Abstract
Glutamate carboxypeptidase II (GCPII) is a membrane-bound binuclear zinc metallopeptidase with the highest expression levels found in the nervous and prostatic tissue. Throughout the nervous system, glia-bound GCPII is intimately involved in the neuron-neuron and neuron-glia signaling via the hydrolysis of N-acetylaspartylglutamate (NAAG), the most abundant mammalian peptidic neurotransmitter. The inhibition of the GCPII-controlled NAAG catabolism has been shown to attenuate neurotoxicity associated with enhanced glutamate transmission and GCPII-specific inhibitors demonstrate efficacy in multiple preclinical models including traumatic brain injury, stroke, neuropathic and inflammatory pain, amyotrophic lateral sclerosis, and schizophrenia. The second major area of pharmacological interventions targeting GCPII focuses on prostate carcinoma; GCPII expression levels are highly increased in androgen-independent and metastatic disease. Consequently, the enzyme serves as a potential target for imaging and therapy. This review offers a summary of GCPII structure, physiological functions in healthy tissues, and its association with various pathologies. The review also outlines the development of GCPII-specific small-molecule compounds and their use in preclinical and clinical settings.
Keywords: Metalloprotease, prostate-specific membrane antigen, glutamate excitotoxicity, prostate cancer, N-acetylaspartylglutamate
1. INTRODUCTION
Glutamate carboxypetidase II (GCPII; EC 3.4.17.21) also known as prostate specific membrane antigen (PSMA), N-acetylated-alpha-linked acidic dipeptidase (NAALADase), and folate hydrolase (FOLH1) is a zinc-dependent peptidase that is increasingly recognized as a target of therapeutic interventions in a variety of neurologic disorders as well as a marker for imaging of and therapy for prostate cancer (PCa). GCPII was identified by different laboratories in different tissues of different species more than 20 years ago; original designations reflected the belief that the identified protein was either a unique entity in a given organ (PSMA) or had a single defined role (NAALADase). Even though original designations still persist in the literature, the term glutamate carboxypeptidase II (GCPII) is now preferred and will be used throughout this review.
2. GCPII STRUCTURE
2.1. Primary Structure
Human GCPII conforms to the pattern typical for type II transmembrane proteins having a short N-terminal cytoplasmic tail (amino acids 1 – 18), a single membrane-spanning helix (amino acids 19 – 43) and a large extracellular part (amino acids 44 – 750).
The N-terminal GCPII tail interacts with several scaffold proteins including clathrin, clathrin adaptor protein 2, filamin A, and caveolin-1. These interactions govern/modulate GCPII endocytosis via different routes, including caveolae-dependent and clathrin-coated pit-dependent mechanisms [1–4]. In the case of clathrin-dependent trafficking, the MXXXL N-terminal motif is indispensable for GCPII internalization and recycling [4]. GCPII is internalized in a constitutive manner, yet the internalization rate is increased by the binding of GCPII-specific antibodies to the extracellular domain of the protein [5]. These findings are being exploited for the development of therapeutic approaches to target the delivery of toxins, drugs, and short-range isotopes to the interior of GCPII-expressing cells.
The bulk of the protein is oriented to the extracellular milieu, where it can act on its natural substrates (Fig. 1; see Chapter 3.3). The extracellular portion of GCPII homodimerizes and the dimerization is believed to be required for GCPII hydrolytic activity [6], even though the active site in each subunit is structurally independent [7]. GCPII is also heavily N- and O-glycosylated (glycans can account for up to 25% of the total molecular weight of the protein); there are ten N-glycosylation sites predicted within the primary sequence of human GCPII and the N-glycosylation is indispensable for GCPII enzymatic activity and stability [8–12]. Furthermore, glycosylation of the protein is implicated in apical sorting, proteolytic resistance and its association with lipid rafts [13,14].
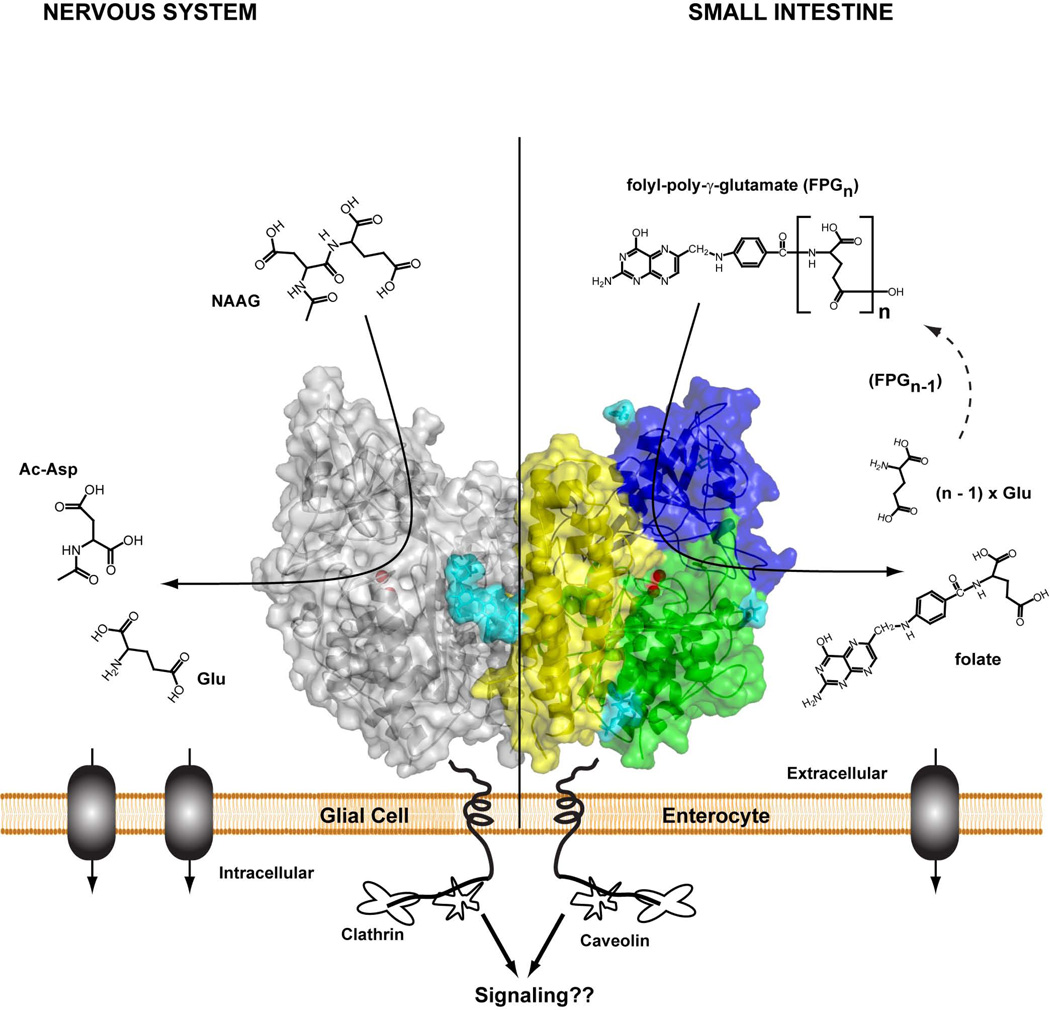
Fig. (1).
Homodimer of human GCPII (crystal structure) tethered to the biological membrane. One monomer shown in semitransparent surface representation with individual domains of the extracellular part colored green (protease domain; amino acids 57 – 116 and 352 – 590), blue (apical domain; amino acids 117 – 351), and yellow (C-terminal; amino acids 591 – 750); the second monomer is colored gray. N-linked sugar moieties are colored cyan, and the active-site Zn2+ ions are shown as red spheres. Left panel – residing at the plasma membrane of astrocytes /schwann cells, GCPII catabolizes NAAG, the most prevalent peptidic neurotransmitter in the mammalian nervous system. N-acetylaspartate and glutamate, the reaction products, are selectively transported into glial cells, metabolized and reused for NAAG synthesis in neurons. Right panel– GCPII (or folate hydrolase) at the plasma membrane of enterocytes in the proximal jejunum sequentially hydrolyzes the C-terminal γ-glutamate tail of dietary folates, finally leaving folate-monoglutamate, which can be then transported transcellularly into the blood stream.
2.2. Tertiary Structure
The 3-dimensional structure of the human GCPII ectodomain was solved by two groups independently [7, 15]. The overall fold closely resembles the structure of the transferrin receptor [16]. The extracellular part of GCPII consists of three distinct domains spanning amino acids 57–116 and 352–590 (the protease domain), 117–351 (the apical domain), and 591–750 (the C-terminal or dimerization domain). Synergetic action of all three domains is required for productive substrate binding and processing, as several residues from each domain contribute to the architecture of the GCPII substrate binding cavity and are involved in ligand recognition [7].
The GCPII substrate binding cavity is divided by the active site (featuring two zinc ions) into two “halves”, designated the S1’ pocket and the S1 pocket, respectively. The binuclear zinc active site, with the two zinc ions coordinated by the side chains of His377, Asp387, Glu425, Asp453, and His553, is indispensable for the GCPII hydrolytic activity [17,18]. It is also exploited for the design of high-affinity inhibitors as every high-affinity GCPII inhibitor includes a zinc-binding group in its structure. Amino acid residues shaping S1 and S1’ pockets dictate GCPII preferences towards physicochemical characteristics of cognate substrates and small-molecule inhibitors.
The S1’ pocket, also termed a pharmacophore pocket, is ‘optimized’ for binding of glutamate and glutamate-like moieties [19–21]. Not surprisingly then, both known natural GCPII substrates (NAAG and folyl-poly-γ-glutamates) feature glutamate as the C-terminal residue. Additionally, the majority of GCPII-specific inhibitors are derived from glutamate (or NAAG) scaffolds to take the advantage of S1’ pocket affinity towards glutamate. It should be noted, however, that although glutamate-like moieties are preferred in the S1’ pocket, the pocket has an amphipathic character, i.e. exploits both polar (hydrogen-bonding, ionic) and non-polar (hydrophobic, van der Waals) interactions to accommodate and stabilize binding of substrate or inhibitor [8,19,22]. Consequently, the glutamate-like functionality can be substituted by a more hydrophobic moiety if an inhibitor is to be used in settings, where decreased polarity is desired (such as brain-penetrating compounds) [21, 23].
The S1 pocket of GCPII is fairly specific for glutamate and aspartate side chains, both negatively charged amino acids. This specificity is conferred by the “arginine patch”, a conspicuous spatial arrangement of Arg534, Arg536 and Arg463 side chains [24]. Even though GCPII selectivity for negatively charged residues in the S1 pocket is virtually absolute in the case of substrates, a wide variety of corresponding distal parts (D-parts) of inhibitors is tolerated here. In contrast to a small (approximate dimensions 8 × 8 × 8 Å) and unyielding S1’ pocket [19], the “extended” S1 pocket can be described as a shape-shifting funnel with a narrow base (~8 Å) at the position of the active-site zinc ions, and the rim of the funnel (an approximate diameter of 20 Å) at a distance 20 Å from the base. Large dimensions of the S1 funnel thus allow for considerable variability of the D-moiety in the inhibitor; these characteristics have been extensively used in inhibitor design [25–31].
3. GCPII IN PHYSIOLOGY
3.1. GCPII Localization
In 1987, the world was simple and straightforward. A newly developed 7E11 antibody (see Chapter 4.2. for its current clinical use) recognizing the N-terminal part of GCPII localized GCPII expression solely to the prostatic tissue (consequently termed prostate specific membrane antigen – PSMA) [32]. Later, however, PSMA was shown to be identical to GCPII or NAALADase as it was termed then [33]. With the development of novel, more sensitive antibodies and more data available, the picture of GCPII expression pattern became more complex [34–40].
Today in healthy human tissue a consensus has been reached for the following sites of GCPII expression: prostate (secretory-acinar epithelium) [35,38–41], nervous system (astrocytes and schwann cells) [42,37], kidney (proximal tubules) [35,36,38,43,44], and small intestine (jejunal brush border membranes) [35,36,38–40]. However, several other studies have pointed towards a broader GCPII expression profile in humans, including expression in heart, pancreas, bladder, skin, breast, liver, lung, colon, testis, etc., although the expression levels in the latter tissues is thought to be much lower compared to “consensus sites” [34,36,39]. To complicate things further, in vitro data (immunoblots, immunohistochemistry) mentioned above do not offer the same picture as in vivo data using GCPII ligands (small molecules and/or labeled antibodies) for imaging studies. In in vivo studies using humans and/or laboratory animals, off-target binding is typically absent, suggesting restricted GCPII expression [45–49]. The underlying reason for these differences is not known at present, though it might be associated with the restricted accessibility of GCPII for small molecules/antibodies in vivo compared to in vitro studies or the epitopes/binding sites being masked by posttranslational modifications or putative interaction partners.
From all malignant tissues, the highest levels of GCPII are found in androgen resistant prostate cancer, with expression levels increasing with increasing cancer grade [41]. Not surprisingly, prostate cancer is the prime target for imaging/therapy by modalities aimed at GCPII (Chapter 4.2.). In addition to prostate cancer, GCPII expression has been noticed in malignancies from kidney, bladder, breast, colon, and schwann cells [34,35,50–52]. Highly promising for imaging/treatment of diverse solid cancers is the fact that GCPII is expressed in neovasculature of solid tumors, but absent from normal vasculature [43,51,53–55]. It remains to be seen whether this phenomenon will be exploited in the clinic.
Upregulated GCPII expression in aggressive tumors implies a role of GCPII in cancer progression. Consistent with this notion, several reports and patent applications [56,57] found strong correlation between GCPII hydrolytic activity and tumor cell growth/invasion. Yao et al reported that GCPII expression increases folate uptake and gives GCPII expressing cells a proliferative advantage [58,59]. Similarly, Conway et al found that the enzymatic activity of GCPII was required for endothelial cell invasion [60], while experiments with murine transgenic prostate tissue suggested that GCPII expression facilitates prostate carcinogenesis [61]. Contrary to these data, however, Ghosh et al found an inverse correlation between GCPII expression and prostate cancer cell invasiveness [62]. It remains to be rigorously tested in more experimental settings, whether or not the manipulation/ inhibition of GCPII enzymatic activity can be beneficial in terms of blocking the invasiveness of prostate cancer or tumor growth.
It should be noted that there are marked differences between humans and rodents that are used as experimental models of human diseases. For example, GCPII is absent from rodent prostatic tissue [36,63] and small intestine [36,64], even though these are major sites of GCPII expression in humans. Additionally, there are no reports of GCPII expression in neovasculature of solid tumors in rodents as compared to human subjects and there are marked differences in binding of small-molecule GCPII ligands to plasma proteins between these species (Pomper, unpublished). All of these differences have to be carefully considered in the course of the development of GCPII targeting modalities.
In conclusion, more detailed studies are needed to clarify data available in the literature. First, detailed characterization of anti-GCPII antibodies is needed, including specificity (GCPII vs. GCPIII), sensitivity and cross-reactivity with non-human GCPII orthologs. Additionally, ultrastructural studies are urgently needed to map precisely GCPII expression to individual cell populations (and subcellular compartments) in healthy organs.
3.2. GCPII Splice Variants, Paralogs, and Related Peptidases
Pertinent to physiological functions of GCPII as well as the development of ligands/antibodies targeting the enzyme can be the existence of several GCPII paralogs (GCPIII, NAALADAse L, NAALADAse L2, PSMAL), splice variants (PSM’, PSM-C – PSM-F), and M28 family members (e.g. plasma glutamate carboxypeptidase).
The best studied GCPII splice variant, designated PSM’, has been first described by Su et al and the authors suggested that the relative ratio of GCPII/PSM’ mRNA in prostate can be used for determining the “tumor index” of the prostatic tissue [65], even though the clinical significance of these findings was later questioned by others [66]. Uncertain is also the existence of the PSM’ protein that would be missing the first 57 amino acids of the full length GCPII, i.e. the intracellular and transmembrane segments, and located in the cytosol of PSM’ expressing cells. Although a similar protein (lacking first 59 amino acids) was indeed later isolated by Grauer et al [67] from the LNCaP cell line, more recent data points towards PSM’ being a product of GCPII endoproteolysis rather that a transcript of the PSM’ splice variant [68]. There is limited information on other GCPII splice variants at the mRNA level, and nothing is known about the existence and function of corresponding protein products.
Out of four GCPII paralogs in humans (NAALADase L, NAALADase L2, PSMAL, and GCPIII), GCPIII is the most relevant when considering the design of GCPII ligands for the treatment of disease. Human GCPIII is a type II integral membrane metallopeptidase comprising 740 amino acids and sharing 67% identity with GCPII at the amino acid level. Human GCPIII was cloned in 1999 (the mouse GCPIII ortholog in 2004) and the expression profile mapping showed mRNA presence predominantly in ovary, testes, spleen, heart, placenta and brain [69,70]. However, the physiological role of GCPIII and protein localization in humans/mice are unknown due to the lack of GCPIII-specific antibodies/ligands. Published studies using both transfected cells and purified proteins show that GCPIII hydrolyzes NAAG with kinetic parameters similar to GCPII [22,71]. Additionally, there is a significant overlap (near identity) in the substrate specificity as well as the pharmacologic profile between the two proteins. These biochemical findings were recently corroborated and rationalized by solving the GCPIII crystal structure [72]. Given the close sequence/structural similarity between GCPII and GCPIII, small-molecule ligands aimed at GCPII are very likely to interact with GCPIII with similar potency. Considering our lack of knowledge of GCPIII physiology/expression pattern, the impact of such cross-reactivity towards the intended use of GCPII-specific compounds is difficult to predict at present. Clearly, such cross-reactivity can be advantageous (e.g. the complete inhibition of NAAG-hydrolyzing activity in brain) in some settings, while undesired in other (e.g. specific GCPII targeting on cancer cells). The development of GCPIII-specific modalities (antibodies, inhibitors) and establishing GCPIII-knockout mice would definitely help in dissecting diverse physiological roles of the two proteins and be beneficial in rationalizing the design of candidate drugs targeting GCPII/GCPIII.
Plasma glutamate carboxypeptidase (PGCP) is a 56 kDa glycoprotein found in high amounts in human plasma. Both GCPII and PGCP belong to the M28 family, share 27% identity at the amino acid level and require zinc ions for proteolytic activity. Although the pharmacological profile as well as substrate specificity of human PGCP differ from GCPII, both enzymes are able to hydrolyze NAAG suggesting some structural/functional overlap between the two [73,74]. In this respect it is interesting to note that in the recent phase I clinical trial aimed at PCa imaging using N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-4-[18F]fluoro-benzyl-L-Cysteine ([18F]DCFBC), a GCPII specific imaging agent [49], an increased signal from the blood pool was observed in human subjects, suggesting potential binding of the compound to an unidentified plasma protein. Ongoing studies shall answer the question whether this observation can be linked to the presence of PGCP or other proteins in human blood.
3.3. GCPII Physiological Functions
Although high expression levels of GCPII in advanced prostate cancer serve as an excellent target for PCa imaging/therapy, its physiological function(s) in this tissue is not known. Similarly, despite substantial expression levels of GCPII in kidneys and (human) neovasculature its physiological role remains to be defined. As a result, only two roles, both requiring GCPII enzymatic activity, are well defined at present – folate-hydrolyzing activity [75,76] and NAAG-hydrolyzing activity [77] in the small intestine and the nervous system, respectively (Fig. 1). In addition to functioning as a hydrolase/peptidase, several reports allude to a non-proteolytic role(s) of GCPII in human physiology. GCPII has been shown to facilitate integrin signaling in epithelial cells [60], to be associated with the anaphase-promoting complex in prostate cancer cells [78] and to activate the NF-κB signaling pathway promoting cell proliferation [79]. However, further studies are required to shed more light on non-enzymatic GCPII functions, including the exact mechanism(s) of transmembrane signaling and the identification of putative physiological interaction partners (if they exist).
3.3.1. GCPII as Folate Hydrolase
Dietary folates consist of a mixture of poly-γ-glutamylated species, but only mono-glutamylated compounds are actively transported through the intestinal wall into the bloodstream [80]. In humans (and pigs), trimming of glutamate tails of dietary folates is performed by GCPII located in the brush border of proximal jejunum – the folate hydrolase activity of GCPII thus plays an important role in folate absorption in humans [81]. It is interesting to note that due to the absence of GCPII expression in rat intestine, dietary folates are hydrolyzed by γ-glutamyl hydrolase, a hydrolase found in pancreatic secretions in rat [64]. The physiological significance (if any) of this difference is not known.
Folate metabolism has been reported to be influenced by polymorphism in the GCPII gene, though existing epidemiologic data are somewhat contradictory. Devlin et al reported 50% decreased activity of the H475Y GCPII naturally occurring variant (corresponding to the C1561T at the nucleotide level) towards folate-poly-γ-glutamates [82]. Several groups have analyzed the influence of GCPII polymorphism on folate/homocysteine concentrations and their association with the incidence of various disorders (neural tube defects, gastric cancer, prostate cancer, cardiovascular disease) [83–88]. So far, unfortunately, the epidemiologic data provide conflicting results that are difficult to reconcile. Clearly, further studies are needed to draw a more detailed picture of GCPII involvement in folate metabolism.
3.3.2. GCPII as NAAG-Hydrolase
N-acetyl-aspartyl-glutamate (NAAG) is the most prevalent and widely distributed peptidic transmitter in the mammalian brain (reaching millimolar concentrations) and its expression pattern overlaps with nearly all major neurotransmitters including glutamate, GABA, acetylcholine, dopamine and serotonin [89,90]. NAAG is synthesized in neurons from glutamate and N-acetyl-aspartate by recently discovered NAAG-synthetase I (Rimkl B) and is stored in synaptic vesicles of presynaptic axon terminals [91]. Upon depolarization, the dipeptide is released in a calcium dependent manner and rapidly diffuses from the synaptic cleft into an extrasynaptic space where it undergoes two different fates [92,93].
Intact NAAG acts as an agonist at the metabotropic glutamate receptor 3 (mGluR3) located at both presynaptic nerve terminals as well as on astrocytes apposed to the synapse. Upon mGluR3 activation, an associated G-protein coupled pathway leads to the decrease in cAMP (and cGMP) second messenger concentrations within the cell. In the presynaptic nerve terminal this action results in the decrease in the amount of glutamate that would be released upon further nerve stimulation, thus dampening the amount of the discharged neurotransmitter [94–96]. In astrocytes mGluR3 activation stimulates production and secretion of transforming growth factor β [97,98]. Both these actions of NAAG can be viewed as neuroprotective. It is therefore not surprising that mGluR3 agonists, such as LY379268, have been found to be neuroprotective in a variety of animal models of neurological conditions [99].
The catabolic arm of the NAAG fate in the extrasynaptic space relies on the presence of GCPII (and possibly GCPIII, though the role of this enzyme in NAAG metabolism is not well defined at present) at the plasma membrane of astrocytes and schwann cells in the CNS and PNS, respectively [37,42]. Here, extrasynaptic NAAG is hydrolyzed into Ac-Asp and glutamate and the reaction products are transported into astrocytes/oligodendrocytes [77,100,101]. The NAAG-hydrolyzing activity of GCPII “inactivates” NAAG, indirectly blocking its effects on mGluR3.
Taken into account processes described above, the following rationale is used for the development of GCPII-specific inhibitors for the treatment of neurologic conditions associated with glutamate excitotoxicity: by inhibiting the NAAG-hydrolyzing activity of GCPII, there is a marked increase of intact NAAG (and the decrease in free glutamate as the reaction product) in the extrasynaptic space leading to the activation of mGluR3 receptors. The mGluR3 activation/signaling results in both the secretion of neuroprotective peptides by astrocytes and the reduction in glutamate release from presynaptic nerve terminals. Lower glutamate concentrations in the synaptic cleft (or extracellular milieu) are beneficial by not hyperactivating ionotropic glutamate channels that would be otherwise associated with serious pathological consequences (chapter 4.1.1.). This rationale has been extensively tested and confirmed in a variety of animal models of PNS and CNS disorders (Chapter 4.1.3.).
4. GCPII AS A THERAPEUTIC TARGET
4.1. CNS and PNS Disorders
4.1.1. Glutamate Excitotoxicity
Excessive glutamate causes neuronal dysfunction and degeneration, a phenomenon known as “glutamate excitotoxicity”. Glutamate is the most prevalent excitatory neurotransmitter in the mammalian nervous system and glutamate excitotoxicity has been inextricably linked to both acute and chronic neuronal disorders, including stroke, traumatic brain injury, amyotrophic lateral sclerosis, Alzheimer’s dementia and Parkinson’s disease. In a simplified view, the mechanisms underlying glutamate excitotoxicity are initiated by non-physiological increase in synaptic glutamate concentrations resulting in hyperactivation of ionotropic glutamate channels, the most notably N-methyl-D-aspartate receptors (NMDAR). Excessive calcium influx via NMDARs triggers a complex and intertwined array of cellular responses, including nitric oxide overproduction, mitochondrial dysfunction associated with free radical generation, caspase activation, or ionic homeostasis imbalance, that ultimately lead to necrotic or apoptotic death of affected neurons (reviewed in ref. [102,103]).
Pharmacologic interventions aimed at attenuating neuronal damage associated with glutamate excitotoxicity mostly concentrate on modifying/blocking downstream effector processes, and utilize ionotropic receptor antagonists, NO synthase inhibitors, free radical scavengers, caspase inhibitors etc. Unfortunately, despite the continued research in this field, there are no pharmacological interventions providing significant neuroprotection at present [102,103].
Given the failure of several NMDAR antagonists, alternative strategies focus on targeting processes upstream of the excitotoxic insult, i.e. blocking glutamate release from presynaptic terminals. The rationale of this approach is that by diminishing the amount of glutamate in the synaptic cleft, it is possible block several diverse downstream pathologic processes at once. Lamotrigin and riluzole are two examples of such strategy. Both drugs are presynaptic sodium channel blockers used in clinical practice to alleviate symptoms of epilepsy and amyotrophic lateral sclerosis, respectively [104,105]. Similarly, a growing body of evidence shows that agonists targeting type II metabotropic glutamate receptors (such as mGluR3) are effective in the treatment of variety of neurological disorders, including pain, addictive disorders, and schizophrenia [99]. In a simple extension, GCPII-specific inhibitors could be equally effective by increasing the extrasynaptic concentration of NAAG and its agonist action at mGluR3 (see below).
4.1.2. Development of GCPII Inhibitors
The first GCPII inhibitors were identified in the early 1990s and included non-hydrolyzable or conformationally restricted NAAG peptide analogs, glutamate derivatives and quisqualic acid [106,107]. Although these inhibitors were weak and lacked specificity, they provided a solid basis for further inhibitor design [29]. Research in the area of inhibitor design expanded during the mid-1990s as more biological information on NAAG became available and the realization that GCPII inhibition had therapeutic value was recognized. A consistent finding throughout the next 15 years of research showed that potent GCPII inhibitors required two functionalities – a glutarate moiety to bind to the C-terminal glutamate recognition site of GCPII and a zinc chelating group to coordinate the divalent zinc atoms at the enzyme’s active site. Employing this inhibitor template has led to the identification of several families of potent and selective GCPII inhibitors, exemplified by 2-PMPA, 2-MPPA, and ZJ43 as detailed below.
2-PMPA and Phosphonate-Based Inhibitors
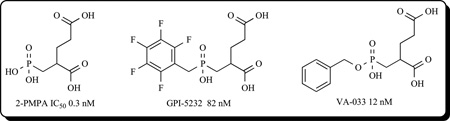
The first potent and selective GCPII inhibitor, termed 2-(phosphonomethyl)pentanedioic acid (2-PMPA), was reported in 1996 by Jackson et al. [108]. The pentanedioic acid portion of the inhibitor was designed to interact with the glutarate recognition site of GCPII while the phosphonate group was utilized to chelate to the active site zinc ions. 2-PMPA was characterized as a competitive inhibitor with an IC50 value of 300 pM with exquisite selectivity having no activity at over 100 different transporter, enzymes and receptors, including several glutamate targets [109]. Subsequently, structure–activity relationship (SAR) studies using 2-PMPA as a template yielded other potent phosphonate-base inhibitors including GPI 5232 [110], VA-033 [111], and phenylalkylphosphonamidates [28].
Phosphinic acid-based GCPII inhibitors have been useful in models of disease to demonstrate the relevance of GCPII as therapeutic target. These inhibitors have shown utility in various preclinical models of disease wherein excess glutamate is presumed pathogenic including ischemia [112–115], pain [116–118], amyotrophic lateral sclerosis [119], seizures [120,121], morphine tolerance [122] and aggression [123]. Unfortunately, poor oral bioavailability and limited brain penetration have precluded this series from advancing to the clinic. Efforts to make 2-PMPA more lipophilic included changes of the γ-acid functionality of glutarate and the preparation of phosphinic acid derivatives. The resulting analogs exhibited lower potency than 2-PMPA even though some inhibited GCPII in the mid nM range. In any case, the lipophilicity of these compounds was still not high enough to improve the pharmacokinetic profile. Current efforts are focused on the synthesis of phosphinic acid prodrugs to enhance oral bioavailability. This strategy has been successfully employed with phosphonate based inhibitors of ACE including Fosinopril [124].
2-MPPA and Thiol-Based Inhibitors
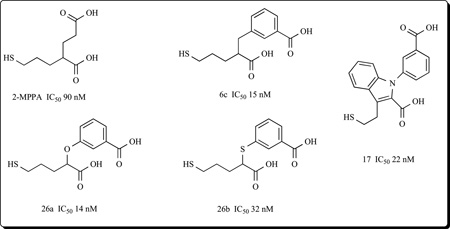
Additional efforts to reduce the polarity of phosphorous-based inhibitors led to thiol-based GCPII inhibitors. Optimization of the number of carbons between the glutarate moiety and the Zn2+ binding thiol group resulted in the discovery of 2-MPPA (2-(3-mercaptopropyl) pentanedioic acid) which exhibited nanomolar level potency (IC50 = 90 nM).
2-MPPA showed selectivity for GCPII inhibition and most important, it exhibited efficacy in several animal models of disease after oral administration. These included neuropathic pain [125], familial amyotrophic lateral sclerosis [126], painful and sensory diabetic neuropathy [127] and cocaine addiction [128]. 2-MPPA was subsequently used in humans in an exploratory clinical study designed to assess safety and tolerability of GCPII inhibition. 2-MPPA did not provoke any adverse CNS effects and was well-tolerated at plasma concentrations where efficacy was expected to be observed based on animal studies. Even though additional safety and pharmacokinetic studies in healthy subjects were justified, no additional studies were carried out because of the relatively low potency of 2-MPPA (estimated human therapeutic dose of ~750 mgs) together with concerns over potential immune reactivity common to thiol containing drugs. Using 2-MPPA as a template, however, more potent thiol based drugs were sought. Thiol inhibitors containing a benzyl moiety at the P1‘ position such as 3-(2-carboxy-5-mercaptopentyl)benzoic acid (6c) were found to be more potent than 2-MPPA (IC50 = 15 nM vs. 90 nM). Phenoxy and phenylsulfanyl analogues such as 3-(1-carboxy-4-mercaptobutoxy) benzoic acid (26a) and 3-[(1-carboxy-4-mercaptobutyl)thio]benzoic acid (26b) were also found to possess potent inhibitory activities (IC50 = 14 and 32 nM, respectively) [23]. Most recently a series of N-substituted 3-(2-mercaptoethyl)-1H-indole-2-carboxylic acids (e.g 17) with IC50 values in the 20–50 nanomolar range were described [129]. Interestingly, these latter compounds represent the first achiral GCPII inhibitors with significant structural differences from NAAG.
ZJ-43 and Urea-Based Inhibitors
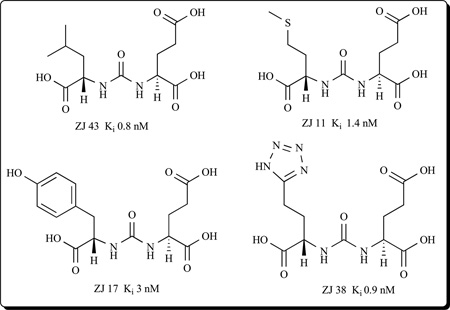
Another group of compounds that yielded potent GCPII inhibitors contain urea as the zinc- binding group [26,130–132]. This class of compounds are based on NAAG-like analogs in which the two amino acids are joined through their NH2 groups by a urea linkage, and utilize both the aspartate binding site (P1 position) in addition to the glutarate moiety (P1’ position) in the enzyme. Noteworthy in this group is ZJ-43 with low nanomolar potency [132] as well as ZJ 11, ZJ 17, and ZJ 38 [26,31,131].
ZJ-43 has shown efficacy in animal models of schizophrenia [132], traumatic brain injury [133], neuropathic pain [134] and inflammatory peripheral pain [135]. Unfortunately, like 2-PMPA and its derivatives, urea-based compounds exhibit very low bioavailability following oral administration and have minimal brain penetration.
Other Classes of Inhibitors
Even though phosphonates, thiols and urea-based compounds have shown limitations as potential clinical candidates, they have invariably demonstrated the relevance of GCPII as a therapeutic molecular target in a variety of diseases that exhibit glutamate excitotoxicity as common denominator. Consequently, robust efforts are being carried out in several laboratories to improve on bioavailability and minimize potential toxicity. These include the development of prodrugs of 2-PMPA derivatives like phosphonate and phosphinate esters [136], more lipophilic and potent thiols [23], and the use of alternative zinc- binding functionalities such as hydroxamates [137]. Additional efforts in our laboratory are focused on creating new GCPII inhibitor structures employing alternative zinc binding groups which when used in other drugs have demonstrated oral bioavailability and drug like character in the clinic. These include 2-aminophenylamides, sulfamides, sulfonamides, imidazoles and other nitrogen containing hetero-cycles.
4.1.3. Utility of GCPII Inhibitors Observed in PNS/CNS Preclinical Models
The utility of GCPII inhibitors has been demonstrated in several animal models of disease whereby enhanced glutamate transmission is presumed pathogenic. These models include inflammatory and neuropathic pain [25,26,116–118,127,134,135,138–145] brain ischemia [25,109,114,146,147] motoneuron disease [148] spinal cord and traumatic brain injury [115,133,149], peripheral neuropathy [127,144,150,151], epilepsy [121] and drug abuse [128,152–157]. These findings are summarized in Table 1.
Table 1.
| INHIBITOR USED | MAJOR FINDING | REF |
|---|---|---|
| PAIN | ||
| 2-PMPA, i.t. | Antinociceptive effect in rat formalin but not hot plate model | [118] |
| 2-PMPA, i.t. | Reduction of mechanical allodynia induced by paw carrageenan injection; no effect on post-op pain or thermal injury | [117] |
| Cmpd 34 | Reduction of thermal hyperalgesia in CCI model of neuropathic pain | [25] |
| 2-PMPA, i.p. | Reduction of allodynia and ectopic discharge activity of afferent nerves in partial nerve ligation model | [116] |
| GPI 5232 i.p. | Reversal of thermal hyperalgesia in BB/W diabetic rats following 6 mo dosing | [144] |
| 2-MPPA, p.o. | Reduction of thermal hyperalgesia in CCI model of neuropathic pain | [140] |
| 2-PMPA, applied directly | Inhibition of input, post-discharge, C- and Aδ-fibre-evoked responses after carrageenan inflammation | [139] |
| Cmpd 8d (urea) i.t. | Reduction of the response to inflammatory pain evoked by footpad injection of formalin | [26] |
| ZJ-11, 17 and 43, i.t. and i.v. | Reduction of mechanical allodynia in formalin and the partial sciatic nerve ligation models blocked by co-administration of a group II mGluR antagonist | [134] |
| ZJ-43, i.p. | Reduction of mechanical allodynia in a bone cancer model; effect was antagonized by group II mGluR antagonist | [145] |
| 2-MPPA, p.o. | Reversal of thermal hyperalgesia in BB/W diabetic rats following 6 mo dosing | [127] |
| 2-PMPA, i.p. | Reduction of allodynia in CCI model with brain levels of 2PMPA (30uM) and NAAG (3uM) | [141] |
| ZJ43 and 2-PMPA, footpad | Reduction of pain responses in carrageenan and formalin models blocked by co-administration of a group II mGluR antagonist | [135] |
| ZJ43 & 2-PMPA i.c.v. | Dose dependent analgesia blocked by co-administration of a group II mGluR antagonist in mouse formalin model | [143] |
| ZJ-43, i.p. | Prevention of the development of mechanical allodynia and reduction of the enhanced current output in the central nucleus of amygdala in mouse formalin model | [138] |
| STROKE / ISCHEMIA | ||
| 2-PMPA, i.p. | Neuroprotection following middle cerebral artery occlusion; concomitant increase in brain NAAG and attenuation of ischemia-induced rise in glutamate | [109] |
| 2-PMPA | Neuroprotection in rat cerebellar neurons against hypoxia and NMDA | [146] |
| 2-PMPA, i.p. | Neuroprotection of retinal ganglion cell survival in an ischemia-reperfusion model of C57BL/6 mouse eyes | [115] |
| GPI 5232, i.v. | Reduction of brain infarction and amelioration of pathological EEG changes following middle cerebral artery occlusion | [147] |
| GPI 5232, i.v. | Neuroprotection in a middle cerebral artery occlusion model of cerebral ischemia | [25] |
| ALS / MOTONEURON DISEASE | ||
| 2-MPPA, p.o. | Protection against motoneuron death in G93A familial ALS transgenic mice | [148] |
| PERIPHERAL NEUROPATHY | ||
| GPI 5232, i.p. | Partial prevention of hyperalgesia, nerve conduction slowing, axonal and morphological abnormalities in BB/W rat model of Type I diabetes | [144] |
| 2-PMPA | Protection against glucose-induced neuronal death and neurite degeneration in dorsal root ganglion neurons; blocked by group II mGluR antagonist | [150] |
| 2-MPPA, p.o. | Improvement of established hyperalgesia, nerve conduction velocity abnormalities and myelinated fiber atrophy in BB/W rat model of Type I diabetes | [127] |
| 2-MPPA, p.o. | Protection of the nerve conduction velocity and morphological abnormalities induced by several chemotherapies including cisplatin, paclitaxel, bortezomib | [151] |
| EPILEPSY / SEIZURES | ||
| 2-PMPA, i.p. | Protection against the development of cocaine-kindled seizures; no effect on acute challenges with pentylenetetrazole, bicuculline, N-methyl-D-aspartate, maximal electroshock | [121] |
| DRUG ABUSE | ||
| 2-PMPA, i.p. | Reduction of ethanol consumption in alcohol-preferring (P) rats | [152] |
| 2-PMPA, i.p. | Attenuation of the development of sensitization to the locomotor-activating effects of cocaine; no antagonism of the acute effects of cocaine | [155] |
| 2-PMPA, i.p. and 2-MPPA, p.o. | Attenuation of the acquisition and expression of the conditioned place preference response to cocaine but not food | [128] |
| 2-MPPA, p.o. | Prevention of the development of morphine tolerance without affecting acute morphine antinociception; blocked by co-administration of a group II mGluR antagonist | [153] |
| 2-PMPA, i.p. | Inhibition of cocaine's rewarding effects as assessed by i.v. cocaine self-administration and intracranial electrical brain-stimulation reward; blocked by co-administration of a group II mGluR antagonist | [156] |
| 2-PMPA, i.p. | Attenuation of cocaine-induced reinstatement of drug-seeking behavior via blocking increases in dopamine and glutamate in the nucleus accumbens; blocked by co-administration of a group II mGluR antagonist | [157] |
| 2-MPPA, p.o. | Attenuation of cocaine-induced reinstatement of drug-seeking behavior in rats; no effect on sucrose-seeking behavior; blocked by co-administration of a group II mGluR antagonist | [154] |
| BRAIN AND SPINAL CORD INJURY | ||
| ZJ-43, i.p. | Protection of degenerating neurons and astrocyte loss following lateral fluid percussion model of traumatic brain injury in rats; effect abolished by co-administration of a group II mGluR antagonist | [133] |
| ZJ-43, i.p. | Increase in dialysate NAAG and reduction in glutamate, aspartate and GABA levels in hippocampus following fluid percussion injury | [149] |
| 2-PMPA | Protection against dynorphin A-induced ischemic spinal cord injury in rats assessed by reduction of cerebrospinal fluid glutamate and improvement in motor scores | [115] |
Importantly the GCPII inhibitor efficacy data have been generated with structurally divergent small molecule compounds including thiol- phosphinic acid-, and urea-based inhibitors as well as NAAG peptide analogs, providing strong evidence for the mechanism of their effect. Studies in GCPII knockout mice also support these inhibitor findings; Bacich et al showed that the knockout mice have decreased sensitivity to both central and peripheral nerve damage [158]. From a mechanistic perspective, many GCPII inhibitor effects elicited in vivo were blocked by the co-administration of selective group II metabotropic receptor antagonists demonstrating that at least part of the GCPII utility is likely mediated via this receptor. Not only has efficacy been observed, but several studies have demonstrated that GCPII inhibition in models of neurological disease is accompanied by a decrease in brain glutamate and a concomitant increase in brain NAAG [109,141]. Importantly in normal animals, however, GCPII inhibition has no effect on basal glutamate transmission [109]. From a therapeutic standpoint, this is ideal. If this holds true in the clinic, GCPII inhibition could limit excess glutamate release and provide neuroprotection without the untoward side effects observed previously with potent glutamate receptor antagonists [159].
4.2. GCPII in Cancer
As stated in previous chapters, GCPII is strongly expressed in human prostatic tissue and with gradual increase in staining intensity from the healthy organ through benign prostate hyperplasia, low-grade adenocarcinoma up to metastatic, androgen-independent disease. Given the extremely high levels of expression, a fairly restricted expression pattern and the membrane localization of GCPII, the protein is an ideal candidate for PCa imaging and therapy.
4.2.1. Current Imaging Techniques for PCa
Many recent reviews covering imaging in general and molecular imaging, specifically, in PCa have been published [160–164]. As with most cancers, anatomic imaging with computed tomography (CT) and/or magnetic resonance (MR) imaging remains the mainstay for staging and therapeutic monitoring. The nuclear medicine technique of bone scanning with [99mTc]methylene diphosphonate (MDP) and single photon emission computed tomography (SPECT), also widely used to detect metastases, is now giving way to [18F]NaF with positron emission tomography (PET), due to the higher sensitivity of the latter [165]. SPECT and PET are considered molecular imaging techniques in that they detect and enable imaging of the underlying metabolism or a specific protein marker associated with the disease. However, PCa specifically is not particularly amenable to the standard clinical method of PET with [18F]fluorodeoxyglucose (FDG), likely due to the relatively metabolically quiescent nature of PCa vs. other malignancies. Accordingly, a wide variety of new molecular imaging agents and techniques are under development for PCa, such as positron-emitting versions of choline [166,167], 1-amino-3-fluorocyclobutane-1-carboxylic acid (FACBC) [168], 2-deoxy-2-fluoro-β-L-arabinofuranosyl)-5-methyluracil) (FMAU) [168,169], and [18F]fluorodihydrotestosterone (FDHT) [170,171]. Each agent and technique has its niche and reports on a different aspect of the disease. A heavily pursued target for imaging and therapy of PCa – using a variety of affinity reagents – is GCPII, which is also known (in the periphery) as the prostate-specific membrane antigen (PSMA) [32,38,48,49,54,130,172–176].
Since 1996 there has been a murine monoclonal antibody to PSMA radiolabeled with 111In, known as [111In]capromab pendetide or ProstaScint™, used to image PCa [177,178]. However, monoclonal antibodies are somewhat cumbersome as imaging agents due to their long circulation times, which cause significant background radioactivity within the blood pool and other non-target tissues. ProstaScint™ must be administered five days prior to imaging, which is inconvenient, and even then significant blood pool remains obscuring potential malignant lesions within the pelvis [179]. One problem with ProstaScint™ is the fact that it targets an intracellular epitope on PSMA such that the cells must be dead or dying for the antibody to gain access to the target.
Several antibodies to extracellular epitopes of PSMA have since been developed and these are believed at least in part to mitigate the problems associated with the use of ProstaScint™. The most frequently cited of these is the J591 antibody (and its derivatives), which has been used productively to image not only PCa but other solid tumors due to the presence of PSMA within the tumor neovasculature [43,54,180,181]. Reagents based on the J591 antibody (or its humanized derivative huJ591) are clinically the most advanced, and J591 conjugates have been shown to be well-tolerated and non-immunogenic in several clinical trials. Advantageously, in addition to the primary tumor, J591-based reagents are also able to target bone and soft tissue metastases efficiently. J591 radiolabeled with 111In has been used for for SPECT; 90Y, 177Lu or 131I for β-therapy; and 213Bi and 225Ac for α-therapy [180,182–189]. Most recently the J591 antibody has been radiolabeled with 89Zr, taking advantage of the 78.4 h half-life of 89Zr to enable productive tumor imaging [190]. The group lead by Pichler reported preparation of a series of high affinity antibodies against PSMA, their labeling with 64Cu and use for high-resolution PET imaging of prostate cancer xenografts [191–193].
One way to address the problem of poor pharmacokinetics of antibodies for imaging is to use aptamers, which are affinity reagents based on nucleic acids [194]. Aptamers to PSMA have been radiolabeled with the positron emitter 64Cu, but images in vivo have not yet been produced [195].
4.2.2. Novel Approaches for GCPII Imaging
Several groups have taken a different approach to circumventing the pharmacokinetic problem by developing low molecular weight agents that bind with high affinity to PSMA, which enable rapid uptake by tumor tissue and washout from non-target sites. The low molecular weight agents fall into two major classes, namely the phosphoramidates and the ureas [31,175,196].
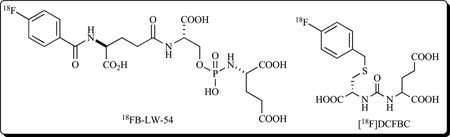
A phosphoramidate peptidomimetic, based on GCPII therapeutics initially developed at ZENECA Pharmaceuticals in the mid 1990’s [108], has been radiolabeled with 18F using the N-succinimidyl-4-18F-fluorobenzoate (18F-SFB) prosthetic group, and was able to delineate PSMA+ LNCaP xenografts on small animal PET with 4:1 target (LNCaP tumor) to non-target (PSMA- PC3 tumor) ratios at 1.24% injected dose per gram (ID/g) in LNCaP tumors at 2 h post-injection (compound FB-LW-54) [175]. Recently, Berkman’s group reported that depending on the physicochemical characteristics of the P1 moiety, several of their phosphoramidate peptidomimetics feature pseudoirreversible binding to GCPII [27]. Pseudoirreversibility in inhibitor binding has been shown to increase the rate of PSMA internalization and this fact can be exploited for transporting drug into the PSMA-positive cells. No such correlation is available for urea-based inhibitors and it remains to be seen whether this interesting distinction will have any connotations for the future selection of zinc binding groups.
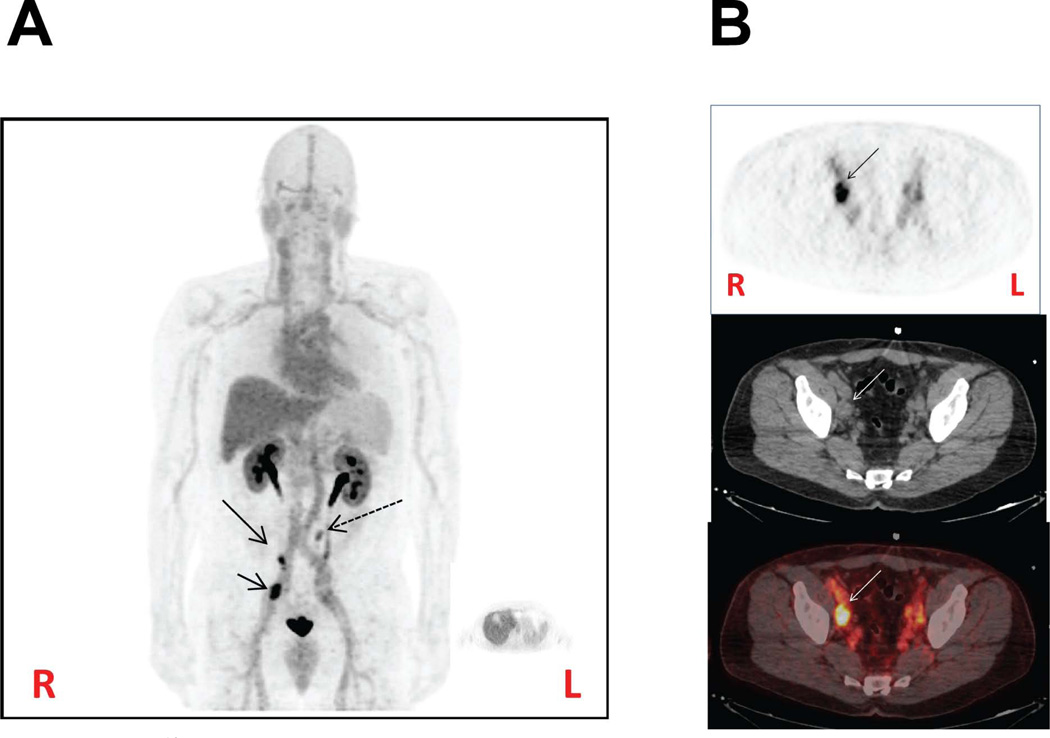
The urea series is originally based on symmetric inhibitors of GCPII discovered by Kozikowski and coworkers [131]. Compounds of the urea class have been radiolabeled with 125I for autoradiography and preclinical imaging, with 123I or 99mTc for SPECT, and with 11C, 68Ga or 18F for PET [48,49,173,174,197, 198]. Compounds of this class tend to provide images with very high target to non-target ratios and high tumor uptake values, occasionally over 40% ID/g using an isogenic PSMA+/− tumor model system. As with the phosphoramidates, the ureas require a glutamate to bind within the pharmacophore pocket, while an additional carboxylate is required in the S1 site. However, because of a tunnel of 20 Å leading to the surface of the enzyme [7], large functional groups, such as chelating or fluorescent moieties, can be appended to the urea, which is considered the PSMA targeting aspect. The linker from the targeting moiety to the chelator can be used for pharmacokinetic manipulation [173,176]. PSMA targeting compounds labeled with fluorescent species, particularly those that emit in the near-infrared region of the spectrum, can be used for intra-operative surgical guidance [46]. PSMA-targeting ureas labeled with 123I and 18F have recently been used in Phase I clinical trials in patients with PCa to good effect (Fig. 2).
Fig. (2).
Human PET imaging with [18F]DCFBC. (A) Coronal view. Note increased radiopharmaceutical uptake in an enlarged right external iliac lymph node (short arrow), a small right external iliac node (long arrow) and a small left periaortic node (dashed arrow). [18F]DCFBC, like all of the hydrophilic GCPII inhibitors of the urea class, is excreted almost exclusively through the urine. The large pelvic focus of uptake in the midline is due to radiotracer in the bladder. (B) Axial view. Clockwise from upper left: PET, CT and fused PET/CT images depicting the right external iliac lymph node (arrow). These foci of increased radiopharmaceutical uptake are most likely due to metastases from prostate cancer. R = right, L = left.
Notably, all successful PSMA-based imaging agents have been used in the periphery for cancer. Because they are highly charged and therefore unable to cross the blood-brain barrier, imaging of GCPII within the central nervous system (CNS) has proved a difficult goal. Nevertheless, efforts to eliminate the tricarboxylic acid motif to enable imaging within the CNS are under way, in analogy with the development of therapeutic agents that penetrate the brain [21]. Motivation for that pursuit derives in part from the ability of N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-S-3-[125I] iodo-L-tyrosine ([125I]DCIT) to distinguish patients with schizophrenia from those with unipolar depression in certain brain regions when using in vitro autoradiography [199].
4.2.3. GCPII in PCa Therapy
An obvious extension of imaging studies is the use of antibodies and fragments thereof in conjugates for cancer therapy. Several of such reagents have been developed and tested primarily settings. The beta-emitting isotope 90Y has been used to replace 111In in ProstaScint™, and the use of the conjugate resulted in slight improvements in the symptomatology of the disease in PCa patients [200]. More impressive antitumor activity, as determined by the decrease in PSA levels, was obtained by the use of 90Y-J591 and 117Lu-J591 conjugates, with the 117Lu-J591 offering better and more predictable responses [182,187].
In a complementary approach, several studies used chemical conjugates of antibodies and immunotoxins to target GCPII-expressing cells. Both in vitro studies and mouse xenograft models were exploited to test the efficacy of J591 conjugates with ricin A, melitin-like peptide 101, monomethylauristatin E, and maytansinoid 1 [201–204]. Results of these studies showed promise for PCa treatment in preclinical animal models that included significant tumor growth inhibition and even complete ablation of implanted tumors. Future clinical applications might be, however, hampered by inherent problems of such conjugates, including their immunogenicity and the difficulties of producing large and homogenous quantities. These problems might, at least in part, be overcome by harnessing the power of recombinant DNA technology to construct less immunogenic and smaller conjugates. Such a conjugate, comprising a single-chain antibody fragment linked to a truncated form of Pseudomonas exotoxin A, has been engineered and its anti-tumor activity demonstrated in C4-2 tumor xenografts in vivo [205,206].
Because GCPII/PSMA is an integral membrane protein with the active site external to the cell, it often serves as a proof-of-principle in developing new nanoparticle-based imaging and therapeutic agents that target tumors with leaky vasculature. One of the first studies to demonstrate nanoparticles targeting an epithelial target used semiconductor quantum dots that employed the J591 antibody to PSMA [207]. Suppression of autofluorescence was a major goal of that study, as the reagents used were for optical imaging. The significance of the study is that particles that could contain a therapeutic payload may be targeted to tumors in vivo, despite the target being in epithelium and not within the vasculature, where it is ostensibly much more readily accessible. A variety of combination imaging and therapeutic (theranostic) agents, using several modalities, have been developed recently, including fluorescent, superparamagnetic nanospheres, aptamer-targeted iron oxide nanoparticles loaded with doxorubicin [208,209], and biocompatible nanoparticles carrying preventive as well as therapeutic agents [208,210], some of which are beginning to be functionalized with low molecular weight urea inhibitors for targeting.
A particularly creative application of PSMA targeting is for antibody recruitment. The idea, developed by Spiegel and coworkers, employs a linker between the urea-based targeting moiety and an anti-dinitrophenol (anti-DNP) antibody binding terminus. The so called antibody recruiting molecule to prostate cancer (ARM-Ps) link the anti-DNP antibody to the prostate tumor cell, where it can be cleared by the immune system by virtue of the immune-stimulating capacity of DNP [211]. Interestingly, in developing the ARM-P technology, a new arene-binding site (for the DNP moiety) was uncovered in PSMA, enabling the synthesis of further high-affinity ligands [30]. This technology provides an excellent example of how deeper mechanistic understanding of the biology of even a well studied system such as GCPII/PSMA in the periphery can inform further chemical synthesis of new drugs and other reagents.
5. FUTURE PERSPECTIVES
Human GCPII has shown its utility in a variety of preclinical and clinical settings ranging from the treatment of neurological disorders to imaging and therapy of prostate cancer and solid malignancies. There are, however, many challenges still lying ahead. In the area of neurological diseases, it is imperative to identify and develop orally available GCPII inhibitors that could efficiently penetrate the blood-brain barrier and target GCPII residing in the CNS. Additionally, isoform-specific small molecule ligands that are able to discriminate between GCPII and its paralogs (e.g. GCPIII) might be needed for truly specific imaging of GCPII in human brain. As for cancer-related applications with the focus on therapy, it would be highly desirable to unravel physiological function(s) of GCPII in cancerous tissues including tumor neovasculature so that a more rational approach could be applied in this area. Additionally, more comprehensive biological and structural information is needed on GCPII paralogs and mammalian orthologs (including interspecies differences) that could again simplify and rationalize the development of modalities targeting GCPII under various (patho)physiological conditions. The development of future GCPII-specific modalities (small-molecule probes as well as macromolecules targeting GCPII) will be instrumental to decipher the role of GCPII in both healthy and diseased tissues. We believe that the unique characteristics of GCPII render this enzyme an attractive target for both basic and applied research.
ACKNOWLEDGEMENT
The financial support from EMBO (Installation grant 1978), IRG (project number 249220) NIH CA 134675, and the IBT (AV0Z50520701) institutional support are gratefully acknowledged.
REFERENCES
- 1.Anilkumar G, Rajasekaran SA, Wang S, Hankinson O, Bander NH, Rajasekaran AK. Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 2003;63:2645–2648. [PubMed] [Google Scholar]
- 2.Anilkumar G, Barwe SP, Christiansen JJ, Rajasekaran SA, Kohn DB, Rajasekaran AK. Association of prostate-specific membrane antigen with caveolin-1 and its caveolae-dependent internalization in microvascular endothelial cells: implications for targeting to tumor vasculature. Microvasc. Res. 2006;72:54–61. doi: 10.1016/j.mvr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Goodman OB, Jr, Barwe SP, Ritter B, McPherson PS, Vasko AJ, Keen JH, Nanus DM, Bander NH, Rajasekaran AK. Interaction of prostate specific membrane antigen with clathrin and the adaptor protein complex-2. Int. J Oncol. 2007;31:1199–1203. [PubMed] [Google Scholar]
- 4.Rajasekaran SA, Anilkumar G, Oshima E, Bowie JU, Liu H, Heston W, Bander NH, Rajasekaran AK. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell. 2003;14:4835–4845. doi: 10.1091/mbc.E02-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, Rahmati R, Bander NH. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 6.Schulke N, Varlamova OA, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, Maddon PJ, Heston WD, Olson WC. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12590–12595. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. EMBO J. 2006;25:1375–1384. doi: 10.1038/sj.emboj.7600969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barinka C, Rinnova M, Sacha P, Rojas C, Majer P, Slusher BS, Konvalinka J. Substrate specificity, inhibition and enzymological analysis of recombinant human glutamate carboxypeptidase II. J. Neurochem. 2002;80:477–487. doi: 10.1046/j.0022-3042.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- 9.Barinka C, Sacha P, Sklenar J, Man P, Bezouska K, Slusher BS, Konvalinka J. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci. 2004;13:1627–1635. doi: 10.1110/ps.04622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castelletti D, Fracasso G, Alfalah M, Cingarlini S, Colombatti M, Naim HY. Apical transport and folding of prostate-specific membrane antigen occurs independent of glycan processing. J. Biol. Chem. 2006;281:3505–3512. doi: 10.1074/jbc.M509460200. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh A, Heston WD. Effect of carbohydrate moieties on the folate hydrolysis activity of the prostate specific membrane antigen. Prostate. 2003;57:140–151. doi: 10.1002/pros.10289. [DOI] [PubMed] [Google Scholar]
- 12.Holmes EH, Greene TG, Tino WT, Boynton AL, Aldape HC, Misrock SL, Murphy GP. Analysis of glycosylation of prostate-specific membrane antigen derived from LNCaP cells, prostatic carcinoma tumors, and serum from prostate cancer patients. Prostate Suppl. 1996;7:25–29. [PubMed] [Google Scholar]
- 13.Castelletti D, Alfalah M, Heine M, Hein Z, Schmitte R, Fracasso G, Colombatti M, Naim HY. Different glycoforms of prostate-specific membrane antigen are intracellularly transported through their association with distinct detergent-resistant membranes. Biochem. J. 2008;409:149–157. doi: 10.1042/BJ20070396. [DOI] [PubMed] [Google Scholar]
- 14.Christiansen JJ, Rajasekaran SA, Inge L, Cheng L, Anilkumar G, Bander NH, Rajasekaran AK. N-glycosylation and microtubule integrity are involved in apical targeting of prostate-specific membrane antigen: implications for immunotherapy. Mol. Cancer Ther. 2005;4:704–714. doi: 10.1158/1535-7163.MCT-04-0171. [DOI] [PubMed] [Google Scholar]
- 15.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5981–5986. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence CM, Ray S, Babyonyshev M, Galluser R, Borhani DW, Harrison SC. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 17.Klusak V, Barinka C, Plechanovova A, Mlcochova P, Konvalinka J, Rulisek L, Lubkowski J. Reaction mechanism of glutamate carboxypeptidase II revealed by mutagenesis, X-ray crystallography, and computational methods. Biochemistry. 2009;48:4126–4138. doi: 10.1021/bi900220s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speno HS, Luthi-Carter R, Macias WL, Valentine SL, Joshi AR, Coyle JT. Site-directed mutagenesis of predicted active site residues in glutamate carboxypeptidase II. Mol. Pharmacol. 1999;55:179–185. doi: 10.1124/mol.55.1.179. [DOI] [PubMed] [Google Scholar]
- 19.Barinka C, Rovenska M, Mlcochova P, Hlouchova K, Plechanovova A, Majer P, Tsukamoto T, Slusher BS, Konvalinka J, Lubkowski J. Structural insight into the pharmacophore pocket of human glutamate carboxypeptidase II. J. Med. Chem. 2007;50:3267–3273. doi: 10.1021/jm070133w. [DOI] [PubMed] [Google Scholar]
- 20.Mesters JR, Henning K, Hilgenfeld R. Human glutamate carboxypeptidase II inhibition: structures of GCPII in complex with two potent inhibitors, quisqualate and 2-PMPA. Acta Crystallogr. D Biol. Crystallogr. 2007;63:508–513. doi: 10.1107/S090744490700902X. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Byun Y, Barinka C, Pullambhatla M, Bhang HE, Fox JJ, Lubkowski J, Mease RC, Pomper MG. Bioisosterism of urea-based GCPII inhibitors: Synthesis and structure-activity relationship studies. Bioorg. Med. Chem. Lett. 2010;20:392–397. doi: 10.1016/j.bmcl.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlouchova K, Barinka C, Klusak V, Sacha P, Mlcochova P, Majer P, Rulisek L, Konvalinka J. Biochemical characterization of human glutamate carboxypeptidase III. J. Neurochem. 2007;101:682–696. doi: 10.1111/j.1471-4159.2006.04341.x. [DOI] [PubMed] [Google Scholar]
- 23.Majer P, Hin B, Stoermer D, Adams J, Xu W, Duvall BR, Delahanty G, Liu Q, Stathis MJ, Wozniak KM, Slusher BS, Tsukamoto T. Structural Optimization of Thiol-Based Inhibitors of Glutamate Carboxypeptidase II by Modification of the P1' Side Chain. J. Med.Chem. 2006;49:2876–2885. doi: 10.1021/jm051019l. [DOI] [PubMed] [Google Scholar]
- 24.Barinka C, Hlouchova K, Rovenska M, Majer P, Dauter M, Hin N, Ko YS, Tsukamoto T, Slusher BS, Konvalinka J, Lubkowski J. Structural basis of interactions between human glutamate carboxypeptidase II and its substrate analogs. J. Mol. Biol. 2008;376:1438–1450. doi: 10.1016/j.jmb.2007.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson PF, Tays KL, Maclin KM, Ko YS, Li W, Vitharana D, Tsukamoto T, Stoermer D, Lu XC, Wozniak K, Slusher BS. Design and pharmacological activity of phosphinic acid based NAALADase inhibitors. J. Med.Chem. 2001;44:4170–4175. doi: 10.1021/jm0001774. [DOI] [PubMed] [Google Scholar]
- 26.Kozikowski AP, Zhang J, Nan F, Petukhov PA, Grajkowska E, Wroblewski JT, Yamamoto T, Bzdega T, Wroblewska B, Neale JH. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J. Med.Chem. 2004;47:1729–1738. doi: 10.1021/jm0306226. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Toriyabe Y, Kazak M, Berkman CE. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry. 2008;47:12658–12660. doi: 10.1021/bi801883v. [DOI] [PubMed] [Google Scholar]
- 28.Maung J, Mallari JP, Girtsman TA, Wu LY, Rowley JA, Santiago NM, Brunelle AN, Berkman CE. Probing for a hydrophobic a binding register in prostate-specific membrane antigen with phenylalkylphosphonamidates. Bioorg Med Chem. 2004;12:4969–4979. doi: 10.1016/j.bmc.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12:767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhang AX, Murelli RP, Barinka C, Michel J, Cocleaza A, Jorgensen WL, Lubkowski J, Spiegel DA. A remote arene-binding site on prostate specific membrane antigen revealed by antibody-recruiting small molecules. J. Am. Chem. Soc. 2010;132:12711–12716. doi: 10.1021/ja104591m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat. Rev. Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 32.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 33.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Natl. Acad. Sci. U. S. A. 1996;93:749–753. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinoshita Y, Kuratsukuri K, Landas S, Imaida K, Rovito PM, Jr, Wang CY, Haas GP. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg. 2006;30:628–636. doi: 10.1007/s00268-005-0544-5. [DOI] [PubMed] [Google Scholar]
- 35.Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50:472–483. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- 36.Rovenska M, Hlouchova K, Sacha P, Mlcochova P, Horak V, Zamecnik J, Barinka C, Konvalinka J. Tissue expression and enzymologic characterization of human prostate specific membrane antigen and its rat and pig orthologs. Prostate. 2008;68:171–182. doi: 10.1002/pros.20676. [DOI] [PubMed] [Google Scholar]
- 37.Sacha P, Zamecnik J, Barinka C, Hlouchova K, Vicha A, Mlcochova P, Hilgert I, Eckschlager T, Konvalinka J. Expression of glutamate carboxypeptidase II in human brain. Neuroscience. 2007;144:1361–1372. doi: 10.1016/j.neuroscience.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 39.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43:150–157. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 40.Troyer JK, Beckett ML, Wright GL., Jr Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 41.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Berger UV, Carter RE, McKee M, Coyle JT. N-acetylated alpha-linked acidic dipeptidase is expressed by non-myelinating Schwann cells in the peripheral nervous system. J. Neurocytol. 1995;24:99–109. doi: 10.1007/BF01181553. [DOI] [PubMed] [Google Scholar]
- 43.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 44.Lopes AD, Davis WL, Rosenstraus MJ, Uveges AJ, Gilman SC. Immunohistochemical and pharmacokinetic characterization of the site-specific immunoconjugate CYT-356 derived from antiprostate monoclonal antibody 7E11-C5. Cancer Res. 1990;50:6423–6429. [PubMed] [Google Scholar]
- 45.Bander NH. Technology insight: monoclonal antibody imaging of prostate cancer. Nat. Clin. Pract. Urol. 2006;3:216–225. doi: 10.1038/ncpuro0452. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem. Biophys. Res. Commun. 2009;390:624–629. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David KA, Milowsky MI, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Nanus DM, Bander NH. Clinical utility of radiolabeled monoclonal antibodies in prostate cancer. Clin. Genitourin. Cancer. 2006;4:249–256. doi: 10.3816/CGC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 48.Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 2005;11:4022–4028. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 49.Mease RC, Dusich CL, Foss CA, Ravert HT, Dannals RF, Seidel J, Prideaux A, Fox JJ, Sgouros G, Kozikowski AP, Pomper MG. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin. Cancer Res. 2008;14:3036–3043. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gala JL, Loric S, Guiot Y, Denmeade SR, Gady A, Brasseur F, Heusterspreute M, Eschwege P, De NP, Van CP, Tombal B. Expression of prostate-specific membrane antigen in transitional cell carcinoma of the bladder: prognostic value? Clin. Cancer Res. 2000;6:4049–4054. [PubMed] [Google Scholar]
- 51.Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Muhlmann G, Ofner D, Zelger B, Ensinger C, Yang XJ, Geley S, Margreiter R, Bander NH. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009;40:1754–1761. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Tavora F, Sharma R, Eisenberger M, Netto GJ. PSMA expression in Schwannoma: a potential clinical mimicker of metastatic prostate carcinoma. Urol. Oncol. 2009;27:525–528. doi: 10.1016/j.urolonc.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 54.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS, Bander NH. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007;25:540–547. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 55.Morris MJ, Pandit-Taskar N, Divgi CR, Bender S, O'Donoghue JA, Nacca A, Smith-Jones P, Schwartz L, Slovin S, Finn R, Larson S, Scher HI. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin. Cancer Res. 2007;13:2707–2713. doi: 10.1158/1078-0432.CCR-06-2935. [DOI] [PubMed] [Google Scholar]
- 56.Slusher BS, Jackson PF. Methods of cancer treatment using NAALADase inhibitors. 5,804,602 U.S. Patent. 1998 Sep 8;
- 57.Slusher BS, Lapidus RG. Pharmaceutical compositions and methods of inhibiting angiogenesis using NAALADase inhibitors. 2003/0064912 A1 U.S. Patent. 2003 Apr 3;
- 58.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. Prostate. 2006;66:867–875. doi: 10.1002/pros.20361. [DOI] [PubMed] [Google Scholar]
- 59.Yao V, Berkman CE, Choi JK, O'Keefe DS, Bacich DJ. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate. 2010;70:305–316. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 60.Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol. Cell Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao V, Parwani A, Maier C, Heston WD, Bacich DJ. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68:9070–9077. doi: 10.1158/0008-5472.CAN-08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh A, Wang X, Klein E, Heston WD. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005;65:727–731. [PubMed] [Google Scholar]
- 63.Aggarwal S, Ricklis RM, Williams SA, Denmeade SR. Comparative study of PSMA expression in the prostate of mouse, dog, monkey, and human. Prostate. 2006;66:903–910. doi: 10.1002/pros.20413. [DOI] [PubMed] [Google Scholar]
- 64.Shafizadeh TB, Halsted CH. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J. Nutr. 2007;137:1149–1153. doi: 10.1093/jn/137.5.1149. [DOI] [PubMed] [Google Scholar]
- 65.Su SL, Huang IP, Fair WR, Powell CT, Heston WD. Alternatively spliced variants of prostate-specific membrane antigen RNA: ratio of expression as a potential measurement of progression. Cancer Res. 1995;55:1441–1443. [PubMed] [Google Scholar]
- 66.Schmittgen TD, Teske S, Vessella RL, True LD, Zakrajsek BA. Expression of prostate specific membrane antigen and three alternatively spliced variants of PSMA in prostate cancer patients. Int. J Cancer. 2003;107:323–329. doi: 10.1002/ijc.11402. [DOI] [PubMed] [Google Scholar]
- 67.Grauer LS, Lawler KD, Marignac JL, Kumar A, Goel AS, Wolfert RL. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSM' protein in the LNCaP prostatic carcinoma cell line. Cancer Res. 1998;58:4787–4789. [PubMed] [Google Scholar]
- 68.Mlcochova P, Barinka C, Tykvart J, Sacha P, Konvalinka J. Prostate-specific membrane antigen and its truncated form PSM'. Prostate. 2009;69:471–479. doi: 10.1002/pros.20894. [DOI] [PubMed] [Google Scholar]
- 69.Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, Rafalski VA, Wroblewska B, Neale JH. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J. Neurochem. 2004;89:627–635. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- 70.Pangalos MN, Neefs JM, Somers M, Verhasselt P, Bekkers M, van der HL, Fraiponts E, Ashton D, Gordon RD. Isolation and expression of novel human glutamate carboxypeptidases with N-acetylated alpha-linked acidic dipeptidase and dipeptidyl peptidase IV activity. J. Biol. Chem. 1999;274:8470–8483. doi: 10.1074/jbc.274.13.8470. [DOI] [PubMed] [Google Scholar]
- 71.Bacich DJ, Ramadan E, O'Keefe DS, Bukhari N, Wegorzewska I, Ojeifo O, Olszewski R, Wrenn CC, Bzdega T, Wroblewska B, Heston WD, Neale JH. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J. Neurochem. 2002;83:20–29. doi: 10.1046/j.1471-4159.2002.01117.x. [DOI] [PubMed] [Google Scholar]
- 72.Hlouchova K, Barinka C, Konvalinka J, Lubkowski J. Structural insight into the evolutionary and pharmacologic homology of glutamate carboxypeptidases II and III. FEBS J. 2009;276:4448–4462. doi: 10.1111/j.1742-4658.2009.07152.x. [DOI] [PubMed] [Google Scholar]
- 73.Gingras R, Richard C, El-Alfy M, Morales CR, Potier M, Pshezhetsky AV. Purification, cDNA cloning, and expression of a new human blood plasma glutamate carboxypeptidase homologous to N-acetylaspartyl-alpha-glutamate carboxypeptidase/prostate-specific membrane antigen. J. Biol. Chem. 1999;274:11742–11750. doi: 10.1074/jbc.274.17.11742. [DOI] [PubMed] [Google Scholar]
- 74.Zajc T, Suban D, Rajkovic J, Dolenc I. Baculoviral expression and characterization of human recombinant PGCP in the form of an active mature dimer and an inactive precursor protein. Protein Expr. Purif. 2011;75:119–126. doi: 10.1016/j.pep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Luthi-Carter R, Barczak AK, Speno H, Coyle JT. Hydrolysis of the neuropeptide N-acetylaspartylglutamate (NAAG) by cloned human glutamate carboxypeptidase II. Brain Res. 1998;795:341–348. doi: 10.1016/s0006-8993(98)00244-3. [DOI] [PubMed] [Google Scholar]
- 76.Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, May F, Mukherjee B, Heston WD. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 77.Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha- linked acidic dipeptidase activity from rat brain. J. Biol. Chem. 1987;262:14498–14506. [PubMed] [Google Scholar]
- 78.Rajasekaran SA, Christiansen JJ, Schmid I, Oshima E, Ryazantsev S, Sakamoto K, Weinstein J, Rao NP, Rajasekaran AK. Prostate-specific membrane antigen associates with anaphase-promoting complex and induces chromosomal instability. Mol. Cancer Ther. 2008;7:2142–2151. doi: 10.1158/1535-7163.MCT-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colombatti M, Grasso S, Porzia A, Fracasso G, Scupoli MT, Cingarlini S, Poffe O, Naim HY, Heine M, Tridente G, Mainiero F, Ramarli D. The prostate specific membrane antigen regulates the expression of IL-6 and CCL5 in prostate tumour cells by activating the MAPK pathways. PLoS. One. 2009;4:e4608. doi: 10.1371/journal.pone.0004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert. Rev. Mol. Med. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halsted CH, Ling EH, Luthi-Carter R, Villanueva JA, Gardner JM, Coyle JT. Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum. Molecular characterization and relation to glutamate carboxypeptidase II. J. Biol. Chem. 1998;273:20417–20424. doi: 10.1074/jbc.273.32.20417. [DOI] [PubMed] [Google Scholar]
- 82.Devlin AM, Ling EH, Peerson JM, Fernando S, Clarke R, Smith AD, Halsted CH. Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum. Mol. Genet. 2000;9:2837–2844. doi: 10.1093/hmg/9.19.2837. [DOI] [PubMed] [Google Scholar]
- 83.Afman LA, Trijbels FJ, Blom HJ. The H475Y polymorphism in the glutamate carboxypeptidase II gene increases plasma folate without affecting the risk for neural tube defects in humans. J. Nutr. 2003;133:75–77. doi: 10.1093/jn/133.1.75. [DOI] [PubMed] [Google Scholar]
- 84.Devlin AM, Clarke R, Birks J, Evans JG, Halsted CH. Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly population. Am. J Clin. Nutr. 2006;83:708–713. doi: 10.1093/ajcn.83.3.708. [DOI] [PubMed] [Google Scholar]
- 85.Gotze T, Rocken C, Rohl FW, Wex T, Hoffmann J, Westphal S, Malfertheiner P, Ebert MP, Dierkes J. Gene polymorphisms of folate metabolizing enzymes and the risk of gastric cancer. Cancer Lett. 2007;251:228–236. doi: 10.1016/j.canlet.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 86.Halsted CH, Wong DH, Peerson JM, Warden CH, Refsum H, Smith AD, Nygard OK, Ueland PM, Vollset SE, Tell GS. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogeneous Norwegian population: the Hordaland Homocysteine Study. Am. J Clin. Nutr. 2007;86:514–521. doi: 10.1093/ajcn/86.2.514. [DOI] [PubMed] [Google Scholar]
- 87.Lievers KJ, Kluijtmans LA, Boers GH, Verhoef P, den HM, Trijbels FJ, Blom HJ. Influence of a glutamate carboxypeptidase II (GCPII) polymorphism (1561C-->T) on plasma homocysteine, folate and vitamin B(12) levels and its relationship to cardiovascular disease risk. Atherosclerosis. 2002;164:269–273. doi: 10.1016/s0021-9150(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 88.Morin I, Devlin AM, Leclerc D, Sabbaghian N, Halsted CH, Finnell R, Rozen R. Evaluation of genetic variants in the reduced folate carrier and in glutamate carboxypeptidase II for spina bifida risk. Mol. Genet. Metab. 2003;79:197–200. doi: 10.1016/s1096-7192(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 89.Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol. Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- 90.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J. Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 91.Becker I, Lodder J, Gieselmann V, Eckhardt M. Molecular characterization of N-acetylaspartylglutamate synthetase. J. Biol. Chem. 2010;285:29156–29164. doi: 10.1074/jbc.M110.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai G, Forloni G, Robinson MB, Stauch BL, Coyle JT. Calcium-dependent evoked release of N-[3H]acetylaspartylglutamate from the optic pathway. J. Neurochem. 1988;51:1956–1959. doi: 10.1111/j.1471-4159.1988.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 93.Zollinger M, Amsler U, Do KQ, Streit P, Cuenod M. Release of N-acetylaspartylglutamate on depolarization of rat brain slices. J. Neurochem. 1988;51:1919–1923. doi: 10.1111/j.1471-4159.1988.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 94.Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J. Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- 95.Wroblewska B, Santi MR, Neale JH. N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24:172–179. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 96.Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J. Neurochem. 2006;96:1071–1077. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- 97.Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J. Neurosci. 1998;18:9594–9600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas AG, Liu W, Olkowski JL, Tang Z, Lin Q, Lu XC, Slusher BS. Neuroprotection mediated by glutamate carboxypeptidase II (NAALADase) inhibition requires TGF-beta. Eur. J Pharmacol. 2001;430:33–40. doi: 10.1016/s0014-2999(01)01239-0. [DOI] [PubMed] [Google Scholar]
- 99.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J. Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- 101.Riveros N, Orrego F. A study of possible excitatory effects of N-acetylaspartylglutamate in different in vivo and in vitro brain preparations. Brain Res. 1984;299:393–395. doi: 10.1016/0006-8993(84)90727-3. [DOI] [PubMed] [Google Scholar]
- 102.Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol. Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 103.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 104.Gordon PH. Amyotrophic lateral sclerosis: pathophysiology, diagnosis and management. CNS. Drugs. 2011;25:1–15. doi: 10.2165/11586000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 105.Syed TU, Sajatovic M. Extended-release lamotrigine in the treatment of patients with epilepsy. Expert. Opin. Pharmacother. 2010;11:1579–1585. doi: 10.1517/14656566.2010.485611. [DOI] [PubMed] [Google Scholar]
- 106.Serval V, Galli T, Cheramy A, Glowinski J, Lavielle S. In vitro and in vivo inhibition of N-acetyl-L-aspartyl-L-glutamate catabolism by N-acylated L-glutamate analogs. J Pharmacol Exp Ther. 1992;260:1093–1100. [PubMed] [Google Scholar]
- 107.Subasinghe N, Schulte M, Chan MY, Roon RJ, Koerner JF, Johnson RL. Synthesis of acyclic and dehydroaspartic acid analogues of Ac-Asp-Glu-OH and their inhibition of rat brain N-acetylated alpha-linked acidic dipeptidase (NAALA dipeptidase) J. Med.Chem. 1990;33:2734–2744. doi: 10.1021/jm00172a009. [DOI] [PubMed] [Google Scholar]
- 108.Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, Trainor DA. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J. Med. Chem. 1996;39:619–622. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- 109.Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat. Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 110.Jackson PF, Slusher BS. Design of NAALADase inhibitors: a novel neuroprotective strategy. Curr Med Chem. 2001;8:949–957. doi: 10.2174/0929867013372797. [DOI] [PubMed] [Google Scholar]
- 111.Ding P, Miller MJ, Chen Y, Helquist P, Oliver AJ, Wiest O. Syntheses of conformationally constricted molecules as potential NAALADase/PSMA inhibitors. Org. Lett. 2004;6:1805–1808. doi: 10.1021/ol049473r. [DOI] [PubMed] [Google Scholar]
- 112.Cai Y, Qiu Y, Wei L, Zhang W, Hu S, Smith PR, Crabtree VP, Tong S, Thakor NV, Zhu Y. Complex character analysis of heart rate variability following brain asphyxia. Med Eng Phys. 2006;28:297–303. doi: 10.1016/j.medengphy.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 113.Cai Z, Lin S, Rhodes PG. Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur J Pharmacol. 2002;437:139–145. doi: 10.1016/s0014-2999(02)01289-x. [DOI] [PubMed] [Google Scholar]
- 114.Harada C, Harada T, Slusher BS, Yoshida K, Matsuda H, Wada K. N-acetylated-alpha-linked-acidic dipeptidase inhibitor has a neuroprotective effect on mouse retinal ganglion cells after pressure-induced ischemia. Neurosci Lett. 2000;292:134–136. doi: 10.1016/s0304-3940(00)01444-0. [DOI] [PubMed] [Google Scholar]
- 115.Long JB, Yourick DL, Slusher BS, Robinson MB, Meyerhoff JL. Inhibition of glutamate carboxypeptidase II (NAALADase) protects against dynorphin A-induced ischemic spinal cord injury in rats. Eur J Pharmacol. 2005;508:115–122. doi: 10.1016/j.ejphar.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 116.Chen SR, Wozniak KM, Slusher BS, Pan HL. Effect of 2-(phosphono-methyl)-pentanedioic acid on allodynia and afferent ectopic discharges in a rat model of neuropathic pain. J. Pharmacol. Exp. Ther. 2002;300:662–667. doi: 10.1124/jpet.300.2.662. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto T, Nozaki-Taguchi N, Sakashita Y. Spinal N-acetyl-alpha-linked acidic dipeptidase (NAALADase) inhibition attenuates mechanical allodynia induced by paw carrageenan injection in the rat. Brain Res. 2001;909:138–144. doi: 10.1016/s0006-8993(01)02650-6. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T, Nozaki-Taguchi N, Sakashita Y, Inagaki T. Inhibition of spinal N-acetylated-alpha-linked acidic dipeptidase produces an antinociceptive effect in the rat formalin test. Neuroscience. 2001;102:473–479. doi: 10.1016/s0306-4522(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 119.Thomas AG, Corse AM, Coccia CF, Bilak MM, Rothstein JD, Slusher BS. NAALADase inhibition protects motor neurons against chronic glutamate toxicity. Eur. J Pharmacol. 2003;471:177–184. doi: 10.1016/s0014-2999(03)01832-6. [DOI] [PubMed] [Google Scholar]
- 120.Luszczki JJ, Mohamed M, Czuczwar SJ. 2-phosphonomethyl-pentanedioic acid (glutamate carboxypeptidase II inhibitor) increases threshold for electroconvulsions and enhances the antiseizure action of valproate against maximal electroshock-induced seizures in mice. Eur J Pharmacol. 2006;531:66–73. doi: 10.1016/j.ejphar.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 121.Witkin JM, Gasior M, Schad C, Zapata A, Shippenberg T, Hartman T, Slusher BS. NAALADase (GCP II) inhibition prevents cocaine-kindled seizures. Neuropharmacology. 2002;43:348–356. doi: 10.1016/s0028-3908(02)00124-7. [DOI] [PubMed] [Google Scholar]
- 122.Popik P, Kozela E, Wrobel M, Wozniak KM, Slusher BS. Morphine tolerance and reward but not expression of morphine dependence are inhibited by the selective glutamate carboxypeptidase II (GCP II, NAALADase) inhibitor, 2-PMPA. Neuropsychopharmacology. 2003;28:457–467. doi: 10.1038/sj.npp.1300048. [DOI] [PubMed] [Google Scholar]
- 123.Lumley LA, Robison CL, Slusher BS, Wozniak K, Dawood M, Meyerhoff JL. Reduced isolation-induced aggressiveness in mice following NAALADase inhibition. Psychopharmacology (Berl) 2004;171:375–381. doi: 10.1007/s00213-003-1610-z. [DOI] [PubMed] [Google Scholar]
- 124.Hecker SJ, Erion MD. Prodrugs of phosphates and phosphonates. J. Med. Chem. 2008;51:2328–2345. doi: 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- 125.Majer P, Jackson PF, Delahanty G, Grella BS, Ko YS, Li W, Liu Q, Maclin KM, Polakova J, Shaffer KA, Stoermer D, Vitharana D, Wang EY, Zakrzewski A, Rojas C, Slusher BS, Wozniak KM, Burak E, Limsakun T, Tsukamoto T. Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: discovery of an orally active GCP II inhibitor. J Med. Chem. 2003;46:1989–1996. doi: 10.1021/jm020515w. [DOI] [PubMed] [Google Scholar]
- 126.Ghadge GD, Slusher BS, Bodner A, Canto MD, Wozniak K, Thomas AG, Rojas C, Tsukamoto T, Majer P, Miller RJ, Monti AL, Roos RP. Glutamate carboxypeptidase II inhibition protects motor neurons from death in familial amyotrophic lateral sclerosis models. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9554–9559. doi: 10.1073/pnas.1530168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang W, Murakawa Y, Wozniak KM, Slusher B, Sima AA. The preventive and therapeutic effects of GCPII (NAALADase) inhibition on painful and sensory diabetic neuropathy. J Neurol Sci. 2006;247:217–223. doi: 10.1016/j.jns.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 128.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 129.Grella B, Adams J, Berry JF, Delahanty G, Ferraris DV, Majer P, Ni C, Shukla K, Shuler SA, Slusher BS, Stathis M, Tsukamoto T. The discovery and structure-activity relationships of indole-based inhibitors of glutamate carboxypeptidase II. Bioorg Med Chem Lett. 2010;20:7222–7225. doi: 10.1016/j.bmcl.2010.10.109. [DOI] [PubMed] [Google Scholar]
- 130.Barinka C, Byun Y, Dusich CL, Banerjee SR, Chen Y, Castanares M, Kozikowski AP, Mease RC, Pomper MG, Lubkowski J. Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J. Med. Chem. 2008;51:7737–7743. doi: 10.1021/jm800765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T, Wroblewska B, Neale JH, Pshenichkin S, Wroblewski JT. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase) J. Med.Chem. 2001;44:298–301. doi: 10.1021/jm000406m. [DOI] [PubMed] [Google Scholar]
- 132.Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S, Wroblewska B, Bzdega T, Vicini S, Barton FB, Neale JH. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–885. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- 133.Zhong C, Zhao X, Sarva J, Kozikowski A, Neale JH, Lyeth BG. NAAG peptidase inhibitor reduces acute neuronal degeneration and astrocyte damage following lateral fluid percussion TBI in rats. J Neurotrauma. 2005;22:266–276. doi: 10.1089/neu.2005.22.266. [DOI] [PubMed] [Google Scholar]
- 134.Yamamoto T, Hirasawa S, Wroblewska B, Grajkowska E, Zhou J, Kozikowski A, Wroblewski J, Neale JH. Antinociceptive effects of N-acetylaspartylglutamate (NAAG) peptidase inhibitors ZJ-11, ZJ-17 and ZJ-43 in the rat formalin test and in the rat neuropathic pain model. Eur J Neurosci. 2004;20:483–494. doi: 10.1111/j.1460-9568.2004.03504.x. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto T, Saito O, Aoe T, Bartolozzi A, Sarva J, Zhou J, Kozikowski A, Wroblewska B, Bzdega T, Neale JH. Local administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in peripheral pain in rats. Eur J Neurosci. 2007;25:147–158. doi: 10.1111/j.1460-9568.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- 136.Harsanyi K, Domany G, Greiner I, Forintos H, Keglevich G. Synthesis of 2-phosphinoxidomethyl- and 2-phosphonomethyl glutaric acid derivatives. Heteroatom Chemistry. 2005;16:562–565. [Google Scholar]
- 137.Stoermer D, Liu Q, Hall MR, Flanary JM, Thomas AG, Rojas C, Slusher BS, Tsukamoto T. Synthesis and biological evaluation of hydroxamate-Based inhibitors of glutamate carboxypeptidase II. Bioorg. Med. Chem. Lett. 2003;13:2097–2100. doi: 10.1016/s0960-894x(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 138.Adedoyin MO, Vicini S, Neale JH. Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Mol Pain. 2010;6:60. doi: 10.1186/1744-8069-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carpenter KJ, Sen S, Matthews EA, Flatters SL, Wozniak KM, Slusher BS, Dickenson AH. Effects of GCP-II inhibition on responses of dorsal horn neurones after inflammation and neuropathy: an electrophysiological study in the rat. Neuropeptides. 2003;37:298–306. doi: 10.1016/j.npep.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 140.Majer P, Jackson PF, Delahanty G, Grella BS, Ko YS, Li W, Liu Q, Maclin KM, Polakova J, Shaffer KA, Stoermer D, Vitharana D, Wang EY, Zakrzewski A, Rojas C, Slusher BS, Wozniak KM, Burak E, Limsakun T, Tsukamoto T. Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: discovery of an orally active GCP II inhibitor. J. Med. Chem. 2003;46:1989–1996. doi: 10.1021/jm020515w. [DOI] [PubMed] [Google Scholar]
- 141.Nagel J, Belozertseva I, Greco S, Kashkin V, Malyshkin A, Jirgensons A, Shekunova E, Eilbacher B, Bespalov A, Danysz W. Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain - relation to brain concentration. Neuropharmacology. 2006;51:1163–1171. doi: 10.1016/j.neuropharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 142.Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Res. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto T, Kozikowski A, Zhou J, Neale JH. Intracerebroventricular administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in inflammatory pain. Mol Pain. 2008;4:31. doi: 10.1186/1744-8069-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang W, Slusher B, Murakawa Y, Wozniak KM, Tsukamoto T, Jackson PF, Sima AA. GCPII (NAALADase) inhibition prevents long-term diabetic neuropathy in type 1 diabetic BB/Wor rats. J Neurol Sci. 2002;194:21–28. doi: 10.1016/s0022-510x(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 145.Saito O, Aoe T, Kozikowski A, Sarva J, Neale JH, Yamamoto T. Ketamine and N-acetylaspartylglutamate peptidase inhibitor exert analgesia in bone cancer pain. Can J Anaesth. 2006;53:891–898. doi: 10.1007/BF03022832. [DOI] [PubMed] [Google Scholar]
- 146.Tortella FC, Lin Y, Ved H, Slusher BS, Dave JR. Neuroprotection produced by the NAALADase inhibitor 2-PMPA in rat cerebellar neurons. Eur J Pharmacol. 2000;402:31–37. doi: 10.1016/s0014-2999(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 147.Williams AJ, Lu XM, Slusher B, Tortella FC. Electroencephalogram analysis and neuroprotective profile of the N-acetylated-alpha-linked acidic dipeptidase inhibitor, GPI5232, in normal and brain-injured rats. J. Pharmacol. Exp. Ther. 2001;299:48–57. [PubMed] [Google Scholar]
- 148.Ghadge GD, Slusher BS, Bodner A, Canto MD, Wozniak K, Thomas AG, Rojas C, Tsukamoto T, Majer P, Miller RJ, Monti AL, Roos RP. Glutamate carboxypeptidase II inhibition protects motor neurons from death in familial amyotrophic lateral sclerosis models. Proc. Natl. Acad. Sci. U S A. 2003;100:9554–9559. doi: 10.1073/pnas.1530168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhong C, Zhao X, Van KC, Bzdega T, Smyth A, Zhou J, Kozikowski AP, Jiang J, O'Connor WT, Berman RF, Neale JH, Lyeth BG. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]
- 150.Berent-Spillson A, Robinson AM, Golovoy D, Slusher B, Rojas C, Russell JW. Protection against glucose-induced neuronal death by NAAG and GCP II inhibition is regulated by mGluR3. J Neurochem. 2004;89:90–99. doi: 10.1111/j.1471-4159.2003.02321.x. [DOI] [PubMed] [Google Scholar]
- 151.Carozzi VA, Chiorazzi A, Canta A, Lapidus RG, Slusher BS, Wozniak KM, Cavaletti G. Glutamate carboxypeptidase inhibition reduces the severity of chemotherapy-induced peripheral neurotoxicity in rat. Neurotox Res. 17:380–391. doi: 10.1007/s12640-009-9114-1. [DOI] [PubMed] [Google Scholar]
- 152.McKinzie DL, Li TK, McBride WJ, Slusher BS. NAALADase inhibition reduces alcohol consumption in the alcohol-preferring (P) line of rats. Addict. Biol. 2000;5:411–416. doi: 10.1111/j.1369-1600.2000.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 153.Kozela E, Wrobel M, Kos T, Wojcikowski J, Daniel WA, Wozniak KM, Slusher BS, Popik P. 2-MPPA, a selective glutamate carboxypeptidase II inhibitor, attenuates morphine tolerance but not dependence in C57/Bl mice. Psychopharmacology (Berl) 2005;183:275–284. doi: 10.1007/s00213-005-0182-5. [DOI] [PubMed] [Google Scholar]
- 154.Peng XQ, Li J, Gardner EL, Ashby CR, Jr, Thomas A, Wozniak K, Slusher BS, Xi ZX. Oral administration of the NAALADase inhibitor GPI-5693 attenuates cocaine-induced reinstatement of drug-seeking behavior in rats. Eur J Pharmacol. 627:156–161. doi: 10.1016/j.ejphar.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 156.Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, Li J, Gardner EL. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bacich DJ, Wozniak KM, Lu XC, O'Keefe DS, Callizot N, Heston WD, Slusher BS. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–323. doi: 10.1111/j.1471-4159.2005.03361.x. [DOI] [PubMed] [Google Scholar]
- 159.Low SJ, Roland CL. Review of NMDA antagonist-induced neurotoxicity and implications for clinical development. Int J Clin Pharmacol Ther. 2004;42:1–14. doi: 10.5414/cpp42001. [DOI] [PubMed] [Google Scholar]
- 160.Carroll PR, Coakley FV, Kurhanewicz J. Magnetic resonance imaging and spectroscopy of prostate cancer. Rev. Urol. 2006;8(Suppl 1):S4–S10. [PMC free article] [PubMed] [Google Scholar]
- 161.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 162.Jadvar H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat. Rev. Urol. 2009;6:317–323. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mease RC. Radionuclide based imaging of prostate cancer. Curr. Top. Med. Chem. 2010;10:1600–1616. doi: 10.2174/156802610793176774. [DOI] [PubMed] [Google Scholar]
- 164.Zaheer A, Cho SY, Pomper MG. New agents and techniques for imaging prostate cancer. J. Nucl. Med. 2009;50:1387–1390. doi: 10.2967/jnumed.109.061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iagaru A, Mittra E, Dick DW, Gambhir SS. Prospective Evaluation of (99m)Tc MDP Scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for Detection of Skeletal Metastases. Mol. Imaging Biol. 2011 doi: 10.1007/s11307-011-0486-2. in press. [DOI] [PubMed] [Google Scholar]
- 166.Igerc I, Kohlfurst S, Gallowitsch HJ, Matschnig S, Kresnik E, Gomez-Segovia I, Lind P. The value of 18F-choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur. J Nucl. Med. Mol. Imaging. 2008;35:976–983. doi: 10.1007/s00259-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 167.Reske SN, Blumstein NM, Glatting G. [11C]choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur. J Nucl. Med. Mol. Imaging. 2008;35:9–17. doi: 10.1007/s00259-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 168.Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, Bowman FD, Issa MM, Goodman MM. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 169.Tehrani OS, Muzik O, Heilbrun LK, Douglas KA, Lawhorn-Crews JM, Sun H, Mangner TJ, Shields AF. Tumor imaging using 1-(2'-deoxy-2'-18F-fluoro-beta-D-arabinofuranosyl)thymine and PET. J. Nucl. Med. 2007;48:1436–1441. doi: 10.2967/jnumed.107.042762. [DOI] [PubMed] [Google Scholar]
- 170.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, Welch MJ. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur. J Nucl. Med. Mol. Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 171.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, Finn RD, Erdi Y, Pentlow K, Dyke J, Squire O, Bornmann W, McCarthy T, Welch M, Scher H. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J. Nucl. Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 172.Bander NH, Nanus DM, Milowsky MI, Kostakoglu L, Vallabahajosula S, Goldsmith SJ. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin. Oncol. 2003;30:667–676. doi: 10.1016/s0093-7754(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 173.Banerjee SR, Foss CA, Castanares M, Mease RC, Byun Y, Fox JJ, Hilton J, Lupold SE, Kozikowski AP, Pomper MG. Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA) J. Med. Chem. 2008;51:4504–4517. doi: 10.1021/jm800111u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pomper MG, Musachio JL, Zhang J, Scheffel U, Zhou Y, Hilton J, Maini A, Dannals RF, Wong DF, Kozikowski AP. 11C-MCG: synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase) Mol. Imaging. 2002;1:96–101. doi: 10.1162/15353500200202109. [DOI] [PubMed] [Google Scholar]
- 175.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. J. Nucl. Med. 2009;50:2042–2048. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents. Mol. Pharm. 2009;6:790–800. doi: 10.1021/mp9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Murphy GP, Maguire RT, Rogers B, Partin AW, Nelp WB, Troychak MJ, Ragde H, Kenny GM, Barren RJ, III, Bowes VA, Gregorakis AK, Holmes EH, Boynton AL. Comparison of serum PSMA, PSA levels with results of Cytogen-356 ProstaScint scanning in prostatic cancer patients. Prostate. 1997;33:281–285. doi: 10.1002/(sici)1097-0045(19971201)33:4<281::aid-pros9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 178.Nagda SN, Mohideen N, Lo SS, Khan U, Dillehay G, Wagner R, Campbell S, Flanigan R. Long-term follow-up of 111In-capromab pendetide (ProstaScint) scan as pretreatment assessment in patients who undergo salvage radiotherapy for rising prostate-specific antigen after radical prostatectomy for prostate cancer. Int. J Radiat. Oncol. Biol. Phys. 2007;67:834–840. doi: 10.1016/j.ijrobp.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 179.Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J. Urol. 2004;172:133–136. doi: 10.1097/01.ju.0000132138.02846.08. [DOI] [PubMed] [Google Scholar]
- 180.Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J. Nucl. Med. 2003;44:610–617. [PubMed] [Google Scholar]
- 181.Yao D, Trabulsi EJ, Kostakoglu L, Vallabhajosula S, Joyce MA, Nanus DM, Milowsky M, Liu H, Goldsmith SJ. The utility of monoclonal antibodies in the imaging of prostate cancer. Semin. Urol. Oncol. 2002;20:211–218. doi: 10.1053/suro.2002.36250. [DOI] [PubMed] [Google Scholar]
- 182.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 183.McDevitt MR, Barendswaard E, Ma D, Lai L, Curcio MJ, Sgouros G, Ballangrud AM, Yang WH, Finn RD, Pellegrini V, Geerlings MW, Jr, Lee M, Brechbiel MW, Bander NH, Cordon-Cardo C, Scheinberg DA. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60:6095–6100. [PubMed] [Google Scholar]
- 184.Pandit-Taskar N, O'Donoghue JA, Morris MJ, Wills EA, Schwartz LH, Gonen M, Scher HI, Larson SM, Divgi CR. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J. Nucl. Med. 2008;49:1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, Bander NH. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237–5243. [PubMed] [Google Scholar]
- 186.Vallabhajosula S, Smith-Jones PM, Navarro V, Goldsmith SJ, Bander NH. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): studies in nude mice. Prostate. 2004;58:145–155. doi: 10.1002/pros.10281. [DOI] [PubMed] [Google Scholar]
- 187.Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky MI, Nanus DM, Bander NH. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin. Cancer Res. 2005;11:7195–7200. doi: 10.1158/1078-0432.CCR-1004-0023. [DOI] [PubMed] [Google Scholar]
- 188.Vallabhajosula S, Goldsmith SJ, Hamacher KA, Kostakoglu L, Konishi S, Milowski MI, Nanus DM, Bander NH. Prediction of myelotoxicity based on bone marrow radiation-absorbed dose: radioimmunotherapy studies using 90Y- and 177Lu-labeled J591 antibodies specific for prostate-specific membrane antigen. J. Nucl. Med. 2005;46:850–858. [PubMed] [Google Scholar]
- 189.Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, Milowski MI, Nanus DM, Bander NH, Goldsmith SJ. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J. Nucl. Med. 2005;46:634–641. [PubMed] [Google Scholar]
- 190.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alt K, Wiehr S, Ehrlichmann W, Reischl G, Wolf P, Pichler BJ, Elsasser-Beile U, Buhler P. High-resolution animal PET imaging of prostate cancer xenografts with three different 64Cu-labeled antibodies against native cell-adherent PSMA. Prostate. 2010;70:1413–1421. doi: 10.1002/pros.21176. [DOI] [PubMed] [Google Scholar]
- 192.Elsasser-Beile U, Reischl G, Wiehr S, Buhler P, Wolf P, Alt K, Shively J, Judenhofer MS, Machulla HJ, Pichler BJ. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J. Nucl. Med. 2009;50:606–611. doi: 10.2967/jnumed.108.058487. [DOI] [PubMed] [Google Scholar]
- 193.Wolf P, Freudenberg N, Buhler P, Alt K, Schultze-Seemann W, Wetterauer U, Elsasser-Beile U. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate. 2010;70:562–569. doi: 10.1002/pros.21090. [DOI] [PubMed] [Google Scholar]
- 194.Javier DJ, Nitin N, Levy M, Ellington A, Richards-Kortum R. Aptamer-targeted gold nanoparticles as molecular-specific contrast agents for reflectance imaging. Bioconjug. Chem. 2008;19:1309–1312. doi: 10.1021/bc8001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Synthesis and radiolabeling of chelator-RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorg. Med. Chem. 2011;19:4080–4090. doi: 10.1016/j.bmc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu T, Wu LY, Kazak M, Berkman CE. Cell-Surface labeling and internalization by a fluorescent inhibitor of prostate-specific membrane antigen. Prostate. 2008;68:955–964. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 197.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Green G, Fox JJ, Horti A, Mease RC, Pomper MG. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J. Med. Chem. 2010;53:5333–5341. doi: 10.1021/jm100623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, Zimmerman CN, Barrett JA, Eckelman WC, Pomper MG, Joyal JL, Babich JW. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guilarte TR, Hammoud DA, McGlothan JL, Caffo BS, Foss CA, Kozikowski AP, Pomper MG. Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophr. Res. 2008;99:324–332. doi: 10.1016/j.schres.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, Becker M, Marquez C, Knox S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289–1297. [PubMed] [Google Scholar]
- 201.Henry MD, Wen S, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64:7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 202.Huang X, Bennett M, Thorpe PE. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate. 2004;61:1–11. doi: 10.1002/pros.20074. [DOI] [PubMed] [Google Scholar]
- 203.Ma JY, Medicherla S, Kerr I, Mangadu R, Protter AA, Higgins LS. Selective p38alpha mitogen-activated protein kinase inhibitor attenuates lung inflammation and fibrosis in IL-13 transgenic mouse model of asthma. J Asthma Allergy. 2008;1:31–44. doi: 10.2147/jaa.s4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Russell PJ, Hewish D, Carter T, Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira D, Molloy PL, Werkmeister JA, Kortt AA. Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivo studies. Cancer Immunol. Immunother. 2004;53:411–421. doi: 10.1007/s00262-003-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wolf P, Alt K, Buhler P, Katzenwadel A, Wetterauer U, Tacke M, Elsasser-Beile U. Anti-PSMA immunotoxin as novel treatment for prostate cancer? High and specific antitumor activity on human prostate xenograft tumors in SCID mice. Prostate. 2008;68:129–138. doi: 10.1002/pros.20684. [DOI] [PubMed] [Google Scholar]
- 206.Wolf P, Alt K, Wetterauer D, Buhler P, Gierschner D, Katzenwadel A, Wetterauer U, Elsasser-Beile U. Preclinical evaluation of a recombinant anti-prostate specific membrane antigen single-chain immunotoxin against prostate cancer. J. Immunother. 2010;33:262–271. doi: 10.1097/CJI.0b013e3181c5495c. [DOI] [PubMed] [Google Scholar]
- 207.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 208.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J. Med. Chem. 2011;54:1321–1332. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 209.Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-Guided Prostate Cancer Therapy Using Aptamer-Functionalized Thermally Cross-Linked Superparamagnetic Iron Oxide Nanoparticles. Small. 2011;7:2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- 210.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Murelli RP, Zhang AX, Michel J, Jorgensen WL, Spiegel DA. Chemical control over immune recognition: a class of antibody-recruiting small molecules that target prostate cancer. J. Am. Chem. Soc. 2009;131:17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]