Abstract
AIM
To study the inhibitory effect of intravitreal captopril on oxygen-induced retinopathy (OIR) in mice.
METHODS
Eighty postnatal day (P)7 C57BL/6J mice were randomly divided into treated group and control group with forty mice in each group. The mice were exposed to 75% ± 2% oxygen for 5 days (P7-P11) and then returned to room air for 5 days (P12-P17) to induce retinal neovascularization (RNV). Beginning on P12, the mice in treated group received daily intravitreal injections of captopril (3.0mL/kg), while those in control group received daily intravitreal injections of phosphate-buffered saline (PBS) (3.0mL/kg) through P17. After anesthetized at P17, one eye was chosen randomly as experimental eye and were enucleated. RNV was examined by Adenosine diphosphate-ase (ADPase) stained retina flat-mounts and was quantitated histologically by counting the neovascular endothelial cell nuclei anterior to inner limiting membrane (ILM). The expressions of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) were measured by immunohistochemical method.
RESULTS
Comparing with control group, more regular distributions, better branch and reduced density of RNV were observed in eyes of treated group. The number of neovascular cell nuclei was less in treated group than that in control group (t=6.135, P<0.01). Stain of MMP-2 and VEGF was weaker in treated group than that in control group.
CONCLUSION
The results indicate that captopril can significantly inhibit RNV in OIR mice.
Keywords: retinal neovascularization, oxygen-induced retinopathy, captopril, intravitreal injection
INTRODUCTION
OIR is a leading cause of blindness among infants in developed and middle-income countries[1]. Although laser photocoagulation or cryotherapy of the retina reduces the incidence of blindness by 25%, the visual outcomes after treatment often are poor[2],[3]. Preventive therapy for OIR is sorely needed.
Much attention has bee directed toward elucidating the molecular mechanisms of OIR. Endothelial cells respond to locally produced angiogenic factors and upregulate the expression of extracellular proteinases. The current research focus, captopril, is the angiotensin-converting enzyme inhibitor that can restrain MMP-2. The research shows that in addition to lowering blood pressure, captopril can also produce anti-tumor blood vessels and corneal neovascularization, so it becomes a new drug candidate to cure vascular proliferative disease[4].
Although several investigators have described a possible role of MMP-2 in retinal neovascularization[5],[6], there has been no conclusive evidence regarding a critical role of captopril in retinal neovascularization. In this study, we sought to determine the effect of captopril on OIR by intravitreal injection of captopril.
MATERIALS AND METHODS
Oxygen Induced Retinopathy in Mice
OIR was induced in newborn mice according to the protocol of Smith et al[7]. At postnatal day (P) 7, mice were placed along with their dam into a custom-built chamber. They were maintained in 75%± 2% oxygen (hyperoxia) up to 5 days (P11), after which they were transferred back to cages in room air (normoxia). Room temperature was maintained at 68°F, and the rooms were illuminated with standard fluorescent lighting on a 12-hour light-dark cycle. Newborn mice were nursed by the dam and given food (standard mouse chow) and water ad libitum. The mice in treated group received daily intravitreal injections of captopril (3.0mL/kg) on P12 through P17, while those in control group were injected with PBS (3.0mL/kg) by intravitreal for 5 days. At P17, the pups were anesthetized.
Observation of RNV
At P17, twenty mice of each group were deeply anesthetized by intraperitoneal (IP) injections into the cardiac left ventricle of ketamine (80mg/kg) and xylazine (15mg/kg), one eye of them was chosen randomly as experimental eye. They were removed and fixed for 1 hour in 10% phosphate-buffered formalin. The cornea and lens were removed, and the entire retina was then carefully dissected from the eye cup, radially cut from the edge of the retina to the equator in all four quadrants, and retinas were processed for magnesium activated adenosine diphosphate (ADPase) staining. ADPase stained retinas were flatmounted on microscope slides with a gelatincoated cover slip. Flatmounts were carefully examined under microscope.
Histologic Quantification of RNV
At P17, twenty mice of each group were killed by IP injections with an overdose of pentobarbital sodium. One eye of them were enucleated, fixed with 4% paraformaldehyde, embedded in paraffin, and prepared for light microscopy. Serial 6μm sections from all eyes were cut sagittally parallel to the optic nerve head. The sections were stained with Hematoxylin Eosin (HE) method. To determine the extent of RNV, we counted all retinal neovascular cell nuclei anterior to the ILM under microscope, along equal lengths of each step section, with a masked protocol. Averaging of the 10 counted sections yielded a mean number of neovascular cell nuclei per section per eye. Ten percent of the eyes exhibited retinal detachment or endophthalmitis and were excluded from the evaluation.
Immunohistochemical Analysis of the Angiogenic Factors
Five unstained 6μm slices were selected randomly from two groups respectively and were performed immunohistochemical analysis of the angiogenesis-related factors MMP-2 and VEGF. The primary antibody and the secondary antibody were provided by Wuhan Boster Biotechnology Company, and the working concentration of antibody was 1:100. The primary antibody was replaced by PBS to make negative control, with diaminobenzidine (DAB) chromogenic. The MMP-2 and VEGF expressed positive cells were cytoplasm or canary and tan particles in nucleus. Select 5 incontinuous high-power field (400 times) randomly from each slice, use MetaMorph/ Evolution MP5.0/BX51 to do grayscale scanning, determine the positive cells integral optical density (IOD) of MMP-2 and VEGF, and use their average as the indicator.
Statistical Analysis
Data were expressed as the mean±SD. Software used was SPSS 13.0. The significance of differences was evaluated by the paired t-test (2 tailed). All P<0.01 were considered statistically significant.
RESULTS
Qualitative assessment of RNV
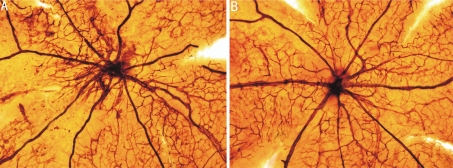
The retina vessels of control group have an obvious dilation pattern extending from the optic nerve and larger regions of nonperfusion. Neovascular tufts extend from the surface of the retina at the junction between the perfused and nonperfused retina (Figure 1A). In contrast, retinas of treated group demonstrated markedly reduced neovascular tissue, despite the presence of comparable peripapillary regions of nonperfusion (Figure 1B).
Figure 1. Observation of RNV by ADP enzyme stained retina flat-mounts (×100).
A: Retina from P17 control group. Neovascular tufts were visible at the junction between the perfused and the nonperfused junction of the retina; B: Retina from P17 treated group. Few neovascular tufts were apparent
Quantitative assessment of RNV
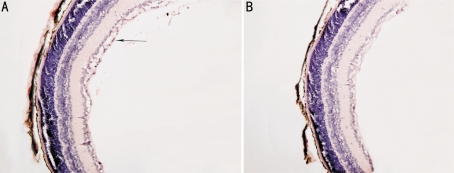
There were neovascular cell nuclei anterior to the ILM into vitreous of both control and treated groups. In control group, an average of (30.25±0.55) nuclei in the each slice (Figure 2A), significantly more than that (4.28±0.72) in treated group (Figure 2B) (t=6.135, P<0.01).
Figure 2. Quantification of RNV by HE staining method (×200).
A: Section of P17 retina from control group. Arrow denotes retinal neovascular cell nuclei anterior to the ILM; B: Section of P17 retina from treated group. A marked reduction in preretinal neovascularization was apparent compared with that in control group
Expression of MMP-2 and VEGF
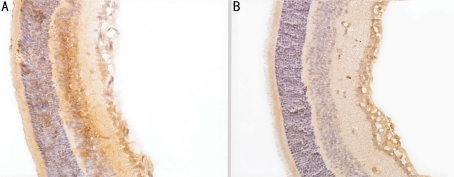
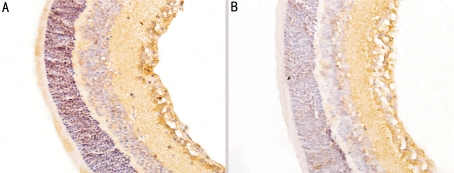
In control group, the expressions of MMP-2 and VEGF were strongly observed in ganglion cell layer, inner plexiform layer, inner nuclear layer and neovascularization breaking through the ILM (Figures 3A, 4A). In treated group, there were no expression of MMP-2 in per layer of the retinal and little expression only in intracytoplasm of some ganglion cell layer (Figure 3B); there were no expression of VEGF in inner nuclear layer and outer nuclear layer and little expression only in intracytoplasm of some ganglion cell layer and inner plexiform layer (Figure 4B). Compared with control group, the protein expressions of MMP-2 and VEGF of treated group were significantly decreased (t1=4.15, t2=5.23, P<0.01). In control group, IOD of MMP-2 and VEGF were (14.25±3.75) and (23.53±2.52), while in treated group, those were (3.56±1.15) and (10.54±2.61), respectively.
Figure 3. Expression of MMP-2 by immunohistochemical method (×400) A: High expression of MMP-2 in P17 control group; B: Low expression of MMP-2 in P17 treated group.
Figure 4. Expression of VEGF by immunohistochemical method (×400).
A: High expression of VEGF in P17 control group; B: Low expression of VEGF in P17 treated group
DISCUSSION
OIR is characterized by an abnormal proliferation of blood vessels in the developing retina and is a frequent complication of blinding retinal diseases. Although oxygen deprivation is an early stimulus for neovascularization and cell injury, the molecular signals for the pathologic development of RNV and neuronal cell death are not fully defined. RNV results from the production of angiogenic factors, cytokines, and cell adhesion molecules that promote angiogenesis through complex interactions that are not fully understood[8],[9].
Induction of angiogenic factors and extracellular matrix degradation stimulate angiogenesis and cell injury[10]. Matrix metalloproteinase (MMPs) is regarded as the necessary condition of the process of RNV, the precise mechanism by which MMPs influence angiogenesis is not clear. The present study demonstrated a critical role for MMP-2, but not for MMP-9, in the development of extraretinal neovascularization[11]. Several important experimental observations suggest the importance of MMP-2 among the various MMPs in the development of angiogenesis[12]. Fang et al[13] has reported that suppression of MMP-2 alone inhibits the transition from the prevascular to the vascular stage during tumor development in an experimental tumor model. Therefore, the application of antiangiogenic drugs, particularly inhibitors of MMP-2 is currently being investigated as potential therapies for ocular neovascularization.
Captopril that widely used in treating the high blood pressure inhibit Matrix metalloproteinase activity by free sulfhydryl forming a chelate with angiotensin-converting enzyme active site Zn2+[14]. Volpert OV's[15] research proved that captopril with specificity could directly act on the capillary endothelial cells to inhibit endothelial cell migration and proliferation, and this inhibition restrained not the effect of angiotensin conversion enzyme (ACE) but the effect of MMP-2 activity. In our study, we used this characteristic to observe the effect of captopril in RNV of OIR models. Also, some reports suggest that there is an interaction between VEGF expression and MMP expression[16]. Although there are some reports that MMPs regulate VEGF expression[17],[18], there are other reports of VEGF-induced upregulation of MMPs[19]-[21].
In this report, we demonstrated that intravitreal captopril could inhibit the expressions of MMP-2 and VEGF protein and RNV. However it didn't completely prevent the RNV. The reason was perhaps that captopril's local concentration was not high enough to completely prevent the RNV. The drug dose could be increased to make captopril's local concentration higher. Meanwhile, the result showed that though MMP-2 and VEGF protein expression of treated group were lower than those of control group, but they couldn't be inhibited completely. So the does used in the experiment was not enough to completely inhibit MMP-2 and VEGF expression.
In summary, experiments in OIR models suggest that MMP-2 may be of particular importance in the regulation of extraretinal neovascularization. Current therapies for OIR include laser photocoagulation or cryoablation that kill small areas of cells and slow the deterioration of vision, but both therapies result in limited vision loss due to the nature of the treatment[22],[23]. In our study, we used intravitreal injection of captopril to inhibit MMP-2 activity to inhibit RNV of OIR effectively. The new technology offers the hope of making current treatment more effective, and the possibility of providing primary therapy without attendant vision loss.
Footnotes
Foundation item: Natural Science Foundation of Liaoning Province, China (No.20052089); Science and Technology Project of Liaoning Province, China (No.2010225034)
REFERENCES
- 1.Sapieha P, Joyal JS, Rivera JC, Kermorvant-Duchemin E, Sennlaub F, Hardy P, Lachapelle P, Chemtob S. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120(9):3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SH, Tan JP, Nederberg F, Fukushima K, Colson J, Yang C, Nelson A, Yang YY, Hedrick JL. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31(31):8063–8071. doi: 10.1016/j.biomaterials.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol. 2009;24(1):82–86. doi: 10.1080/08820530902800322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Li C, Baciu PC. Expression of integrins and MMPs duringalkaline burn induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2002;43(4):955–962. [PubMed] [Google Scholar]
- 5.Majka S, McGuire P, Colombo S, Das A. The balance between proteinases and inhibitors in a murine model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2001;42(1):210–215. [PubMed] [Google Scholar]
- 6.Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced Retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(12):5370–5375. doi: 10.1167/iovs.03-0249. [DOI] [PubMed] [Google Scholar]
- 7.Smith LEH, Wesolowski E. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 8.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006;47(6):2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 9.Woo Taek Kim, Eok Soo Suh. Retinal Protective effects of resveratrol via modulation of nitric oxide synthass on oxygen-induced retinopathy. Korean J Ophthalmol. 2010;24(2):108–118. doi: 10.3341/kjo.2010.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siao CJ, Tsirka SE. Extracellular proteases and neuronal cell death. Cell Mol Biol (Noisy-le-grand) 2002;48(2):151–161. [PubMed] [Google Scholar]
- 11.Di Y, Chen XL. Expression and significance of MMP-2 and VEGF in retinal neovascularization. Yanke Yanjiu. 2009;2(12):1089–1093. [Google Scholar]
- 12.Barnett JM, Mccollum GW, Fowler JA, Duan JJ, Kay JD, Liu RQ, Bingaman DP, Penn JS. Pharmacologic and genetic manipulation of MMP-2 AND -9 affects retinal neovascularization in rodent models of OIR. Invest Ophthalmol Vis Sci. 2007;48(2):907–915. doi: 10.1167/iovs.06-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA. 2000;97(8):3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surace EM, Balaggan KS, Tessitore A, Mussolino C, Cotugno A. Inhibition of ocular neovascularization by hedgehog blockade. Mol Ther. 2006;13(3):573–579. doi: 10.1016/j.ymthe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Volpert OV, Ward WF, Lingen MW, Chesler L, SOLT db, Johnson MD, Molteni A, Polverini PJ, Bouck NP. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest. 1996;98(3):671–679. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009;116(9):1599–1603. doi: 10.1016/j.ophtha.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Deryungina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62(2):580–588. [PubMed] [Google Scholar]
- 18.Sonmez K, Drenser KA, Capone A, Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115(6):1065–1070. doi: 10.1016/j.ophtha.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells. Circ Res. 1998;83(8):832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 20.Xiang N, Zhao MJ, Li XY, Zheng HH, Li GG, Li B. Redundant mechanisms for vascular growth factors in retinopathy of prematurity in vitro. Ophthalmic Res. 2010;45(2):92–101. doi: 10.1159/000316134. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Ni WJ. Inhibition of GM6001 on retinal neovascularization in neonatal rats with retinopathy. Guoji Yanke Zazhi. 2008;8(2):268–271. [Google Scholar]
- 22.Kusaka S, Shima C, Wada K, Arahori H, Shimojyo H, Sato T, Fujikado T. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008;92(11):1450–1455. doi: 10.1136/bjo.2008.140657. [DOI] [PubMed] [Google Scholar]
- 23.Chen TC, Tsai TH, Shih YF, Yeh PT, Yang CH, Hu FC, Lin LL, Yang CM. Long-term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci. 2010;51(12):6140–6148. doi: 10.1167/iovs.10-5234. [DOI] [PubMed] [Google Scholar]