Abstract
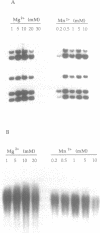
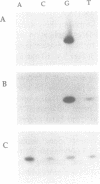
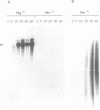
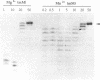
Bacteriophage PRD1 replicates its DNA by means of a protein-primed replication mechanism. Compared to Mg2+, the use of Mn2+ as the metal activator of the phage DNA polymerase results in a great stimulation of the initiation reaction. The molecular basis of the observed stimulatory effect is an increase in the velocity of the reaction. The phage DNA polymerase is also able to catalyze the formation of the initiation complex in the absence of DNA template. Although the presence of Mn2+ does not affect either the polymerization activity or the processivity of the DNA polymerase, this metal is unable to activate the overall replication of the phage genome. This can be explained by a deleterious effect of Mn2+ on the 3'-5'-exonucleolytic and/or the strand-displacement activity, the latter being an intrinsic function of the viral DNA polymerase required for protein-primed DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSMAN M. J., LEHMAN I. R., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. II. General properties of the reaction. J Biol Chem. 1958 Jul;233(1):171–177. [PubMed] [Google Scholar]
- Bamford D. H., Mindich L. Characterization of the DNA-protein complex at the termini of the bacteriophage PRD1 genome. J Virol. 1984 May;50(2):309–315. doi: 10.1128/jvi.50.2.309-315.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D. H., Rouhiainen L., Takkinen K., Söderlund H. Comparison of the lipid-containing bacteriophages PRD1, PR3, PR4, PR5 and L17. J Gen Virol. 1981 Dec;57(Pt 2):365–373. doi: 10.1099/0022-1317-57-2-365. [DOI] [PubMed] [Google Scholar]
- Bamford D., McGraw T., MacKenzie G., Mindich L. Identification of a protein bound to the termini of bacteriophage PRD1 DNA. J Virol. 1983 Aug;47(2):311–316. doi: 10.1128/jvi.47.2.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford J. K., Hänninen A. L., Pakula T. M., Ojala P. M., Kalkkinen N., Frilander M., Bamford D. H. Genome organization of membrane-containing bacteriophage PRD1. Virology. 1991 Aug;183(2):658–676. doi: 10.1016/0042-6822(91)90995-n. [DOI] [PubMed] [Google Scholar]
- Bernad A., Blanco L., Lázaro J. M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989 Oct 6;59(1):219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Blasco M. A., Salas M. A general structure for DNA-dependent DNA polymerases. Gene. 1991 Apr;100:27–38. doi: 10.1016/0378-1119(91)90346-d. [DOI] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Esteban J. A., Salas M. DNA-independent deoxynucleotidylation of the phi 29 terminal protein by the phi 29 DNA polymerase. J Biol Chem. 1992 Jan 15;267(2):1225–1230. [PubMed] [Google Scholar]
- Blanco L., Prieto I., Gutiérrez J., Bernad A., Lázaro J. M., Hermoso J. M., Salas M. Effect of NH4+ ions on phi 29 DNA-protein p3 replication: formation of a complex between the terminal protein and the DNA polymerase. J Virol. 1987 Dec;61(12):3983–3991. doi: 10.1128/jvi.61.12.3983-3991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J Biol Chem. 1979 Aug 10;254(15):6889–6893. [PubMed] [Google Scholar]
- Caldentey J., Bamford J. K., Bamford D. H. Structure and assembly of bacteriophage PRD1, and Escherichia coli virus with a membrane. J Struct Biol. 1990 Jul-Sep;104(1-3):44–51. doi: 10.1016/1047-8477(90)90056-i. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Joyce C. M., Beardsley G. P. Novel blunt-end addition reactions catalyzed by DNA polymerase I of Escherichia coli. J Mol Biol. 1987 Nov 5;198(1):123–127. doi: 10.1016/0022-2836(87)90462-1. [DOI] [PubMed] [Google Scholar]
- Clark J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988 Oct 25;16(20):9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J. A., Bernad A., Salas M., Blanco L. Metal activation of synthetic and degradative activities of phi 29 DNA polymerase, a model enzyme for protein-primed DNA replication. Biochemistry. 1992 Jan 21;31(2):350–359. doi: 10.1021/bi00117a006. [DOI] [PubMed] [Google Scholar]
- Garmendia C., Bernad A., Esteban J. A., Blanco L., Salas M. The bacteriophage phi 29 DNA polymerase, a proofreading enzyme. J Biol Chem. 1992 Feb 5;267(4):2594–2599. [PubMed] [Google Scholar]
- Gutiérrez C., Sogo J. M., Salas M. Analysis of replicative intermediates produced during bacteriophage phi 29 DNA replication in vitro. J Mol Biol. 1991 Dec 20;222(4):983–994. doi: 10.1016/0022-2836(91)90589-x. [DOI] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- LEHMAN I. R., RICHARDSON C. C. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. IV. AN EXONUCLEASE ACTIVITY PRESENT IN PURIFIED PREPARATIONS OF DEOXYRIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:233–241. [PubMed] [Google Scholar]
- Leith I. R., Hay R. T., Russell W. C. Adenovirus subviral particles and cores can support limited DNA replication. J Gen Virol. 1989 Dec;70(Pt 12):3235–3248. doi: 10.1099/0022-1317-70-12-3235. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström K. H., Bamford D. H., Palva E. T., Lounatmaa K. Lipid-containing bacteriophage PR4: structure and life cycle. J Gen Virol. 1979 Jun;43(3):583–592. doi: 10.1099/0022-1317-43-3-583. [DOI] [PubMed] [Google Scholar]
- Lyra C., Savilahti H., Bamford D. H. High-frequency transfer of linear DNA containing 5'-covalently linked terminal proteins: electroporation of bacteriophage PRD1 genome into Escherichia coli. Mol Gen Genet. 1991 Aug;228(1-2):65–69. doi: 10.1007/BF00282449. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McGraw T., Yang H. L., Mindich L. Establishment of a physical and genetic map for bacteriophage PRD1. Mol Gen Genet. 1983;190(2):237–244. doi: 10.1007/BF00330646. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Siak J. S., Gray R. H. Characteristics of PRD1, a plasmid-dependent broad host range DNA bacteriophage. J Virol. 1974 Sep;14(3):689–699. doi: 10.1128/jvi.14.3.689-699.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula T. M., Caldentey J., Serrano M., Gutierrez C., Hermoso J. M., Salas M., Bamford D. H. Characterization of a DNA binding protein of bacteriophage PRD1 involved in DNA replication. Nucleic Acids Res. 1990 Nov 25;18(22):6553–6557. doi: 10.1093/nar/18.22.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Bamford D. H. Linear DNA replication: inverted terminal repeats of five closely related Escherichia coli bacteriophages. Gene. 1986;49(2):199–205. doi: 10.1016/0378-1119(86)90280-5. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Caldentey J., Bamford D. H. Bacteriophage PRD1 terminal protein: expression of gene VIII in Escherichia coli and purification of the functional P8 product. Gene. 1989 Dec 21;85(1):45–51. doi: 10.1016/0378-1119(89)90462-9. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Caldentey J., Lundström K., Syväoja J. E., Bamford D. H. Overexpression, purification, and characterization of Escherichia coli bacteriophage PRD1 DNA polymerase. In vitro synthesis of full-length PRD1 DNA with purified proteins. J Biol Chem. 1991 Oct 5;266(28):18737–18744. [PubMed] [Google Scholar]
- Shiue S. Y., Hsieh J. C., Ito J. Mapping of the DNA linking tyrosine residue of the PRD1 terminal protein. Nucleic Acids Res. 1991 Jul 25;19(14):3805–3810. doi: 10.1093/nar/19.14.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Metal activation of DNA synthesis. Biochem Biophys Res Commun. 1976 Jun 7;70(3):812–817. doi: 10.1016/0006-291x(76)90664-1. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A. The properties and host range of male-specific bacteriophages of Pseudomonas aeruginosa. J Gen Microbiol. 1974 Oct;84(2):332–342. doi: 10.1099/00221287-84-2-332. [DOI] [PubMed] [Google Scholar]
- Wong F. H., Bryan L. E. Characteristics of PR5, a lipid-containing plasmid-dependent phage. Can J Microbiol. 1978 Jul;24(7):875–882. doi: 10.1139/m78-145. [DOI] [PubMed] [Google Scholar]