Abstract
Adults with autism exhibit inhibitory deficits that are often manifested in behavioral modifications, such as repetitive behaviors, and/or sensory hyper-responsiveness. If such behaviors are the result of a generalized deficiency in inhibitory neurotransmission, then it stands to reason that deficits involving localized cortical-cortical interactions – such as in sensory discrimination tasks – could be detected and quantified. This study exemplifies a newly developed method for quantifying sensory testing metrics. Our novel sensory discrimination tests may provide (a) an effective means for biobehavioral assessment of deficits specific to autism and (b) an efficient and sensitive measure of change following treatment. The sensory discriminative capacity of 10 subjects with autism and 10 controls was compared both before and after short duration adapting stimuli. Specifically, vibrotactile amplitude discriminative capacity was obtained both in the presence and absence of 1 sec adapting stimuli that were delivered 1 sec prior to the comparison stimuli. Although adaptation had a pronounced effect on the amplitude discriminative capacity of the control subjects, little or no impact was observed on the sensory discriminative capacity of the subjects with autism. This lack of impact of the adapting stimuli on the responses of the subjects with autism was interpreted to be consistent with the reduced GABAergic mediated inhibition described in previous reports. One significant aspect of this study is that the methods could prove to be a useful and efficient way to detect specific neural deficits and monitor the efficacy of pharmacological or behavioral treatments in autism.
Keywords: autism, inhibition, adaptation, habituation, amplitude discrimination, sensory discrimination
Introduction
It has been well established that conditioning stimulation – or prolonged pre-exposure to sensory stimulation – significantly modifies discriminative capacity and alters the ability of both peripheral and CNS neurons to process sensory information. Less widely appreciated is the fact that primary sensory cortical mechanisms undergo transient and significant alterations in response to even a brief exposure (1 sec or less) to sensory stimulation. For example, both visual and somatosensory cortical pyramidal neurons undergo prominent use-dependent modifications of their receptive fields and response properties. Such modifications attain full development within a few tens of milliseconds of stimulus onset, and disappear within seconds after stimulus termination (visual cortical neurons: (Bredfeldt and Ringach, 2002; Celebrini et al., 1993; Das and Gilbert, 1995; DeAngelis et al., 1995; Dinse and Kruger, 1990; Pack and Born, 2001; Pettet and Gilbert, 1992; Ringach et al., 1997; Shevelev et al., 1998; Shevelev et al., 1992; Sugase et al., 1999); for review of short-term primary somatosensory cortical neuron dynamics see (Kohn and Whitsel, 2002)). However, in the cortex of an individual with autism, the impact of sensory history on CNS mechanisms may deviate significantly from the norm. We recently reported both the failure of prior history of tactile stimulation to alter tactile spatial localization in adults with autism, and the better-than-normal tactile spatial localization performance of adults with autism when the period of adaptation was short, which were concluded to be attributable to the deficient cerebral cortical GABAergic inhibitory neurotransmission characteristic of this disorder (Tommerdahl et al., 2007a). These results led the authors to consider whether the lack of an adaptation effect on localization performance in subjects with autism was unique to spatial discrimination, or rather that adaptation may have a differential effect on this population under the implementation of other protocols as well that target GABA-mediated synaptic neurotransmission.
In a previous report, we demonstrated that a subject’s ability to discriminate between two simultaneously delivered vibrotactile stimuli – differing only in amplitude – was very robust (low inter-subject variability) in healthy subjects and was very sensitive to varying conditions of pre-exposure to sensory stimuli (Tannan et al., 2007b). Changing the duration of a conditioning stimulus delivered to one of the two sites before the amplitude discrimination task significantly altered a subject’s ability to determine the actual difference between the two stimuli. One significant finding of that study was that specific durations of conditioning stimuli altered the subject’s amplitude discriminative capacity in a predictive and quantifiable fashion. In this study, we sought to determine if these conditioning stimuli would have a similar impact on the amplitude discriminative capability of subjects with autism.
Methods
The subjects were ten males clinically diagnosed with autism (i.e., Autistic Disorder or Asperger Disorder; DSM-IV-TR; (APA, 2000)), all naïve both to the study design and issue under investigation. Control data used for comparison has been reported in a previous study (Tommerdahl et al., 2007b). Autism subjects were recruited from the University of North Carolina Neurodevelopmental Disorders Research Center Subject Registry. All ten individuals had been previously tested with the Autism Diagnostic Interview – Revised (ADI-R; (LeCouteur et al., 2003)), the Autism Diagnostic Observation Schedule - Module 4 (ADOS; (Lord et al., 1999)), as well as the Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, 1999)), and met the diagnostic criteria for autism on the ADI-R. Education levels were as follows: one subject completed the 11th grade, and the remaining nine subjects completed high school. Participants were screened for co-morbid psychiatric diagnoses, peripheral injury, and other conditions that would affect somatosensation. The average ages were 26.1 ± 6.3 yrs for the autism group and 23.5 ± 3.1 yrs for the control group (mean ± stdev). The average IQ scores were as follows: for the autism group, Verbal = 102.3 ± 17.8, Performance = 103.5 ± 18.7, Full-4 = 102.8 ± 17.7; for the control group, Verbal = 116.0 ± 14.0, Performance = 117.3 ± 6.0, Full-4 = 118.3 ± 7.4. No statistical differences were observed between the two groups for either age or IQ. The subjects gave informed consent and were paid $25/hour for their time. All procedures were reviewed and approved in advance by an institutional review board.
A two alternative forced-choice (2AFC) tracking protocol was used to evaluate the amplitude discriminative capacity of each of the ten right hand dominant subjects. This protocol was implemented and described in detail in a previous study (Tannan et al., 2007b). The subject was seated comfortably in a chair with the right arm resting on a table surface. The subject’s right hand was placed under a portable vibrotactile dual-site stimulator (CM-1; for full description, see (Tannan et al., 2007a)). Two probe tips (5 mm diameter) were positioned 30 mm apart along a transversally oriented linear axis along the hand dorsum. The hand dorsum was selected to receive the stimulation because: 1) innervation density across this skin region remains relatively constant, 2) the surface is easily accessible and permits convenient stimulator placement, 3) the surface is relatively flat, reducing confounds of skin curvature present at other potential sites of stimulation, 4) it permits positioning of the subject’s arm and hand in a comfortable and stable position for the full duration of an experimental session and, 5) perhaps most importantly, there is very little, if any, between-subject use-dependent changes in sensitivity at this particular site. Previous studies have shown that human subjects demonstrate very consistent performance with similar discrimination tasks on the hand dorsum (Tannan et al., 2005a; Tannan et al., 2005b; Tannan et al., 2007a; Tannan et al., 2007b; Tannan et al., 2006; Tommerdahl et al., 2007a). One significant aspect of those previous studies was that consistent results were obtained although stimulus positions were randomly located on a trial-by-trial basis, and thus, the relatively large size of the probe tip apparently compensates for the differential distribution of bone vs. muscle across the hand dorsum.
Visual cueing was provided with a computer monitor during the experimental run. Specifically, an array of LEDs was used to indicate to the subject when the stimulus was on and when the subject was to respond. The subject was not given performance feedback or knowledge of the results during the data acquisition until all sessions were completed. At the start of each run, the two probe tips were driven towards the skin until each tip registered a force of 0.1 g, as determined by a closed-loop algorithm in the CM-1 stimulator feedback system. The tips were then further indented into the skin by 500 µm to ensure good contact with the skin. An audiometer was used to insure that no auditory cues were emitted from the stimulator during delivery of the range of stimuli used in this study.
The tracking protocol in each experimental run, 40 trials total, consisted of 2 sequential blocks. In the first block, a vibrotactile test stimulus (25 Hz, amplitude between 105–200 µm) was delivered to one skin site at the same time that a standard stimulus (25 Hz, amplitude fixed at 100 µm) was applied to the other skin site. Previous studies have demonstrated that, for 25 Hz flutter stimuli, (i) the distance at which the two stimuli were positioned apart on the hand dorsum (30 mm) is well outside a subject’s two point limen (Tannan et al., 2005a; Tannan et al., 2005b; Tannan et al., 2006) and (ii) at a 30 mm probe separation there is no difference in the ability of a subject to detect a difference in the amplitudes of flutter stimulation applied simultaneously or sequentially to the two skin sites (Tannan et al., 2007a). The loci of the test and standard stimuli were randomly selected on a trial-by-trial basis. Stimulus duration was 0.5 sec, followed by subject response (the subject was queried to select, using a two-button switchbox, the skin site that received the most intense stimulus) and a 5 sec delay before onset of the next trial. At the beginning of the experimental run, the test stimulus was 200 µm (peak-to-peak amplitude) and the standard was 100 µm. In the initial 10 trials, the amplitude of the test stimulus was modified based on the subject’s response to the preceding trial, accomplished using a 1-up/1-down forced-choice tracking protocol. This approach was selected because it enabled rapid determination (“tracking”) of each subject’s minimally detectable difference in the amplitudes of two-site skin flutter stimulation (Tannan et al., 2007a). The difference in the amplitudes of the test and standard stimuli delivered on each of these initial 10 trials was adjusted on the basis of the subject’s response in the preceding trial (the discrepancy in amplitude was decreased if the subject’s response in the preceding trial was correct; it was increased if the response was incorrect). After the initial 10 trials were completed, test stimulus amplitude was modified using a 2-up/1-down protocol – in these trials two correct/one incorrect subject response(s) resulted in a decrement/increment, respectively, in the amplitude difference between the test and standard stimuli. The step size was held constant throughout all experimental runs at 10 µm.
In the second block, delivery of the test and standard stimuli was preceded by adapting stimulation. Specifically, a 25 Hz 100 µm adapting stimulus at the location of the test stimulus was delivered 1 sec prior to the presentation of the test and standard stimuli. By presenting the adapting stimulus to the same site as the test stimulus, it was possible to quantify the effect of reduced perceived intensity due to adapting stimulation. The duration of adapting stimulation delivered was 1 sec. A 2- up/1-down protocol was used in Block #2 to track the subject’s ability to determine the most intense stimulus. The initial conditions of Block #2 were the final conditions of Block #1. A series of training trials, each consisting of a pair of stimuli differing in amplitude by 100 µm (200 µm vs. 100 µm), were conducted prior to the first session. The subject was provided with feedback only during training trials and was allowed to continue on to the experimental run after answering correctly 5 times in a row. A single run took approximately 4 min.
Results
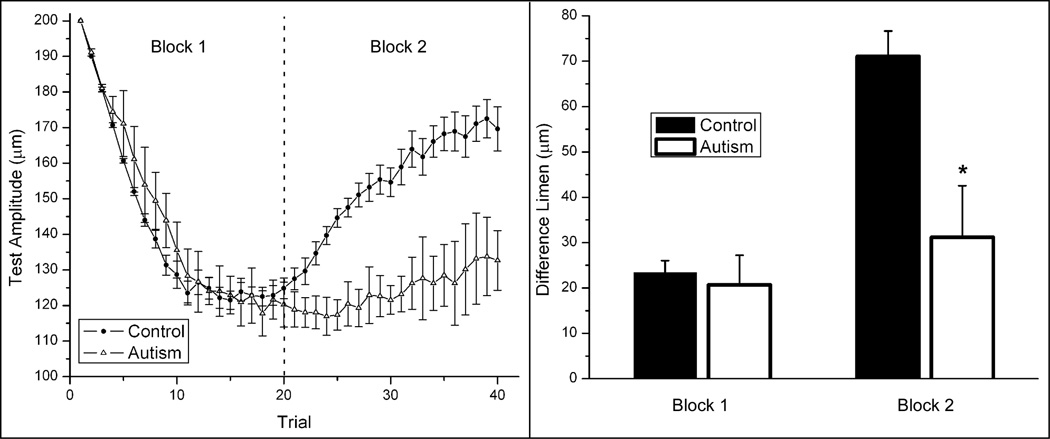
A two alternative forced-choice (2AFC) tracking protocol was used to determine capacities to discriminate between the amplitudes of two simultaneously delivered vibrotactile stimuli in subjects with autism and to directly compare subjects’ discriminative capacities under the condition of adapting stimulation. Tracking plots averaged across each group are shown in the left panel of Figure 2. Difference limens (DLs) for each subject were determined by averaging the last 5 tracking values for each block. The right panel of Figure 2 summarizes the average across-subject (n = 10) performance during both blocks of the protocol for the control and autism groups. Note that in Block 1, during which amplitude discrimination without adaptation was measured, there was no significant difference between the control and autism groups (repeated measures ANOVA; p = 0.7826). However, as the bars indicate for Block 2, adaptation resulted in a significant difference in performance between the two groups (p < 0.01) such that the control group difference limen was greater than that of the autism group. Furthermore, when compared to the unadapted condition (Block 1), amplitude discrimination performance was significantly degraded (the DL was elevated; Block 2) in the control group (p < 0.01) whereas no significant change due to adaptation was observed in the autism group (p = 0.1057).
Figure 2.
Left Panel: Comparison of averaged tracking response (with s.e. bars) for the control group and the autism group. No adapting stimulus was applied to either stimulus site in Block 1. The test condition in Block 2 was preceded by an adapting stimulus (1sec in duration) at the site of the test stimulus. Right panel: Comparison of the difference limen (obtained by averaging the outcome of the last 5 trials for each condition) for the control group and the autism group. Note that in Block 2, performance between the groups was significantly different (* ANOVA; p < 0.01).
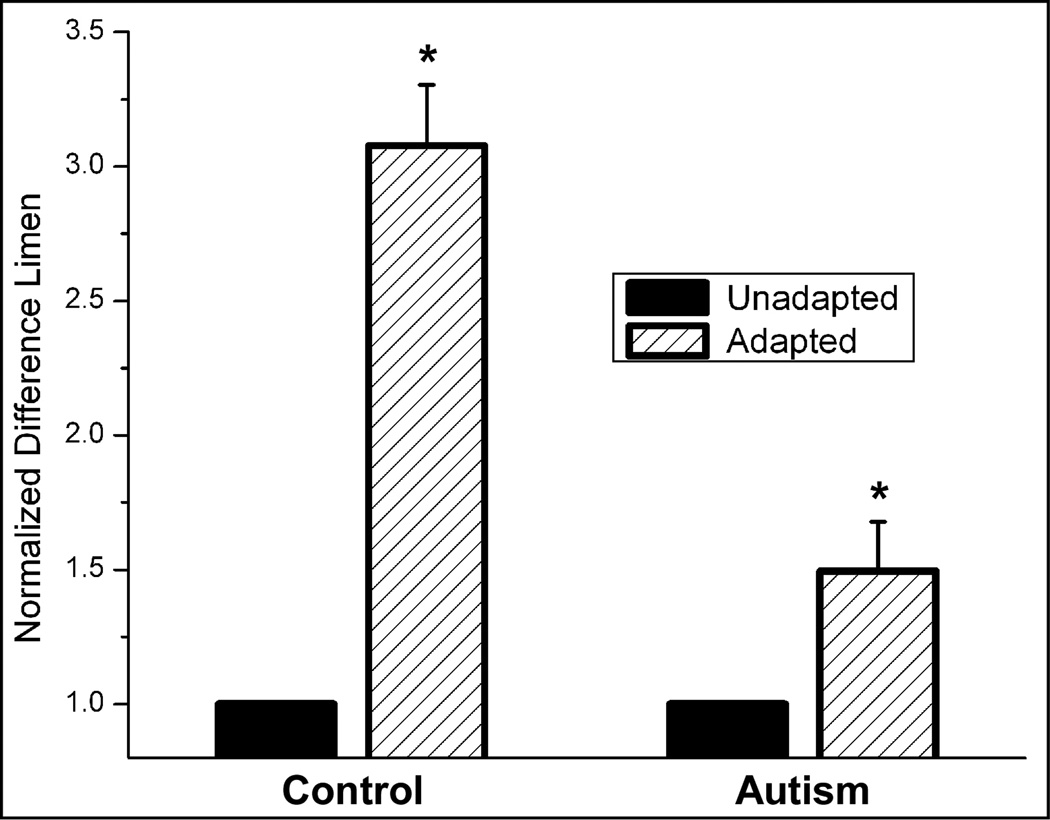
In order to determine whether the differential effects of adaptation observed between the groups were consistent within subjects, the data was normalized to the unadapted condition (shown in Figure 3). The 1 sec adapting stimulus significantly impaired amplitude discrimination capacity by nearly 45% for the control group (p < 0.01). The normalized plot reveals that the autism group performance was in fact significantly degraded with adaptation as well (p < 0.01), although to a much lesser degree (~ 5%) than the control group (p < 0.01). Relatively small error bars in the normalized plot confirm that the change in performance due to adaptation was consistent across all the subjects who participated in the study.
Figure 3.
Comparison of difference limen (with s.e. bars) normalized to the unadapted condition. Note that for both the Control and the Autism group, 1 sec adaptation resulted in an elevated difference limen (* ANOVA; p < 0.01). The Control group showed a greater impairment with adaptation (~45%) than the Autism group (~5%).
Discussion
The present study evaluated the ability of adults with autism to discriminate amplitudes of two simultaneous 25 Hz flutter stimuli on the hand dorsum. While amplitude discrimination capacity was not significantly different between the control and autism groups, the effects of adaptation on performance during this task were remarkably different for each group. Specifically, on average, the difference limen for the previously-reported control group was significantly elevated when a 1 sec adapting stimulus was delivered to one of the test sites (thus directly comparing a stimulus at a pre-exposed stimulus site with a stimulus at an un-exposed site), whereas the autism group showed no significant change under the same conditions in a group-wise analysis. Normalization of the data for each subject to the unadapted condition demonstrated that individuals with autism do in fact adapt during this amplitude discrimination task, though to a significantly smaller extent (~5% reduction in performance) than the controls (~45%).
In our previous report, using the same methodology with 20 healthy control subjects, we demonstrated that the perceived intensity of a stimulus was reduced significantly and robustly in a timedependent fashion. Several durations of adaptation were delivered to the skin (0.2, 0.5, 1 and 2 sec), and all had impact on the perceived intensity of the subsequently delivered stimulus (Tannan et al., 2007b). The effects of delivering adapting stimuli on the perception of subsequent test stimuli – particularly the reduction in sensation – has been characterized in some detail (Delemos and Hollins, 1996; Gescheider et al., 1995; Goble and Hollins, 1993; Laskin and Spencer, 1979; Tannan et al., 2007b; Tommerdahl et al., 2005b; Tommerdahl et al., 2007a; Verrillo, 1985; Verrillo and Gescheider, 1977). In our study of healthy control subjects, short (≤ 2 sec) stimulus durations were adequate to evoke robust changes in discriminative capacity, and we demonstrated that increasing durations of adapting stimulation systematically reduced a subject’s amplitude discriminative capacity. One of the conclusions of that study was that it would be difficult to ascribe the differences in sensory performance with short adapting stimuli to changes that occur in the periphery. Although prolonged stimulation leads to a reduction in the response of neurons to subsequent stimuli at both the peripheral and central levels of neural processing (Bensmaia et al., 2005; Chung et al., 2002; O'Mara et al., 1988), the change in response of peripheral afferents at the stimulus durations used in this study (≤ 2 sec) do not evoke changes that could account for significant decreases in sensory performance (see (Whitsel et al., 2000) for full discussion). Thus, the decreases in performance observed in healthy subjects that are absent in individuals with autism are most likely attributed to centrally mediated mechanisms.
There have been other studies that describe the dynamic perceptual differences between healthy adults and subjects with autism that implicate centrally mediated mechanistic deficiencies in the autism population. Tannan et al (Tannan et al., 2006) demonstrated that the effects of short-duration adaptation change the performance of a healthy subject’s tactile discriminative capacity. In that study, adaptation with a 5 sec stimulus resulted in an approximately 2-fold improvement in spatial localization performance over that achieved with a 0.5 sec adapting stimulus, and it was proposed that this observed improvement in spatial discrimination was due to the enhanced spatial funneling of the population-level response of contralateral primary somatosensory cortex (SI) – a robust phenomenon that is at least in part due to GABAergic inhibitory neurotransmission and has been shown using comparable stimulus conditions in neuroimaging studies of anesthetized non-human primates (Juliano et al., 1989). Using the same methodology in a subsequent report, Tommerdahl and colleagues demonstrated that adults with autism showed no improvement in the spatial localization task with a 5.0 sec adapting stimulus although the individuals with autism outperformed healthy controls under the shorter duration 0.5 sec adaptation condition (Tommerdahl et al., 2007a). One possible reason given for the outcome in that study was the observation that autism is associated with a mutation in regions centered around the GABAA-β3 receptor subunit gene (Buxbaum et al., 2002; DeLorey, 2005; Shao et al., 2003). A number of researchers (Belmonte et al., 2004; Collins et al., 2006; Polleux and Lauder, 2004) have suggested that the neocortical dysfunction in autism may be attributable to a deficiency during early development in GABA-mediated synaptic neurotransmission (see (Tommerdahl et al., 2007a) for full discussion).
How do the measures derived from the previous spatial localization study (Tommerdahl et al., 2007a) compare to the results of this current study? First, it is important to note that spatial localization and amplitude discrimination are two very different entities, and one of our underlying goals was to see if the specific effect that adaptation had on spatial localization could be generalized to cover other metrics of cortical information processing capacity. Second, adaptation has been shown to have an effect on both the gain as well as the contrast of a stimulus (Kohn, 2007). While the percept of a stimulus that follows an adapting stimulus is notably (normally) turned down in gain (i.e., it feels weaker), it also improves the contrast of that signal (i.e., spatial perception of that signal is improved). The previous report focused on the change in contrast that normally occurs with adaptation, while this report focuses on the change in gain. In both cases, it appears that autism has a significant impact on the effect of adaptation.
A number of other neurophysiological studies have demonstrated that individuals with autism have either a reduction in inhibition or an imbalance in excitation to inhibition. Recently, Perry et al (Perry et al., 2007) showed that adults with autism evoke a reduced pre-pulse inhibition (PPI) to the human startle response (in comparison with healthy controls) and interpreted their results to indicate a deficiency in sensorimotor gating and inhibitory processing in autism. Similarly, several other studies have demonstrated PPI deficits in children with autism (Ornitz et al., 1993), adults with Asperger’s syndrome (McAlonan et al., 2002) and fragile X syndrome (Frankland et al., 2004). These deficits in PPI could be associated with clinical features that are often observed in individuals with autism “such as restrictive and repetitive behaviors, reflect problems with inhibitory mechanisms” (Perry et al., 2007) and we would interpret these findings as consistent with the lack of inhibition observed in the response to adapting stimuli.
One possible explanation for the decreased inhibition that is evident in autism could be the differences that exist between cortical minicolumns in autism and neurotypical populations. The inhibitory processes between minicolumns are a necessary component for the moment-to-moment changes that normally occur in cerebral cortex with repetitive stimulation (Chiu et al., 2005; Favorov and Kelly, 1994a; b; Kohn et al., 2000; Tommerdahl et al., 1993; Tommerdahl et al., 2005a), and Casanova and colleagues have developed a large body of evidence that demonstrates that the cerebral cortex of autism subjects is significantly modified at the minicolumnar level (Casanova et al., 2002). Casanova also suggests that this aberrant minicolumnar structure results in the disruption of the inhibitory architecture (Casanova et al., 2003) that is required for normal function in local neural circuitry. In other words, disruption of functional connectivity at the local minicolumnar level could be responsible for or strongly correlated with the dysfunctional connectivity that leads to a degradation of the normal response to repetitive stimulation in which cortical ensembles decrease in response with increasing repetition. Thus, a generalized lack of inhibition – or disinhibition – in the GABA mediated cortical circuitry of individuals with autism could be one of the principle factors that leads to the differences observed between the control and autism population in this study. We view this idea as consistent with (i) the recommendation that drugs that promote GABAergic CNS synaptic neurotransmission be considered for early intervention and as potential therapeutic agents for autism (Belmonte et al., 2004; Bethea and Sikich, 2007); and (ii) the neuromechanistic proposal that an increased ratio of excitation to inhibition in CNS information processing systems accounts for some of the principal features of autism (Rubenstein and Merzenich, 2003).
One overriding goal that we have in the development of all of our quantitative sensory testing protocols is the ultimate utility of providing not only the accurate assessment of sensory cortical function, but also an efficient process that could be useful in a clinical and/or clinical research environment. For example, the inherent advantage that comes with monitoring the percepts evoked by comparison of two simultaneously presented stimuli rather than single-site stimulation appears to be significant. The temporal confounds normally presented by stimuli delivered sequentially in a single skin site protocol could be prominent, given that short duration adapting stimuli normally have a significant impact on amplitude discriminative capacity. It should be noted that the protocol described in this report takes approximately 2–4 minutes per subject to complete, a duration that contrasts sharply with many traditional single-site tactile stimulation psychophysical techniques which usually require significantly longer periods of time and are difficult, if not impossible, to implement in a clinical setting. Perceptual metrics – such as the one described in this report – could prove useful for evaluating the efficacy of behavioral and/or pharmacological treatments of individuals with autism. For instance, if the measures obtained from individuals with autism approach those of control values after being administered drugs that promote GABAergic CNS synaptic neurotransmission, it will lend further quantitative support to the idea that such therapeutic agents are beneficial to individuals with autism. New studies are currently evaluating this interesting possibility.
Figure 1.
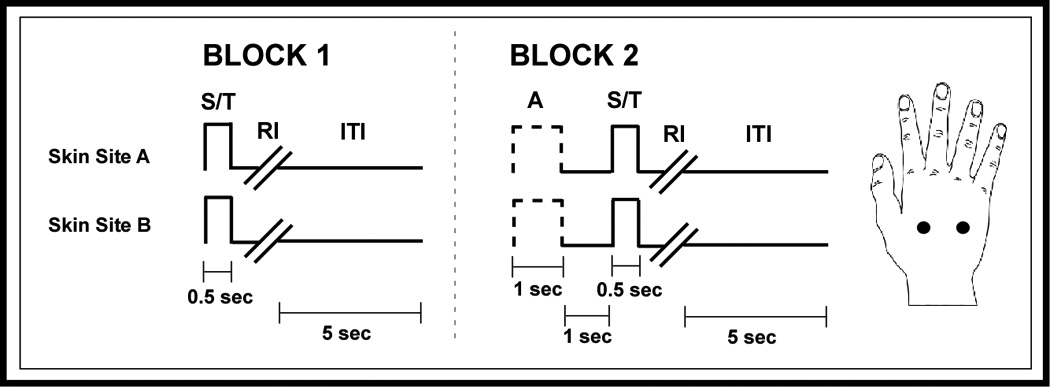
Schematic of the protocols used for amplitude discrimination. Two blocks of stimulus delivery were employed. In the first block, two 25 Hz vibrotactile stimuli, the standard (S) and test (T), were delivered at the same time for 0.5 sec. A 5 sec delay (excluding subject response interval (RI)) was imposed before onset of the next trial. In the second block, single-site adapting stimulation was first delivered for 1 sec, followed by a 1 sec inter-stimulus interval, and then the standard and test stimuli.
Acknowledgements
This work was funded, in part, by the Cure Autism Now Foundation (M. Tommerdahl, PI) and the Department of Defense (Contract # W81XWH-07-1-0287, M. Tommerdahl, PI).
References
- APA. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmaia SJ, Leung YY, Hsiao SS, Johnson KO. Vibratory adaptation of cutaneous mechanoreceptive afferents. J Neurophysiol. 2005;94:3023–3036. doi: 10.1152/jn.00002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea TC, Sikich L. Early pharmacological treatment of autism: a rationale for developmental treatment. Biol Psychiatry. 2007;61:521–537. doi: 10.1016/j.biopsych.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredfeldt CE, Ringach DL. Dynamics of spatial frequency tuning in macaque V1. J Neurosci. 2002;22:1976–1984. doi: 10.1523/JNEUROSCI.22-05-01976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autisim. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Celebrini S, Thorpe S, Trotter Y, Imbert M. Dynamics of orientation coding in area V1 of the awake primate. Vis Neurosci. 1993;10:811–825. doi: 10.1017/s0952523800006052. [DOI] [PubMed] [Google Scholar]
- Chiu JS, Tommerdahl M, Whitsel BL, Favorov OV. Stimulus-dependent spatial patterns of response in SI cortex. BMC Neurosci. 2005;6:47. doi: 10.1186/1471-2202-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gilbert CD. Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J Neurophysiol. 1995;74:779–792. doi: 10.1152/jn.1995.74.2.779. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Anzai A, Ohzawa I, Freeman RD. Receptive field structure in the visual cortex: does selective stimulation induce plasticity? Proc Natl Acad Sci U S A. 1995;92:9682–9686. doi: 10.1073/pnas.92.21.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delemos KA, Hollins M. Adaptation-induced enhancement of vibrotactile amplitude discrimination: the role of adapting frequency. J Acoust Soc Am. 1996;99:508–516. doi: 10.1121/1.414509. [DOI] [PubMed] [Google Scholar]
- DeLorey TM. GABRB3 gene deficient mice: a potential model of autism spectrum disorder. Int Rev Neurobiol. 2005;71:359–382. doi: 10.1016/s0074-7742(05)71015-1. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Kruger K. Contribution of area 19 to the foreground-background-interaction of the cat: an analysis based on single cell recordings and behavioural experiments. Exp Brain Res. 1990;82:107–122. doi: 10.1007/BF00230843. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates: I Development of afferent connections. Cereb Cortex. 1994a;4:408–427. doi: 10.1093/cercor/4.4.408. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates: II. Emergent functional properties. Cereb Cortex. 1994b;4:428–442. doi: 10.1093/cercor/4.4.428. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Gescheider GA, Santoro KE, Makous JC, Bolanowski SJ. Vibrotactile forward masking: effects of the amplitude and duration of the masking stimulus. J Acoust Soc Am. 1995;98:3188–3194. doi: 10.1121/1.413808. [DOI] [PubMed] [Google Scholar]
- Goble AK, Hollins M. Vibrotactile adaptation enhances amplitude discrimination. J Acoust Soc Am. 1993;93:418–424. doi: 10.1121/1.405621. [DOI] [PubMed] [Google Scholar]
- Juliano SL, Whitsel BL, Tommerdahl M, Cheema SS. Determinants of patchy metabolic labeling in the somatosensory cortex of cats: a possible role for intrinsic inhibitory circuitry. J Neurosci. 1989 Jan;9(1):1–12. doi: 10.1523/JNEUROSCI.09-01-00001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Kohn A, Metz C, Quibrera M, Tommerdahl MA, Whitsel BL. Functional neocortical microcircuitry demonstrated with intrinsic signal optical imaging in vitro. Neuroscience. 2000;95:51–62. doi: 10.1016/s0306-4522(99)00385-1. [DOI] [PubMed] [Google Scholar]
- Kohn A, Whitsel BL. Sensory cortical dynamics. Behav Brain Res. 2002;135:119–126. doi: 10.1016/s0166-4328(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Laskin SE, Spencer WA. Cutaneous masking. I. Psychophysical observations on interactions of multipoint stimuli in man. J Neurophysiol. 1979;42:1048–1060. doi: 10.1152/jn.1979.42.4.1048. [DOI] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, Rutter M. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles: Western Psychological Corporation; 2003. [Google Scholar]
- Lord C, Rutter M, Dilavore P, Risi S. The Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Corporation; 1999. [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- O'Mara S, Rowe MJ, Tarvin RP. Neural mechanisms in vibrotactile adaptation. J Neurophysiol. 1988;59:607–622. doi: 10.1152/jn.1988.59.2.607. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Lane SJ, Sugiyama T, de Traversay J. Startle modulation studies in autism. J Autism Dev Disord. 1993;23:619–637. doi: 10.1007/BF01046105. [DOI] [PubMed] [Google Scholar]
- Pack CC, Born RT. Temporal dynamics of a neural solution to the aperture problem in visual area MT of macaque brain. Nature. 2001;409:1040–1042. doi: 10.1038/35059085. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pettet MW, Gilbert CD. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992;89:8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Hawken MJ, Shapley R. Dynamics of orientation tuning in macaque primary visual cortex. Nature. 1997;387:281–284. doi: 10.1038/387281a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Cuccaro ML, Hauser ER, Raiford KL, Menold MM, Wolpert CM, Ravan SA, Elston L, Decena K, Donnelly SL, Abramson RK, Wright HH, DeLong GR, Gilbert JR, Pericak-Vance MA. Fine mapping of autistic disorder to chromosome 15q11-q13 by use of phenotypic subtypes. Am J Hum Genet. 2003;72:539–548. doi: 10.1086/367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelev IA, Eysel UT, Lazareva NA, Sharaev GA. The contribution of intracortical inhibition to dynamics of orientation tuning in cat striate cortex neurons. Neuroscience. 1998;84:11–23. doi: 10.1016/s0306-4522(97)00363-1. [DOI] [PubMed] [Google Scholar]
- Shevelev IA, Volgushev MA, Sharaev GA. Dynamics of responses of V1 neurons evoked by stimulation of different zones of receptive field. Neuroscience. 1992;51:445–450. doi: 10.1016/0306-4522(92)90328-y. [DOI] [PubMed] [Google Scholar]
- Sugase Y, Yamane S, Ueno S, Kawano K. Global and fine information coded by single neurons in the temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- Tannan V, Dennis R, Tommerdahl M. A novel device for delivering two-site vibrotactile stimuli to the skin. J Neurosci Methods. 2005a;147:75–81. doi: 10.1016/j.jneumeth.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tannan V, Dennis RG, Tommerdahl M. Stimulus-dependent effects on tactile spatial acuity. Behav Brain Funct. 2005b;1:18. doi: 10.1186/1744-9081-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Dennis RG, Zhang Z, Tommerdahl M. A portable tactile sensory diagnostic device. J Neurosci Methods. 2007a;164:131–138. doi: 10.1016/j.jneumeth.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Simons S, Dennis RG, Tommerdahl M. Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res. 2007b doi: 10.1016/j.brainres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Whitsel BL, Tommerdahl MA. Vibrotactile adaptation enhances spatial localization. Brain Res. 2006;1102:109–116. doi: 10.1016/j.brainres.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Favorov O, Whitsel BL, Nakhle B, Gonchar YA. Minicolumnar activation patterns in cat and monkey SI cortex. Cereb Cortex. 1993;3:399–411. doi: 10.1093/cercor/3.5.399. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Favorov OV, Whitsel BL. Effects of high-frequency skin stimulation on SI cortex: mechanisms and functional implications. Somatosens Mot Res. 2005a;22:151–169. doi: 10.1080/08990220500084461. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Hester KD, Felix ER, Hollins M, Favorov OV, Quibrera PM, Whitsel BL. Human vibrotactile frequency discriminative capacity after adaptation to 25 Hz or 200 Hz stimulation. Brain Res. 2005b;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel B. Response of SI cortex to ipsilateral, contralateral and bilateral flutter stimulation in the cat. BMC Neurosci. 2005c;6:29. doi: 10.1186/1471-2202-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res. 2007a;1154:116–123. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Zachek M, Holden JK, Favorov OV. Effects of stimulus-driven synchronization on spatial localization. Behav Brain Res. 2007b doi: 10.1186/1744-9081-3-61. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrillo RT. Psychophysics of vibrotactile stimulation. J Acoust Soc Am. 1985;77:225–232. doi: 10.1121/1.392263. [DOI] [PubMed] [Google Scholar]
- Verrillo RT, Gescheider GA. Effect of prior stimulation on vibrotactile thresholds. Sens Processes. 1977;1:292–300. [PubMed] [Google Scholar]
- Wechsler D. WASI Manual. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Whitsel BL, Kelly EF, Delemos KA, Xu M, Quibrera PM. Stability of rapidly adapting afferent entrainment vs responsivity. Somatosens Mot Res. 2000;17:13–31. doi: 10.1080/08990220070265. [DOI] [PubMed] [Google Scholar]