Health Research (CIHR) Grant INO-77909 (to M. C.).
- DRG
- dorsal root ganglion/ganglia
- TTX-S
- tetrodotoxin-sensitive
- TTX-R
- tetrodotoxin-resistant.
REFERENCES
- 1. Cummins T. R., Sheets P. L., Waxman S. G. (2007) The roles of sodium channels in nociception: implications for mechanisms of pain. Pain 131, 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dib-Hajj S. D., Binshtok A. M., Cummins T. R., Jarvis M. F., Samad T., Zimmermann K. (2009) Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res. Rev. 60, 65–83 [DOI] [PubMed] [Google Scholar]
- 3. Rush A. M., Cummins T. R., Waxman S. G. (2007) Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 579, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black J. A., Dib-Hajj S., McNabola K., Jeste S., Rizzo M. A., Kocsis J. D., Waxman S. G. (1996) Spinal sensory neurons express multiple sodium channel α subunit mRNAs. Brain Res. Mol. Brain Res. 43, 117–131 [DOI] [PubMed] [Google Scholar]
- 5. Dib-Hajj S. D., Tyrrell L., Black J. A., Waxman S. G. (1998) NaN, a novel voltage-gated sodium channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc. Natl. Acad. Sci. U.S.A. 95, 8963–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amaya F., Decosterd I., Samad T. A., Plumpton C., Tate S., Mannion R. J., Costigan M., Woolf C. J. (2000) Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol. Cell. Neurosci. 15, 331–342 [DOI] [PubMed] [Google Scholar]
- 7. Ho C., O'Leary M. E. (2011) Single-cell analysis of sodium channel expression in dorsal root ganglion neurons. Mol. Cell. Neurosci. 46, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isom L. L., Scheuer T., Brownstein A. B., Ragsdale D. S., Murphy B. J., Catterall W. A. (1995) Functional coexpression of the β1 and type IIA α subunits of sodium channels in a mammalian cell line. J. Biol. Chem. 270, 3306–3312 [DOI] [PubMed] [Google Scholar]
- 9. Catterall W. A. (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25 [DOI] [PubMed] [Google Scholar]
- 10. Isom L. L. (2001) Sodium channel β subunits: anything but auxiliary. Neuroscientist 7, 42–54 [DOI] [PubMed] [Google Scholar]
- 11. Isom L. L. (2002) The role of sodium channels in cell adhesion. Front. Biosci. 7, 12–23 [DOI] [PubMed] [Google Scholar]
- 12. Coward K., Jowett A., Plumpton C., Powell A., Birch R., Tate S., Bountra C., Anand P. (2001) Sodium channel β1 and β2 subunits parallel SNS/PN3 α subunit changes in injured human sensory neurons. Neuroreport 12, 483–488 [DOI] [PubMed] [Google Scholar]
- 13. Isom L. L., De Jongh K. S., Patton D. E., Reber B. F., Offord J., Charbonneau H., Walsh K., Goldin A. L., Catterall W. A. (1992) Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science 256, 839–842 [DOI] [PubMed] [Google Scholar]
- 14. Morgan K., Stevens E. B., Shah B., Cox P. J., Dixon A. K., Lee K., Pinnock R. D., Hughes J., Richardson P. J., Mizuguchi K., Jackson A. P. (2000) β3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. U.S.A. 97, 2308–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takahashi N., Kikuchi S., Dai Y., Kobayashi K., Fukuoka T., Noguchi K. (2003) Expression of auxiliary β subunits of sodium channels in primary afferent neurons and the effect of nerve injury. Neuroscience 121, 441–450 [DOI] [PubMed] [Google Scholar]
- 16. Oh Y., Sashihara S., Black J. A., Waxman S. G. (1995) Na+ channel β1 subunit mRNA: differential expression in rat spinal sensory neurons. Brain Res. Mol. Brain Res. 30, 357–361 [DOI] [PubMed] [Google Scholar]
- 17. Shah B. S., Stevens E. B., Gonzalez M. I., Bramwell S., Pinnock R. D., Lee K., Dixon A. K. (2000) β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is up-regulated in the chronic constriction injury model of neuropathic pain. Eur. J. Neurosci. 12, 3985–3990 [DOI] [PubMed] [Google Scholar]
- 18. Yu F. H., Westenbroek R. E., Silos-Santiago I., McCormick K. A., Lawson D., Ge P., Ferriera H., Lilly J., DiStefano P. S., Catterall W. A., Scheuer T., Curtis R. (2003) Sodium channel β3, a new disulfide-linked auxiliary subunit with similarity to β2. J. Neurosci. 23, 7577–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lampert A., O'Reilly A. O., Reeh P., Leffler A. (2010) Sodium channelopathies and pain. Pflugers Arch. 460, 249–263 [DOI] [PubMed] [Google Scholar]
- 20. Fischer T. Z., Waxman S. G. (2010) Familial pain syndromes from mutations of the NaV1.7 sodium channel. Ann. N.Y. Acad. Sci. 1184, 196–207 [DOI] [PubMed] [Google Scholar]
- 21. Zhao J., O'Leary M. E., Chahine M. (2011) Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary β subunits. J. Neurophysiol. 106, 608–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vijayaragavan K., Powell A. J., Kinghorn I. J., Chahine M. (2004) Role of auxiliary β1, β2, and β3 subunits and their interaction with Nav1.8 voltage-gated sodium channel. Biochem. Biophys. Res. Commun. 319, 531–540 [DOI] [PubMed] [Google Scholar]
- 23. Zhao J., Ziane R., Chatelier A., O'Leary M. E., Chahine M. (2007) Lidocaine promotes the trafficking and functional expression of Nav1.8 sodium channels in mammalian cells. J. Neurophysiol. 98, 467–477 [DOI] [PubMed] [Google Scholar]
- 24. Messner D. J., Catterall W. A. (1985) The sodium channel from rat brain. Separation and characterization of subunits. J. Biol. Chem. 260, 10597–10604 [PubMed] [Google Scholar]
- 25. Roberts R. H., Barchi R. L. (1987) The voltage-sensitive sodium channel from rabbit skeletal muscle. Chemical characterization of subunits. J. Biol. Chem. 262, 2298–2303 [PubMed] [Google Scholar]
- 26. Sutkowski E. M., Catterall W. A. (1990) β1 subunits of sodium channels. Studies with subunit-specific antibodies. J. Biol. Chem. 265, 12393–12399 [PubMed] [Google Scholar]
- 27. Sangameswaran L., Delgado S. G., Fish L. M., Koch B. D., Jakeman L. B., Stewart G. R., Sze P., Hunter J. C., Eglen R. M., Herman R. C. (1996) Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J. Biol. Chem. 271, 5953–5956 [DOI] [PubMed] [Google Scholar]
- 28. Sangameswaran L., Fish L. M., Koch B. D., Rabert D. K., Delgado S. G., Ilnicka M., Jakeman L. B., Novakovic S., Wong K., Sze P., Tzoumaka E., Stewart G. R., Herman R. C., Chan H., Eglen R. M., Hunter J. C. (1997) A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J. Biol. Chem. 272, 14805–14809 [DOI] [PubMed] [Google Scholar]
- 29. Vijayaragavan K., O'Leary M. E., Chahine M. (2001) Gating properties of Nav1.7 and Nav1.8 peripheral nerve sodium channels. J. Neurosci. 21, 7909–7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azouz R., Gray C. M. (2000) Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc. Natl. Acad. Sci. U.S.A. 97, 8110–8115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henze D. A., Buzsaki G. (2001) Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience 105, 121–130 [DOI] [PubMed] [Google Scholar]
- 32. Platkiewicz J., Brette R. (2010) A threshold equation for action potential initiation. PLoS Comput. Biol. 6, e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colbert C. M., Pan E. (2002) Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 5, 533–538 [DOI] [PubMed] [Google Scholar]
- 34. Hu W., Tian C., Li T., Yang M., Hou H., Shu Y. (2009) Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat. Neurosci. 12, 996–1002 [DOI] [PubMed] [Google Scholar]
- 35. Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. (1979) The steady-state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflugers Arch. 379, 137–142 [DOI] [PubMed] [Google Scholar]
- 36. Harty T. P., Dib-Hajj S. D., Tyrrell L., Blackman R., Hisama F. M., Rose J. B., Waxman S. G. (2006) Nav1.7 mutant A863P in erythromelalgia: effects of altered activation and steady-state inactivation on excitability of nociceptive dorsal root ganglion neurons. J. Neurosci. 26, 12566–12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rush A. M., Dib-Hajj S. D., Liu S., Cummins T. R., Black J. A., Waxman S. G. (2006) A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc. Natl. Acad. Sci. U.S.A. 103, 8245–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dib-Hajj S. D., Cummins T. R., Black J. A., Waxman S. G. (2010) Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 33, 325–347 [DOI] [PubMed] [Google Scholar]
- 39. Chen C., Cannon S. C. (1995) Modulation of Na+ channel inactivation by the β1 subunit: a deletion analysis. Pflugers Arch. 431, 186–195 [DOI] [PubMed] [Google Scholar]
- 40. McCormick K. A., Isom L. L., Ragsdale D., Smith D., Scheuer T., Catterall W. A. (1998) Molecular determinants of Na+ channel function in the extracellular domain of the β1 subunit. J. Biol. Chem. 273, 3954–3962 [DOI] [PubMed] [Google Scholar]
- 41. McCormick K. A., Srinivasan J., White K., Scheuer T., Catterall W. A. (1999) The extracellular domain of the β1 subunit is both necessary and sufficient for β1-like modulation of sodium channel gating. J. Biol. Chem. 274, 32638–32646 [DOI] [PubMed] [Google Scholar]
- 42. Qu Y., Rogers J. C., Chen S. F., McCormick K. A., Scheuer T., Catterall W. A. (1999) Functional roles of the extracellular segments of the sodium channel α subunit in voltage-dependent gating and modulation by β1 subunits. J. Biol. Chem. 274, 32647–32654 [DOI] [PubMed] [Google Scholar]
- 43. Zimmer T., Benndorf K. (2002) The human heart and rat brain IIA Na+ channels interact with different molecular regions of the β1 subunit. J. Gen. Physiol. 120, 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qu Y., Curtis R., Lawson D., Gilbride K., Ge P., DiStefano P. S., Silos-Santiago I., Catterall W. A., Scheuer T. (2001) Differential modulation of sodium channel gating and persistent sodium currents by the β1, β2, and β3 subunits. Mol. Cell. Neurosci. 18, 570–580 [DOI] [PubMed] [Google Scholar]
- 45. Lopez-Santiago L. F., Brackenbury W. J., Chen C., Isom L. L. (2011) Na+ channel Scn1b gene regulates dorsal root ganglion nociceptor excitability in vivo. J. Biol. Chem. 286, 22913–22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lopez-Santiago L. F., Pertin M., Morisod X., Chen C., Hong S., Wiley J., Decosterd I., Isom L. L. (2006) Sodium channel β2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J. Neurosci. 26, 7984–7994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meadows L. S., Chen Y. H., Powell A. J., Clare J. J., Ragsdale D. S. (2002) Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary β1, β2, and β3 subunits. Neuroscience 114, 745–753 [DOI] [PubMed] [Google Scholar]