Abstract
In this study, we used a validated psychophysical tool, the hedonic general magnitude scale (hedonic gLMS), to examine whether hedonic responsivity after repetitive tasting of a sweet-tasting liquid follows a habituation pattern that is independent of adaptation to the sweet taste at the orosensory level, and whether the pattern of response is different between obese (N=22) and lean (N=32) women. The perceived sweet intensity and hedonic value of a 24% w/v sucrose solution was measured with the gLMS and sucrose preferences with the Monell two-series, forced-choice tracking method. Although women perceived the same intensity of sweetness across trials, obese women responded with a slower rate of habituation to the liking of repetitive sweet-taste orosensory stimulation than did lean women. Therefore, the decreased hedonic response observed in obese women cannot be explained by adaptation processes at the orosensory level or by differential perception of taste intensity or scale bias between the groups. The groups did not differ in the level of sweetness preferred. Because obesity was associated with slower patterns of habituation to the palatability elicited by sweetness in women, this characteristic could contribute to slower satiation rates, prolongation of eating episodes, and excessive food consumption in obese women.
Keywords: habituation, sweet taste, obesity, hedonics, food pleasure, palatability, sensory perception
Introduction
During the course of a meal, a food that is palatable at the beginning becomes progressively less palatable as satiation is attained. At this time, the consumption of this particular food generally terminates. Nevertheless, on many occasions, even after eating one food to satiation, there is still “room for dessert”. This phenomenon, where the pleasantness induced by a food that has been recently eaten decreases more than the pleasantness of uneaten foods, has been coined the term “sensory-specific satiety” (B. J. Rolls, Rolls, Rowe, & Sweeney, 1981). That the decline in pleasantness occurs within minutes of finishing a meal, irrespective of the energy density of the food (Epstein, Temple, Roemmich, & Bouton, 2009), and that chewing the food without swallowing is sufficient to produce sensory-specific satiety (E. T. Rolls & Rolls, 1997) suggest that external signals (e.g., sensory properties of food) play a more important role than do postabsorptive effects.
An alternative to the model of sensory-specific satiety is the theory of habituation (Epstein et al., 2009). Habituation, one of the simplest forms of learning, is defined as “a response decrement as a function of stimulus repetition which does not result from either receptor adaptation or effectors fatigue” (Thompson & Spencer, 1966). This form of learning not only explains the decline of salivation, motivated responding (e.g., working to obtain food in a computer task), and the decline in how much food is consumed after the repetitive stimulation with food stimuli that takes place as the eating episode progresses (Hetherington, 1996),(Raynor & Epstein, 2001), but also provides a theoretical basis to understand recovery of the response, that is, the resumption of eating, or dishabituation (i.e., recovery of salivation and motivated responding), when a new food is presented (Epstein et al., 2009). Habituation may be a basic mechanism underlying sensory-specific satiety (Hetherington, 1996; Raynor & Epstein, 2001).
Slower patterns of habituation to food are associated with greater energy intake (Epstein et al., 2009). Because foods that are liked are often overconsumed (B. J. Rolls, Rowe, & Rolls, 1982; Sorensen, Moller, Flint, Martens, & Raben, 2003), recent investigations have focused on whether there are differences in sensory-specific satiety and habituation among obese and lean individuals (Brondel et al., 2007). Although obese subjects exhibited slower rates of habituation of salivation and motivated responding for food than did their lean peers (Epstein, Paluch, & Coleman, 1996; Epstein et al., 2008; Bond et al., 2009), lean and obese subjects did not differ when studied with sensory-specific satiety paradigms (Brondel et al., 2007; Snoek, Huntjens, Van Gemert, De Graaf, & Weenen, 2004): pleasantness ratings for the taste of an eaten food declined significantly more than did pleasantness ratings of an uneaten food for both groups. Furthermore, the degree of reduction in pleasantness (a measure of the sensitivity to sensory-specific satiety) was similar between the groups (Brondel et al., 2007; Snoek et al., 2004).
Noteworthy, when measuring liking, both sensory-specific satiety and habituation paradigms have used traditional labeled scales (e.g., category, visual analogue scale). However, recent evidence suggests that the intensity labels used in these traditional scales do not denote the same perceived intensities or perceived liking of foods in obese and lean individuals (Bartoshuk, Duffy, Hayes, Moskowitz, & Snyder, 2006). A newer psychophysical tool, the hedonic general magnitude scale (hedonic gLMS), takes into consideration that the perceived intensity or hedonics denoted by labels vary because they depend on experiences (Bartoshuk et al., 2006). To this end, in the hedonic gLMS, subjects are asked to rate the liking for foods in the context of all hedonic experiences rather than in the narrow context of hedonic experiences related to just food (Lanier, Hayes, & Duffy, 2005). The use of this tool has revealed that obese individuals live in different hedonic worlds than do the nonobese (Bartoshuk et al., 2006). Specifically, obese individuals have a more intense liking for foods. Therefore, the finding that obese and lean individuals do not differ in their sensitivity to sensory-specific satiety may be an artifact caused by using an invalidated instrument to assess liking.
In the study here described, we used the hedonic gLMS to compare habituation patterns to the pleasure elicited by sweetness in lean and obese women. We hypothesized that hedonic responses to repetitive stimulation with sweet taste would follow a habituation pattern and that the habituation rate to the pleasantness elicited by sweetness would be slower in obese than in lean individuals. We studied only women because women respond with stronger cortical reactivity and lower cognitive control to gustatory food-related stimuli than do men (Uher, Treasure, Heining, Brammer, & Campbell, 2006; Wang et al., 2009).
Methods and Procedures
Participants
Women were recruited from advertisements in local newspapers. During initial telephone screening, subjects were excluded if they had diabetes, were pregnant or lactating, had a history of chronic rhinitis or food allergies, or were taking any medication except birth control pills. The study population consisted of 54 women who were between 21 and 40 years of age and who reported having a body mass index (BMI) between 18.5 and 24.9 kg/m2 or >29.9 kg/m2. To corroborate subjects’ self-reports, height and weight were measured with women wearing light clothing and no shoes (Detecto model 439 physician scale; Detecto, Webb City, MO). BMI was < 25.0 kg/m2 for 32 women (hereafter referred to as the lean group) and > 29.9 kg/m2 for the remaining 22 women (hereafter referred to as the obese group).
All procedures were approved by the Office of Regulatory Affairs at the University of Pennsylvania, and each woman gave informed written consent.
Procedures
Each woman was tested during the morning hours on 2 separate days a week apart. Subjects underwent a battery of sensory tests at the Monell Center, including sucrose and monosodium glutamate detection thresholds and suprathreshold perception, the data for which are published elsewhere (Pepino, Finkbeiner, Beauchamp, & Mennella, 2010). To standardize testing procedures and the level of hunger/satiation at the time of testing, subjects were asked to abstain from food and from smoking (if they were smokers, N=24) for 12 h before coming to Monell. To enhance compliance, participants were aware that blood glucose levels would be measured from a capillary blood sample taken by finger-prick (OneTouch®, LifeScan, Inc., Milpitas, CA), to ensure they had fasted, and that carbon monoxide levels would be measured using a Vitalograph (Vitalograph, Inc., Lenexa, KS), to ensure they had abstained from smoking, if they were smokers. Upon arrival to Monell, participants were provided and consumed a standard breakfast, which consisted of a protein bar (180 kcal) and 14 ounces of orange juice (190 kcal). At 1.5 h after eating breakfast, we measured their subjective perceptions after repetitive tasting of a 24% sucrose solution on one testing day and determined the intensity of sweetness most preferred on the other.
General Labeled Magnitude Scales
Before testing, each subject was trained in the use of the general Labeled Magnitude Scale (gLMS)(Lanier et al., 2005). The gLMS is a computerized psychophysical tool that requires subjects to rate the perceived intensity along a vertical axis lined with adjectives that are spaced semi-logarithmically, based upon experimentally determined intervals to yield ratio-quality data (Green et al., 1996). The gLMS shows only adjectives, not numbers, to the subjects, but the experimenter receives numerical data from the computer program that display the scales. For intensity ratings, the top of the scale represents the “strongest imaginable sensation of any kind”, and participants are trained that this refers to all kind of sensations, including pain(Lanier et al., 2005). For hedonic ratings, the scale ranges from “strongest imaginable unpleasant experience of any kind” (−100 mm) to “strongest imaginable pleasant experience of any kind” (+100 mm), including a “neutral” sensation in the middle (0 mm) (Lanier et al., 2005). Participants were asked to rate the hedonic experience of tasting the sweet solution in the context of the full range of pleasure and displeasure that they have experienced in their life.
Sweet Taste Reactivity (Habituation) Test
Subjects were instructed to sip and taste without swallowing 13 samples (one every 2 min, with no interstimulus rinse). The samples were presented in medicine cups and contained 10 ml of a 24% w/v sucrose solution for trials 1–10 and 12–13 and 10 ml of a 0.88% w/v NaCl solution on trial 11 (dishabituation stimulus). They were asked to taste the sample for 10 s and then to spit it out. Subjects rated changes in intensity and hedonic responses immediately after spitting out each solution, by answering three questions: 1) How pleasant was the taste? 2) How strong is your desire for a different taste? 3) How intense was the taste? Subjects rated the first question using the hedonic general Label Magnitude Scale (gLMS), the second question using a gLMS (for intensity of their “desire for a different taste”), and the third question using a gLMS scale for each of the five basic tastes (i.e., one scale for each taste quality: sweet, salty, bitter, sour, and umami).
Sucrose Preferences
To determine the level (intensity) of sweetness most preferred, we used the Monell two-series, forced-choice tracking technique (Mennella, Lukasewycz, Griffith, & Beauchamp, 2011). In brief, subjects were presented with pairs of solutions that differed in sucrose concentration (3%, 6%, 12%, 24%, 36% w/v) (see Pepino, Finkbeiner, Beauchamp, & Mennella, 2010) for more details). The first pair of samples presented was from the middle range (6% w/v vs. 24% w/v). Subjects tasted, without swallowing, each solution and then pointed to which of the pair they liked better. Each subsequent pair was then determined by the subject’s preceding preference choice. The procedure continued until the subject chose either a given concentration of sucrose when it was paired with both a higher and lower concentration, or the highest (36% w/v) or lowest (3% w/v) solution two consecutive times. Subjects performed the forced-choice series twice: in the first series they were presented with the weaker stimulus first; then the entire task was repeated with the stronger stimulus presented first. The geometric mean of the sucrose concentrations chosen during the two trial series provided the estimate of sucrose preference.
Statistical Analysis
The primary dependent variables were changes in response to the repetitive presentation of 24% w/v sucrose (i.e., degree of pleasantness from tasting it, degree of desire for a new taste, and taste intensity) and the intensity of sucrose most preferred (geometric mean). Since half of the women were smokers, and a history of smoking might affect patterns of habituation to food stimuli (Epstein, Caggiula, Perkins, Mitchell, & Rodefer, 1992), preliminary analysis of the data included both smoking status (nonsmoker, smoker) and body weight group (lean, obese) as factors. Because a history of smoking did not significantly affect rate of habituation to the sweet taste (either hedonic or sensory variables) or sucrose preferences, smoking groups were collapsed and all the subsequent analyses focused on body weight grouping.
The data on intensity of sucrose most preferred were analyzed by a one-way analysis of variance (ANOVA) with body weight group (lean, obese) as the between-subjects variable. Changes in responses (i.e., degree of pleasantness, desire for a new taste) to the repetitive presentation of the sweet stimuli were analyzed by a separate mixed repeated-measures ANOVA with group (lean, obese) as the between-subjects factor and trial number (1–13) as the within-subjects factor. Changes in measures of taste intensity were examined with mixed repeated-measures ANOVA with group (lean, obese) and taste quality (sweet, salty) as the between-subjects factors and the 13 trials as the within-subjects factor. Sour, bitter, and savory were not included in the analyses because values scored on these scales were almost nil or had no variance. When the ANOVAs revealed significant interactions, post-hoc Fisher least significant difference analyses were conducted. The critical value for significance was P<0.05. Greenhouse-Geisser corrections were used to correct violations of the assumption of sphericity.
Results
Subject Characteristics
As shown in Table 1, there were no significant differences between lean and obese women in age, race, years of education, income level, or the percentage who were smokers (all p-values>0.24).
Table 1.
Subject characteristicsa
| Lean Women (n=34) | Obese Women (n=22) | p-Value | |
|---|---|---|---|
| Age (years) | 27.4 ± 1.1 | 27.6 ± 1.3 | 0.92 |
| BMI (kg/m2) | 22.1 ± 0.8 | 38.4 ± 1.0 | 0.0001 |
| Yearly Income (%) | |||
| <$15,000 | 25.8% | 40.9% | |
| $15,000 – $35,000 | 29.0% | 18.2% | 0.45 |
| >$35,000 | 45.2% | 40.9% | |
| Years of Education | 14.8 ± 0.4 | 14.0 ± 0.5 | 0.24 |
| Race (%) | |||
| White | 50.0% | 36.4% | |
| Black | 43.8% | 59.1% | 0.54 |
| Other/Mixed | 6.3% | 4.6% | |
| Smokers (%) | 41% | 50% | 0.46 |
BMI, body mass index. Significant p-values are in boldface.
Sweet Taste Reactivity Test
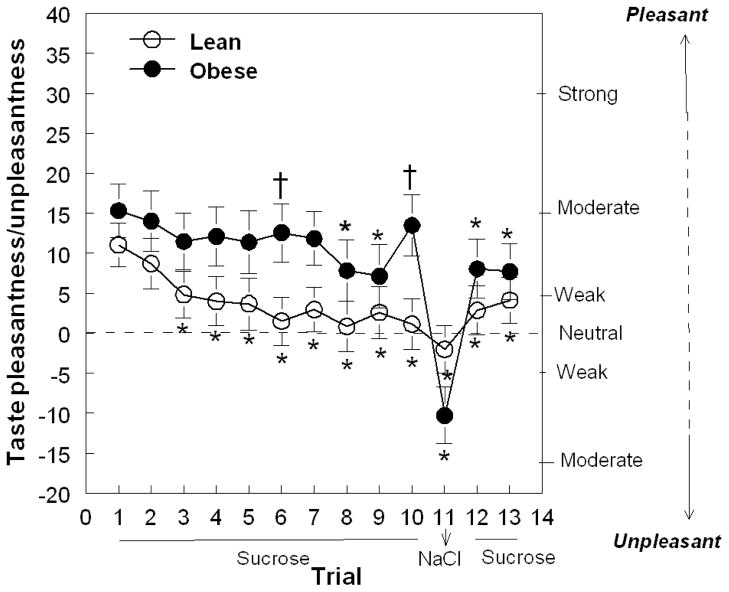
Both obese and lean women habituated to the pleasantness elicited by a sweet taste (F(12,624)= 7.98; p<0.0001). However, obese women habituated at a slower rate than did lean women (F(12,624)= 2.43; p=0.03). As shown in Figure 1, although both obese and lean women experienced similar levels of pleasure when first tasting the sweet solution, obese women perceived the sweet taste as pleasant for a longer period of time (16 min) than did lean women (6 min).
Figure 1.
Hedonic values reported by obese and lean women when tasting an unswallowed solution across 13 trials (mean ± SE). The solutions presented during trials 1–10 and 12–13 were 24% w/v sucrose; during trial 11 it was 0.88% w/v NaCl. *Significant difference from trial 1 within each group. †Significant difference between lean and obese women.
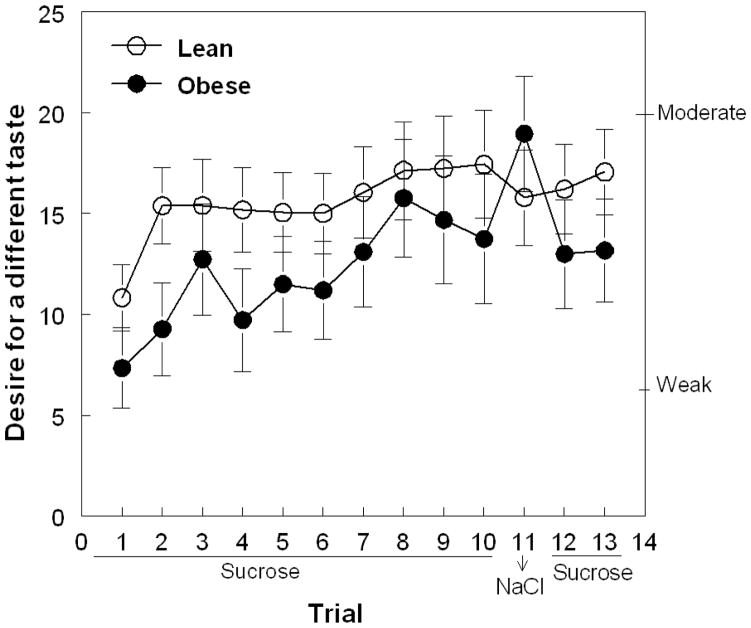
The unexpected presentation of the salty solution on trial 11 also resulted in differential hedonic responses between obese and lean women. The change of hedonic value from positive to negative between trial 10 and trial 11 was significantly greater in obese than in lean women (lean: −3.1±4.8; obese: −23.8±5.8; F(1,52) =7.44; p=0.009). There were no differences between groups in their desire for a different taste, which similarly increased across trials for all women (F(1,624)=3.83; p<0.0001; Figure 2).
Figure 2.
Desire for a different taste reported by women when tasting an unswallowed solution across 13 trials using gLMS (mean ± SE). The solutions presented during trials 1–10 and 12–13 were 24% w/v sucrose; during trial 11, it was 0.88% w/v NaCl (dishabituation stimulus). The y-axis labels represent gLMS notation: barely detectable, weak and moderate.
Sensory Properties
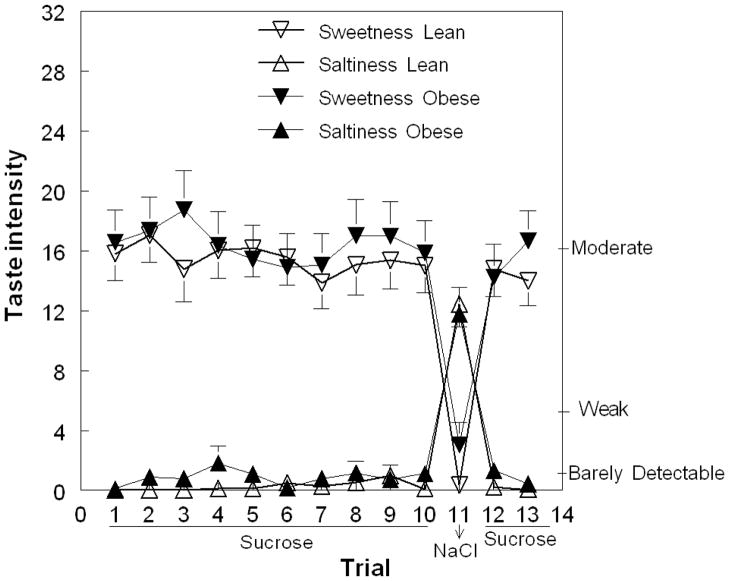
There was a main effect of taste (F(1,52)=124.5; p<0.0001) and a significant interaction between taste and trials (F(12,624)=50.69; p<0.0001) but no main effect of, nor interaction with, body weight group (all p-values>0.70) across trials. Both obese and lean women perceived the sweetness of the 24% w/v sucrose concentration equally intense across trials 1–10 and 12–13. For both groups, the solution tasted in trial 11 (dishabituation stimulus) was perceived as salty with no detectable sweetness (Figure 3).
Figure 3.
Perceived sweetness and saltiness reported by obese and lean women when tasting an unswallowed solution across 13 trials using gLMS (mean ± SE). The solutions presented during trials 1–10 and 12–13 were 24% w/v sucrose; during trial 11 it was 0.88% w/v NaCl (dishabituation stimulus). The y-axis labels represent gLMS notation: barely detectable, weak, and moderate.
Sucrose Preferences
There were no statistical differences on the intensity of sucrose preferred by the two groups of women (p=0.58). Lean women most preferred 16.1±2.0% w/v sucrose solution, and obese women most preferred 17.9±2.4 % w/v sucrose solution.
Discussion
Changes in the liking for a sweet-tasting liquid that is repetitively tasted followed habituation patterns that differed between obese and lean women. While we found no differences in the intensity of sucrose most preferred or the degree of pleasantness experienced when initially tasting sweetness, obese women differed from lean women in their hedonic responses when sweetness was repeatedly tasted, as happens during the course of a meal. Compared with their lean counterparts, obese women responded with a slower pattern of habituation to the pleasantness elicited by sweetness. It is noteworthy that obese and lean woman judged the sweetness intensity of the 24% w/v sucrose solution similarly, at a level that remained relatively constant across trials. Therefore, decreased appetitive responses to the sweet taste were not explained by adaptation processes occurring in the oral cavity, and differences observed between obese and lean women were not due to differential perception of taste intensity or scale bias between the groups.
The present findings are consistent with previous research showing slower decrements in salivary responses to repeated presentation of food cues in obese than in lean adults (Epstein et al., 1996; Bond et al., 2009) and further suggest that the observed differences in salivation patterns between the two groups result from a slower decrease in the hedonic value of the stimulus rather than in the sensory response to repeated presentation of food cues. Noteworthy is the phenomenon of negative alliesthesia, which describes how the hedonic value of a sensory stimulus changes as a function of the internal state of an individual (Cabanac 1971). However, unlike our method, where post-ingestive effects of the sweet stimuli can be ruled out, the study of gustatory alliesthesia requires that subjects repetitively consume a sweet stimulus and hedonic responses to sweetness are examined before and several times after subjects consume a glucose load (Cabanac, 1971; Cabanac & Duclaux, 1970). Remarkably similar to our findings, there is an absence of satiety aversion to sucrose, or negative alliesthesia, in obese compared to lean adults (Cabanac & Ducleaux 1970). The lack of negative alliesthesia in obese subjects has been interpreted as an indication of reduced sensitivity to internal signals in the control of food intake in obese people (Cabanac & Ducleaux 1970). Our findings suggest that, at least in part, the observed differences in negative alliesthesia between lean and obese subjects result from a slower decrease in the hedonic value of the stimulus at the central level, likely relying on neuronal circuitry of reward, rather than in a reduced sensitivity to post-prandial internal signals. That obese women experience more pleasure from repeatedly tasting sweetness than do their lean peers is also in agreement with the hypothesized association between some forms of obesity and a heightened sensitivity to food reward (Davis, 2009). Recent studies have shown that individuals with this hypersensitivity, and especially those with low levels of inhibitory control, are more likely to engage in overeating, which in turn increases their risk of becoming obese (Rollins, Dearing, & Epstein 2010; Appelhans et al. 2011). Further, our results concur with and complement findings from neuroimaging studies that revealed that, compared to lean individuals, obese individuals showed increased activation of brain areas associated with food reward when tasting or viewing a picture of a palatable, high-calorie meal (DelParigi et al., 2004; Rothemund et al., 2007; Stoeckel et al., 2008; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008). Included within the typical brain areas associated with food reward is the orbitofrontal cortex. Notably, activation of this brain area correlates with subjective pleasantness experienced while eating a meal and tracks changes in pleasantness for food caused by sensory-specific satiety (Kringelbach, O’Doherty, Rolls, & Andrews, 2003).
It is important to emphasize that, contrary to the proposed association between obesity and hypersensitivity to reward, a positron emission tomography (PET) study in humans (Wang et al., 2001) and several animal models studies of obesity (Hamdi, Porter, & Prasad, 1992; Huang et al., 2006; Thanos, Michaelides, Piyis, Wang, & Volkow, 2008) suggest that severe obesity is associated with a reduced level of striatal dopaminergic (D2) receptors. Because dopamine is one of the primary messengers of reward (i.e., eating a favorite meal elevates striatal dopamine levels in a dose-dependent manner such that the higher the pleasure elicited by the taste of the food, the more dopamine is released) (Small, Jones-Gotman, & Dagher, 2003), these PET findings suggest that obese individuals may be less sensitive to reward than their nonobese peers (Wang et al., 2001). Wang and colleagues hypothesized that the dopamine deficiency observed in these individuals would increase their vulnerability to develop addictive behaviors, including overeating, as a means to “self-medicate” or compensate for the reduced activity in brain reward circuits. However, because association studies do not imply causation, they cautioned that the alternative explanation, that reduced brain dopamine D2 receptor availability is the result of neuroadaptations developed after chronic overstimulation from overeating, cannot be ruled out (Wang et al., 2001).
Recent findings from a neuroimaging study appear to reconcile these seemly opposite hypotheses of obesity being associated with a “higher” and a “lower” sensitivity to reward (Stice et al., 2008). They found that obese adolescent girls show both (a) more activation of brain areas associated with sensory pleasure while anticipating and receiving a palatable food and (b) less activation in the striatum after consuming the food. That normal-weight adolescents who are at high risk for obesity because of parental obesity, displayed greater activation of both striatal and somatosensory brain areas when anticipating or after consuming a palatable food suggest that the blunted striatal response observed after obese individuals consume a palatable food may be a consequence of overstimulation of the dopaminergic system (Stice, Yokum, Burger, Epstein, & Small 2011).
In the present study, subjects tasted but did not swallow the sweet-tasting solution. Therefore, the differences observed between obese and lean women most probably relate to differences in anticipatory responses. That obese women experience a greater anticipated reward from tasting sweetness than do lean women is also suggested by their differential response when unexpectedly receiving a salty solution on trial 11. Unlike lean women, who by this trial were habituated to the pleasantness of sucrose and described both the sweet and the salty stimuli as hedonically neutral, obese women still perceived the sweet stimulus as moderately pleasant and then when surprised with the salty stimulus described it as weakly to moderately unpleasant. However, because we do not have hedonic ratings for the salty solution outside this habituation paradigm, whether obese women are truly responding with a greater negative contrast effect or dislike the salty solution more than lean women is unknown.
Perhaps because of the universal pleasant reaction elicited by sweetness, most research studies of reward system pathways use sweet taste as stimulus. In animal models, sucrose and other sweeteners induce dopamine release in the nucleus accumbens (Hajnal, Smith, & Norgren, 2004), (de Araujo et al., 2008), and pharmacological blockage of dopamine receptors suppresses sweet consumption (Xenakis & Sclafani, 1981). Several research studies have investigated whether obese individuals respond to sweet stimuli differently than do nonobese individuals, with conflicting results: some studies found that obese subjects preferred (or consumed) higher levels of sweetness than did lean subjects, whereas others found opposite or no effects (McDaniel & Reed, 2004). As suggested in the introduction, the inconsistent results in the literature may be due to the different methods used (Bartoshuk et al., 2006). Here we used a method that opens a different dimension in sweet liking: liking over time. This method may avoid some of the social desirability biases encountered when testing the hedonic value of foods in laboratory settings and, perhaps because subjects are unaware of the real question under study, the data have more real-world relevance. It should be mentioned that for simplicity and design clarity, we examined hedonic responses to pure sweet taste stimulation, but future studies should examine whether similar findings are observed when more ecologically relevant stimuli, such as sweet flavors (i.e. stimuli containing smell texture or other sensory stimulation in addition to taste), are used.
One of the major arguments against the theory that habituation is the mechanism underlying sensory-specific satiety is that changes in liking, or pleasantness ratings, the core outcome in the model of sensory specific satiety, fail to associate with changes in others responses that show habituation patterns such as salivary responses. Further, while habituation patterns of salivation and motivated responding for food differ between obese and lean individuals, changes in pleasantness elicited by a repetitively tasted, or eaten, food do not (Epstein et al., 2009). By using a scaling method that makes the individual judge sensations based on all experiences, the present data suggest that the use of the hedonic gLMS may reveal previously missed differences in sensitivity to sensory-specific satiety between obese and lean individuals. Moreover, because our findings with hedonic responses are parallel to previous reported differences in habituation patterns of physiological or motivational responses between obese and lean individuals, they add support to the hypothesis that habituation is the very basic mechanism underlying sensory-specific satiety.
In conclusion, obesity is associated with slower patterns of habituation to the palatability elicited by sweet taste in women. This characteristic could contribute to slower satiation rates, prolongation of eating episodes, and excessive sweet food consumption among the obese. Measuring liking over time with the hedonic gLMS may help reveal important aspects of underlying mechanisms in obesity and point to future areas of research to examine contributions of hedonic responses to overeating and other types of eating patterns.
Obese and lean woman judge the sweetness intensity of a 24% w/v sucrose solution similarly
There are no differences in the intensity of sucrose most preferred by obese and lean women
Obese and lean women experienced similar levels of pleasure when first tasting a sweet solution
However, obese women experience more pleasure from repeatedly tasting sweetness than do their lean peers
Obesity is associated with slower patterns of habituation to the palatability elicited by sweet taste in women
Acknowledgments
This research was supported in part by a grant from the Pennsylvania Department of Health and National Institutes of Health (NIH) grant DC011287. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. This publication was also made possible by grant UL1 RR024992, subaward KL2RR024994, from the NIH-National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and by the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. We acknowledge and thank the expert technical assistance of Susana Finkbeiner and Ms. Patricia Watson for editorial comments on an earlier version of the manuscript.
Footnotes
Disclosure: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting Food Reward: Delay Discounting, Food Reward Sensitivity, and Palatable Food Intake in Overweight and Obese Women. Obesity. 2011 Apr 7; doi: 10.1038/oby.2011.57. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, Raynor HA, Vithiananthan S, Sax HC, Pohl D, Roye GD, Wing RR. Differences in salivary habituation to a taste stimulus in bariatric surgery candidates and normal-weight controls. Obes Surg. 2009;19:873–878. doi: 10.1007/s11695-009-9861-3. [DOI] [PubMed] [Google Scholar]
- Brondel L, Romer M, Van Wymelbeke V, Walla P, Jiang T, Deecke L, Rigaud D. Sensory-specific satiety with simple foods in humans: no influence of BMI? Int J Obes (Lond) 2007;31:987–995. doi: 10.1038/sj.ijo.0803504. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Duclaux R. Obesity: Absence of satiety aversion to sucrose. Science. 1970;168:496–497. doi: 10.1126/science.168.3930.496. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Davis C. Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (Lond) 2009;33(Suppl 2):S49–53. doi: 10.1038/ijo.2009.72. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Caggiula AR, Perkins KA, Mitchell SL, Rodefer JS. Abstinence from smoking decreases habituation to food cues. Physiol Behav. 1992;52:641–646. doi: 10.1016/0031-9384(92)90391-e. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch R, Coleman KJ. Differences in salivation to repeated food cues in obese and nonobese women. Psychosom Med. 1996;58:160–164. doi: 10.1097/00006842-199603000-00011. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A, Nadbrzuch R. Sensitization and habituation of motivated behavior in overweight and non-overweight children. Learn Motiv. 2008;39:243–255. doi: 10.1016/j.lmot.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Roemmich JN, Bouton ME. Habituation as a determinant of human food intake. Psychol Rev. 2009;116:384–407. doi: 10.1037/a0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- Hetherington MMR, BJ . Sensory-specific satiety: theoretical framework and principle characteristics. In: Capaldi ED, editor. The Psychology of Eating. Washington DC: American Psychological Association; 1996. pp. 267–290. [Google Scholar]
- Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Deng C. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;83:821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- McDaniel AH, Reed DR. The human sweet tooth and its relationship in obesity. In: Moustaid-Moussa N, Berdanier C, editors. Genomics and Proteomics in Nutrition. New York, NY: Marcel Dekker; 2004. pp. 49–67. [Google Scholar]
- Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem Senses. 2011;36:345–355. doi: 10.1093/chemse/bjq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring) 2010;18(5):959–965. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychol Bull. 2001;127:325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55(3):420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rowe EA, Rolls ET. How sensory properties of foods affect human feeding behavior. Physiol Behav. 1982;29(3):409–417. doi: 10.1016/0031-9384(82)90259-1. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls JH. Olfactory sensory-specific satiety in humans. Physiol Behav. 1997;61:461–473. doi: 10.1016/s0031-9384(96)00464-7. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Snoek HM, Huntjens L, Van Gemert LJ, De Graaf C, Weenen H. Sensory-specific satiety in obese and normal-weight women. Am J Clin Nutr. 2004;80:823–831. doi: 10.1093/ajcn/80.4.823. [DOI] [PubMed] [Google Scholar]
- Sorensen LB, Moller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord. 2003;27:1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31(12):4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–442. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]