Abstract
Diffusion tensor imaging (DTI) is highly sensitive in detecting brain structure and connectivity phenotypes in autism spectrum disorders (ASD). Since one of the core symptoms of ASD is reduced sociability (reduced tendency to seek social interaction), we hypothesized that DTI will be sensitive in detecting neural phenotypes that correlate with decreased sociability in mouse models. Relative to C57BL/6J (B6) mice, juvenile BALB/cJ mice show reduced sociability. We performed social approach test in a three-chambered apparatus and in-vivo longitudinal DTI at post-natal days 30, 50 and 70 days-of-age in BALB/cJ (n=32) and B6 (n=15) mice to assess the correlation between DTI and sociability and to evaluate differences in DTI parameters between these two strains. Fractional anisotropy (FA) and mean diffusivity (MD) values from in-vivo DTI data were analyzed from white matter (corpus callosum, internal and external capsule) and gray matter (cerebral cortex, frontal motor cortex, hippocampus, thalamus and amygdaloid) regions based on their relevance to ASD. A moderate but significant (p<0.05) negative correlation between sociability and FA in hippocampus and frontal motor cortex was noted for BALB/cJ mice at 30 days-of-age. Significant differences in FA and MD values between BALB/cJ and B6 mice were observed in most white and gray matter areas at all three time points. Significant differences in developmental trajectories of FA and MD values from thalamus and frontal motor cortex were also observed between BALB/cJ and B6, indicating relative under-connectivity in BALB/cJ mice. These results indicate that DTI may be used as an in-vivo, non-invasive imaging method to assess developmental trajectories of brain connectivity in mouse models of neurodevelopmental and behavioral disorders.
Keywords: Autism spectrum disorders, Mouse models, Diffusion tensor imaging, Fractional anisotropy, Mean diffusivity, Social behavior
1. Introduction
Autism spectrum disorders (ASDs) comprise of a heterogeneous set of neurodevelopmental disorders characterized by impairments in social behaviors, communication, and restricted and repetitive behaviors (Anagnostou and Taylor, 2011; APA, 2001). Abnormal brain under-connectivity, in which multiple networks throughout the brain are affected (Palmen et al., 2004), may contribute to reduce sociability (tendency to seek social interaction). Childhood-onset reduction in sociability is one of the most common symptoms in ASD and tends to impair development of social skills and social cognition (Belmonte et al., 2004; Dawson et al., 2002; Geschwind and Levitt, 2007; Grelotti et al., 2002; Schultz, 2005). The specific neural circuitry underlying sociability development is poorly understood, probably due to the etiological heterogeneity, variable age range and severity of human ASD. These gaps in knowledge are typical of many neuropsychiatric disorders.
Some of the above mentioned variability can be addressed by performing studies in genetically homogeneous animal models and rodent models with etiological and face validity for some ASD phenotypes have been established (Brodkin et al., 2004; Crawley, 2007; Fairless et al., 2008; Moy et al., 2009). These models allow us to study the developmental neurobiology of important behavior patterns under controlled experimental conditions over the entire life-span of the animal, which may ultimately shed light on the neurobiology of ASD (Brodkin et al., 2004). Juvenile BALB/cJ mice show reduced sociability and social reward and have been suggested to display some core pheno-types relevant to ASD. (Brodkin et al., 2004; Brodkin, 2007; Fairless et al., 2008; Fairless et al., 2012 Panksepp et al., 2007; Panksepp and Lahvis, 2007; Sankoorikal et al., 2006). Thus, this strain may be a useful model to study the relationship between sociability and brain phenotypes using imaging techniques across brain development.
Diffusion tensor imaging (DTI) is a non-invasive magnetic resonance imaging (MRI) technique that measures water diffusion in the brain and is sensitive to integrity, orientation and connectivity of the nerve fibers and it is commonly used as a quantitative technique for characterization of abnormalities in the brain including ASD (Alexander et al., 2007; Ameis et al., 2011; Barnea-Goraly et al., 2004; Bode et al., 2011; Ingalhalikar et al., 2011; Ke et al., 2009; Lange et al., 2010; Sundaram et al., 2008), schizophrenia and other neuro-developmental and psychiatric disorders (Tang et al., 2011; Walther et al., 2011), stroke (Jang, 2011; Molko et al., 2001), and tumor (Hygino da Cruz et al., 2011; Wang et al., 2011).
Mouse models are useful in testing hypotheses about the relationships between behaviors and brain connectivity using DTI (Lim and Helpern, 2002). Hu et al. (Hu et al., 2009), have reported that diffusion-weighted MRI can detect changes in brain structure earlier than the appearance of behavioral symptoms in a mouse model of Sandhoff’s disease. Recently Kim et al. (Kim et al., 2012), have also reported an association between DTI and sociability using ex-vivo high resolution DTI in the BALB/cJ mouse model.
The current study was performed to test the hypothesis that in-vivo DTI can be used as a surrogate imaging marker to assess social-behavioral abnormality in the BALB/cJ mouse model. We also assessed the utility of DTI in detecting the developmental trajectory of DTI parameters in various white matter (WM) and gray matter (GM) regions of the brain in BALB/cJ mice in comparison to the more social C57BL/6J (B6) mice.
2. Results
On the basis of sniffing time (social approach test), we observed that BALB/cJ were less sociable (p <0.05, Student’s t-test) at 30 day-of-age in comparison to the B6 mice (Fig. 1). There was no significant difference in the sniffing time between BALB/cJ and B6 mice at 50 and 70 day time point (p >0.05), which is consistent with a previously published study (Fairless et al., 2012).
Fig. 1.
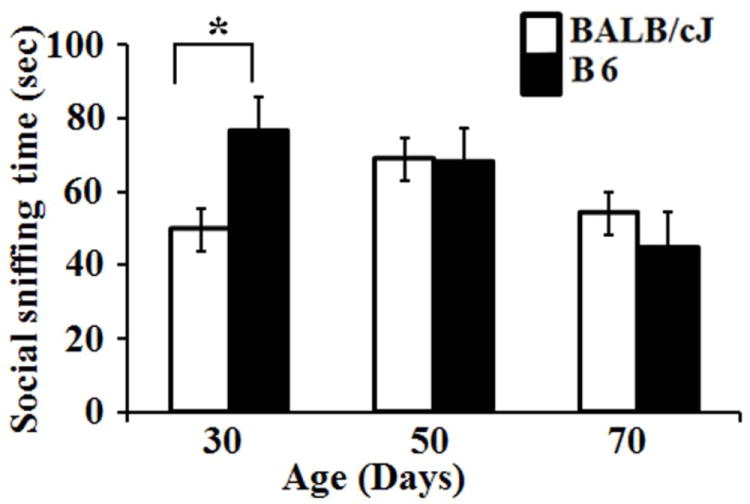
Bar diagram showing the mean and standard error in social sniffing time between BALB/cJ and B6 at different time points. Asterisk (*) represents significant differences with a p-value of ≤ 0.05.
2.1. Correlation between sociability and DTI
In-vivo DTI data from most of the animals was without any substantial motion artifacts and in general, the signal-to-noise ratio (SNR) from the cortical region of the brain was over 100 (139.76±16.91) for the non-diffusion weighted (b0) images and it was over 75 (76.66±6.79) for the diffusion weighted (b786.73 s/mm2) images. This resulted in excellent quality of the in-vivo DTI data as evident by the MD, FA and FA weighted color coded maps, shown in figure 2. A moderate but significant positive correlation was observed between MD values from the EC and social sniffing time of 30 day-old BALB/cJ mice (r =0.519; p =0.007). A moderate negative correlation was also observed between FA values from the Hippo (r =-0.523; p =0.006) and the FMC (r =-0.458; p =0.037) and sociability in these mice. None of the other areas demonstrated significant correlation between FA or MD and sociability of 30, 50 or 70 day old BALB/cJ mice (supplementary data Table S1). Similarly, the B6 mice did not demonstrate any significant correlation between sociability and FA or MD values at any age (Supplementary data, Table S2).
Fig. 2.
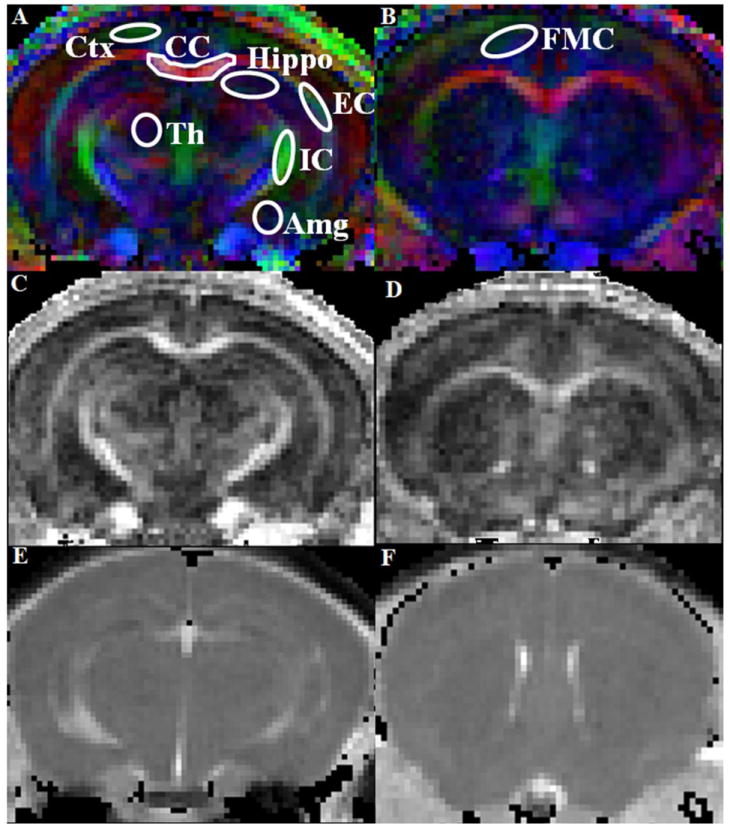
Representative FA weighted color maps from the mid brain (A) and frontal (B) region of a 30-day-old BALB/cJ mouse. The region of interests (ROIs) on different gray and white matter regions are shown overlaid on the color maps. The color represents the direction of the white matter fibers in which red is right-left, green is anterior-posterior, and blue is superior-inferior direction. The ROIs were placed bilaterally in all regions except CC where a single ROI was placed at the medial region. [Ctx =Cerebral cortex, CC =Corpus callosum, Hippo =Hippocampus, EC =External capsule, IC =Internal capsule, Th =Thalamus, Amg =Amygdaloid, FMC =Frontal motor cortex]. The FA (C, D) and MD (E, F) maps corresponding to the color map slices demonstrate the quality of the in-vivo DTI data.
2.2. Longitudinal changes in white matter regions in BALB/cJ mice
Significantly higher FA from the CC and EC was observed at 50 and 70 day in comparison to the 30 day time point (Table 1). However there were no significant differences between 50 and 70 day time points. When a linear mixed model was used to assess the differences in the growth pattern of WM areas, significant difference in the growth trajectory of the FA from the EC was observed in BALB/cJ mice in comparison to B6 mice (Table 2, Fig. 3). The negative z score of the trajectory indicates that BALB/cJ mice exhibit a slower rate of change in FA values during this growth period (30-70 days) in the EC region in comparison to the B6 mice (Fig. 3).
Table 1.
Longitudinal and cross sectional changes at different time points in FA and MD (×10-3mm2/s) values in white matter regions in BALB/cJ and B6 mice
| BALB/cJ | ||||||
|---|---|---|---|---|---|---|
| Age (days) | Fractional Anisotropy (FA) | Mean Diffusivity (MD) | ||||
| CC | IC | EC | CC | IC | EC | |
| 30 | 0.48±0.07*$ | 0.41±0.06 | 0.27±0.05*$$# | 0.81±0.06# | 0.81±0.06 | 0.72±0.12 |
| 50 | 0.52±0.05 | 0.41±0.05 | 0.29±0.04## | 0.80±0.04# | 0.80±0.10 | 0.74±0.08 |
| 70 | 0.53±0.06# | 0.43±0.07 | 0.30±0.03 | 0.79±0.11 | 0.82±0.06 | 0.70±0.12# |
| B6 | ||||||
| 30 | 0.44±0.06 | 0.42±0.07 | 0.32±0.04 | 0.78±0.10 | 0.82±0.05 | 0.75±0.12 |
| 50 | 0.48±0.06 | 0.42±0.07 | 0.36±0.04 | 0.74±0.04 | 0.77±0.08 | 0.71±0.14 |
| 70 | 0.49±0.05 | 0.46±0.07 | 0.32±0.05 | 0.74±0.04 | 0.82±0.04 | 0.76±0.05 |
CC= corpus callosum, IC= internal capsule, EC= external capsule.
Significant difference between 30 and 50 day;
significant between 30 and 70 day with in group (longitudinal) (BALBc/J or B6).
Mark # represents significant differences between BALBc/J and B6 (cross sectional) at different age (30, 50 and 70 day), respectively.
Single marks (*, $ and #) represent statistically significant difference with uncorrected p value ≤ 0.05 while double marks represent statistically significant differences after Bonferroni correction with p value ≤ 0.003.
Table 2.
Longitudinal and cross sectional changes at different time points in FA and MD (10-3mm2/s) values in gray matter regions in BALB/cJ and B6 mice
| BALB/cJ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fractional Anisotropy (FA) | Mean diffusivity (MD) | |||||||||
| Age (days) | Ctx | Hippo | FMC | Th | Amg | Ctx | Hippo | FMC | Th | Amg |
| 30 | 0.20±0.03*$ | 0.18±0.03 | 0.18±0.03$ | 0.25±0.04 | 0.22±0.03 | 0.73±0.05 | 0.76±0.09 | 0.75±0.06 | 0.77±0.06 | 0.77±0.06 |
| 50 | 0.18±0.02 | 0.18±0.02 | 0.18±0.02† | 0.26±0.04 | 0.23±0.03## | 0.71±0.09 | 0.79±0.04## | 0.73±0.09 | 0.75±0.03## | 0.74±0.05 |
| 70 | 0.17±0.04 | 0.18±0.02## | 0.16±0.03 | 0.26±0.04 | 0.24±0.04 | 0.72±0.05 | 0.80±0.06 | 0.76±0.07 | 0.75±0.05 | 0.74±0.05 |
| B6 | ||||||||||
| 30 | 0.19±0.03 | 0.18±0.03 | 0.21±0.05 | 0.22±0.04 | 0.23±0.03** | 0.73±0.03 | 0.78±0.05 | 0.73±0.09 | 0.74±0.10 | 0.81±0.15 |
| 50 | 0.18±0.05 | 0.18±0.03 | 0.19±0.05 | 0.23±0.04 | 0.27±0.02†† | 0.65±0.12 | 0.75±0.04 | 0.70±0.05 | 0.72±0.03 | 0.72±0.04 |
| 70 | 0.17±0.04 | 0.16±0.02 | 0.20±0.06 | 0.22±0.06 | 0.25±0.03 | 0.71±0.04 | 0.76±0.07 | 0.72±0.04 | 0.74±0.04 | 0.85±0.15 |
Ctx=cerebral cortex; Hippo= hippocampus; FMC=frontal motor cortex; Th= thalamus, Amg= amygdaloid.
Significant difference between 30 and 50 day;
significance between 50 and 70 day;
significance between 30 and 70 day with in group (longitudinal) (BALBc/J or B6).
Mark # represents significant differences between BALBc/J and B6 (cross sectional) at different age (30, 50 and 70 day), respectively.
Single marks (*, †, $ and #) represent statistically significant difference with uncorrected p value ≤ 0.05 using uncorrected while double marks represent statistically significant differences after Bonferroni correction with p value ≤ 0.003.
Fig. 3.
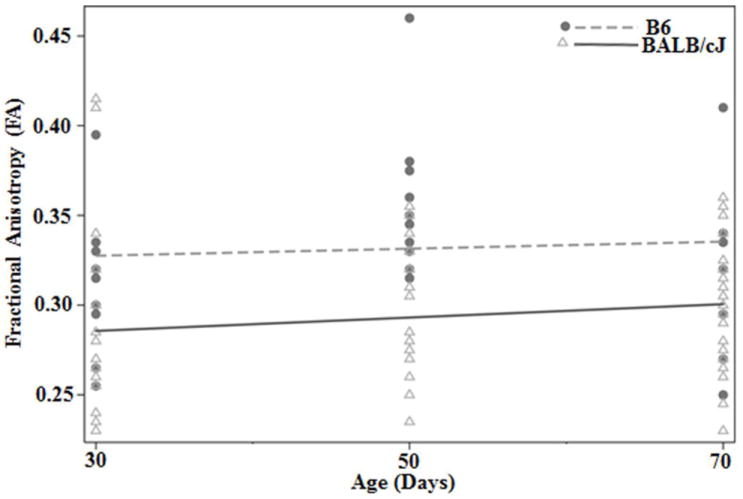
Growth trajectory of the FA values from external capsule in BALB/cJ and B6 mice. Higher FA value was observed in B6 mice at all-time points as compared to BALB/cJ mice. Black circles demonstrate the data points from B6 mice and open triangle represents data from BALB/cJ at each time points. The dotted line represents the average growth trajectory of B6 mice and the dark solid line represents the average growth trajectory of BABL/cJ mice.
2.3. Longitudinal changes in gray matter regions in BALB/cJ
The FA values from the Ctx and FMC at 30 day were significantly higher than the values at 50 and 70 day (Table 2). Significantly higher FA was also noted from the FMC at 50 day in comparison to the 70 day time points. When the differences in growth trajectories were assessed between BALB/cJ and B6 mice, significantly different trajectories of FA values from the Hippo, FMC and Th were observed (Table 3). Significantly positive developmental trajectories in Hippo and Th were noted in BALB/cJ mice, which demonstrate faster rate of change in FA values over the time in these regions in BALB/cJ as compared to B6 mice. On the other hand, a negative developmental trajectory in FMC demonstrated slower rate of change in FA values over time in BALB/cJ as compared to B6. These results indicate that different regions of the brain have different developmental trajectories in BALB/cJ mice.
Table 3.
Differences in the developmental trajectories of FA and MD (×10-3mm2/s) values in different gray and white matter regions between BABL/cJ and B6 using a multilevel model for trajectory analysis
| White matter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Fractional anisotropy (FA) | Mean diffusivity (MD) | ||||||||
| CC | IC | EC | CC | IC | EC | |||||
| Z-score | 2.42# | -1.21 | -4.17## | 2.68# | -0.51 | 0.105 | ||||
| Gray matter | ||||||||||
| Parameters | Ctx | Hippo | FMC | Th | Amg | Ctx | Hippo | FMC | Th | Amg |
| Z- score | 0.34 | 2.45# | -3.37## | 3.9## | -2.51 | 1.33 | 2.38# | 1.3 | 1.18# | -2.5# |
CC=corpus callosum, IC= internal capsule, EC= external capsule, Ctx= cerebral cortex, Hippo= hippocampus, FMC= frontal motor cortex, Th= thalamus, Amg= amygdaloid.
A single mark (#) represents uncorrected p value difference while double marks represent level of significance after Bonferroni correction. A p value ≤ 0.5 was taken as level of significant for uncorrected while ≤ 0.003 for Bonferroni correction in all statistical computations.
2.4. Longitudinal changes in B6 mice at different time points
No significant differences in the WM regions were observed at any time point (Table 1). In the GM regions, the Amg region demonstrated lower FA at 30 days in comparison to the 50 day time point. The FA value was significantly higher at 50 day in comparison to both the 30 and 70 day time point. No significant difference in FA or MD values was observed from any other GM regions (Table 2).
2.5. Cross-sectional analysis of FA and MD values between BALB/cJ and B6 mice at different time points
Among the WM areas, significantly lower FA from the EC was observed in the BALB/cJ mice than B6 mice at 30 and 70 days (Table 1 and Fig. 4A). Additionally, the FA from the CC in BALB/cJ mice was higher than the B6 mice at 70 days. The MD values from the CC of BALB/cJ mice were higher than B6 mice at 30 and 50 days-of-age (Table 1). Among the GM areas, significantly lower FA from the Amg at 50 days was noted in BALB/cJ mice. However, the hippocampal FA values in BALB/cJ mice were significantly higher at 70 days. The hippocampal MD values in BALB/cJ mice at 50 days were also significantly higher than the B6 mice (Table 2).
Fig. 4.
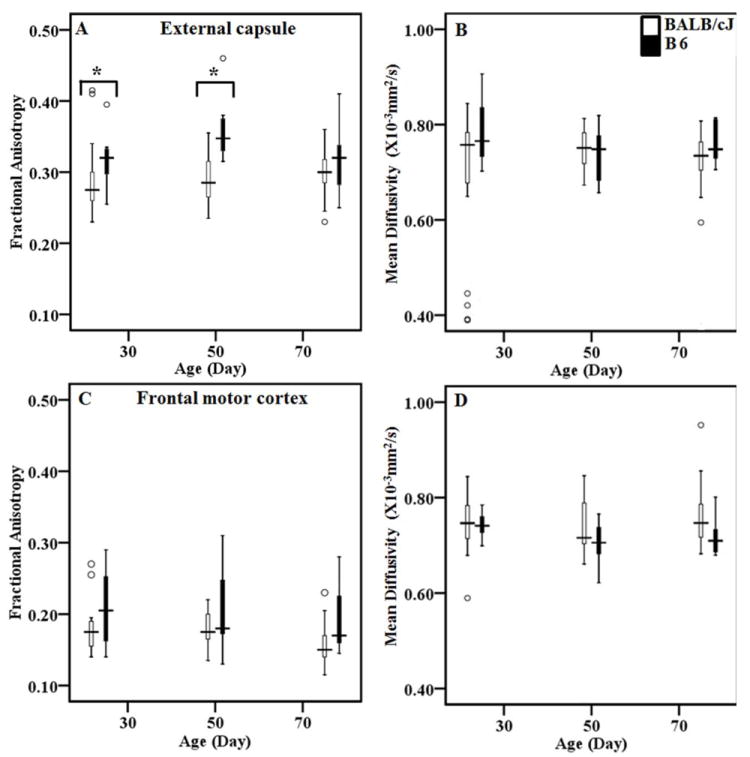
Box plots demonstrating the FA and MD (×10-3mm2/s) values from the external capsule (A and B) and frontal motor cortex (C and D) in BALB/cJ and B6 at 3 time points. Figure (A, C) show FA and (B, D) show MD values at 3 time points from BALB/cJ and B6. White boxes represent data from BALB/cJ while black boxes represent values from B6 mice. The upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile. The line in the box indicates median value of the data. The data ranges are represented with lines extending the boxes, while the circles indicate outliers. Asterisk (*) represents statistically significant differences.
2.6. Histopathological Finding
Histological staining (H&E and Luxol fast blue) was performed from 2 BALB/cJ and 2 B6 mice at each time point. Due to the limited number of samples used, only a descriptive comparison between the staining pattern of BALB/cJ and B6 samples was performed. In comparison to BALB/cJ, the B6 brain samples demonstrated compact and darkly stained vacuolated nucleoli in EC and FMC on H&E stained sections (data not shown). When the LFB stained samples from 30-day-old brains were compared, the EC region of the brains in B6 mice appeared to have more compact and longer WM fibers than the BALB/cJ, in which the WM fibers appeared to be dispersed and comparatively smaller in size (white arrows, Fig. 5, M and N). This difference was also observed from the samples taken from the 50 and 70 day-old animals (Fig. 5, P, Q, and R, S).
Fig. 5.
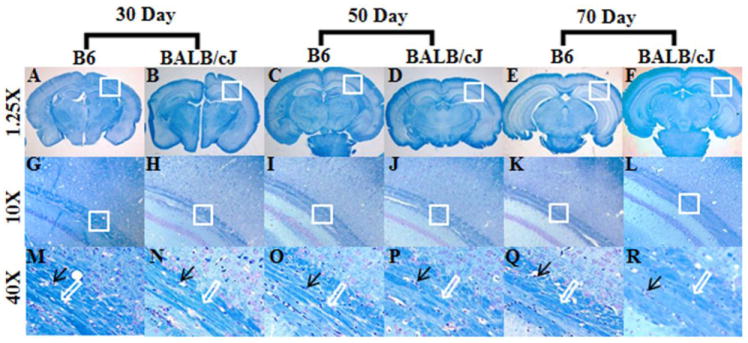
Representative luxol-fast blue (LFB) stained sections from the external capsule region in B6 and BALB/cJ brain samples. Figure A-F represents the brain sections at 1.25x resolution. Figure G-L showing images from the rectangular box from external capsule at a magnification of the 10X and figure M-R demonstrates further magnification at 40X from the rectangular box shown in G-L. Black arrow demonstrating the vacuolated nucleoli from the axonal fibers, while the white arrow indicates to the relative compactness of the white matter fibers.
3. Discussion
3.1. Sociability and DTI
Significant differences in the sociability score between BALB/cJ and B6 mice only at 30 day-of-age indicate that BALB/cJ mice are less social only during pre-pubescence. These observations are in agreement with a recent study in which only the juvenile BALB/cJ mice demonstrated lower sociability scores than the B6 mice (Fairless et al., 2012). These results also parallel clinical observations in ASD patients, some of which show social behavioral improvements during adolescence and adulthood, particularly an increasing interest in social interactions (APA, 2001; Fecteau et al., 2003; Piven et al., 1996; Rumsey et al., 1985). However, despite a gradual increase in sociability, these individuals often have deficits in social skills and social cognition that persist through much or all of their lifespan.
We observed a moderate but significantly positive correlation between sociability and MD values from the EC of BALB/cJ mice at 30 days. A recent ex-vivo DTI study in BALB/cJ mice also reported significant correlations between DTI and sociability at 30-days-of-age (Kim et al., 2012). These investigators reported significantly positive regression between FA and sociability in thalamus, substantia nigra, somatosensory and entorhinal cortex while significantly negative regression between sociability and MD in sensory cortex, motor cortex, external capsule and amygdaloid region were reported. In the current study, significant differences in FA and MD values from several WM and GM areas were observed between BALB/cJ and B6 mice at all ages even though the sociability scores were not significantly different at older ages. These findings suggest that DTI parameters may be more sensitive in assessing underlying connectivity disorders than tests for social behaviors.
3.2. Developmental changes in BALB/cJ and B6 mice
We also observed significant differences in FA and MD values between BALB/cJ and B6 mice in the CC and EC at all ages. In addition, when the growth trajectories in FA and MD values were compared between B6 and BALB/cJ mice, significantly different developmental trajectories in CC, EC, Amg, FMC, Hippo and Th (Table 3) were observed between the two groups indicating reduced brain connectivity in BALB/cJ mice that continued at all ages despite changes in sociability scores. The relatively lower FA values in the EC of the BALB/cJ mice may be due to differences in axonal density, size, axonal loss or abnormal myelination. Some of these differences can be explained by the scattered, less dense and relatively shorter WM fibers seen on LFB stains of the BALB/cJ brains (Fig. 5). In a longitudinal MRI study in autistic subjects, Courchesne et al. (Courchesne et al., 2011), reported markedly different pathological trajectories in brain size with overgrowth evident in early life but an accelerated rate of decline marking adult life. Recently, Lazar et al. (Lazar et al., 2011), demonstrated atypical GM and WM maturation in patients with ASD over time using diffusion kurtosis, a measure of non-gaussian diffusion. The reduced kurtosis in the GM of ASD patients in that study suggests delay or failure to develop a proper synaptic network which may be responsible for some of the functional deficits reported in ASD (Lazar et al., 2011). Although BALB/cJ mice demonstrate only some behavioral phenotypes that parallel human ASD, it is interesting to note that BALB/cJ mice exhibit differences from B6 mice in the growth trajectory of FA and MD values similar to the difference found between humans with ASD and typically developing humans. This indicates that our approach of using a developmental growth trajectory to analyze in-vivo longitudinal DTI will be advantageous over ex-vivo DTI or postmortem studies in assessing other putative models relevant to ASD, because the in-vivo longitudinal DTI approach provides a non-invasive method of tracking developmental trajectories or monitoring effects of therapeutic intervention over time in the same individual.
3.3. Differences in WM areas between BALB/cJ and B6 mice
Low FA and higher MD values often indicate impaired WM connectivity/or impaired myelination. Unusually high or low DTI values in a pathological state indicate abnormal function (Thomason and Thompson, 2011). Several studies have reported reduced FA and increased MD in different GM and WM regions of the brain in ASD patients who also exhibited abnormal behavior (Lange et al., 2010; Noriuchi et al., 2010; Shukla et al., 2011). Barnea-Goraly et al. (Barnea-Goraly et al., 2004) reported lower FA in ventromedial prefrontal cortex, anterior cingulate, temporal lobe and CC in ASD children with an average age of 14 years. Others have also reported lower FA in CC and IC of ASD patients including children and adults of age 10-35 years (Alexander et al., 2007; Just et al., 2004; Keller et al., 2007). Taken together, these studies suggest that disruption of the WM tracts between regions implicated in social functioning may contribute to impaired social cognition in ASD. In the present study, we also observed significantly higher MD in CC and reduced FA in EC in BALB/cJ mice at 30 days-of-age suggesting abnormal brain development and WM under-connectivity in these regions. The higher MD from the CC in BALB/cJ indicates reduced cell density, increased extracellular space and lower axonal density which is suggestive of inter-hemispheric under-connectivity that plays an important role in transferring information between the hemispheres and socio-behavioral development. Reduced FA in EC in BALB/cJ, as compared to B6 mice, indicates reduced myelination as seen on histology (Fig. 5). The impaired myelination may be responsible for altered social behavior in these animals as demyelination leads to impairments in abilities that demand cooperation between different brain areas (Skranes et al., 2007).
Although, in general, our results are similar to results in recent DTI studies in patients with ASD, there are differences in the anatomical location of brain areas in mice vs. humans (Alexander et al., 2007; Ameis et al., 2011; Barnea-Goraly et al., 2004; Brito et al., 2009; Keller et al., 2007; Lange et al., 2010; Shukla et al., 2011). These differences could be attributable to a variety of factors: the fact that the BALB/cJ mice exhibit only some behavioral phenotypes that are similar to human ASD; the high level of etiological heterogeneity within ASD; and the structural differences between the human and the mouse brain. The heterogeneity in age of human subjects and degree of severity of ASD in human studies may have also accounted for this variability.
3.4. Differences in GM areas of BALB/cJ and B6 mice
Increased cerebral cortical volumes and micro and macroscopic neuroanatomical changes in children with ASD have been reported using MRI (Carper et al., 2002). The abnormalities in GM may be related to malformations of the WM, especially in ASD considering the fact that behavioral and cognitive deficit is most likely due to impaired integrative processing through intra-hemispheric and interhemispheric transfer of information (Just et al., 2004; Minshew et al., 1997). Among several brain regions implicated in the pathogenesis of ASD, findings in the limbic system, which is involved in social and emotional behaviors, have been reported fairly consistently in ASD (Amaral et al., 2008; Courchesne, 1997; Palmen et al., 2004). Postmortem studies of both adults and children with ASD have reported reduced neuronal size and increased cell packaging in the hippo and Amg (Bauman and Kemper, 2005; Palmen et al., 2004). We also observed significantly increased MD in Hippo and decreased FA in Amg in BALB/cJ as compared to B6 at 50 day-of-age. An earlier study by Keller et al. (Keller et al., 2007), reported reductions in the structural integrity of WM, which supports short and long range anatomical and functional connectivity in cortical areas in ASD. In ASD patients, reduced FA in Amg was shown to be indicative of deficits in social cognition (Barnea-Goraly et al., 2004). Chung et al. (Chung et al., 2004) have also reported lower WM density (an index for neural connectivity) in the genu, rostrum, and splenium of the CC in patients with ASD and suggested that this reduction might result in impaired interhemispheric connectivity in frontal, temporal and occipital regions. We have also observed significantly higher FA in FMC in 30-day-old BALB/cJ mice in comparison to 50 and 70 day old BALB/cJ mice. The higher FA in FMC at 30 day may be indicative of abnormally higher neural connectivity which, combined with under-connectivity in CC may contribute to behavioral abnormalities in these animals (Just et al., 2004). Therefore, it is possible that abnormalities in the sub regions of the CC could disrupt the functional connectivity among cortical regions in the two hemispheres. We also observed a negative slope in developmental trajectory of FA in this region, which indicates that a much slower maturation in BALB/cJ occurs as compared to B6 mice. This abnormal development of the FMC may contribute to deficits in behavioral abnormalities of BALB/cJ mice, particularly in the juvenile period.
Neuropathological observations of GM areas in human ASD point towards early pre and post-natal developmental abnormalities that involve multiple regions of the brain, including the Ctx, cortical WM, Amg, brain stem and cerebellum (Schmitz and Rezaie, 2008). The BTBR strain of mice, which also exhibit behavioral deficits mimicking the core deficits of autism, exhibit abnormal cellular and anatomic features that correlate with behavioral deficits and callosal abnormalities (Stephenson et al., 2011). A qualitative analysis of the LFB stained histological sections demonstrated presence of dispersed and shorter WM fibers from EC in BALB/cJ, in comparison to B6 mice, which may be due to axonal loss or due to abnormal myelination. The concordance between DTI and histology data demonstrate that abnormality in different brain regions persist during later stage of life even when there were no significant differences in sociability in these animals. These results further suggest that DTI may be used as a non-invasive imaging technique in studying neuro-behavioral and developmental disorders.
Although promising, we acknowledge certain limitations of our study. FA values from the gray matter are typically lower than the WM and are hence sensitive to the signal to noise (SNR) of the raw images. An accurate and precise quantification of FA values in GM requires substantially more SNR than the quantification of WM FA values (Farrell et al. 2007). Since the SNR of our b0 images was well over 100 and the diffusion weighted images had SNR over 75, we believe that the FA values from the GM in our study are less sensitive to noise in the data. Furthermore, since an ROI approach was used to analyze the data, we believe that these values reliable since an earlier study suggested that ROI-based measures of FA and MD are more reproducible than voxel-wise measures and are less sensitive to noise levels (Farrell et al., 2007). Additionally, motion-induced artifacts can impact on the sensitivity of the FA and MD values and cardiac gating has been suggested to reduce these effects in DTI studies of the rat brain (Kim et al., 2010). Since we primarily relied on restraining the animal’s head with a three-point apparatus, and did not use cardiac gating, we occasionally encountered motion artifacts in our study. This resulted in the loss of a few data points from each group and also limited the analysis of the gray and white matter to a few of the 15 imaging slices acquired. The cerebellar region has been highly implicated in ASD and it would have been interesting to study this region in the BALB/cJ mice. However, breathing induced motion artifacts were typically observed in cerebellar region and hence we could not analyze this region.
While differences in DTI were noted between BALB/cJ and B6 mice at all ages studied, the DTI abnormalities had a modest correlation with reduced sociability. This raises the possibility that DTI is only detecting structural differences between the two strains of mice studied that are not directly relevant to sociability. To further confirm or disprove the correlation of DTI with social behavioral abnormalities, future studies involving additional mouse models relevant to ASD (Crawley, 2007; McFarlane et al., 2008; Moy et al., 2009; Tabuchi et al., 2007) and assays that test additional behavioral phenotypes relevant to ASD (Brodkin, 2007; Crawley, 2007; Fairless et al., 2008; Silverman et al., 2010) may be necessary.
While decreased myelination in the BALB/cJ mice explains, to some degree, the reduced FA in these animals, it is well known that other factors, such as fiber orientation, also affect FA values. Future correlative studies of DTI and electron microscopy (Laitinen et al., 2010; Shereen et al., 2011) may further help in elucidating the mechanism of changes observed in these animals.
In conclusion, our findings support the hypothesis of abnormal microstructural integrity and connectivity of WM as well as cortical networks of the brain in BALB/cJ mice and abnormal social behavior that seem to parallel in some phenotypes observed in human ASD.
4. Experimental Procedure
4.1. Animal model
Three week old male BALB/cJ (n= 32) and B6 (n= 15) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). For the social-choice test, A/J mice (n= 10, Jackson Labs, Bar Harbor, ME, USA) were used as “stimulus mice”. The stimulus mice were gonadectomized prior to puberty to minimize the extent to which they would elicit sexual and aggressive motivations from the test mice. Food and water were available ad libitum, and the animals were maintained in a room maintained on a 12 hour light and dark cycle (lights on at 7:00 am). All animal procedures, including behavior assays and imaging experiments were conducted in strict compliance with the National Institutes of Health guidelines for the care and use of laboratory animals and the study was approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
4.2. Measures of Social Behavior
Social behavior in mice was assayed on 28, 48 and 68 days-of-age corresponding to pre-pubescence, post-pubescence and early adulthood (Adriani et al., 2004; Dye et al., 2011). Since some studies in human ASD have reported increased interest in social interaction of ASD individuals as they grow into adulthood (Fecteau et al., 2003; Piven et al., 1996; Rumsey et al., 1985) and since increased sociability with increasing age in BALB/cJ mice has also been reported (Fairless et al., 2012; Sankoorikal et al., 2006), we evaluated the correlation of sociability with DTI in this study to look for developmental and behavioral changes over three major developmental stages of the mouse [pre-pubescence (30 days), post-pubescence (50 days) and early adulthood (70 days)]. A three-chamber apparatus was used to perform the social-choice test as described previously (Brodkin et al., 2004; Brodkin, 2007; Fairless et al., 2008; Kim et al., 2012; Panksepp et al., 2007; Panksepp and Lahvis, 2007; Sankoorikal et al., 2006). Briefly, the test mouse (BALB/cJ or B6) was initially placed in a three-chambered box to get acclimated to its surroundings for 10 minutes. A “stimulus” A/J mouse and a paper-weight were then simultaneously introduced in the two clear Plexiglas cylinders located on either side of the three-chambered apparatus, each of which had multiple small holes in them. The chamber with stimulus mouse was labeled as the ‘social chamber” while the chamber with paper-weight was labeled as the “non-social” chamber. The interaction of the test BALB/cJ or B6 mouse with the A/J mouse was then recorded for five minutes and the time spent sniffing the cylinder with A/J mouse was denoted as the social sniffing time. In order to minimize the habituation or familiarity of the test mouse to the A/J mouse, the A/J mice were randomly chosen (from a group of 10 mice) for each test and the longitudinal social approach tests were separated by 20 days.
4.3. Diffusion Tensor Imaging
4.3.1. Animal Preparation
For the in-vivo MRI studies, the animal was anesthetized with 3% isoflurane in oxygen and mounted on a cradle. The head of the mouse was secured with a nose cone and an in-house developed restraining device to minimize motion induced artifacts. Subdural needle electrodes were placed in the forelimbs and a respiration pillow was placed on the dorsal side of the body. A thermister was inserted into the rectum to monitor the body temperature. The electrodes, respiration pillow and the thermister were connected to a small animal monitoring device (SA Instruments, NY, USA) to monitor vital signs including the electrocardiogram, respiration and core body temperature. The cradle with the animal in position was then inserted inside a 20 mm inner diameter transmit-receive quadrature volume coil (M2M Imaging, Cleveland, OH, USA) and the coil was placed in the center of the magnet. During the scan, anesthesia was maintained at 1-1.5% isoflurane and the animal body temperature was regulated at 37 (±1) °C by blowing warm air into the magnet bore via a hose connected to a thermostatically controlled warm air device (SA Instruments, NY, USA).
4.3.2. In-vivo DTI
To reduce stress-induced effects on imaging from handling the mice for the social approach test, in-vivo DTI studies were performed 48 hours after the social approach test. Thus the three time points for the in-vivo DTI studies were at 30, 50 and 70 days-of-age. All in-vivo DTI studies were performed on a Varian 9.4 T horizontal bore magnet equipped with 25 G/cm gradients interfaced to a Varian Direct Drive console (Agilent, Palo Alto, CA, USA) operating the Varian software version vnmrj 2.3.C. After initial scout and T2-weighted images in the axial plane, DTI images were acquired using a multi-slice diffusion-weighted spin echo sequence. Diffusion-weighting was applied along six directions optimally selected for anisotropy measurement (Jones et al., 1999) using a b-value of 786.73 s/mm2. The b-value was selected to optimize the SNR of the parametric maps. The compromise reached was due to a combination of the shortest possible echo time and SNR of the diffusion weighted image. A total of 15 imaging slices in the axial plane were acquired to cover most of the brain parenchyma. Consistent localization of imaging slices was maintained by selecting the imaging slices starting from olfactory bulb and ending in the cerebellum (on the mid-sagittal scout image) on all imaging studies. Imaging parameters for DTI sequence included: TR =2s; TE =33ms; field of view =20 × 20mm, matrix size =128 × 128, number of averages =; slice thickness =0.8mm with interleaved slice acquisition order without any cardiac or respiratory gating. Total acquisition time for DTI data was 2 hours.
After the end of the experiment, animals were taken back to the housing facility for longitudinal studies. In order to perform histological correlation, some animals were randomly selected and sacrificed at each time point by means of transcardiac perfusion. At day 30 (BALB/cJ =4, B6 =4), at 50 day time point (BALB/cJ =4, B6 =4) and at the end of the last imaging study (70 days, BALB/cJ =24, B6 =7) mice were sacrificed for these studies. After perfusion fixation, the brains were removed and stored at 4°C in 4% paraformaldehyde solution till the time for histology.
4.4. Histology
After brain fixation, 12 brain samples (2 from each time point i.e. 30, 50 and 70 days-of-age) from BALB/cJ and B6 mice were processed for histological study. The brain samples were cut into 2 mm thick axial blocks with 5-6 blocks per brain. All tissue blocks were processed for paraffin-wax embedding and were cut in a series of 5μm thick axial sections. Staining was performed according to standard methods, using hematoxylin and eosin (H&E) for morphology and a Kluver-Barrera method for myelin staining. In the Kluver-Barrera technique, Luxol fast blue (LFB) is used to stain myelinated axons and cresyl violet (Nissl) is used to stain the neuronal somata (cell bodies) (Kluver and Barrera, 1953).
4.5. Image processing
Image reconstruction from acquired Varian FID files was performed offline using in-house custom software developed in the IDL programming environment (ITT Visual Information Solutions, Boulder, CO, USA). A Gaussian filter (width 0.5) was used to smooth the data and remove some noise from the images. DT I-Studio (www.mristudio.org) was used to generate fractional anisotropy (FA) and mean diffusivity (MD×10-3mm2/s) maps. The raw images were used and no eddy current compensation or motion correction algorithms from the DTI-Studio were used in post-processing of the data. The FA color maps were used to draw region-of-interests (ROIs) from 5 GM and 3 WM regions of the brain in BALB/cJ and B6 mice at each time point. ROIs were chosen based on the relevance of particular brain regions in mediating sociability (Amaral et al., 2008) or other ASD-relevant social behaviors, and on prior ex-vivo DTI study in BALB/cJ mice (Kim et al., 2012). Two of the 15 slices were used for placement of the ROI in different GM and WM regions of the brain, since these slice locations were typically free from motion artifacts and encompassed most of the ROIs selected. A single ROI was placed at the dorsal 3rd lateral ventricle level on the mid axial slice of the color coded map for the corpus callosum (CC). Bilateral ROIs were used for the external capsule (EC), internal capsule (IC), cerebral cortex (Ctx), hippocampus (Hippo), thalamus (Th), and amygdaloid (Amg) regions (Fig. 2A). The ROI from the frontal motor cortex (FMC) was placed at the level of central sulcus and caudal regions as shown in figure 2B. Placement of the ROIs was determined on the basis of anatomical location as described in mouse brain atlas (Franklin and Paxinos, 2007).
4.6. Statistical analysis
4.6.1. Correlation between sociability and DTI
All statistical computations were performed using statistical package for social sciences (SPSS, version 16.0 SPSS, Inc, Chicago, USA) and Stata 11 (Stata Corp LP, College Station, Texas). Pearson correlation was performed to assess the correlation between FA or MD and sociability at different time points in BALB/cJ and B6 mice. An uncorrected p value of ≤ 0.05 was considered to be statistically significant. Additionally, to account for multiple comparisons, Bonferroni correction was also applied to get an adjusted p value of ≤ 0.003.
4.6.2. Cross sectional and longitudinal comparison of FA and MD values at different age
Some of the animals died during longitudinal scanning while motion artifacts precluded inclusion of the DTI data from a few animals at each time point. Thus after removal of the data from these animals the following number of animals were used at each time point: At 30 days-of-age (BALB/cJ, n =28; B6, n =12), at 50 days-of-age (BABL/cJ, n =27; B6, n =11) and at 70 days-of-age (BALB/cJ, n =28; B6, n =7). An independent Student’s t-test was performed for comparison between and within groups (cross sectional and longitudinal analysis) in BALB/cJ and B6 mice at different ages.
4.6.3. Prediction of growth trajectories from FA and MD values
A linear mixed model for multilevel model analysis (Singer and Willett, 2003) was also used to look for changes in FA and MD that could predict the developmental growth trajectories in BALB/cJ and B6 mice. This method has been used previously to demonstrate patterns of restricted and repetitive behaviors and interests developed over time in patients with ASD (Esbensen et al., 2009; Richler et al., 2010; Schumann et al., 2010). The advantages of this multilevel approach are that it allows modeling changes within and between subjects; unbalanced design and variably spaced measurements; and flexibility in specification of dependency of the outcome variable on independent variables. Details of the multilevel model have been published previously (Singer and Willett, 2003). Briefly, the model assesses changes over a period of time using the following equation:
| (1) |
where Yij is the outcome variable of interest for animal i at time point j; GROUP denotes the group-membership variable (0 -B6 and 1 -BALB/cJ). γ00 and γ10 represent the population average initial status and rate of change, respectively; ζ0i and ζ1i are residuals, εij is the random measurement error. We assumed that εij ~ N (0, σ2), and ζ0i and ζ1i follow a joint Gaussian distribution with a mean of 0 and covariance matrix Σ. For this analysis, we focused on testing whether γ10 is zero (the null hypothesis). If γ10 =0, there would be no group effect on the variable of interest. That is, the population trajectories for B6 (GROUP =0) and BALB/cJ (GROUP =1) are the same.
Supplementary Material
Highlights.
-
➢
BALB/cJ mice are less social at 30 days-of-age in comparison to B6 mice
-
➢
Significant differences in fractional anisotropy of external capsule between BALB/cJ and B6 mice
-
➢
Significant differences in developmental trajectories of FA from external capsule and frontal motor cortex between BALB/cJ and B6 mice
-
➢
Results indicate the potential of FA and MD as surrogate imaging markers for assessing brain under-connectivity
Acknowledgments
This study was funded by the NIH grants R01MH080718 (ESB) and R21HD058237 (HP) and utilized the Small Animal Imaging Facility of the University of Pennsylvania.
List of Abbreviations
- ASD
Autism spectrum disorders
- Amg
Amygdaloid
- B6
C57BL/6J
- CC
Corpus callosum
- Ctx
Cerebral cortex
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- EC
External capsule
- FMC
Frontal motor cortex
- GM
Gray matter
- H&E
Hematoxylin and eosin
- Hippo
Hippocampus
- IC
Internal capsule
- LFB
Luxol fast blue
- MD
Mean diffusivity
- MRI
Magnetic resonance imaging
- SNR
Signal to noise ratio
- Th
Thalamus
- WM
White matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: Studies with nicotine. Neuropsychopharmacology. 2004;29:869–78. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, Wang AT, Hollander E, Anagnostou E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS One. 2011;6:e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol Autism. 2011;2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. American Psychiatric Association: Diagnostic and statistical manual of mental disorders. Washington, DC: 2001. [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–7. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode MK, Mattila ML, Kiviniemi V, Rahko J, Moilanen I, Ebeling H, Tervonen O, Nikkinen J. White matter in autism spectrum disorders - evidence of impaired fiber formation. Acta Radiol. 2011;52:1169–74. doi: 10.1258/ar.2011.110197. [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues Lde S, Gasparetto EL, Calcada CA. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–43. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–7. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–51. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–78. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–45. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–59. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73:700–17. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye CA, El Shawa H, Huffman KJ. A lifespan analysis of intraneocortical connections and gene expression in the mouse II. Cerebral Cortex. 2011;21:1331–50. doi: 10.1093/cercor/bhq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam KS, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. J Autism Dev Disord. 2009;39:57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–7. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, Morton EA, Tan J, Berrettini WH, Li H, Abel T, Brodkin ES. Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res. 2012 doi: 10.1016/j.bbr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, Mori S. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J Magn Reson Imaging. 2007;26:756–67. doi: 10.1002/jmri.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Mottron L, Berthiaume C, Burack JA. Developmental changes of autistic symptoms. Autism. 2003;7:255–68. doi: 10.1177/1362361303007003003. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2007. [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev Psychobiol. 2002;40:213–25. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Hu L, Sun Y, Villasana LE, Paylor R, Klann E, Pautler RG. Early changes in the apparent diffusion coefficient (ADC) in a mouse model of Sandhoff’s disease occur prior to disease symptoms and behavioral deficits. Magn Reson Med. 2009;62:1175–84. doi: 10.1002/mrm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygino da Cruz LC, Jr, Vieira IG, Domingues RC. Diffusion MR imaging: an important tool in the assessment of brain tumors. Neuroimaging Clin N Am. 2011;21:27–49. vii. doi: 10.1016/j.nic.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Parker D, Bloy L, Roberts TP, Verma R. Diffusion based abnormality markers of pathology: toward learned diagnostic prediction of ASD. Neuroimage. 2011;57:918–27. doi: 10.1016/j.neuroimage.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation. 2011;28:345–52. doi: 10.3233/NRE-2011-0662. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, Zhou Z, Ruan Z, Lu Z, Tao G, Liu Y. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–7. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–7. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kim S, Pickup S, Fairless AH, Ittyerah R, Dow HC, Abel T, Brodkin ES, Poptani H. Association between sociability and diffusion tensor imaging in BALB/cJ mice. NMR Biomed. 2012;25:104–12. doi: 10.1002/nbm.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Pickup S, Poptani H. Effects of cardiac pulsation in diffusion tensor imaging of the rat brain. J Neurosci Methods. 2010;194:116–21. doi: 10.1016/j.jneumeth.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–3. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Sierra A, Pitkanen A, Grohn O. Diffusion tensor MRI of axonal plasticity in the rat hippocampus. Neuroimage. 2010;51:521–30. doi: 10.1016/j.neuroimage.2010.02.077. [DOI] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3:350–8. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M, Miles L, Donaldson J, Jensen JH, Ming JC. International Society for Magnetic Resonance in Medicine. Vol. 19. International Society for Magnetic Resonance in Medicine; Montral, Canada: 2011. Atypical gray and white matter microstructure in autism spectrum disorders; p. 350. [Google Scholar]
- Lim KO, Helpern JA. Neuropsychiatric applications of DTI - a review. NMR Biomed. 2002;15:587–93. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–16. [PubMed] [Google Scholar]
- Molko N, Pappata S, Mangin JF, Poupon C, Vahedi K, Jobert A, LeBihan D, Bousser MG, Chabriat H. Diffusion tensor imaging study of subcortical gray matter in cadasil. Stroke. 2001;32:2049–54. doi: 10.1161/hs0901.094255. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Young NB, Demyanenko GP, Maness PF. Impaired sociability and cognitive function in Nrcam-null mice. Behav Brain Res. 2009;205:123–31. doi: 10.1016/j.bbr.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–9. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–83. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–71. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry. 1996;35:523–9. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Rapoport JL, Sceery WR. Autistic children as adults: psychiatric, social, and behavioral outcomes. J Am Acad Child Psychiatry. 1985;24:465–73. doi: 10.1016/s0002-7138(09)60566-5. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–23. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34:4–11. doi: 10.1111/j.1365-2990.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen A, Nemkul N, Yang D, Adhami F, Dunn RS, Hazen ML, Nakafuku M, Ning G, Lindquist DM, Kuan CY. Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia-ischemia-induced thrombotic stroke. J Cereb Blood Flow Metab. 2011;31:1155–69. doi: 10.1038/jcbfm.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, Muller RA. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49:1378–82. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism. 2011;2:7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–65. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–6. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CY, Friedman JI, Carpenter DM, Novakovic V, Eaves E, Ng J, Wu YW, Gottlieb S, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, McGinn TG, Flanagan L, Davis KL. The effects of hypertension and body mass index on diffusion tensor imaging in schizophrenia. Schizophr Res. 2011;130:94–100. doi: 10.1016/j.schres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, Strik W, Muller TJ. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42:276–83. doi: 10.1016/j.nbd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Wang S, Kim S, Chawla S, Wolf RL, Knipp DE, Vossough A, O’Rourke DM, Judy KD, Poptani H, Melhem ER. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2011;32:507–14. doi: 10.3174/ajnr.A2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


