Abstract
OBJECTIVE:
Nonadherence to antiretroviral therapy among children/youth with HIV often is associated with disease progression. This study examined the agreement between child and caregiver perceptions of barriers to adherence and factors associated with these barriers.
METHODS:
Children/youth with perinatally acquired HIV and their parents/caregivers (n = 120 dyads) completed a questionnaire about 19 potential barriers to adherence to the child’s antiretroviral therapy regimen. Agreement between the 2 reports was measured via the kappa statistic. Factors associated with the barriers were assessed by using multiple logistic regression.
RESULTS:
Of the 120 children, 55% were African American, 54% were boys, and the average age was 12.8 years. The most frequently reported barrier by either the caregiver or youth was “forgot.” There were varying degrees of agreement between child and caregiver on the following barriers: “forgot,” “taste,” “child was away from home,” “child refused,” and “child felt good.” Children who knew their HIV status were more likely to report logistical barriers, such as scheduling issues. Children with a biological parent as their caregiver were more likely to report regimen or fear of disclosure as a barrier.
CONCLUSIONS:
Lack of agreement was observed for more than half of the studied barriers, indicating discrepancies between children’s and caregivers’ perceptions of factors that influence medication-taking. The findings suggest a need for interventions that involve both child and caregiver in the tasks of remembering when to administer the child’s medications, sustaining adherence, and appropriately transitioning medication responsibility to the youth.
KEY WORDS: children, HIV, adherence, adolescents, pediatric
What’s Known on This Subject:
Nonadherence to antiretroviral therapy among children and youth with HIV is a frequent problem that can result in treatment failure and disease progression for this population. Children and adolescents face different barriers to adherence than adults infected with HIV.
What This Study Adds:
Few studies have examined specific barriers to adherence as reported by children with perinatally acquired HIV and their caregivers. This report examines the agreement between child and caregiver perceptions of adherence barriers and the factors associated with these barriers.
Antiretroviral therapy (ART), specifically highly active antiretroviral therapy (HAART), has allowed children and youth with HIV to prolong survival well into adulthood1; however, nonadherence to ART is associated with disease progression and development of resistant strains that are impervious to available ART medications.2 At least 95% adherence is necessary for the best therapeutic outcomes in adults,3 which is significantly higher than adherence rates required for effective management of many other chronic medical conditions. ART regimens often are complicated, involving special dosing instructions and toxicities.4 Youth with HIV face different barriers to adherence than adults, because of factors such as the role of the caregiver, child’s age and knowledge of HIV status, and the transition through adolescence to adulthood. Researching for Excellence in Adolescent Care and Health, an observational study that included adolescents infected with HIV via high-risk behavior, reported only 28.3% were consistently adherent to their HAART regimen, whereas clinical trials of ART therapies in children and adolescents reported adherence as high as 70%.5,6 Nevertheless, adherence in youth is far below the levels recommended to sustain virologic control. Therefore, health care providers need to appreciate the barriers to ART adherence in children/youth with perinatally acquired HIV.
Few studies have examined specific barriers to adherence reported by children with perinatally acquired HIV and their caregivers. In an adult study, the most common reasons for nonadherence included not wanting to be reminded of HIV, not wanting others to notice illness, and not remembering to ask health care providers questions about the regimen.7 The Researching for Excellence in Adolescent Care and Health study found that the major barriers to adherence among adolescents with HIV were medication-related adverse effects and complications in day-to-day routines.5
Data from the Pediatric AIDS Clinical Trials Group (PACTG) P1042S, a multisite observational study, indicated that child or caregiver endorsement of even 1 barrier to adherence was associated with higher child viral load.8 After adjusting for child age at study entry, primary caregiver type, and child’s knowledge of his or her HIV status, caregiver report of no barriers was significantly associated with lower viral load. Significant child-caregiver inter-rater agreement was observed in reporting at least 1 barrier to adherence, contrary to a prior report of low child-caregiver agreement on medication adherence, particularly when youth were older and assumed more responsibility for their medications.9 Another study found child-caregiver agreement about medication responsibility predicted better adherence.10
Younger children typically assume less responsibility than older children for medication-taking and may not know their HIV diagnosis.11 For many reasons, including disclosing the biological mother’s HIV status, stigma, emotional impact, and/or discrimination, caregivers may wait until early adolescence to disclose HIV status to the child, which may adversely affect adherence to ART.11 As children enter adolescence, they often assume increasing responsibility for medication administration, with the primary caregiver’s role reduced.12 Caregiver report of greater youth responsibility has been associated with poorer adherence, suggesting that transition of responsibility is not always successful and reflecting the need to better understand and facilitate this process.13 Knowledge about barriers to adherence to antiretroviral (ARV) medications, as independently reported by children and caregivers, may help clarify factors contributing to difficulty transitioning responsibility and inform potential interventions to improve ART adherence. This article examined agreement between child and caregiver perceptions of individual adherence barriers and associations of these barriers with ART regimen, medication responsibility, knowledge of HIV status, type of caregiver, and child age.
Methods
PACTG P1042S is a longitudinal substudy of PACTG P219C, a multicenter cohort study that followed HIV-infected and uninfected, perinatally HIV-exposed children in the United States from September 2000 to May 2007.14 For P1042S, 159 perinatally infected children and adolescents (ages 8 to <19 years old) were randomly selected from P219C participants who were prescribed ART, were primarily English or Spanish speaking, and had completed at least 1 P219C neurocognitive assessment. The main purpose of P1042S was to examine cognitive and behavioral correlates of adherence to ART, assessed by pill count and child/adolescent and parent/caregiver self-report measures of adherence. Data collected at entry included barriers to medication adherence (child and caregiver reports), demographic data, and relevant child health status markers gathered within P219C. Centers for Disease Control and Prevention clinical classification was determined by the presence of any severely symptomatic diagnoses in children <13 years of age, and the revised surveillance case definition of AIDS-defining conditions for adolescents ≥13 years of age.15,16
P1042S Child/Adolescent Questionnaire and Parent/Caregiver Questionnaire
Reported barriers were obtained from the P1042S Child/Adolescent and Parent/Caregiver Questionnaires (Table 1). Questionnaires were completed independently by children and caregivers, and collected data on medication adherence, barriers to adherence, and responsibility for health care tasks. Interviewer-assisted administration occurred only when participant(s) had difficulty completing the questionnaire independently. HIV was mentioned only in the parent/caregiver questionnaire, which allowed children who did not know their HIV status to participate. Both questionnaires contained items about 19 different barriers to adherence to the child’s ART medication regimen asking the respondent how often each barrier occurred in the preceding month. Barriers were classified as logistical, regimen, child, disclosure, and emotional (Table 1).
TABLE 1.
Questions on the Child/Adolescent and the Parent/Caregiver Questionnaire
| People may miss their medications for various reasons. In the past month, how often have you/your child missed taking medication because of the following reasons? Circle 1 number for each question. Response options are: Never or Rarely, Sometimes, Often, Mostly or Always | |||
|---|---|---|---|
| Type | Barrier | Question to Child | Question to Caregiver |
| Logistical | Child could not get meds | Couldn’t get medication (drugstore doesn’t have supply) | Couldn’t get medication (drugstore doesn’t have supply) |
| Did not refill | Didn’t refill; ran out | Didn’t refill; ran out | |
| Forgot | Forgot | Forgot | |
| Schedule interfered | Scheduling interferes with lifestyle (meals, school, sleep) | Scheduling interferes with child’s lifestyle (meals, school, sleep) | |
| Multiple caregivers | Multiple caregivers | Multiple caregivers | |
| Child away | Were away from home | Child was away from home | |
| Busy with other things | Were busy with other things | We were busy with other things | |
| Change in daily routine | Had a change in daily routine | We had a change in daily routine | |
| Slept through dose time | Fell asleep or slept through dose time | Caregiver fell asleep or slept through dose time | |
| Regimen | Cannot keep down | Taste, can’t get it down, or keep it down (pill or liquid) | Taste, can’t get it down, or keep it down (pill or liquid) |
| Too much medication | Had too many pills to take | Child had too much medication to take | |
| Avoid side effects | Wanted to avoid side effects | We wanted to avoid side effects | |
| Toxicity | Felt like medication was toxic or harmful | Caregiver felt like medication was toxic or harmful | |
| Problems taking as directed | Had problem taking pills as directed, for example, with meals or on an empty stomach | Child had problem taking pills as directed, for example, with meals or on an empty stomach | |
| Child | Child refused | Just didn’t want to | Child refuses |
| Child felt sick | Felt sick or ill | Child felt sick or ill | |
| Child felt good | Felt good | Child felt good | |
| Disclosure | Child concerned that others notice medications | Did not want others to notice medication | Child did not want others to notice medication |
| Emotional | Felt depressed | Felt depressed or overwhelmed | Caregiver felt depressed or overwhelmed |
The questionnaires also included a 10-item, study-specific adaptation of the Diabetes Family Responsibility Questionnaire17 to determine who assumed primary responsibility for health care tasks, such as remembering when to take medicine and refilling prescriptions when needed. Degree of medication responsibility was obtained by averaging the coded responses over the 4 medication-related task items.13 Caregiver-reported medication responsibility was used for the analyses reported herein because of previous significant correlation with adherence.13
Statistical Methods
Responses were dichotomized defining a barrier as having occurred in the past month if the respondent endorsed “sometimes,” “often,” or “mostly or always”; it was defined as not present when described as “never or rarely.” Frequency of reported barriers was compared across age, medication responsibility, and knowledge of HIV status groups by using a Fisher’s exact test. Consistency of caregiver and child responses to the barriers within each barrier type was evaluated by using Cronbach’s α. Inter-rater agreement (between child and caregiver) was measured via the κ statistic and assessed separately for age, medication responsibility, and knowledge of HIV status groups. Because of item differences in the respective child and caregiver questionnaires, agreement on “slept through dose time” and “felt depressed” was not assessed. Differences between child and caregiver in reported barrier frequencies were assessed with McNemar’s test.
Multiple logistic regression was used to assess the associations of reported barriers with knowledge of HIV status, medication responsibility (primary caregiver not fully responsible versus primary caregiver fully responsible), type of caregiver (biological parent versus other), child’s age (<12 vs ≥12 years), and ARV regimen (HAART with protease inhibitor [PI], HAART without PI, or non-HAART ARV). (Exact logistic regression was used for the logistical barriers due to small cell counts.) The child-reported barriers and the caregiver-reported barriers were modeled separately. All statistical tests were 2-sided, done at the .05 significance level, and were conducted by using SAS version 9 (SAS Institute, Cary, NC).
Results
Participant Characteristics
For P1042S, 159 perinatally infected children and youth were randomly selected from potentially eligible P219C participants and agreed to participate. Among those enrolled, 151 were considered evaluable. Eight were considered nonevaluable because of an eligibility disqualification after enrollment (n = 4), withdrawal of consent before study completion (n = 2), missed clinic/study visits (n = 1), and a randomization error (n = 1; consent obtained for P219C data extraction but not for P1042S data collection). Eligibility disqualifications consisted of being outside the window for required 219C NP testing, not being on ART, and discontinuation from 219C study. Of the 151, 120 child/caregiver dyads had complete barriers to adherence data. Child participants were primarily African American (55%) or Hispanic (28%), 54% were boys, and the mean age at enrollment was 12.8 years (SD = 2.39, range 8 to 18 years, median = 12.35, interquartile range = 3.63). Forty-eight children (40%) were <12 years old and the remaining were 12 to 18 years old. At study entry, 34% had previous Centers for Disease Control and Prevention Class C classification; mean CD4% was 29.2% (range 2% to 54%); mean viral load was 2.9 log10 copies/mL (range 1.1 to 5.9 log10copies/mL); and 40% had detectable viral load (>400 copies/mL). Most youth had knowledge of their HIV status (76%) and spoke English as their primary language (87%). Twenty-six percent of youth had repeated a grade in school, and 40% required special assistance or special education classes.
At baseline, most youth were on HAART (93%). The distribution of ART regimens was as follows: nucleoside reverse transcriptase inhibitor (NRTI) and PI regimens (56%); non-nucleoside reverse transcriptase inhibitor (NNRTI), NRTI, and a PI (19%); NNRTIs and NRTIs (17%); and different regimens (8%). Most were on a PI-containing regimen (77%) and were taking medications twice daily (94%).
Of the 120 dyads, 66 children (55%) and 73 caregivers (61%) reported the child had missed a dose in the preceding month. Of children with available pill count data (n = 96), 49% were considered nonadherent (consumed <90% of prescribed medications).
Fewer than half (42%) of the youth had a biological parent as their primary caregiver. Two-thirds of primary caregivers (68%) had completed a high school education or higher. For youth whose caregiver rated their degree of medication responsibility (n = 119), 62 (52%) had their caregiver fully responsible for medications, 43 (36%) shared responsibility with their parent/caregiver, and 14 (12%) were fully responsible for their medications.
Barriers to Adherence
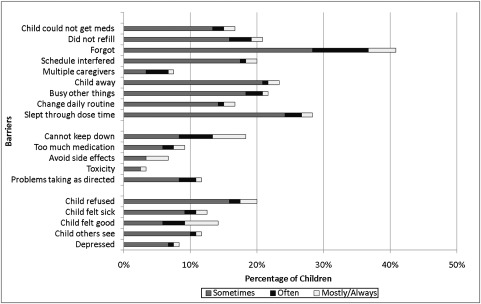
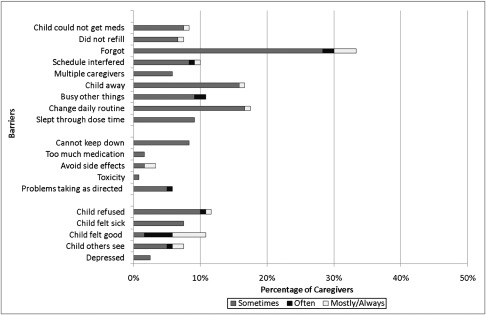
Figures 1 and 2 illustrate the distribution of barriers to adherence reported by either the child/adolescent (Fig 1) or parent/caregiver (Fig 2), respectively. Logistical issues were the most common barrier type cited by both children (62%) and caregivers (47%). Children more frequently cited regimen issues (29%) and child-related issues (33%), compared with caregivers who cited regimen issues (13%) and child-related issues (25%) less often. The most commonly cited logistical issue was “child forgot to take medications.” Other frequent child/adolescent-reported barriers were logistical in nature and included “slept through dose time” (28%), “child away” (23%), “busy with other things” (22%), and “did not refill” (21%). Additional frequent parent/caregiver-reported barriers were either logistical or child-related, including “change in daily routine” (18%), “child away” (17%), “child refused” (12%), “child felt good” (11%), and “busy with other things” (11%).
FIGURE 1.
Child-reported barriers to adherence (n = 120).
FIGURE 2.
Caregiver-reported barriers to adherence (n = 120).
Both children and caregivers were consistent in their responses to different logistical barriers (Cronbach’s α: 0.84 and 0.74, respectively). Child responses to different regimen barriers were also consistent (Cronbach’s α = 0.80); however, child and caregiver responses within other types of barriers were not consistent.
Agreement Between Child and Caregiver Report
Children more often than caregivers reported having at least 1 barrier to adherence (P = .008). There was little to no agreement between children and caregivers for more than half (63%) of the 19 barriers (κ < 0.2 in Table 2). There was significant agreement between child and caregiver report of the following barriers: “forgot” (κ = 0.41, P < .001), “taste/cannot get it down” (κ = 0.44, P < .001), “child was away from home” (κ = 0.38, P < .001), “child refused” (κ = 0.26, P = .01), and “child felt good” (κ = 0.24, P = .02) (Table 2). There was significant but weak agreement for the barrier “child/caregiver was busy with other things” (κ = 0.19, P = .03). Children and caregivers tended to agree about the type of barrier, but did not agree on the specific barriers within a type.
TABLE 2.
Type of Barriers and Inter-rater Agreement on Barriers to Medication Adherencea
| Type | Barrier | κ | 95% CI for κ | Exact P Value |
|---|---|---|---|---|
| Logistical issues | Any logistical issue | 0.44 | (0.29, 0.59) | <.001 |
| Child could not get meds | −0.05 | (–0.19, 0.09) | .70 | |
| Did not refill | 0.14 | (−0.05, 0.33) | .09 | |
| Forgot | 0.41 | (0.25, 0.58) | <.001 | |
| Schedule interfered | 0.10 | (−0.09, 0.30) | .26 | |
| Multiple caregivers | 0.06 | (−0.17, 0.29) | >.99 | |
| Child away | 0.38 | (0.18, 0.58) | <.001 | |
| Busy with other things | 0.19 | (−0.01, 0.39) | .03 | |
| Change in daily routine | 0.15 | (−0.06, 0.35) | .19 | |
| Regimen issues | Any regimen issue | 0.35 | (0.17, 0.53) | <.001 |
| Cannot keep down | 0.44 | (0.21, 0.66) | <.001 | |
| Too much medication | −0.03 | (−0.06, 0.01) | >.99 | |
| Avoid side effects | −0.05 | (−0.08, −0.01) | >.99 | |
| Toxicity | −0.01 | (−0.04, 0.01) | >.99 | |
| Problems taking as directed | 0.12 | (−0.11, 0.36) | .19 | |
| Child Issues | Any child issue | 0.24 | (0.06, 0.42) | .01 |
| Child refused | 0.26 | (0.05, 0.47) | .01 | |
| Child felt sick | 0.08 | (−0.13, 0.29) | .60 | |
| Child felt good | 0.24 | (0.00, 0.48) | .02 | |
| Disclosure issues | Child concerned that others notice medications | 0.19 | (−0.06, 0.44) | .07 |
CI, confidence interval.
The barriers, “depression” and “slept through dose time,” were not included in this table because the questions were different for child and caregiver.
When the sample was stratified by age, medication responsibility, or knowledge of HIV status, agreement results for some strata differed from those for the entire sample. For younger children (ages 8 to 12 years), there was significant and moderate agreement on the barrier “busy with other things” (κ = 0.33, P = .05) and no significant agreement on the barrier “child felt good”. For older children (ages 12 to 18 years), we did not find significant agreement on the barriers “cannot keep down”, “child refused” and “busy with other things”. Among dyads where the caregiver was not fully responsible for medication, there was significant agreement on the barrier “did not refill” (κ = 0.46, P = .003) but there was no significant agreement found on the barriers “cannot keep down,” “child refused,” “child felt good” and “busy with other things”. Among dyads where the caregiver was fully responsible, we found significant agreement on the barriers “problems taking as directed” and “child concerned that others notice medications” (κ = 0.30, P = .02 and κ = 0.41, P = .008, respectively) but found no significant agreement on the barriers “child away,” “child felt good” and “busy with other things.” Children who did not know their HIV status had significant agreement on the barriers “child concerned that others notice medications” (κ = 1, P = .003) but had no significant agreement on the barriers “forgot,” “child refused” and “busy with other things.” Children who knew their HIV status had no significant agreement on the barrier “busy with other things.”
Correlates of Child-Reported Barriers to Adherence
Older children reported the barrier “forgot” more often than younger children (49% vs 29%, P = .04). Children who shared responsibility reported both “forgot” and “busy with other things” more often than children whose caregiver was fully responsible (58% vs 26%, P < .001 and 30% vs 15%, P = .05, respectively). Children who knew their HIV status reported the barrier “forgot” and “slept through dose time” more often than children who did not know their status (48% vs 18%, P = .004 and 35% vs 11%, P = .02).
After adjusting for child’s age, type of caregiver, and medication responsibility, children who knew their HIV status had 3 times the odds of reporting a logistical barrier (P = .01). Children with a biological parent as their primary caregiver had twice the odds of reporting a regimen barrier (P = .05), 8 times the odds of reporting disclosure as a barrier (P = .001), and 4 times the odds of reporting depression as a barrier (P = .05).
Correlates of Caregiver-Reported Barriers to Adherence
Caregivers who shared responsibility reported “forgot” more often than dyads where the caregiver was fully responsible (47% vs 21%, P = .003). Similarly, caregivers of children who knew their HIV status reported the barrier “forgot” more often than those who did not know their status (42% vs 11%, P = .003). In multiple logistic regression models, medication responsibility, knowledge of HIV status, child age, and type of caregiver were not significantly associated with any of the caregiver-reported barrier types. Similarly, type of ARV regimen was not significantly associated with caregiver report of a regimen barrier.
Discussion
Farley and colleagues8 found significant inter-rater agreement in reporting at least 1 barrier to adherence, by using data from the same cohort of children analyzed here. In this analysis, we looked more closely at barriers to adherence by examining inter-rater agreement with respect to each barrier and with respect to the different barrier types. If both child and caregiver reported an obstacle to taking medications, it is more likely that the barrier truly exists or that the child and caregiver share perceptions regarding the difficulties inherent in maintaining daily adherence to a complex medication regimen.
There are many barriers to HIV medication adherence, and children/adolescents and their caregivers do not perceive them consistently. Forgetting to take medications was the most commonly reported barrier to ART medication adherence (41% for children; 33% for caregivers). In a 2006 meta-analysis of 18 studies of adherence to HAART, which included both children and adults, simply forgetting to take medications was commonly reported as a barrier (37%).18 Health care providers should work with families to find the most palatable/tolerable regimen for the child (ie, formulary, taste, pill size, number of pills), develop appropriate dose-timing schedules (ie, linked to realistic daily activities and times of day), and incorporate medication reminders and social supports.
Both children and caregivers were consistent in their responses to questions about logistical barriers. Children reporting some logistical barriers actually may be dealing with a number of other significant home life issues. These children often face a multitude of life stressors, such as poverty, family instability, and transfer among multiple residences, which can impede their ART access and adherence.19 The child’s circumstances should be assessed in their entirety, and appropriate supports put in place, if possible.
Child refusal was a commonly cited barrier, as was the child’s report of having concerns about disclosure. These findings highlight the importance of integrated mental health services coordinated within HIV care settings. Incorporating these services with the child’s HIV care could facilitate a better understanding of the child’s mental health needs and perceptions of his or her HIV disease for both the caregiver and provider.
The lack of agreement observed for many barriers suggests caregivers and children have different perceptions of the factors that affect adherence. This finding highlights the utility of talking with both youth and caregivers, separately if needed, in routine clinical care. For example, when children are considered by their parents or caregivers to be ready to assume more responsibility for their own medication-taking, the child and caregiver might participate together in a training that not only addresses the importance of adherence, but also guides the development of the plan they will follow to achieve and sustain adherence. During the transition of responsibility, children and caregivers should meet regularly with their health care provider(s) to monitor adherence, discuss their approach to adherence, and modify the plan as needed. If the child/adolescent is not ready to take full responsibility, caregiver support may be continued and phased out gradually once the youth can take on this responsibility independently.
The lack of significant inter-rater agreement with respect to some barriers may be attributed to lack of statistical power owing to the low reported frequency of occurrence. We should be cautious in our interpretations about the lack of significant agreement with respect to some of these barriers.
This study is not without limitations. The child questionnaire did not mention HIV status or HIV medications, in particular. For this analysis, the barriers perceived were assumed to be with regard to ART medications, but it is possible child respondents referred to other medications. Because this is a secondary analysis, the results reported may be underpowered particularly within subgroups, and therefore, we may not be detecting all differences that may be of clinical value. A future, adequately powered analysis that also considers the preservation of the overall significance level is recommended for the hypotheses generated herein.
Conclusions
Although moderate inter-rater agreement was observed for several adherence barriers, lack of agreement was observed for more than half, suggesting significant discrepancies between children’s and caregiver’s perceptions of factors that influence medication-taking. Such discrepancies may result in inadequate adherence support for the child and/or difficulty transitioning medication responsibility from caregiver to child. Thus, the findings of this study indicate a need for interventions that involve both child and caregiver to provide support, sustain adherence, improve communication, and appropriately transition responsibility. These findings also suggest that providers should explicitly assess both the children’s and caregivers’ perceptions of barriers to adherence and encourage caregivers to discuss these barriers with their children and develop a collaborative plan together to address them.
Glossary
- ART
antiretroviral therapy
- ARV
antiretroviral
- HAART
highly active antiretroviral therapy
- NRTI
nucleoside reverse transcriptase inhibitor
- PACTG
Pediatric AIDS Clinical Trials Group
- PI
protease inhibitor
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr Garvie completed this work while on faculty at St Jude Children's Research Hospital; participation is continued as an independent consultant to the project.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health (AI068632). This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group and #1 U01 AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development International and Domestic Pediatric and Maternal HIV Clinical Trials Network (contract number N01-DK-9-001/HHSN267200800001C). Funded by the National Institutes of Health (NIH).
COMPANION PAPER: A companion to this article can be found on page e1324, online at www.pediatrics.org/cgi/doi/10.1542/peds.2012-0526.
References
- 1.Patel K, Hernán MA, Williams PL, et al. Pediatric AIDS Clinical Trials Group 219/219C Study Team . Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515 [DOI] [PubMed] [Google Scholar]
- 2.Ballif M, Ledergerber B, Battegay M, et al. Swiss HIV Cohort Study . Impact of previous virological treatment failures and adherence on the outcome of antiretroviral therapy in 2007. PLoS ONE. 2009;4(12):e8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–390 [DOI] [PubMed] [Google Scholar]
- 4.Brogly S, Williams P, Seage GR, III, Oleske JM, Van Dyke R, McIntosh K, PACTG 219C Team . Antiretroviral treatment in pediatric HIV infection in the United States: from clinical trials to clinical practice. JAMA. 2005;293(18):2213–2220 [DOI] [PubMed] [Google Scholar]
- 5.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR, Adolescent Medicine HIV/AIDS Research Network . Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–255 [DOI] [PubMed] [Google Scholar]
- 6.Van Dyke R, Lee S, Johnson G, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109(4). Available at: www.pediatrics.org/cgi/content/full/109/4/e61 [DOI] [PubMed]
- 7.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124–133 [PubMed] [Google Scholar]
- 8.Farley JJ, Montepiedra G, Storm D, et al. PACTG P1042S Team . Assessment of adherence to antiretroviral therapy in perinatally HIV-infected children and youth using self-report measures and pill count. J Dev Behav Pediatr. 2008;29(5):377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolezal C, Mellins C, Brackis-Cott E, Abrams EJ. The reliability of reports of medical adherence from children with HIV and their adult caregivers. J Pediatr Psychol. 2003;28(5):355–361 [DOI] [PubMed] [Google Scholar]
- 10.Martin S, Elliott-DeSorbo DK, Wolters PL, et al. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis J. 2007;26(1):61–67 [DOI] [PubMed] [Google Scholar]
- 11.Mellins CA, Brackis-Cott E, Richards A, Abrams E, Nicholas S. Patterns of HIV status disclosure to perinatally HIV-infected children and subsequent mental health outcomes. Clin Child Psychol Psychiatry. 2003;7:101–114 [Google Scholar]
- 12.Mellins CA, Brackis-Cott E, Dolezal C, Abrams EJ. The role of psychosocial and family factors in adherence to antiretroviral treatment in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23(11):1035–1041 [DOI] [PubMed] [Google Scholar]
- 13.Naar-King S, Montepiedra G, Nichols S, et al; PACTG 1042S Team. Allocation of family responsibility for illness management in pediatric HIV. J Pediatr Psychol. 2009;34(2):187–194 [DOI] [PMC free article] [PubMed]
- 14.Williams PL, Storm D, Montepiedra G, et al. PACTG 219C Team . Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118(6). Available at: www.pediatrics.org/cgi/content/full/118/6/e1745. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep. 1994;43(RR-12):1–10 [Google Scholar]
- 16.Centers for Disease Control and Prevention . 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19 [PubMed] [Google Scholar]
- 17.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Ped Psych. 1990;15:477–492 [DOI] [PubMed] [Google Scholar]
- 18.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havens J, Mellins C, Hunter J. Psychiatric aspects of HIV/AIDS in childhood and adolescence. In: Rutter M, Taylor E, eds. Child and Adolescent Psychiatry. 4th ed. Oxford, UK: Blackwell; 2002:828–841 [Google Scholar]