Abstract
The mammary gland is one of the best-studied examples of an organ whose structure and function are influenced by reciprocal signalling and communication between cells and their microenvironment. The mammary epithelial cell microenvironment includes stromal cells and the extracellular matrix (ECM). Abundant evidence shows that the ECM and growth factors cooperate to regulate cell cycle progression, and that the ECM is altered in breast tumors. In particular, mammographically dense breast tissue is a significant risk factor for developing breast carcinomas. Dense breast tissue is associated with increased stromal collagen and epithelial cell content. In this article we overview recent studies addressing the effects of ECM composition on the breast cancer cell cycle. While the normal breast ECM keeps the mammary epithelial cell cycle in check, the ECM remodeling associated with breast cancer positively regulates the mammary epithelial cell cycle. ECM effects on the downstream biochemical and mechanosignalling pathways in both normal and tumorigenic mammary epithelial cells will be reviewed.
Introduction
Breasts, or mammary glands, are derived from the epidermis and are a distinguishing feature of mammals. As in all epithelial lined organs and glands, communication between mammary epithelial cells (MECs) and their environment governs tissue morphogenesis during development, homeostasis in the adult, and neoplastic progression in the cancerous state (Kenny and Bissell, 2003; Zhan et al., 2008; Lyons et al., 2011). During mammary gland development, the microenvironment is permissive for cell proliferation, branching, and invasion through the stroma to promote organogenesis (Fata et al., 2004a). When proper organ size, shape, and function are achieved, these behaviors cease. In the adult, homeostasis is achieved through maintenance of the differentiated state, coupled with epithelial cell renewal (Rizki and Bissell, 2004). During cancer progression, the tissue microenvironment fails to exert control over epithelial proliferation and differentiation, resulting in uncontrolled growth and invasion (Maffini et al., 2004). Several studies have begun to elucidate the mechanisms by which the tissue microenvironment exerts direct control over MEC proliferation, as well as the molecular basis for loss of proliferation control in cancer.
The mammary gland is one of the best-studied examples of an organ whose structure and function are influenced by reciprocal signalling and communication between cells and their microenvironment. The mammary gland contains epithelium and stroma. Mammary epithelial cells form collecting ducts, and during pregnancy and lactation, milk-secreting alveoli. The stroma includes stromal cells and the extracellular matrix (ECM). Abundant evidence shows that the ECM, cytokines and growth factors cooperate to regulate MEC survival, differentiation, migration, and proliferation, and that the ECM is altered in breast tumors (Muschler and Streuli, 2010; Polyak and Kalluri, 2010; Lyons et al., 2011; Schedin and Keely, 2011). Dense breast tissue is associated with increased stromal collagen and epithelial cell content (Guo et al., 2001; Alowami et al., 2003; Gill et al., 2006), and mammographically dense breast tissue is a significant risk factor for developing breast carcinomas (Gill et al., 2006; McCormack and dos Santos Silva, 2006; Boyd et al., 2009b; Boyd et al., 2010). In this article we overview recent studies addressing the effects of ECM composition on the breast cancer cell cycle. The ECM remodeling associated with breast cancer appears to be a positive regulator of the cell cycle in breast cancer cells, but not always in nontumorigenic breast epithelial cells. This review will focus on the role of the ECM, including its composition and mechanical properties, in proliferation control that contributes to breast cancer.
The mammary epithelial cell microenvironment
The mammary gland ECM
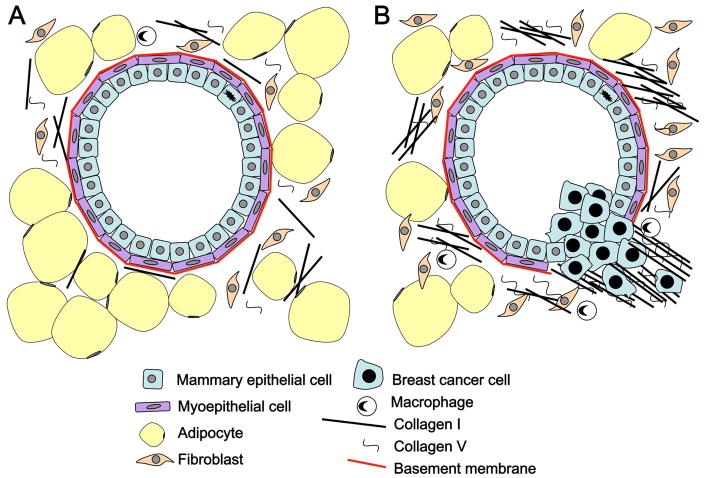
The mammary epithelium is bilayered, with inner luminal cells facing a central cavity and surrounded by a basal layer of myoepithelial cells (Figure 1A). The ECM in contact with this bilayer is the basement membrane, which separates the epithelium from the stroma. Myoepothelial cells produce the basement membrane, a thin sheet composed of collagen IV, laminins, entactin, and proteoglycans (Yurchenco and Patton, 2009). The stroma physically supports the epithelium, providing structure, nutrients, blood, and immune defense. Correspondingly, the stroma contains a variety of cells (including fibroblasts, adipocytes, endothelial cells, smooth muscle, innate and adaptive immune cells, and nerve cells), as well as ECM proteins (Shekhar et al., 2003; Stein et al., 2004). The stromal cells secrete paracrine factors, growth factors and cytokines, influencing MEC function and development. Stromal matrix components include collagens type I and III, which are the major structural proteins, as well as proteoglycans, hyaluronic acid, fibronectin and tenascins. The composition of the ECM varies during mammary gland development and pregnancy (Schedin et al., 2004; O’Brien et al., 2010; Lyons et al., 2011). Cells adhere to the ECM through several different receptors, with the best-studied being integrins (Hynes, 2002; Katsumi et al., 2004). Integrins are αβ heterodimers, with specifc αβ subunit combinations serving as receptors for different ECM components (Muschler and Streuli, 2010). In particular, β1 integrins are implicated in maintaining normal MEC phenotype (White et al., 2004; Taddei et al., 2008; Streuli, 2009; Streuli and Akhtar, 2009). Knockout of β1 integrin disrupts proliferation, differentiation and alveolar development (Li et al., 2005a; Naylor et al., 2005). Knockout of α2 integrin, a key receptor for collagens in the mammary gland, leads to loss of branching complexity (Chen et al., 2002). Although much remains to be discovered about how ECM receptors such as integrins link extracellular mechanical signals to biochemical signals within the cell, accumulating evidence implicates these interactions in the development and progression of breast cancer in response to alterations in the ECM (Muschler and Streuli, 2011; Schedin and Keely, 2011).
Figure 1.
Model of a mammary epithelial cell duct in a compliant versus stiff collagen matrix. (A) In a compliant matrix, MECs organize into polarized acini and ductal structures. (B) A collagen dense matrix promotes MEC proliferation and migration.
Mechanical properties of the ECM
The influence of ECM on gene expression in MECs was described as early as 1981 (Bissell, 1981; Emerman et al., 1981; Bissell et al., 1982) and more recently it was shown that 3D culture in Matrigel™ influences epigenetics, specifically histone modification patterns (Le Beyec et al., 2007) The additional findings that functional differentiation depends on 3-dimensional (3D) context (Hall et al., 1982; Hall and Bissell, 1986) and that MECs differentiate and produce milk in 3D reconstituted basement membranes (Matrigel™) but not in 2D culture (Petersen et al., 1992; Howlett and Bissell, 1993) suggest that at least some of these cellular responses are mediated via mechanosignaling. Mechanosignaling converts a mechanical event into a biochemical event, such as in signal transduction.
Integrin-mediated focal complexes, which are assembled in response to mechanical stress and stiffness, function as mechano-sensors (Katsumi et al., 2004; Katsumi et al., 2005). Integrins undergo conformational changes upon ligand binding, and integrin-cytoskeletal linkages are enhanced when force is exerted on integrins. These events suggest that binding sites within the complex are exposed under strain (Choquet et al., 1997). The polarity of MECs and their adherence via α6β4 integrin to the basement membrane at hemidesmosomes, and the tight juctions and adherens junctions between cells, likely contribute to mechanosignalling in MECs as they do in other cell types (Wozniak and Chen, 2009). Myoepithelial cells also likely participate in mechanical events within the mammary gland that impact the behavior of the luminal cells. Despite this, the mechanical properties of the basement membrane are yet to be described.
Mechanical properties of the stromal ECM, and more specifically its major structural protein, fibrillar collagen, are under intense scrutiny for their role in regulating gene expression as recently reviewed (Schedin and Keely, 2011), including regulation of the cell cycle (Klein et al., 2009). Mechanics of the stromal ECM are regulated by growth factors, which stimulate fibroblasts to deposit stroma and arrange collagen. Changes in ECM composition, such as occurs during mammary gland development and tumor progression, is characterized by increased ECM deposition and significantly changes the mechanical environment of MECs. Part of this is due to increases in collagen V, which change the structure of collagen I fibrils (Barsky et al., 1982; Wenstrup et al., 2004; Breuls et al., 2009); and increases in fibronectin and proteoglycans, which affect the organization of the collagen fibers. The cross-linking of collagen fibers and collagen arrangement increase matrix stiffness and therefore change the mechanical properties of the ECM (Raub et al., 2008; Raub et al., 2010) as do matrix proteases (Wolf and Friedl, 2009).
Differences in matrix stiffness change MEC cellular response, as shown by in vitro studies. MECs cultured in lower concentration 3D collagen gels organize into polarized acinar and ductal structures (Figure 1A), while those in higher concentration (stiffer) collagen gels, lose polarization and are locally invasive (Figure 1B). An increase in collagen concentration from 1.0 to 4.0 mg/ml represents a range from soft or pliable to a stiff matrix, with elastic moduli ranging from ~170 to 1600 Pascals (Pa) when measured by compression, and 10 to 44kPawhen measured by tensional forces (Roeder et al., 2002; Paszek et al., 2005; Gehler et al., 2009; Provenzano and Keely, 2009). A stiff matrix also provides traction for the cell to proliferate. MECs cultured in stiffer matrices increase proliferation and change their overall gene expression profiles (Wozniak et al., 2003; Paszek et al., 2005; Provenzano et al., 2009).
Cells sense stiffness of their environment in part by pulling against the ECM using intracellular contractile mechanisms, derived from actin-myosin interactions. In a less stiff matrix, cells pull the matrix towards them, while in a stiffer matrix, cells generate forces that result in focal adhesions and stress fiber formation (Wozniak et al., 2003; Gehler et al., 2009). The Rho-ROCK pathway has been linked to MEC response to matrix stiffness. Rho-GTP is decreased by a compliant matrix (Wozniak et al., 2003), while activation of this pathway by a stiff matrix results in phosphorylation and activation of the myosin regulatory light chain (MLC) (Wozniak et al., 2003; Paszek et al., 2005; Wyckoff et al., 2006). MLC can be phosphorylated by both ROCK and MLC-kinase (Amano et al., 1996), but the Rho-ROCK pathway appear to be more important in MEC response to matrix stiffness (Wozniak et al., 2003; Paszek et al., 2005; Wyckoff et al., 2006). The forces generated result in tension across adhesion receptors and focal complexes. This in turn strengthens adhesion, recruits signalling molecules and results in focal adhesion signalling (Schedin and Keely, 2011). Focal adhesion complex proteins implicated in mechanosensing include talin, vinculin, FAK, Src, and p130Cas (Felsenfeld et al., 1999; Wang et al., 2001; Li et al., 2002; Giannone et al.,2003; Jiang et al., 2003; Frame, 2004; Kostic and Sheetz, 2006; Sawada et al., 2006).
Regulation of normal cell cycle progression by the ECM
The cell cycle
Abundant evidence shows that the ECM and growth factors cooperate to regulate cell proliferation in MECs, as well as in many other cell types. Soluble mitogens and ECM proteins together regulate progression through G1-phase of the cell cycle via induction of G1 cyclins, including D-type cyclins (D1, D2 and D3) and E-type cyclins (E1 and E2) (Sherr and Roberts, 2004). The extracellular environment is best known to control cyclin D1 transcription in mid-G1, following induction of the transcription factor c-myc. Cyclin D binds to and activates cdk4 or cdk6. Active cyclin D-cdk4/6 phosphorylates the G1 checkpoint protein Rb, resulting in the release of E2F, a transcription factor sequestered and inhibited by Rb (Musgrove et al., 2011). E2F induces transcription of cyclin E and other genes required for S-phase. Cyclin Ebinds to and activates cdk2 and the complex is imported into the nucleus to phosphorylate proteins necessary to initiate DNA replication in S-phase. Cyclin E also has roles in histone biosynthesis, centrosome duplication, S-phase transcription, pre-mRNA splicing, oncogenic transformation and re-entry into the cell cycle from quiescence (Caldon and Musgrove, 2010).
ECM regulation of the cell cycle
ECM proteins induce cyclin D1 expression and down regulate the cdk inhibitorsp21 and p27by binding to and activating integrins (Assoian and Schwartz, 2001). In vitro studies have shown that integrin-dependent signalling events upstream of cyclin D1 induction are the activation of ERK MAP kinases, Rho family GTPases, and FAK(Assoian and Klein, 2008). If and how these pathways are activated in physiologically relevant mechanical microenvironments was only recently studied (Klein et al., 2009). Integrin-regulated events are typically studied in cultured cells after blockade of ECM-integrin binding (by incubating cells in suspension or on poly-lysine coated dishes) or by preventing integrin clustering (e.g. by disrupting the actin cytoskeleton with depolymerizing drugs or inhibitors of Rho GTPase activation or signaling), both of which result in drastic changes unlikely to occur physiologically. In addition, cells are usually cultured on non deformable substrata (culture dishes or coverslips) unlike the compliant ECM that cells interact with in vivo. Relative to cells grown on 2D ECM, proliferation of normal MECs is suppressed when cultured within 3D ECM composed of soft collagen I (Hotary et al., 2003) or Matrigel (Weaver et al., 1997b; Wang et al., 2002). Some studies have used collagen gels to study the effect of compliant matrixes on integrin signalling and the cell cycle. In cells other than MECs, culture on free-floating or soft collagen gels results in high levels of cdk inhibitors, unphosphorylated ERK, and no cyclin D1 expression (Koyama et al., 1996; Rosenfeldt and Grinnell, 2000; Fringer and Grinnell, 2001; Wall et al., 2007). FAK autophosphorylation, ERK activity, and Rho activation are also impaired when fibroblasts (Fringer and Grinnell, 2001) or MECs (Paszek et al., 2005) are cultured in soft collagen matrices or Matrigel™. These studies suggest that changes in matrix stiffness recapitulate the effects seen upon complete adhesion blockade. Although collagen gels (and Matrigel™) are often softer than many physiological tissues:10–50 Pa vs. 100–10,000 Pa (Bao and Suresh, 2003; Willits and Skornia, 2004), soft collagen I (1 mg/ml) has similar stiffness as normal mammary gland, both about 170 Pa (Paszek et al., 2005).
Similar to cyclin D1, cyclin E1expression is also responsive to extracellular signals (Koyama et al., 1996; Henriet et al., 2000; Cho et al., 2005). Proliferation of a MEC line on 2D collagen was positively regulated via β1 integrin induction of c-myc through activation of the Src and MAPK signaling pathways (Benaud and Dickson, 2001b). c-myc upregulated cyclin E1 expression, downregulated p27, and increased phosphorylation of Rb by cyclin E-cdk2 complexes (Benaud and Dickson, 2001a). Similarly, in normal primary human MECs, α2β1 integrin interaction with a 2D collagen matrix stimulated expression of cyclin E1 and cdk2 as well as cyclinE1-cdk2 complex formation (Klekotka et al., 2001). In contrast, when cultured in a soft 3D collagen matrixas compared to on 2D collagen or tisue culture plastic, MECs downregulate cyclin E1, arrest in G1 phase, and subsequently undergo apoptosis (YW and RSH, unpublished observations).
ECM alteration in breast development and cancer
ECM alteration during mammary gland development
ECM composition changes during both mammary gland development and in breast cancer. During mammary development, the main driver of ductal morphogenesis is epithelial cell proliferation and migration at the endbud, both of which are dependent on cell-matrix interactions. The endbud is covered with a thin basement membrane, which is remodeled as the cells invade the adjacent stroma. Local growth factor interactions between the MECs and stromal cells are required for proliferation. For example, stromal FGF and epithelial FGFR2 are required (Lu et al., 2008); while HGF-met also playsa key role (Liu et al., 2009). As discussed above, β1 integrins are required for MEC cell proliferation in 2D or 3D cultures, and function perturbing anti-β1 integrin antibodies eliminate most endbuds (Klinowska et al., 1999). Integrins may influence expression of FGFR, or may directly cooperate with FGFR to activate signalling and the cell cycle, thus advancing the endbud. Integrins may also regulate c-met signalling by binding laminin within the basement membrane (Liu et al., 2009). Matrix remodeling by matrix metalloproteinases (MMPs) is required for branching and endbud progression as well (Page-McCaw et al., 2007). MMP-3 induces secondary and tertiary branching of mammary ducts, while MMP-2 promotes endbud invasion into the stroma by suppressing MEC apoptosis (Wiseman et al., 2003). Membrane bound MMPs, including MT2-MMP in MECs, MT3-MMP in stromal cells, and MT1-MMP in both cell types are also likely required for mammary morphogenesis (Klinowska et al., 1999).
Basement membrane evasion
Changes in cell-matrix interactions are also integral to the development and progression of cancers (Bissell and Radisky, 2001). Early breast cancer lesions develop in the context of an intact basement membrane. The basement membrane suppresses tumorigenesis by controlling tissue architecture, including epithelial polarity (Weaver et al., 2002; Zhan et al., 2008). The basement membrane also retains in situ carcinomas within its boundaries (Chin et al., 2004; Hwang et al., 2004; Allred et al., 2008). Mechanisms controlling invasion through the basement membrane include increased activity of MMPs, altered cell adhesion, and ECM signaling (Mercurio et al., 2001; Hood and Cheresh, 2002). Increased MMP activity can occur via increased MMP activation or a change in the ratio of MMPs to their inhibitors (tissue inhibitors of metalloprotenases or TIMPs), with the MMP/TIMP ratio altered during both breast cancer progression and normal breast development (Baker et al., 2002; Fata et al., 2004b; Figueira et al., 2009). Other factors that drive cancer progression include changes in myoepithelial cells and alteration of the stromal cell population including cancer associated fibroblasts, macrophages, and other infiltrating leukocytes, as well as changes to the stromal matrix (Orimo and Weinberg, 2006; Hu and Polyak, 2008; Butcher et al., 2009; Qian et al., 2009; Lyons et al., 2011).
Stromal alteration during breast cancer development
Once the basement membrane has been evaded, breast cancer cells encounter the changed stromal matrix, characterized by increased cellularity and ECM deposition, a process known as “desmoplasia.” Collagen V is increased in desmoplasia (Barsky et al., 1982), which mechanically changes the structure of collagen I fibrils (Wenstrup et al., 2004; Berendsen et al., 2006; Breuls et al., 2009). Proteins such as fibronectin and proteaglycans bind to increased collagens and affect collagen fiber organization while increased lysal oxidase activity mediates collagen cross-linking, both of which affect mechanical properties of the ECM (Levental et al., 2009). Increases in collagen production, organization and cross-linking produce a stiffer matrix that imparts distinct biochemical and mechanical influences that contribute to malignancy (Levental et al., 2009). Increased collagen deposition and stiffness of the stromal matrix may be linked to increased breast density, which is a significant risk factor for breast cancer (Li et al., 2005b; Martin and Boyd, 2008). Mammographic density, expressed as either a percentage of the area of the breast (PMD) or as the area of dense tissue (cm2) in a mammogram are both positively associated with risk of breast cancer, but PMD is the stronger association (Byrne et al., 1995). PMD has a consistently strong influence on breast cancer risk, is independent of other risk factors, has a larger gradient in risk than most other risk factors, has an increased risk that extends for at least 10 years after the mammogram used to classify density, and carries a risk not explained by masking (Boyd et al., 2009a).
Evidence for the potent influence of stromal organization and function on invasion, metastasis, and proliferation of breast tumors is growing (Boyd et al., 2001; McCormack and dos Santos Silva, 2006; Boyd et al., 2009a; Lyons et al., 2011). Several studies have shown that MMPs are highly expressed in breast cancers, and that invasive breast cancer growth is preceded by an increase in MMP activity (Duffy et al., 2000). Furthermore, invasive breast cancer cells are partially growth suppressed in pliable 3D collagen culture (Hotary et al., 2003; Wu et al., 2011), suggesting that the presentation of an intact ECM is at least one mechanism to control both invasion and MEC proliferation. Recent studies corroborate these finding by showing that tumor adjacent but histologically normal tissues from breast adenocarcinoma patients show signs of fibrosis and ECM remodeling, including increased collagens I and VI, MMP2 and TGFβ3, among other changes in gene expression (Trujillo et al., 2010). In addition, gene expression profiling of patient matched stroma from both normal tissue and tumors, show ECM components including collagens and fibronections, MMPs, and cell-cycle genes are highly upregulated in tumor-associated stroma (Ma et al., 2009). Although these studies are based on a limited number of patient samples, the findings support the association between increased matrix density, malignancy, and upregulation of proliferation, as discussed below.
Effects of ECM alteration on the breast cancer cell cycle
Stiff matrices positively influence the cell cycle
The ECM remodeling associated with breast cancer appears to be a positive regulator of the cell cycle in MECs, primarily due to mechanical signalling stimulated by increased ECM stiffness (Provenzano and Keely, 2011). As discussed earlier, 3Dculture studies emphasize the importance of matrix compliance for normal tissue differentiation and imply that matrix stiffness regulates cell fate by modulating growth factor signalling and Rho GTPase function. Rho activity is often upregulated in “stiff” tumors (Fritz et al., 1999) and activating ROCK induces tumor dissemination (Croft et al., 2004). Tumors are rigid because they have a stiff stroma, and elevated Rho-dependent cytoskeletal tension associated with stiff stroma drives focal adhesions, disrupts adherens junctions, pertubs tissue polarity, enhances proliferation, and hinders lumen formation in 3D cultures of MECs (Paszek et al., 2005). Matrix stiffness results in integrin clustering, which enhances EGF-dependent ERK activation and increases ROCK-generated contractility and focal adhesions. Thus, ERK and Rho appear to be part of an integrated mechanoregulatory circuit that functions to link physicalcues from the stromal ECM through integrin adhesions to molecular pathways that control cell growth. A chronic increase in cytoskeletal tension such as that mediated by matrix stiffness could drive the assemby and or stabilization of focal adhesions to enhance growth and perturb organization and promote malignant transformation (Paszek et al., 2005).
MECs cultured in stiffer matrices have increased proliferation and changes in gene expression compared to cells in less stiff matrices (Wozniak et al., 2003; Paszek et al., 2005; Provenzano et al., 2009), as do other cell types (Chen et al., 1997; Wang et al., 2000; Discher et al., 2005). MECs in a stiffer matrix upregulate proliferative signals including cyclin D1 (Klein et al., 2009) and an entire set of genes identified as a proliferation signature in human breast carcinoma (Provenzano et al., 2009). This pathway is linked to a FAK-Rho-ERK pathway, as ERK inhibition reverses expression of the prolferation signature induced by rigid substrates (Assoian and Klein, 2008; Provenzano et al., 2009).
β1 integrin positively regulates MEC proliferation both in vitro and in vivo (Taddei et al., 2003), and is necessary for formation of mammary tumors in murine models (White et al., 2004). Disruption of β1 integrin function decreases MEC proliferation in a transgenic mouse model (Faraldo et al., 1998) and in both 2D and 3D tissue culture models (Pasqualini and Hemler, 1994; Wang et al., 1998), as well as decreases proliferation of invasive MDA-MB-231 breast cancer cells cultured on 2D collagen or in 3D Matrigel™ (Park et al., 2006; Wu et al., 2011) and reverts the malignant phenotype of HMT3522-T4-2 breast cancer cells (Weaver et al., 1997a). In contrast, nontumorigenic MECs that form acini-like structures in Matrigel™, are resistant to β1 integrin inhibition (Park et al., 2006). Neither inhibiting apoptosis nor enhancing proliferation blocks lumen formation in these epithelial structures, indicating that nontumorigenic MECs and breast cancer cells exert different mechanisms to maintain their phenotype in the same environment (Debnath et al., 2002).
Pliable collagen inhibits cell cycle progression
In contrast to 3D Matrigel™ and stiff collagen gels, culturing breast cancer cells within pliable3D collagen inhibits proliferation and blocking β1 integrin function rescues this inhibition (Wu et al., 2011). The mechanism is at least partly via direct regulation of cyclin E1, as β1 integrin inhibition restores cyclin E1 expression as well as expression of c-myc and phosphorylation of Rb without influencing cyclin D1expression. These studies implicate c-myc in transcriptional regulation of cyclin E1in addition to its established regulation of cyclin D1. Results similar to these were reported for arterial smooth muscle cells, which arrest in G1 inpliable collagen I and proliferate on monomer collagen (Koyama et al., 1996). In this study, the cdk inhibitorp27 was induced and suppressed cyclin E1-cdk2 without changing cyclin E1 level. 3D collagen likewise arrested glomerular mesangial cells at G0-G1 phase (Tsuboi et al., 2000), associated with downregulation of E2Fand its targets, including c-myc, and dephosphorylation of Rb. Thus in pliablecollagen gels, β1 integrin interaction with collagen I can lead to negative regulation of the cell cycle via direct regulation of cyclin E1. Interestingly, cyclin D1 relocalizes from the nucleus to the cytoplasmin response to culture in pliable3D collagen. At the G1-S boundary, cyclin D1 is usually phosphorylated by GSK3β followed by nuclear export to the cytoplasm, for ubiquitination and degradation by the 26S proteasome (Barbash et al., 2008). Thus, cyclin D1 may be phosphorylated by GSK3β in response to pliable3D collagen, resulting in its inactivation by nuclear export. Cyclin D1 overexpression in cancer is often caused by disruption of its degradation (Pontano and Diehl, 2008), which has been shown to accelerate mammary carcinogenesis.
Summary
Mechanosignalling and the MEC cell cycle
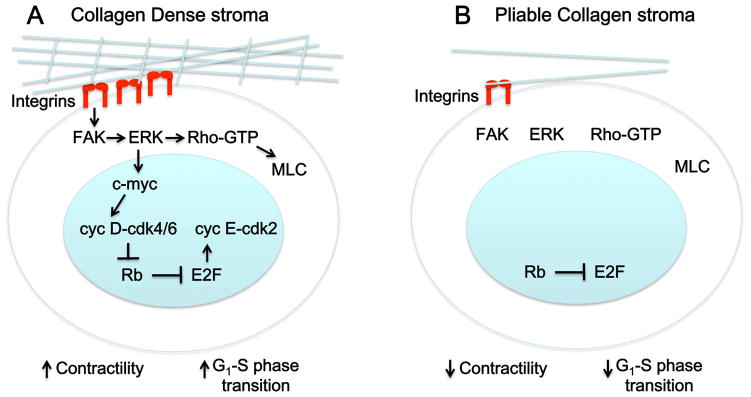
An increasing number of studies show that mechanical signaling regulates MEC entry into and progression through the cell cycle. Three-dimensional culture systems suggest that changes in this signaling, due to increased rigidity caused by collagen deposition and other changes in the ECM, likely enhance proliferation via upregulation of cyclin D and contribute to tumorigenesis (Figure 2A). In contrast, in a less rigid3D environment composed of pliable collagen I, the cell cycleis inhibited due to E2F inhibition by Rb (Figure 2B). This regulation is through β1 integrin-activated signaling pathways, with ERK and Rho functioning downstream to link physical cues from the stromal ECM to the molecular circuitry of the cell cycle. The downstream signaling pathways as well as the control of β1 integrin function in differentially affecting proliferation need to be further elucidated, as do the inherent differences in breast cancer matrix rigidity and their effect on β1 integrin function and signalling.
Figure 2.
Model for cell cycle regulation of mammary epithelial cells by increased ECM density/stiffness. (A) As cells encounter increased resistance to contractility from stiff matrices such as a collagen dense stroma (blue lines), they respond by integrin clustering (red), which leads to phosphorylation and activation of focal adhesion kinase (FAK). This activates a FAK-ERK-Rho signaling loop that induces transcription factors such as c-myc, which stimulate transcription of G1 cyclins (cyclins D and E). Cyclin D (cyc D) and cyclin E (cyc E) activate cdks and facilitate progression through G1 into S phase. Phosphorylation of myosin light chain downstream of Rho-GTP increases cellular contractility, generating tension that is also required for proliferation. (B) In a pliable stroma, integrin clustering is not induced thus downstream signalling pathways are not activated.
Future perspectives
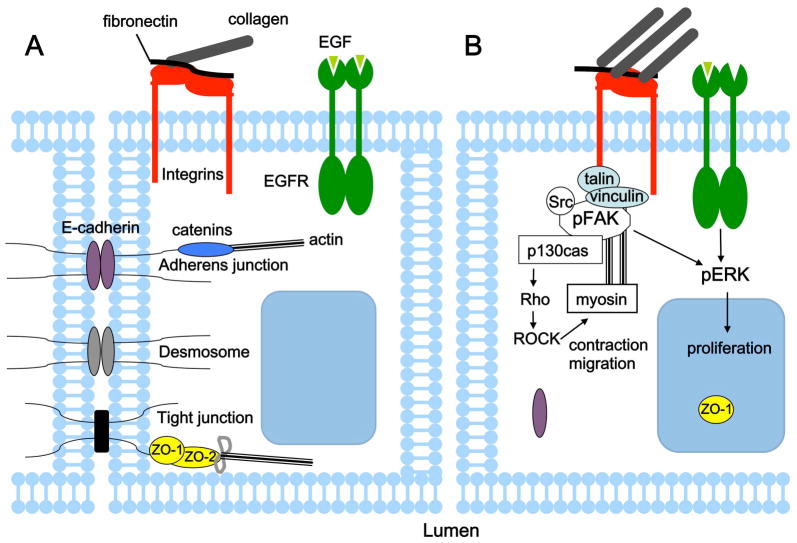
Previous reports have highlighted that, to overcome contact inhibition of cell proliferation, levels of epidermal growth factor (EGF) have to exceed a certain threshold (Kim et al., 2009). It was recently shown that ECM stiffening lowers this threshold nearly 100-fold (Kim and Asthagiri, 2011). With increased substratum stiffness, cells shift from contact-inhibited to contact-independent growth at lower EGF concentrations. Furthermore, this effect involves attenuating the efficiency with which cell–cell contacts inhibit cell cycle entry. The increased stiffness disrupts maturation of these cell–cell contacts by reducing the recruitment of E-cadherin and ZO-1 to cell junctions. Consistent with existing evidence, these results suggest that it is the interplay between the three types of environmental cues –growth factors, cell–cell contacts and ECM – that regulates cell proliferation (Figure 3). This observation has important implications for cancer development and progression, and therefore for developing effective therapies to prevent its development and hinder progression. The importance of understanding cancer as a context-dependent pathology where tumor cells not only interact with but also respond to their microenvironment is gaining widespread recognition. This is evidenced by the update of Hanahan and Weinberg’s “Hallmarks of Cancer” review (Hanahan and Weinberg, 2000) recently published in Cell (Hanahan and Weinberg, 2011). This review notes that the tumor microenvironment contributes to the acquisition of hallmark traits of cancer, one of which is sustaining proliferative signaling. The full extent of microenvironmental effects on the cell cycle, as well as other hallmark cancer traits remains to be determined.
Figure 3.
Integration of environmental cues including growth factors, cell–cell contacts and ECM regulates proliferation. (A) A compliant ECM and contact with neighboring cells impose constraints on epithelial cell proliferation. (B) Increased ECM stiffness reduces the threshold amount of EGF needed to override contact inhibition and stimulates proliferation. Loss of contact inhibition coincides with disruption of cell-cell contacts, change in localization of EGFR and ZO-1, and enchanced ERK signalling (Kim and Asthagiri, 2011). MECs also respond to increased matrix stiffness in a FAK-dependent manner by developing mature focal adhesions, upregulating a FAK-Rho signalling loop and ERK activation, which stimulates contraction, migration and proliferation.
Acknowledgments
Grant sponsors: Rauth Family Foundation and the DHHS/NIH/NCRR from The University of New Mexico Clinical and Translational Science Center; Grant number: #1 UL1RR031977-01
RSH was supported by a Rauth Basic Scientist Research Award from the Rauth Family Foundation and by DHHS/NIH/NCRR grant #1 UL1RR031977-01, The University of New Mexico, Clinical and Translational Science Center (CTSC).
Literature cited
- Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee S, Perou CM, Mohsin SK, O’Connell P, Tsimelzon A, Medina D. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–378. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Baker EA, Stephenson TJ, Reed MW, Brown NJ. Expression of proteinases and inhibitors in human breast cancer progression and survival. Mol Pathol. 2002;55:300–304. doi: 10.1136/mp.55.5.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, Lu F, Rustgi AK, Diehl JA. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer cell. 2008;14:68–78. doi: 10.1016/j.ccr.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Rao CN, Grotendorst GR, Liotta LA. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol. 1982;108:276–283. [PMC free article] [PubMed] [Google Scholar]
- Benaud CM, Dickson RB. Adhesion-regulated G1 cell cycle arrest in epithelial cells requires the downregulation of c-Myc. Oncogene. 2001a;20:4554–4567. doi: 10.1038/sj.onc.1204609. [DOI] [PubMed] [Google Scholar]
- Benaud CM, Dickson RB. Regulation of the expression of c-Myc by beta1 integrins in epithelial cells. Oncogene. 2001b;20:759–768. doi: 10.1038/sj.onc.1204152. [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Bronckers AL, Smit TH, Walboomers XF, Everts V. Collagen type V enhances matrix contraction by human periodontal ligament fibroblasts seeded in three-dimensional collagen gels. Matrix Biol. 2006;25:515–522. doi: 10.1016/j.matbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ, Stone J, Hopper JL. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009a;472:343–360. doi: 10.1007/978-1-60327-492-0_15. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Yaffe M, Minkin S. Mammographic density. Breast Cancer Res. 2009b;11(Suppl 3):S4. doi: 10.1186/bcr2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuls RG, Klumpers DD, Everts V, Smit TH. Collagen type V modulates fibroblast behavior dependent on substrate stiffness. Biochem Biophys Res Commun. 2009;380:425–429. doi: 10.1016/j.bbrc.2009.01.110. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010;5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, Kuo WL, Ljung BM, Chew K, Myambo K, Miranda M, Krig S, Garbe J, Stampfer M, Yaswen P, Gray JW, Lockett SJ. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- Cho MK, Suh SH, Lee CH, Kim SG. Bovine type I collagen inhibits Raw264.7 cell proliferation through phosphoinositide 3-kinase- and mitogen-activated protein kinase-dependent down-regulation of cyclins D1, A and B1. Biochim Biophys Acta. 2005;1744:47–57. doi: 10.1016/j.bbamcr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Croft DR, Sahai E, Mavria G, Li S, Tsai J, Lee WM, Marshall CJ, Olson MF. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 2004;64:8994–9001. doi: 10.1158/0008-5472.CAN-04-2052. [DOI] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JT, Bartley JC, Bissell MJ. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981;134:241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004a;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004b;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin--cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol. 1999;1:200–206. doi: 10.1038/12021. [DOI] [PubMed] [Google Scholar]
- Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 2009;9:20. doi: 10.1186/1471-2407-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Fringer J, Grinnell F. Fibroblast quiescence in floating or released collagen matrices: contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem. 2001;276:31047–31052. doi: 10.1074/jbc.M101898200. [DOI] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, Eliceiri KW, Weaver VM, Calderwood DA, Keely PJ. Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell. 2009;20:3224–3238. doi: 10.1091/mbc.E08-12-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Jiang G, Sutton DH, Critchley DR, Sheetz MP. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J Cell Biol. 2003;163:409–419. doi: 10.1083/jcb.200302001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Maskarinec G, Pagano I, Kolonel LN. The association of mammographic density with ductal carcinoma in situ of the breast: the Multiethnic Cohort. Breast Cancer Res. 2006;8:R30. doi: 10.1186/bcr1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–248. [PubMed] [Google Scholar]
- Hall HG, Bissell MJ. Characterization of the intermediate filament proteins of murine mammary gland epithelial cells. Response to collagen substratum. Exp Cell Res. 1986;162:379–389. doi: 10.1016/0014-4827(86)90343-5. [DOI] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci U S A. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10026–10031. doi: 10.1073/pnas.170290997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer. 2008;44:2760–2765. doi: 10.1016/j.ejca.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, DeVries S, Chew KL, Moore DH, 2nd, Kerlikowske K, Thor A, Ljung BM, Waldman FM. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res. 2004;10:5160–5167. doi: 10.1158/1078-0432.CCR-04-0165. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–695. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kushiro K, Graham NA, Asthagiri AR. Tunable interplay between epidermal growth factor and cell-cell contact governs the spatial dynamics of epithelial growth. Proc Natl Acad Sci U S A. 2009;106:11149–11153. doi: 10.1073/pnas.0812651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekotka PA, Santoro SA, Ho A, Dowdy SF, Zutter MM. Mammary epithelial cell-cycle progression via the alpha(2)beta(1) integrin: unique and synergistic roles of the alpha(2) cytoplasmic domain. The American journal of pathology. 2001;159:983–992. doi: 10.1016/s0002-9440(10)61774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005a;24:1942–1953. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A. 2002;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005b;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Ewald AJ, Martin GR, Werb Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal endbuds during mammary gland branching morphogenesis. Dev Biol. 2008;321:77–87. doi: 10.1016/j.ydbio.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Bachelder RE, Chung J, O’Connor KL, Rabinovitz I, Shaw LM, Tani T. Integrin laminin receptors and breast carcinoma progression. J Mammary Gland Biol Neoplasia. 2001;6:299–309. doi: 10.1023/a:1011323608064. [DOI] [PubMed] [Google Scholar]
- Muschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol. 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, Man YG, Borges V, Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Hemler ME. Contrasting roles for integrin beta 1 and beta 5 cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2:a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontano LL, Diehl JA. Speeding through cell cycle roadblocks: Nuclear cyclin D1-dependent kinase and neoplastic transformation. Cell division. 2008;3:12. doi: 10.1186/1747-1028-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Keely PJ. The role of focal adhesion kinase in tumor initiation and progression. Cell Adh Migr. 2009;3:347–350. doi: 10.4161/cam.3.4.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub CB, Putnam AJ, Tromberg BJ, George SC. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010;6:4657–4665. doi: 10.1016/j.actbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub CB, Unruh J, Suresh V, Krasieva T, Lindmo T, Gratton E, Tromberg BJ, George SC. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J. 2008;94:2361–2373. doi: 10.1529/biophysj.107.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki A, Bissell MJ. Homeostasis in the breast: it takes a village. Cancer Cell. 2004;6:1–2. doi: 10.1016/j.ccr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124:214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H, Grinnell F. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem. 2000;275:3088–3092. doi: 10.1074/jbc.275.5.3088. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Keely PJ. Mammary Gland ECM Remodeling, Stiffness, and Mechanosignaling in Normal Development and Tumor Progression. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Pauley R, Heppner G. Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5:130–135. doi: 10.1186/bcr580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH. Integrins and cell-fate determination. J Cell Sci. 2009;122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA. Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia. 2003;8:383–394. doi: 10.1023/B:JOMG.0000017426.74915.b9. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Heaphy CM, Mai M, Vargas KM, Jones AC, Vo P, Butler K, Joste N, Bisoffi M, Griffith JK. Markers of fibrosis and epithelial to mesenchymal transition demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2010 doi: 10.1002/ijc.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi N, Yoshida H, Kawamura T, Furukawa Y, Hosoya T, Yamada H. Three-dimensional matrix suppresses E2F-controlled gene expression in glomerular mesangial cells. Kidney international. 2000;57:1581–1589. doi: 10.1046/j.1523-1755.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- Wall SJ, Zhong ZD, DeClerck YA. The cyclin-dependent kinase inhibitors p15INK4B and p21CIP1 are critical regulators of fibrillar collagen-induced tumor cell cycle arrest. J Biol Chem. 2007;282:24471–24476. doi: 10.1074/jbc.M702697200. [DOI] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997a;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. 1997b;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521–1531. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo X, Brandt Y, Hathaway HJ, Hartley RS. Three-dimensional collagen represses cyclin E1 via beta1 integrin in invasive breast cancer cells. Breast Cancer Res Treat. 2011;127:397–406. doi: 10.1007/s10549-010-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]