The intellectual paths some scientists have followed toward behavioral experimentation have traversed the physical sciences, as well as biology and other academic territories. Those who have studied physics may well be disposed to strive for a universal lawfulness, expressed quantitatively, in the demonstration of stimulus-response connections. This endeavor can be hard, because of the complexity of some stimuli and certain behavioral responses and because of the need to recognize and control the relevant environmental variables.
Even harder is the delineation of causal relations between particular genes and specific behaviors (1). The pleiotropy of individual genes and overlapping functions between genes make trouble to begin with. Our lack of understanding of mechanisms for penetrance of dominant alleles make it difficult to construct meaningful gene dose/response relationships (2). Interpretations of gene knockout data can stumble over unexpected compensations, effects on other gene products, altered endocrine and neuronal feedback loops, and a lack of control over the temporal and spatial impact of the genetic manipulation.
Nevertheless, a significant body of mouse behavior genetics that depends on modern molecular manipulations has emerged. When the experimenter chooses well-controlled stimuli and biologically important responses of enough simplicity, and their connections are driven by identified neuroendocrine or neurochemical influences of sufficient power, reliable and interesting knowledge can be gained. Therefore, it was surprising when Crabbe et al. (3) reported that they got somewhat different results on different campuses during analyses of mouse strain differences in behavior. More disturbing were the echoes in secondary sources that seemed to reinforce an old stereotype that many behavioral results are unreliable. Thus, I have collected here just a few examples of recent bodies of behavioral data, reliable products of a new mouse functional genomics.
Mutations with Motor Performance Phenotypes
Brutally clear are the effects of genes whose alterations lead to developmental pathologies in the cerebellum or other sensory-motor control regions. A deletion within the RORα gene produces the staggerer mouse, showing abnormalities in cerebellar Purkinje cells associated with severe motor ataxia (4). This phenotype can be contrasted with the whole-body action tremors characteristic of the vibrator mouse (5). Here the pathology is due to a retroposon insertion in an intron of the phosphatidylinositol transfer proten α gene, preventing a normal accumulation of its RNA.
Fletcher and his colleagues (6, 7) found that mutation of the subunit of a voltage-gated calcium channel could produce the tottering phenotype, which includes both ataxia and paroxysmal dyskinesis. Lurcher is a mutation that causes postnatal abnormalities in the mouse cerebellum. The heterozygotes live long enough to display an unsteady, swaying gait and a tendency to walk backward (8, 9). The weaver mouse, which suffers from severe ataxia, is a result of a substitution of a serine for a glycine in a G protein-gated inwardly rectifying potassium channel (10, 11).
Genes for Nuclear Receptors
Predicted from neural and molecular analysis (12), the complete loss of normal female reproductive behavior after a functional knockout of the classical estrogen receptor gene (ER-α), initially was reported as early as 1996 (13). That discovery has been thoroughly replicated (14) and extended by the use of ovariectomized female mice given controlled administrations of estradiol and progesterone, and then assayed for sexual and aggressive behaviors (15). Overall, comparing results of mice bearing the ER-α knockout with those bearing a knockout of the novel ER-β gene, it has become apparent that different patterns of mating responses require different patterns of ER gene activation (16).
Genes for Neuropeptides and Their Receptors
Behavioral studies of oxytocin knockout and vasopressin receptor transgenic mice provide excellent examples of experiments using simple, quantifiable behaviors that examine the role of these genes in the regulation of social behaviors. Oxytocin is a neuropeptide associated with various aspects of affiliative behaviors, social attachment, and social memory. For example, pharmacological studies have demonstrated a role for oxytocin in the formation of long-lasting pairbonds in monogamous rodent species and it is involved in the olfactory memory of the ewe for her lamb (17, 18). Results with the oxytocin knockout mice have shown a role for the oxytocin gene in modulating social behavior. Male oxytocin knockout mice fail to display social recognition (19). They do not have the ability to recognize individuals that they have previously encountered. Although wild-type (WT) and oxytocin knockout mice spend similar amounts of time investigating a novel female, WTs consistently exhibit a decline in olfactory investigation after repeated exposures to the same female, whereas oxytocin knockout males do not. A variety of control experiments proved that the memory deficit was specific for the recognition of socially relevant odors.
Social recognition in oxytocin mutant mice can be restored by a single injection of oxytocin into the brain just before the initial exposure, demonstrating that the deficit in social recognition was not due to a neurological problem caused by the lack of oxytocin during development. Conversely, a specific oxytocin receptor antagonist prevents social recognition in WT mice (19). More recent studies in these mice have used c-Fos immunocytochemistry to identify a region of the amygdala that fails to become activated in the oxytocin knockout mice, compared with WT mice, during a social encounter.† Pharmacological studies have demonstrated that injection of picogram amounts of peptide directly into this region, which is rich in oxytocin receptors, fully restores social recognition. Thus, the behavioral phenotype of these mice has remained consistent among several types of experiments.
Developmental behavioral studies in these mice also have produced results consistent with pharmacological data. Rodent pups emit ultrasonic vocalizations when separated from their mother. Oxytocin decreases the numbers of isolation calls in rat pups, which has led to the hypothesis that oxytocin produces the calming effects of social contact, and therefore decreases the numbers of calls (21). Oxytocin knockout pups produce significantly fewer ultrasonic calls protesting isolation compared with WT pups (22).
Vasopressin, with only two of nine amino acids different from oxytocin (perhaps resulting from a gene duplication), also has been implicated in the regulation of social behaviors (23). Vasopressin facilitates pair bonding in monogamous prairie voles and increases social interactions in this highly social species (18). Interestingly, montane voles, which are nonmonogamous and less social, have similar levels of vasopressin, but differ in the neuroanatomical distribution of vasopressin receptor gene expression (24). Transgenic mice were created by using a genomic vasopressin receptor clone containing the 5′ flanking region of the prairie vole (25). These mice displayed a pattern of vasopressin receptor expression that was remarkably similar to that of the prairie voles, but quite distinct from WT mice. When treated with vasopressin, the transgenic mice displayed an elevation in the duration of social contact, exactly as occurs in prairie voles. In contrast, the identical vasopressin treatment had no effect on social contact in WT mice. This experiment has provided strong proof for the association between localized expression of a gene and social behavior.
Galanin.
Recently, galanin-overexpressing transgenic mice have been shown to display cognitive deficits, e.g., in the Morris water maze, which could be considered reminiscent of Alzheimer's disease symptoms (26). These findings have been replicated within the same batch of mice at three ages and are consistent with well-documented pharmacologic results in which galanin has been administered intraventricularly (27–29). Within and between labs, galanin molecular pharmacology seems to exhibit a high degree of reliability.
Enkephalin.
A collaborative study involving three laboratories examined mice with knockout of a gene for the opioid peptide enkephalin (30). Three different phalanxes of knockout and WT mice were assayed, over a period of 18 months, including different seasons of the year. In every epoch of testing, the knockout mice displayed exaggerated responses to situations evoking fear or anxiety. In fact, the enkephalin knockout mouse may comprise a useful genetic model of chronic fear and anxiety. While the results were being written up, the authors learned of a paper by Filliol et al. (31) analyzing the phenotype of mice with knockouts of the delta opioid receptor. This is the neuropeptide receptor through which only enkephalins can act effectively. The great similarity of results with the delta opioid receptor knockout to those obtained with the enkephalin knockout further supported the reliability of findings in this important area of neurobiology.
Genes for Neurotransmitter Receptors
Dopamine Receptors.
A useful example of the need for careful and complex thinking about the analysis of a seemingly simple behavior is the effect of loss-of-function dopamine D2 receptor mutations on locomotor activity. Baik et al. (32) reported the first D2R knockout mouse strain. Grid crossings were reduced to such a degree that the mice were nearly akinetic, consistent with the phenotype predicted by pharmacological blockade of D2 receptors, and prompting the eponymic description “Parkinsonian-like.” Kelly et al. (33, 34) generated a second strain of D2R knockout mice. Their data indicated a more subtle locomotor dysfunction characterized by a 50% reduction in horizontal distance traveled over a 30-min period, which could largely be attributed to decreased initiation of bouts of locomotor activity, particularly in the earlier blocks of time when the testing environment was the most novel (or unfamiliar). This group further reported that the nominal distanced traveled by D2R knockout mice highly depended on genetic background (greatest on the active C57BL/6 strain and least on the indolent 19S6 strain) although the percentage reduction from controls of the same background remained constant at 50%.
The mutations introduced into the D2dr gene by each laboratory differed in the choice of deleted exon. Although both groups showed comparable evidence for a functionally null allele by the loss of specific radioligand binding (32, 33, 35), absence of receptor imunoreactivity with specific antisera and associated neurochemical alterations such as increased striatal enkephalin expression (32, 35) the possibility exists that either mutant allele might express some cryptic receptor fragment that subtly alters function by mechanisms such as receptor heterodimerization. Further, genetic background of the original F2 animals differed, and housing/environmental circumstances were different between laboratories.
Since these initial publications, both strains of mice have been used in many other collaborative studies that have included additional behavioral analyses. These data reveal a pattern of striking agreement among the results from multiple investigators. Basal activity was consistently decreased ≈50% in all of these studies, and the differences between D2dr−/− and D2dr+/+ mice were greatest when the mice were tested under the more stressful conditions of a novel environment and nearly disappeared when testing was performed in familiar environments or after habituation (35–38). Finally, with D2dr mutants in the Waddington laboratory (39), the same set of ethologically based procedures was used to study the two strains of mice and found essentially identical profiles of behaviors within the natural repertoire of the species, including initial modest reductions in locomotion.
Serotonin (5-HT) Receptors.
In 1998 three groups reported the generation of a 5-HT1A receptor knockout mouse on different strain backgrounds (ref. 40, Swiss–Webster background; ref. 41, 129sv background; ref. 42, C57 background). All three groups reported that the mutant mice demonstrate consistently enhanced anxiety-type behaviors alongside reduced immobility in the forced swim test (40) or tail suspension test (41, 42), indicating an antidepressant-like effect. These data indicate that the phenotype may not be significantly affected by genetic modifiers. It should be noted that other 5-HT receptor genes may not be as easy to deal with as the 5-HT1A receptor.
Nitric Oxide Synthase
To examine the specific behavioral roles of neuronal NO synthase (nNOS), homologous recombination was used to create nNOS gene knockout (nNOS−/−) mice. The combination of the missing nNOS gene and isolated housing conditions facilitated extreme aggressiveness when the animals were subsequently housed in groups of five. Male nNOS−/− residents engaged in 3–4 times more aggressive encounters than WT mice in the intruder-resident model (43). Nearly 90% of the aggressive encounters were initiated by the nNOS−/− animals. Similar results were obtained in diadic or group encounters in neutral arenas. In all test situations, male nNOS−/− mice rarely displayed submissive behaviors.
Behavioral studies of mice with targeted deletion of specific genes suffer from the criticism that the gene product is not only missing during the testing period, but missing throughout development when critical ontogenetic processes, including activation of compensatory mechanisms, may be affected (44). To address this criticism, mice were treated with 7-nitroindazole (7-NI) (50 mg/kg ip) that specifically inhibits nNOS formation in vivo (45). Mice treated with 7-NI displayed substantially increased aggression in two different tests of aggressiveness as compared with control animals (45). Drug treatment did not affect nonspecific locomotor activities. Importantly, NOS activity in brain homogenates was reduced >90% in 7-NI-treated mice. Similarly, immunohistochemical staining for citrulline revealed a dramatic reduction in 7-NI-treated animals (45). These pharmacological results confirm and extend the results obtained in nNOS−/− mice.
Plasma androgen concentrations influences aggression. nNOS−/− and WT mice do not differ in blood testosterone concentrations either before or after agonistic encounters (43). However, recent data on castrated nNOS−/− males suggest that testosterone is necessary, if not sufficient, to promote increased aggression in these mutants (46). Castrated nNOS−/− mice displayed low levels of aggression that was equivalent to the reduced aggression observed among castrated WT males. Androgen-replacement therapy restored the elevated levels of aggression in nNOS−/− mice. Elevated aggression in nNOS−/− males was contingent on individual housing, and aggression increased lawfully in response to aggressive experience (47).
Recently, the excessive aggressiveness and impulsiveness of nNOS knockout mice was shown to be caused by selective decrements in 5-HT turnover and deficient 5-HT(1A) and 5-HT(1B) receptor function in brain regions regulating emotion (47). The consistently high levels of aggression observed in nNOS−/− mice could be reversed by 5-HT precursors and by treatment with specific 5-HT1A and 5-HT1B receptor agonists. Taken together, these results indicate an important role for NO in normal brain 5-HT function and may have significant implications for the treatment of psychiatric disorders characterized by aggressiveness and impulsivity.
For comparison, endothelial NO synthase (eNOS)−/− mice were tested by using two behavioral paradigms (48). In the resident-intruder paradigm, eNOS−/− mice displayed virtually no aggressive encounters and a dramatically decreased duration of aggression relative to WT mice. Second, when tested in a neutral arena with a WT stimulus male, eNOS−/− mice displayed many fewer attacks and a greatly increased latency to attack the stimulus male relative to WT mice (48). Thus, the two isoforms of NOS have balanced, opposite effects: to increase (eNOS) and decrease (nNOS) aggressive behavior.
Circadian Behaviors
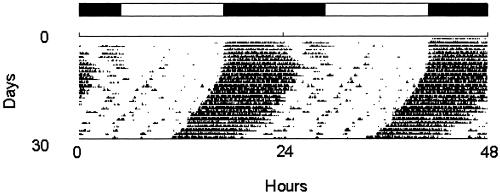
Circadian rhythms are one of the best examples of precision in mouse behavior. Wheel-running behavior in mice is exquisitely timed (e.g., Fig. 1). The daily onset of activity varies less than a few minutes per cycle (≈0.2%), and the circadian period of a normal mouse in constant darkness can be accurately measured by recording many cycles in a cumulative manner. These quantitative features allowed Takahashi and his colleagues (49) to use this behavior to mutagenize, screen, and isolate the circadian Clock mutation in mice. The Clock mutant mouse then was used to positionally clone the Clock gene using both transgenic rescue and molecular analysis (50, 51). In addition to these single-gene experiments, mouse circadian behavior will be useful for analyzing more subtle features such as strain differences and quantitative trait loci.
Figure 1.
Representative wheel-running activity record of a C57BL/6J mouse. By convention, activity records are double-plotted with 48 hr across, so that each day's record is both to the right of and beneath the preceding day. Times of activity are indicated by black marks; the height of the mark indicates the intensity of activity. For the first 7 days shown, the mouse was kept on a light/dark cycle as illustrated by the bar above the activity record. Activity onset occurs very near the time of lights-off, providing a precisely timed behavioral indication of the beginning of night, or a phase reference point. In the absence of a light/dark cycle to provide time cues, activity onset acts as a precise phase reference point, indicating the beginning of the animal's subjective night. In the record shown after 7 days, lights were allowed to go out at the usual time, but not to come on the following morning. In continuous darkness, the cycle of activity persists, but with a period slightly less than 24 hr, resulting in the slope to the left. (Figure kindly supplied by Martha Hotz Vitaterna and Joseph S. Takahashi, Northwestern University, Evanston, IL.)
Outlook
Surely the behavioral aspects of functional genomics can fall prey to the complexities of modifier genes and subtle environmental variables. Effects of genetic background, strain differences, and cosegregating genes may be encountered even in straightforward neurochemical/behavioral systems (refs. 52 and 53; cf. refs. 54–56). As well, a familial pattern of behavior change can be routed through nongenomic transmission, which depends on variations in maternal care (57). Nevertheless, when the right level of neurobiological problem is identified—simple enough to be analyzable, while still embodying emergent behavioral phenomena—an interesting science of behavior genetics comes forth. In 1941 and 1942 Beadle and Tatum (20, 58), working with fungus Neurospora, made discoveries that yielded the classical “one gene-one enzyme” hypothesis. It may fairly be said that modern neurobiologists are struggling to move one step beyond Beadle and Tatum, to show patterns of genes controlling patterns of behavioral responses. In sum, the findings quoted above amply illustrate that when experimenters have control over the relevant variables, reliability and precision can be expected in this area of functional genomics, the delineation of causal relations between particular genes, and mouse behavioral responses.
Footnotes
Ferguson, J. N., Winslow, J. T., Aldag, J. M., Insel, T. R. & Young, L. J. (2000) Soc. Neurosci. Abstr. 373, 8.
References
- 1.Crawley J N. What's Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley; 2000. [Google Scholar]
- 2.Pfaff D W, Berrettini W, Joh T, Maxson S, editors. Genetic Influences on Neural and Behavioral Functions. Boca Raton, FL: CRC; 1999. [Google Scholar]
- 3.Crabbe J C, Wahlsten D, Dudek B C. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton B A, Frankel W N, Kerrebrock A W, Hawkins T L, FitzHuge W, Kusumi K, Russell L B, Mueller K L, van Berkel V, Birren B W, et al. Nature (London) 1996;379:736–739. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton B A, Smith D J, Muller K L, Kerrebrock A W, Bronson R T, van Berkel V, Daly M J, Kruglyak L, Reeves M P, Nemhauser J L, et al. Neuron. 1997;18:711–722. doi: 10.1016/s0896-6273(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher C F, Frankel W N. Hum Mol Genet. 1999;8:1907–1912. doi: 10.1093/hmg/8.10.1907. [DOI] [PubMed] [Google Scholar]
- 7.Caddick S J, Wang C, Fletcher C F, Jenkins N A, Copeland N G, Hosford D A. J Neurophysiol. 1999;81:2066–2074. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- 8.Norman D J, Fletcher C F, Heintz N. Genomics. 1991;9:147–153. doi: 10.1016/0888-7543(91)90232-4. [DOI] [PubMed] [Google Scholar]
- 9.Zuo J, De Jager P L, Takahashi K A, Jiang W, Linden D J, Heintz N. Nature (London) 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
- 10.Slesinger P A, Patil N, Liao Y J, Jan Y N, Jan L Y, Cox D R. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 11.Patil N, Cox D R, Bhat D, Faham M, Myers R M, Peterson A S. Nat Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 12.Pfaff D W. Drive: Neural and Molecular Mechanisms for Sexual Motivation. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 13.Ogawa S, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 14.Rissman E, Early A, Taylor J, Korach K, Lubahn D. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Eng V, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Chester A E, Hewitt S C, Walker V R, Gustafsson J-A, Smithies O, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. . (First Published December 12, 2000, 10.1073/pnas.250473597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insel T R, Young L J. Curr Opin Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 18.Insel T R, Young L J. Nat Rev Neurosci. 2001;2:129–135. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson J N, Young L J, Hearn E F, Insel T R, Winslow J T. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 20.Tatum E L, Beadle G W. Proc Nat Acad Sci USA. 1942;28:234–243. doi: 10.1073/pnas.28.6.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insel T R, Winslow J T. Eur J Pharmocol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- 22.Winslow J T, Hearn E F, Ferguson J, Young L J, Matzuk M M, Insel T R. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 23.Young L J. Horm Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- 24.Young L J, Winslow J T, Nilsen R, Insel T R. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- 25.Young L J, Nilsen R, Waymire K G, MacGregor G R, Insel T R. Nature (London) 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 26.Steiner A, Hohmann J G, Holmes A, Wrenn C C, Cadd G, Jureus A, Clifton D K, Luo M, Gutshall M, Ma S Y, et al. Proc Natl Acad Sci USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson J K, Crawley J N. Behav Neurosci. 1993;107:458–467. doi: 10.1037//0735-7044.107.3.458. [DOI] [PubMed] [Google Scholar]
- 28.McDonald M P, Crawley J N. Behav Neurosci. 1996;110:1025–1032. doi: 10.1037//0735-7044.110.5.1025. [DOI] [PubMed] [Google Scholar]
- 29.Gleason T C, Dreiling J L, Crawley J N. Neuropeptides. 1999;33:265–270. doi: 10.1054/npep.1999.0044. [DOI] [PubMed] [Google Scholar]
- 30.Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar R J, Pfaff D W. Proc Natl Acad Sci USA. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. . (First Published February 6, 2001, 10.1073/pnas.041598498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filliol D, Ghozland S, Chluba J, Martin M, Matthes H W D, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, et al. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 32.Baik J H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Nature (London) 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 33.Kelly M A, Rubinstein M, Asa S L, Zhang G, Saez C, Bunzow J R, Allen R G, Hnasko R, Ben-Jonathan N, Grandy D K, Low M J. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 34.Kelly M A, Rubinstein M, Phillips T J, Lessov C N, Burkhart-Kasch S, Zhang G, Bunzow J R, Fang Y, Gerhardt G A, Grandy D K, Low M J. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J F, Moratalla R, Impagnatiello F, Grandy D K, Cuellar B, Rubinstein M, Beilstein M A, Hackett E, Fink J S, Low M J, et al. Proc Natl Acad Sci USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham C L, Howard M A, Gill S J, Rubinstein M, Low M J, Grandy D K. Pharmacol Biochem Behav. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado R, Saiardi A, Valverde O, Samad T A, Roques B P, Borrelli E. Nature (London) 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 38.Phillips T J, Brown K J, Burkhart-Kasch S, Wenger C D, Kelly M A, Rubinstein M, Grandy D K, Low M J. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 39.Clifford J J, Usiello A, Vallone D, Kinsella A, Borrelli E, Waddington J L. Neuropharmacology. 2000;39:382–390. doi: 10.1016/s0028-3908(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 40.Parks C L, Robinson P S, Sibille E, Shenk T, Toth M. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heisler L K, Chu H M, Brennan T J, Danao J A, Bajwa P, Parsons L H, Tecott L H. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramboz S, Oosting R, Aitamara D, Kung H F, Blier P, Mendelsohn M, Mann J J, Brunner D, Hen R. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson R J, Demas G E, Huang P, Fishman M C, Dawson V, Dawson T M, Snyder S H. Nature (London) 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 44.Nelson R J. Horm Behav. 1997;31:188–196. doi: 10.1006/hbeh.1997.1381. [DOI] [PubMed] [Google Scholar]
- 45.Demas G E, Eliasson M J L, Dawson T M, Dawson V L, Kriegsfeld L J, Nelson R J, Snyder S H. Mol Med. 1997;3:611–617. [PMC free article] [PubMed] [Google Scholar]
- 46.Kriegsfeld L J, Dawson T M, Dawson V L, Nelson R J, Snyder S H. Brain Res. 1997;769:66–70. doi: 10.1016/s0006-8993(97)00688-4. [DOI] [PubMed] [Google Scholar]
- 47.Chiavegatto S, Dawson V L, Mamounas L A, Koliatsos V E, Dawson T M, Nelson R J. Proc Natl Acad Sci USA. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. . (First Published January 16, 2001, 10.1073/pnas.031487198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demas G E, Kriegsfeld L J, Blackshaw S, Huang P, Gammie S C, Nelson R J, Snyder S H. J Neurosci. 1999;19:1–5. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitaterna M H, King D P, Chang A-M, Kornhauser J M, Lowrey P L, McDonald J D, Dove WF, Pinto L H, Turek F W, Takahashi J S. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoch M P, Song E-J, Chang A-M, Vitaterna M H, Zhao Y, Wilsbacher L D, Sangoram A M, King D P, Pinto L H, Takahashi J S. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King D P, Zhao Y, Sangoram A M, Wilsbacher L D, Tanaka M, Antoch M P, Steeves T D L, Vitaterna M H, Kornhauser J M, Lowrey P L, et al. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 53.Lathe R. Trends Neurosci. 1996;19:183–186. doi: 10.1016/s0166-2236(96)20022-0. [DOI] [PubMed] [Google Scholar]
- 54.Kolesnikov Y, Jain S, Wilson R, Pasternak G W. J Pharmacol Exp Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- 55.King M A, Pan Y-X, Mei J, Chang A, Xu J, Pasternak G W. Eur J Pharmacol. 1997;331:R5–R7. doi: 10.1016/s0014-2999(97)01064-9. [DOI] [PubMed] [Google Scholar]
- 56.Pan Y X, Mei J F, Xu J, Wan B L, Zuckerman A, Pasternak G W. J Neurochem. 1998;70:2279–2285. doi: 10.1046/j.1471-4159.1998.70062279.x. [DOI] [PubMed] [Google Scholar]
- 57.Francis D, Diorio J, Liu D, Meaney M J. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 58.Beadle G W, Tatum E L. Proc Nat Acad Sci USA. 1941;27:499–506. doi: 10.1073/pnas.27.11.499. [DOI] [PMC free article] [PubMed] [Google Scholar]