Abstract
The aims of this study were designed to determine whether liraglutide, a long-acting glucagon-like peptide, could reverse the adverse effects of a diet high in fat that also contained trans-fat and high-fructose corn syrup (ALIOS diet). Specifically, we examined whether treatment with liraglutide could reduce hepatic insulin resistance and steatosis as well as improve cardiac function. Male C57BL/6J mice were pair fed or fed ad libitum either standard chow or the ALIOS diet. After 8 wk the mice were further subdivided and received daily injections of either liraglutide or saline for 4 wk. Hyperinsulinemic-euglycemic clamp studies were performed after 6 wk, revealing hepatic insulin resistance. Glucose tolerance and insulin resistance tests were performed at 8 and 12 wk prior to and following liraglutide treatment. Liver pathology, cardiac measurements, blood chemistry, and RNA and protein analyses were performed. Clamp studies revealed hepatic insulin resistance after 6 wk of ALIOS diet. Liraglutide reduced visceral adiposity and liver weight (P < 0.001). As expected, liraglutide improved glucose and insulin tolerance. Liraglutide improved hypertension (P < 0.05) and reduced cardiac hypertrophy. Surprisingly, liver from liraglutide-treated mice had significantly higher levels of fatty acid binding protein, acyl-CoA oxidase II, very long-chain acyl-CoA dehydrogenase, and microsomal triglyceride transfer protein. We conclude that liraglutide reduces the harmful effects of an ALIOS diet by improving insulin sensitivity and by reducing lipid accumulation in liver through multiple mechanisms including, transport, and increase β-oxidation.
Keywords: fatty acid binding protein, fatty acids, microsomal triglyceride transfer protein
obesity, type 2 diabetes (T2DM), and related comorbidities are at epidemic levels and pose a major public health concern. According to data collected from the National Health and Nutrition Examination Survey, the number of Americans classified as obese (body mass index ≥30) continues to increase, with the greatest increases occurring in adult men (30.4%) and among children aged 6 through 19 yr (16.0%) (16). The prevalence of T2DM has dramatically increased, affecting ∼20.8 million people in the United States, and it is estimated that by the year 2050 persons diagnosed with diabetes will increase by 165% (7, 26). Accompanying obesity and insulin resistance is a constellation of metabolic derangements, including nonalcoholic fatty liver disease (NAFLD), hypertension, hypertriglyceridemia, low high-density lipoproteins, that are collectively referred to as metabolic syndrome.
Epidemiological studies in the United States have suggested that a Western diet (high in saturated fat, trans-fat, and sugar) is strongly associated with the increasing prevalence of NAFLD and cardiovascular disease (CVD) (32). In addition, diets containing trans-fat and high-fructose corn syrup (HFCS) have been shown independently to increase insulin resistance, fat mass, and liver lipid accumulation in rodents and humans (29, 30, 32, 44). Although dietary changes and moderate exercise are effective in treating obesity and diabetes, pharmacological treatments that target multiple aspects of insulin resistance/obesity-induced metabolic abnormalities including NAFLD are warranted.
Until recently there was not a suitable mouse model that mimicked the Western diet-induced pathophysiology observed in human populations. Tetri et al. (45) reported that the American lifestyle-induced obesity syndrome (ALIOS) model resulted in C57BL/6J mice at 16 wk having severe hepatic necroinflammatory damage. No data, however, were provided on the effect on the cardiovascular system. Tetri's diet was composed of macronutrients similar to what is contained in an American fast-food diet, with 45% of calories derived from fat. Additional calories are derived from HFCS.
Recently our laboratory published studies that demonstrated glucagon-like peptide 1 (GLP-1) and its cognate receptor GLP-1R are present and functional on human hepatocytes (15). Moreover, we demonstrated that exendin-4, a long-acting GLP-1 analog, increased phosphorylation of PDK-1, AKT, and PKC-ζ. These data suggest that, in addition to its pancreatic properties previously described, GLP-1 analogs have direct insulin-sensitizing effects in hepatocytes (15). The aim of the present study was to determine whether liraglutide (LG), a GLP-1 long-acting analog, could reverse the effects of the ALIOS diet as it relates to the development of metabolic syndrome in mice. Furthermore, our data provide new information related to the role of GLP-1 proteins, which promote improved cardiac function, as well as new information as to how GLP-1 improves hepatocyte insulin resistance by increasing hepatic lipid flux.
In this study we demonstrate that 1) hepatic insulin resistance in ALIOS-fed mice antedates peripheral insulin resistance and cardiac injury; 2) ALIOS mice develop cardiac hypertrophy within 12 wk of feeding; and 3) LG treatment in ALIOS-fed mice reversed diet-induced metabolic dysfunction by improved hepatic lipid handling and restoring insulin sensitivity.
RESEARCH DESIGN AND METHODS
Animal studies.
Fifty male C57BL/J6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). The 4- to 5-wk-old animals were cared for in accordance with protocols approved by the Animal Care and Use Committee of Emory University. For hyperinsulinemic-euglycemic clamp studies, mice were cared for in accordance with the protocols approved by the Vanderbilt Animal Care and Use Committee. Animals were housed in laboratory cages at 23°C under a 12-h light-dark cycle.
Mice were fed either standard chow or the ALIOS diet as described by Tetri et al. (45). The ALIOS diet derives 45% of its calories from fat, with 30% of the fat in the form of partially hydrogenated vegetable oil (28% saturated; 57% monounsaturated fatty acids; 13% polyunsaturated fatty acids; trans-fat custom diet TD06303, Harlan Teklad) (45). ALIOS mice were also given HFCS equivalents in their drinking water at 42 g/l (55% fructose, 45% glucose by weight). Food and water consumption was measured by weighing new and remaining food and water three times weekly.
Following 8 wk on the diet, mice were injected intraperitoneally with saline or LG (200 μg/kg) dissolved in saline (Novo Nordisk, Princeton, NJ) daily for 4 wk. At the onset and termination of the study, fasting blood samples were obtained. At necropsy liver and heart samples were snap frozen in liquid nitrogen and stored at −80°C.
Pathology.
Tissue was prepared as describe by Ding et al. (11). Briefly, liver and heart were removed, weighed, and divided into three sections: cryosections, formalin-fixed sections, and frozen samples. Hematoxylin and eosin stain (formalin fixed embedded in paraffin) and immunohistochemistry (IHC) were performed on the cryosections. Frozen samples were used for RNA analysis and Oil Red O staining. Visceral fat was removed, weighed, and stored at −80°C (11, 15). Heart tissue was excised with great vessels and atria trimmed away before cardiac weights were obtained. The tibias were removed and their length was measured in millimeters.
Glucose and insulin tolerance test.
To obtain glucose levels, mice were fasted for 6–8 h. Glucose (2 g/kg) was then administered intraperitoneally via a 27-gauge insulin syringe. Glucose levels were measured at 0, 15, 30, 60, 90, and 120 min by tail vein sampling with portable glucometer. Insulin tolerance was measured as described previously (45). Briefly, mice were fasted for 6 h and injected intraperitoneally with 0.6 units/kg human regular insulin at a concentration of 0.2 units/ml with a 27-gauge insulin syringe. Glucose levels were measured by tail vein sampling with portable glucometer at 0, 15, 30, 45, and 60 min. Glucose levels were measured and insulin tolerance tests were conducted prior to the administration of LG and saline and at the termination of the study.
Hyperinsulinemic-euglycemic clamp.
The hyperinsulinemic-euglycemic clamp procedures were performed by the National Institutes of Health Mouse Metabolic Phenotyping Center at Vanderbilt University as described by others (3, 34). Briefly, a euglycemic-hyperinsulinemic clamp study was performed in mice fed either standard or ALIOS diet for 6 wk. Catheters were implanted in the carotid artery and the jugular vein of mice for sampling and infusions 5 days before a study. Only mice that returned to within 10% of presurgical body weight were studied. Insulin clamps were performed on 5-h fasted mice (3, 19). [3-3H] glucose was primed (0.8 μCi) and continuously infused for a 100-min equilibration period (0.04 μCi/min) for 2-h to achieve a rate of infusion of (0.08 μCi/min). Baseline blood or plasma parameters were determined in blood samples collected at time (t) − 10 and 0 min. After the sample at t = 0 was taken an insulin infusion (2.5 mU·kg−1·min−1) was started and continued for 120 min. Blood glucose was clamped at ∼120 mg/dl using a variable rate of glucose infusion (GIR). Mice received heparinized saline-washed erythrocytes from donors (5 μl/min) to prevent a fall of hematocrit. Blood glucose was monitored every 10 min, and the GIR was adjusted as needed. Blood was taken at 80–120 min for the determination of [3-3H]glucose. Insulin was determined at t = 0 and 120 min.
Blood chemistry.
Blood drawn from the submandibular vein was used to measure alanine aminotransferase (ALT), aspartate aminotransferase, cholesterol, adiponectin, and triglycerides. Lipids were measured on the CX7 chemistry autoanalyzer (Beckman Coulter Diagnostics, Miami, FL). Adiponectin was measured by ELISA (Immuno-Biological Laboratories, Minneapolis, MN).
Echocardiography and blood pressure.
Mice were anesthetized with 1.5% isoflurane, and echocardiography was performed with ultrasonography (Sequoia 512, Acuson 15L8, Oceanside, CA). A 15-MHz linear ultrasound transducer was used. The 2D guided M-mode measurements of the left ventricular internal diameter were taken from three beats and averaged. Blood pressures were measured by tail cuff by use of Visitech Systems BP-2000 (Apex, NC).
RNA analysis.
Total RNA was extracted from liver tissue using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). An equal amount of total RNA was used to synthesize the first DNA strand by iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Real-time PCR was performed with the Eppendorf Realplex4 (Hauppauge, NY), by SYBR green detection. All samples were run in triplicate, PCR products were normalized to 18s rRNA expression levels.
Immunoblot.
Equal amounts of protein from tissue lysates were resolved on SDS-PAGE (38), transblotted, and subjected to immunodetection using primary antibody for microsomal triglyceride transfer protein (MTTP; Santa Cruz), liver fatty acid binding protein (LFABP; Santa Cruz), and medium-chain acyl-CoA dehydrogenase (MCAD) and long-chain acyl-CoA dehydrogenase (LCAD) (Abcam, Cambridge, MA).
Statistical analysis.
The data are presented as means ± SE. Statistical analysis was performed by using JMP v.8.01 (SAS Institute, Cary, NC). Means were compared by Student's t-test. The Tukey-Kramer test was also used to determine whether any groups differed significantly from each other.
RESULTS
ALIOS diet increased weight and adiposity in mice independent of caloric intake.
The caloric intake between the groups was not significantly different (Table 1). Despite relatively similar caloric intake patterns between the groups, the ALIOS-fed mice gained significantly more weight (Table 1) P < 0.0001. In a separate experiment mice were pair-fed (ALIOS mice were calorically matched to control mice), and the ALIOS mice still gained significantly more weight than the control-fed animals (data not shown). The ALIOS-fed mice group gained an average of 8.2% of their previous week's weight compared with the control-fed animals, which gained an average of 5.4% of their previous week's weight.
Table 1.
Energy consumption and body weight before liraglutide treatment administration
| Average Calories | Body Weight at 8 wk | |
|---|---|---|
| Control | 160.97 ± 14.0 kcal/wk | 25.99 ± 0.33 g |
| ALIOS | 167.78 ± 7.949 kcal/wk | 32.78 ± 0.74 g* |
Values are means ± SE (n = 14 per group).
ALIOS, American lifestyle-induced obesity syndrome diet.
Student's t-test was used to analyze differences;
Statistically significant difference, P < 0.0001.
LG reduces adiposity and liver weight but does not significantly affect body weight loss or adiponectin levels.
During the 4-wk treatment period with LG or saline all mouse cohorts except the ALIOS+saline group lost a small amount weight (Fig. 1). The ALIOS+saline group continued to gain weight during the first 21 days of saline injections; however, this cohort lost weight during the last week of injections (Fig. 1), which may have been due to increased handling. The ALIOS+LG group had no significant change in weight during the 4 wk of injections. Consequently the significant difference in weights observed (Table 2) between the ALIOS groups (saline vs. LG) is a result of the ALIOS+saline group continuing to gain weight throughout the treatment period. There was no significant difference between control+saline and the control+LG groups (Table 2).
Fig. 1.
Liraglutide (LG) injections did not significantly reduce weight. A: during injections all groups lost a small amount of weight except the American lifestyle-induced obesity syndrome diet (ALIOS) + saline-injection group, which continued to gain weight for the majority of the treatment period. The rate of weight gain, however, was not significant for any of the 4 cohorts. Student's t-test was used to analyze difference (n = 7 per group).
Table 2.
Liraglutide reduces visceral adiposity and liver weight, but has no effect on adiponectin levels, in mice fed the ALIOS diet
| Final Body Weight, g | Visceral Fat Weight, g | Liver Weight, g | Adiponectin, μg/ml | |
|---|---|---|---|---|
| Control saline | a26.04 ± 1.13 | a1.805 ± 0.15 | a0.81 ± 0.07 | a16.8 ± 1.17 |
| Control liraglutide | a27.90 ± 0.56 | a1.99 ± 0.16 | a0.86 ± 0.05 | a15.2 ± 0.67 |
| ALIOS saline | b35.06 ± 1.06 | b4.78 ± 0.07 | b1.2 ± 0.04 | b12.4 ± 1.04 |
| ALIOS liraglutide | c31.2 ± 0.25 | c3.60 ± 0.06 | c1.06 ± 0.02 | ab13.8 ± 0.9 |
Values are means ± SE (n = 7 per group).
Superscript letters indicate significant differences between treatment groups; P < 0.001.
Groups with the same letter indicate no significant difference.
Student's t-test was used to analyze differences.
The ALIOS diet resulted in a significant increase in visceral adipose tissue (VAT) (Table 2). The ALIOS+LG group had significantly less VAT compared with the ALIOS+saline-treated mice (P < 0.0001). However, both ALIOS groups had significantly larger VAT than the control chow cohorts, regardless of treatment or placebo (Table 2). A similar pattern was observed in the liver weights of the animals (Table 2). The ALIOS+saline mouse livers were significantly heavier than the ALIOS+ LG mouse livers (P < 0.001). The ALIOS+saline group had a significantly lower level of serum adiponectin compared with controls (P < 0.01, Table 2); LG treatment did not significantly increase the level of serum adiponectin in mice.
ALIOS-fed mice developed hepatic insulin resistance before systemic insulin resistance.
Using hyperinsulinemic-euglycemic clamp studies, the gold standard for detecting hepatic insulin resistance, we determined whether the development of hepatic insulin resistance in mice preceded systemic insulin resistance. Insulin sensitivity/action was assessed in conscious mice by using the insulin clamp (Fig. 2).
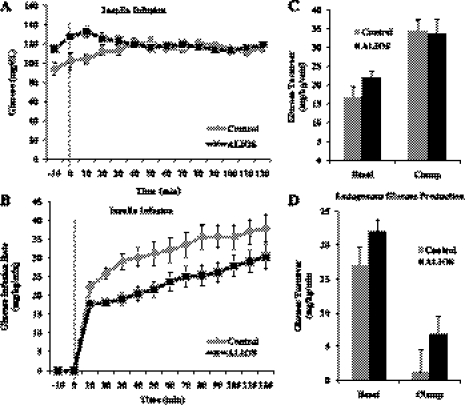
Fig. 2.
Mice fed the ALIOS diet develop hepatic insulin resistance within 6 wk. A: glucose turnover. B: arterial blood glucose during clamp: no significant difference. ALIOS mice required significantly less glucose infusion (P < 0.01). C: endogenous glucose turnover: no significant difference. D: glucose infusion rate during clamp study: ALIOS group required significantly higher amounts of insulin (P < 0.05). Data are presented as means ± SE. Student's t-test was used to analyze difference (n = 11 per group).
At 6 wk, ALIOS mice had significantly higher fasting glucose levels than controls (Fig. 2A) (ALIOS: 121 ± 3 mg/dl vs. control: 98 ± 8.0 mg/dl; P < 0.01); however, there was no difference in their basal levels of insulin (control: 1.8 ± 0.3 ng/dl; ALIOS 2.5 ± 0.3 ng/dl). During the clamp (Fig. 2B), the ALIOS mice required significantly less glucose infusion (∼30% less) compared with the control group (P < 0.01).
The glucose infusion rate is used to measure the ability of insulin to suppress hepatic glucose production and to increase glucose uptake by tissues. In examining the total glucose turnover rate (Fig. 2C) there was no significant difference between any treatment group during the basal and clamp portion of the study, suggesting that insulin sensitivity in peripheral tissues could not explain the lower glucose requirements during the clamp.
Assessing hepatic insulin resistance.
Basal endogenous glucose turnover was 24% higher in the ALIOS group; however, this result did not reach statistical significance (Fig. 2C). During the clamp ALIOS mice required significantly higher levels of insulin compared with control mice (ALIOS: 5.3 ± 0.5 ng/dl vs. control: 3.3 ± 0.6 ng/dl, P < 0.05), and the ability of insulin to suppress endogenous glucose production was impaired (Fig. 2D), indicating that the liver was resistant to the action of insulin. Thus failure of insulin to adequately suppress hepatic glucose production during the clamp explains the lower glucose requirements during the clamp.
Since insulin concentration during the clamp was higher in the ALIOS group but whole body glucose utilization was not increased, the ALIOS diet may modestly attenuate peripheral insulin action.
LG significantly improved glucose handling and insulin sensitivity in ALIOS-treated mice.
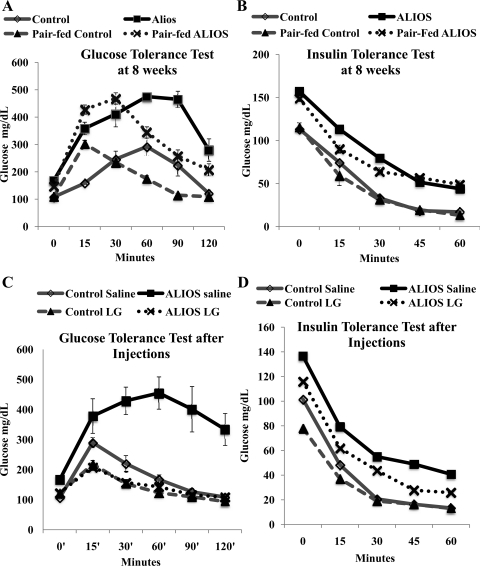
At 8 wk of feeding the ALIOS mice had significantly higher fasting glucose levels compared with control mice (Fig. 3A) (167 ± 10.15 vs. 107.5 ± 4.32 mg/dl P < 0.0001). In pair-fed groups this trend persisted (147 ± 3.069 vs. 108.37 ± 4.17 mg/dl P < 0.0005), indicating this was a caloric-independent observation. The glucose and insulin tolerance tests demonstrated at the start of the treatment period that the ALIOS mice had impaired glucose handling and were insulin insensitive (Fig. 3, A and B). Pair feeding did not result in a significant changes glucose tolerance or insulin sensitivity (Fig. 3, A and B).
Fig. 3.
Liraglutide treatment dramatically improves glucose uptake and insulin sensitivity. A and B: glucose and insulin tolerance tests performed at 8 wk. Mice were fed ad libitum (control or ALIOS diet n = 7 per group) or pair-fed (ALIOS mice received the same amount of kcal as the control group consumed, n = 8) for 8 wk. Both the ad libitum and pair-fed ALIOS groups had significantly higher fasting glucose levels compared with their control counterparts (P < 0.001). Area under the curve (AUC) analysis revealed no significant difference between ad libitum and pair-fed groups. The ALIOS-fed mice had significantly larger AUC than their control counterparts for both the glucose and insulin tolerance tests. C and D: glucose and insulin tolerance tests at the end of the study period (12 wk). The ALIOS-fed liraglutide-treated mice had significantly reduced fasting blood glucose compared with the ALIOS-fed saline-treated mice (P < 0.0001). In the glucose tolerance test there was significant reduction in AUC for the ALIOS-liraglutide group compared with the ALIOS-saline group (P < 0.001). Data are presented as means ± SE.
LG treatments (ALIOS+LG) significantly improved fasting serum glucose levels compared with ALIOS+saline-injected mice (Fig. 3C) (122.2 ± 6.17 vs. 166 ± 50.73 mg/dl, P < 0.0001) and significantly improved glucose tolerance (P < 0.05). As expected, mice receiving LG had significantly higher insulin levels than their saline counterparts: control+saline vs. control+LG (585.16 ± 80.7 vs. 3,106.64 pg/dl ± 1,094.3, P < 0.05); ALIOS+saline vs. ALIOS+LG (1,459.82 ± 618.9 vs. 3,823.66 ± 564.1 pg/dl, P < 0.05). However, there was no significant difference in insulin levels between the control+LG and ALIOS+LG, yet the ALIOS+LG had significantly improved insulin sensitivity (P < 0.05).
LG treatment prevented ALIOS-induced pathology and lipid accumulation in the liver.
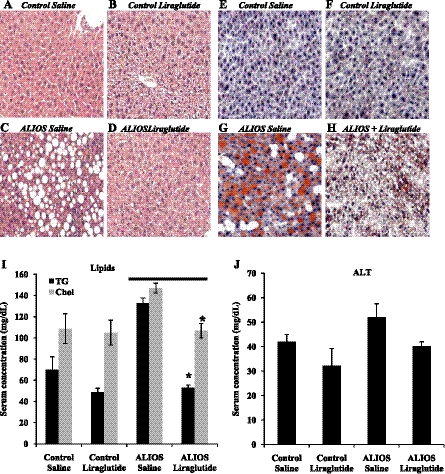
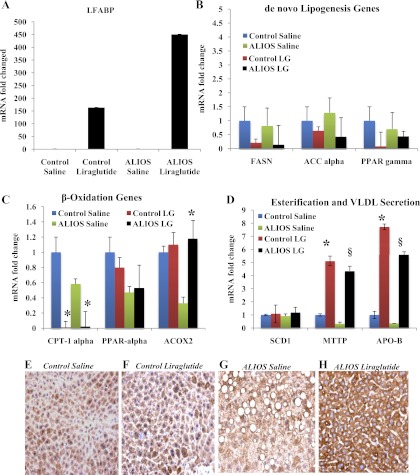
Control mice that were fed standard chow and received either saline or LG injections for 12 wk (Fig. 4, A and B) revealed no evidence of steatosis or nonalcoholic steatohepatitis (NASH). As anticipated in mice fed standard chow, there was no significant difference between either the LG-treated or the saline-treated mice (Fig. 4, A and B). In contrast ALIOS mice injected with saline showed significant pathology (Fig. 4C) including marked hepatic steatosis and ballooning degeneration of hepatocytes, and Oil Red O staining revealed significant lipid accumulation (Fig. 4G). LG treatment significantly reduced hepatic lipid accumulation in the ALIOS+LG-fed mice (Fig. 4H). The ALIOS+saline group had significantly higher serum triglyceride and cholesterol levels (Fig. 4I) (132.5 ± 5.29 and 147 ± 4.58 mg/dl, respectively). LG treatment reduced serum triglyceride and cholesterol levels in the ALIOS-fed mice (52.88 ± 2.78 mg/dl, P < 0.001 and 106.8 ± 6.82 mg/dl, P < 0.05, respectively). The ALIOS+saline group had higher ALT values than control saline; LG treatment reduced ALT values in both control and ALIOS-fed mice (P = 0.09).
Fig. 4.
Liraglutide reduces hepatic steatosis and improves liver function. Hepatic steatosis was observed using Oil Red O stain and liver function detected by lipid and ALT concentrations in serum. A–D: liver histology; hematoxylin and eosin stain (×200). Mice fed the ALIOS diet for 12 wk showed significant steatosis (C). ALIOS mice receiving liraglutide show dramatically reduced steatosis (D). E and F: there is little lipid accumulation in livers of mice fed control diet. ALIOS mice injected with saline had significant amount of lipid accumulation (G), whereas liraglutide treatment (H) significantly reduced the amount of lipid found in ALIOS-fed mice. I: liraglutide treatment significantly reduced serum levels of triglyceride (TG) and total cholesterol (Chol) (n = 7 per group). ALIOS-fed liraglutide group had significantly lower serum triglycerides (*P < 0.001) and total cholesterol (*P < 0.05) compared with ALIOS-fed saline-treated mice. J: there was no difference in alanine aminotransferase (ALT) values between control-fed groups, whereas ALIOS liraglutide group trended downward, but this reduction was not significant (P = 0.09) (n = 7 per group).
LG treatment reduced hypertension and cardiac hypertrophy.
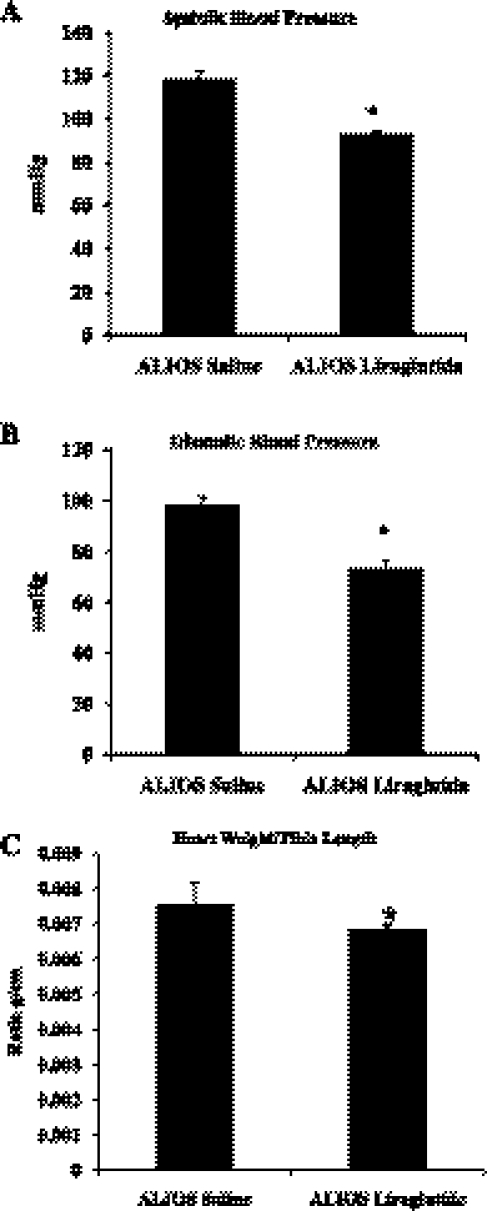
At termination, the ALIOS+saline-treated mice were found to have significantly higher systolic and diastolic blood pressure (Fig. 5, A and B, respectively). LG treatment significantly reduced systolic blood pressure (117.7 ± 3.40 vs. 92.46 ± 3.11 mmHg, P < 0.0001) and diastolic blood pressure (98.32 ± 4.27 vs. 73.30 ± 1.60 mmHg, P < 0.0001). ALIOS+saline-treated mice also had cardiac hypertrophy (Fig. 5C). The mean cardiac weight of the ALIOS+saline mice was significantly heavier than the control saline group (0.00759 ± 0.00027 vs. 0.00651 ± 0.00016 g/cm, P < 0.001). LG treatment significantly reduced cardiac hypertrophy in the ALIOS mice (0.00651 ± 0.00014 g/cm, P < 0.05).
Fig. 5.
Liraglutide treatment reduces blood pressure and reduces cardiac hypertrophy. A: systolic blood pressure. B: diastolic blood pressure. C: cardiac hypertrophy: mouse heart weight was normalized to tibia length to account for growth. ALIOS-fed mice treated with saline had significantly increased heart weight than all other groups (*P < 0.05). Liraglutide treatment of ALIOS-fed mice significantly reduce their heart weights (*P < 0.05) (n = 7 per group).
LG treatment significantly increased mRNA and proteins related to fatty acid uptake, peroxisomal β-oxidation, and VLDL transport.
Quantitative RT-PCR was performed on RNA harvested from mouse livers. IHC and Western blots were also performed. LG treatment resulted in a significant increase mRNA in LFABP, a protein critical for fatty acid uptake (Fig. 6A), in both control and ALIOS-fed mice (P < 0.0001). IHC of liver samples (Fig. 6, E and F), revealed that LG strongly upregulated LFABP compared with saline-treated ALIOS-fed mice. LG reduced mRNA of genes associated with de novo lipogenesis including FASN, ACC-α, and PPAR-γ; however, these reductions were not statistically significant (Fig. 6B). LG increased genes and proteins responsible for peroxisomal fatty acid β-oxidation (ACOX2) (Fig. 6C) in ALIOS-fed mice (P < 0.05), whereas decreasing genes related to mitochondrial β-oxidation (CPT-1α) in both control and ALIOS-fed mice (P < 0.05).
Fig. 6.
Liraglutide increases genes related to fatty acid uptake, β-oxidation, and VLDL transport. A: liraglutide treatment resulted in a significant increase of liver fatty acid binding protein (LFABP) in both control and ALIOS-fed mice (P < 0.0001). B: fatty acid synthase (FASN), acyl CoA-carboxylase-α (ACC-α), and PPAR-γ were all downregulated following liraglutide treatment in ALIOS-fed mice although these did not reach statistical significance compared with the ALIOS-fed saline-treated cohort. C: CPT-1α was downregulated after liraglutide treatment in both the control and ALIOS-fed groups. In the ALIOS-fed liraglutide group ACOX2 was strongly upregulated (*P < 0.05). D: there was no significant change in the expression of SCD1 between control and liraglutide treated groups; however, ALIOS-fed liraglutide mice had significant increase in both microsomal triglyceride transfer protein (MTTP) and ApoB mRNA expression (*P < 0.0001; §P < 0.0001) when treated with liraglutide. E–H: LFABP was evaluated by immunohistochemistry. Liraglutide treatment resulted in a significant increase in LFABP expression in liver tissue.
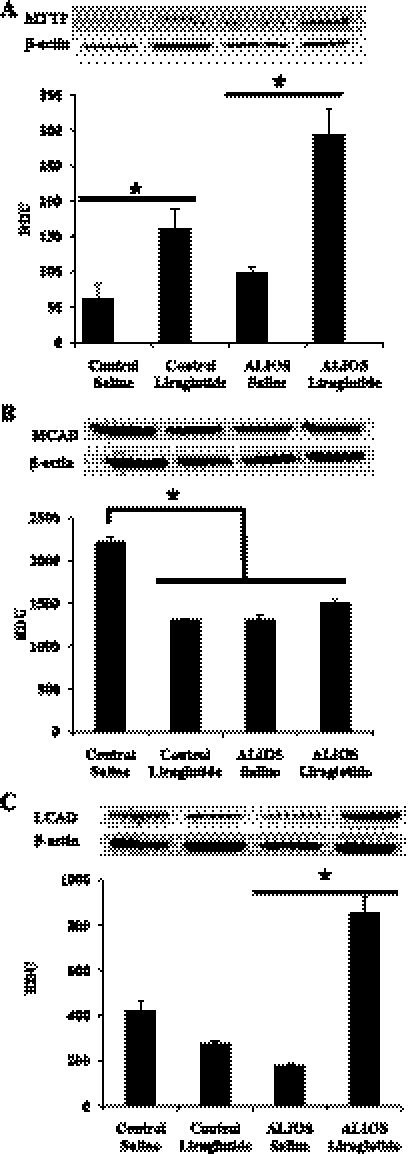
LG treatment also increased mRNA expression and protein for MTTP, a key gene involved in lipid transport (Fig. 6D) (mRNA: P < 0.0001) and (Fig. 7A) (protein: P < 0.001). There was similar mRNA expression of ApoB (P < 0.0001).
Fig. 7.
Liraglutide increases protein related to fatty acid uptake, β-oxidation, and VLDL transport. A: liraglutide treatment significantly increased MTTP expression in both the control and ALIOS-fed groups and also significantly increased MTTP mRNA protein expression in the ALIOS-fed liraglutide-treated mice compared with saline-treated ALIOS-fed mice (*P < 0.001). B: liraglutide has no effect on medium-chain acyl-CoA dehydrogenase (MCAD) expression; control had a significantly higher level of expression than all other groups (*P < 0.05). C: liraglutide treatment significantly increased very long-chain acyl-CoA dehydrogenase (LCAD) expression in the ALIOS-fed group (*P < 0.0001). RDU, relative densitometry units.
Because the ALIOS diet included trans-fat we examined MCAD and LCAD (both critical for desaturation of fatty acids) protein expression (Fig. 7, B and C). Control-fed animals had significantly higher levels of MCAD than all other groups (P < 0.05) (Fig. 7B). LG treatment significantly increased LCAD protein expression in ALIOS-fed mice compared with ALIOS-saline mice (P < 0.0001) (Fig. 7C).
DISCUSSION
The increased incidence of NAFLD and CVD patients closely parallels the increased consumption of HFCS and trans-fat in the United States (32). The present study provides new insights into the dysregulation of lipid handling as a result of a Western diet and potential mechanistic insights whereby the long-acting GLP-1 analog LG can reverse several critical failures associated with metabolic syndrome.
As anticipated, the ingestion of the ALIOS diet resulted in ALIOS-induced insulin resistance; cardinal features of metabolic syndrome, including dyslipidemia, obesity, glucose intolerance, and hepatic steatosis; and previously unreported cardiac damage, including hypertension and cardiac hypertrophy. LG significantly improved all major parameters associated with metabolic syndrome as well as insulin resistance. Importantly, we have demonstrated several additional findings worthy of discussion including the finding that hepatocyte insulin resistance predates the development of systemic insulin resistance; we have begun to elucidate potential pathways to account for recently published data that GLP-1 analogs are capable of reducing hepatocyte free fatty acid (FFA) stores in vitro (in the absence of insulin) and in vivo, as we and others have recently described (11, 15).
Our present work demonstrates that hepatic insulin resistance appears to precede the clinicopathological features of systemic insulin resistance including steatosis and peripheral glucose intolerance (20). Accumulating data indicate hepatic insulin resistance is a pivotal pathophysiological mediator in the development of systemic insulin resistance and metabolic syndrome (8, 24). The findings of this study are the first to demonstrate hepatic insulin resistance within 6 wk of ALIOS administration. Although the exact mechanism by which this may occur is beyond the scope of this article, there is relatively little existing data regarding the temporal relationship between systemic insulin resistance and hepatic insulin resistance in the context of diet-induced obesity (23).
An important pathophysiological change attributed to hepatic insulin resistance is perturbations in VLDL production (25, 43). Increased production of VLDL particles in response to increased adipocyte-derived FFA is a hallmark of T2DM; however, the role of VLDL secretion in NAFLD and NASH is not entirely clear. Previous studies in Japanese men and women who suffered from NAFLD or NASH (13) demonstrated that NASH patients had significantly lower expression of apoB100 and lower serum VLDL-triglyceride. In the same study MTTP mRNA levels were significantly lower (13). Similar findings were reported in an intervention study in obese adolescents (10). For those patients at a higher risk for developing NASH, our data indicate that GLP-1 analogs may be a viable treatment option.
The ALIOS diet resulted in essential hypertension and myocardial hypertrophy within 12 wk of feeding. The finding that LG improves cardiac function is in agreement with early and emerging studies that have shown that GLP-1 and its analogs protect against myocardial ischemia perfusion injury and increases left ventricular function through GLP-1 receptors found in myocardial cells (5, 6, 28).
The model also demonstrated how primary genes involved in hepatocyte lipid handling can be manipulated by LG. These results have significant implications for work we performed recently in vitro in primary human hepatocyte culture demonstrating the GLP-1 analog exendin-4 significantly reduced triglyceride and FFA stores (11, 15). mRNA for genes involved in de novo lipogenesis were significantly suppressed by LG in ALIOS-fed mice. This is in agreement with a recent study by Shlomo et al. (42) showing that GLP-1 reduces hepatic lipogenesis via activation of AMPK.
Another important result was the dramatic increase in LFABP and MTTP (Figs. 7 and 8). This is the first published study linking GLP-1 analogs with increased LFABP and MTTP expression. The critical role of these proteins in the progression of NAFLD may be explained by the emerging concept of FFA lipotoxicity and hepatic flux. Lipotoxicity is defined as cellular toxicity that occurs in nonadipose tissue in the presence of FFA (21, 22, 27) that leads to hepatocyte death by apoptosis. There is growing support for the concept that triglyceride accumulation is actually a protective mechanism used to abate the toxic effects of FFAs (27). Recent evidence suggests that FFA exposure and overload may be potentially more hazardous to hepatocytes and can lead to a dysfunctional unfolded protein response and diminished capacity for the endoplasmic reticular (ER) pathway to prevent apoptosis (21, 22, 27). Hepatic flux or lipid equilibrium is maintained by four interrelated mechanisms: uptake of FFA, de novo lipogenesis, secretion of VLDL, and β-oxidation (12, 35, 46). A defect in any one of these mechanisms leads to aberrant storage of lipid and potential exposure to FFA (33, 35).
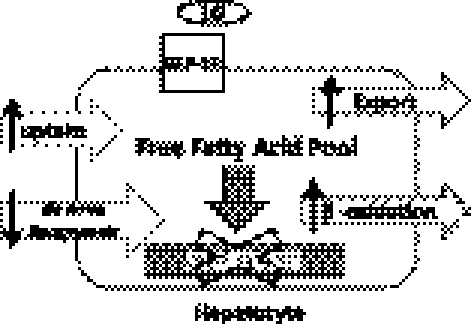
Fig. 8.
Proposed mechanism by which liraglutide improved hepatic lipid flux. Liraglutide appears to affect uptake, export, and β-oxidation of lipid within the hepatocyte, while also decreasing the amount of de novo lipogenesis. ↑, increase; ↓, decrease.
Dietary fat and glucose have both been shown to upregulate LFABP expression (1, 17, 39). LFABP enhances uptake of long-chain fatty acids (LCFA), binding both LCFA and respective metabolites targeting them for peroxisomal β-oxidation, or the ER for increased VLDL packaging and secretion (4, 9, 17). Increasing LFABP may be a compensatory mechanism used to dispose of excessive FFA, whereby the liver serves as a “sink” for toxic FFA (22). We have shown the expression of LCAD and ACOX2, proteins directly involved in β-oxidation, were also upregulated in the LG-treated ALIOS-fed mice, which should increase the cell's capacity to oxidize fatty acids more rapidly. These data support in vitro work we recently published (40) in primary human hepatocytes. We demonstrated that exendin-4 induces fatty acid β-oxidation in hepatocytes fat loaded with either palmitic or elaidic acid (40). These new data provide a plausible mechanism for the in vitro data demonstrating rapid reduction in FFA in primary human hepatocyte culture (15, 40).
In sum, we demonstrate increased FABP, diminished expression of DNL genes, and increased expression of genes associated with oxidation in LG-treated ALIOS-fed mice.
MTTP is an essential heterodimer protein located in the lumen of the ER (23, 31, 47). MTTP facilitates the transfer of neutral lipid to ApoB (31, 48). If FFA induces lipotoxicity- then MTTP expression is critical to preventing FFA overload of the ER which reduces FFA-induced lipotoxic cellular damage. It should be noted that MTTP levels in NASH are suppressed and patients with abetalipoproteinemia have defects in their MTTP activity, which results in steatotic hepatocytes and minimal circulation of ApoB (13, 31, 47). Experiments in fatty liver Shionogi (FLS) mice, which exhibit NASH lesions, also demonstrated that hepatic induction of MTTP resulted in a reduction in NASH lesions, improvement in VLDL export, and improved glucose tolerance (41). In the work presented here, LG treatment significantly increased MTTP, which is critical for the packaging of fats safely within the hepatocytes, averting potential for lipotoxicity.
From our previous work it is clear that GLP-1 is able to reduce lipid accumulation in hepatocytes in the absence of insulin (15). However, the upregulation of LFABP, MTTP, LCAD, ACOX2, PPAR-α, and ApoB when taken together imply increased hepatic capacity to handle FFA (Fig. 8) and timely safe disposal of hepatic triglycerides. Since insulin is known to inhibit MTTP expression, as well as the rate of ApoB synthesis, it is very unlikely that the effects observed in the present studies are derived from the insulin-stimulating feature of GLP-1 (18, 23, 31).
In conclusion, the present investigation provides a robust model to further study not only NAFLD but nonhepatic parameters of metabolic syndrome in a nongenetic, nonartificial diet. These data, and recent data by laboratories throughout the world, substantiate the multiple and direct effects of GLP-1 analogs that go beyond the function as incretins (2, 5, 11, 42). Finally, this work along with other recent work provides a plausible working hypothesis that suggests that GLP-1 analogs markedly improve hepatocyte handling of FFAs by improving hepatocyte transport and β-oxidation. Such beneficial actions may improve the ability of hepatocytes to more effectively handle FFA, thereby preventing or reversing potential lipotoxic effects including a dysfunctional unfolded protein response and hepatocyte apoptosis events associated with the progression of nonalcoholic liver disease to steatohepatitis and cirrhosis (14, 36, 37).
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R24 DK064399, RO1 DK062092, and RO1 DK 075397 and by National Science Foundation Award no. 0450303 (I-66-606-63).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Owen McGuinness (Vanderbilt Mouse Metabolic Phenotyping Center Metabolic Pathophysiology Core) for hyperinsulinemic-euglycemic clamp studies and analysis.
J.E.M developed and designed the study, conducted the experiments, analyzed the data, wrote the manuscript, and edited the manuscript for intellectual content. P.P.F maintained the animals used the study, performed experiments. S.S. conducted experiments, analyzed data, edited the manuscript for intellectual content. D.O. assisted in the development of the study and edited the manuscript. L.C. performed the cardiac measurements for the study. J.A.H reviewed and analyzed data, edited the manuscript for intellectual content. N.K.S. assisted in the development and design of the study, obtained funding for the study, and edited the manuscript for intellectual content. D.S. assisted in the development and design of the study, was responsible for the cardiac data presented in the study, and obtained funding for the study. F.A.A. developed and designed the study, edited the manuscript for intellectual content, obtained the majority of the funding for the study, and had primary responsibility for final content.
Parts of this study were presented in abstract form at The 61st Annual Meeting of the American Association for the Study of Liver Diseases, October 29–November 2, 2010, Boston, MA.
REFERENCES
- 1. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem 21: 1015– 1032, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150: 1155– 1164, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390– 397, 2006. . [DOI] [PubMed] [Google Scholar]
- 4. Binas B, Erol E. FABPs as determinants of myocellular and hepatic fuel metabolism. Mol Cell Biochem 299: 75– 84, 2007. . [DOI] [PubMed] [Google Scholar]
- 5. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54: 146– 151, 2005. . [DOI] [PubMed] [Google Scholar]
- 6. Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther 19: 9– 11, 2005. . [DOI] [PubMed] [Google Scholar]
- 7. Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 24: 1936– 1940, 2001. . [DOI] [PubMed] [Google Scholar]
- 8. Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559– 569, 1998. . [DOI] [PubMed] [Google Scholar]
- 9. Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47: 39– 48, 2006. . [DOI] [PubMed] [Google Scholar]
- 10. de Piano A, Tock L, Carnier J, Oyama LM, Oller do Nascimento CM, Martinz AC, Foschini D, Sanches PL, Ernandes RM, de Mello MT, Tufik S, Damaso AR. Negative correlation between neuropeptide Y/agouti-related protein concentration and adiponectinemia in nonalcoholic fatty liver disease obese adolescents submitted to a long-term interdisciplinary therapy. Metabolism 59: 613– 619, 2010. . [DOI] [PubMed] [Google Scholar]
- 11. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173– 181, 2006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis 30: 391– 401, 2010. . [DOI] [PubMed] [Google Scholar]
- 13. Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 50: 772– 780, 2009. . [DOI] [PubMed] [Google Scholar]
- 14. Gao H, Wang X, Zhang Z, Yang Y, Yang J, Li X, Ning G. GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3–L1 adipocytes. Endocrine 32: 90– 95, 2007. . [DOI] [PubMed] [Google Scholar]
- 15. Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51: 1584– 1592, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847– 2850, 2004. . [DOI] [PubMed] [Google Scholar]
- 17. Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and peroxisome proliferator-activated receptor alpha. J Lipid Res 51: 3103– 3116, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol 19: 277– 284, 2008. . [DOI] [PubMed] [Google Scholar]
- 19. Irimia JM, Meyer CM, Peper CL, Zhai L, Bock CB, Previs SF, McGuinness OP, DePaoli-Roach A, Roach PJ. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J Biol Chem 285: 12851– 12861, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol 47: 142– 156, 2007. . [DOI] [PubMed] [Google Scholar]
- 21. Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28: 360– 369, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClain CJ, Barve S, Deaciuc I. Good fat/bad fat. Hepatology 45: 1343– 1346, 2007. . [DOI] [PubMed] [Google Scholar]
- 23. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 42: 1331– 1346, 2009. . [DOI] [PubMed] [Google Scholar]
- 24. Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87– 97, 2000. . [PubMed] [Google Scholar]
- 25. Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV, Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res 49: 2038– 2044, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 290: 1884– 1890, 2003. . [DOI] [PubMed] [Google Scholar]
- 27. Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52: 774– 788, 2010. . [DOI] [PubMed] [Google Scholar]
- 28. Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58: 975– 983, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism 47: 1089– 1096, 1998. . [DOI] [PubMed] [Google Scholar]
- 30. Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism 53: 454– 457, 2004. . [DOI] [PubMed] [Google Scholar]
- 31. Qiu W, Taghibiglou C, Avramoglu RK, Van Iderstine SC, Naples M, Ashrafpour H, Mhapsekar S, Sato R, Adeli K. Oleate-mediated stimulation of microsomal triglyceride transfer protein (MTP) gene promoter: implications for hepatic MTP overexpression in insulin resistance. Biochemistry 44: 3041– 3049, 2005. . [DOI] [PubMed] [Google Scholar]
- 32. Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc 110: 585– 592, 2010. . [DOI] [PubMed] [Google Scholar]
- 33. Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol 24: 830– 840, 2009. . [DOI] [PubMed] [Google Scholar]
- 34. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117: 739– 745, 2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanal MG. The blind men ‘see’ the elephant-the many faces of fatty liver disease. World J Gastroenterol 14: 831– 844, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sancho V, Nuche B, Arnes L, Cancelas J, Gonzalez N, Diaz-Miguel M, Martin-Duce A, Valverde I, Villanueva-Penacarrillo ML. The action of GLP-1 and exendins upon glucose transport in normal human adipocytes, and on kinase activity compared with morbidly obese patients. Int J Mol Med 19: 961– 966, 2007. . [PubMed] [Google Scholar]
- 37. Sancho V, Trigo MV, Gonzalez N, Valverde I, Malaisse WJ, Villanueva-Penacarrillo ML. Effects of glucagon-like peptide-1 and exendins on kinase activity, glucose transport and lipid metabolism in adipocytes from normal and type-2 diabetic rats. J Mol Endocrinol 35: 27– 38, 2005. . [DOI] [PubMed] [Google Scholar]
- 38. Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J 18: 1612– 1614, 2004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock D, Landrock KK, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 43: 1– 17, 2008. . [DOI] [PubMed] [Google Scholar]
- 40. Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One 6: e25269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shindo N, Fujisawa T, Sugimoto K, Nojima K, Oze-Fukai A, Yoshikawa Y, Wang X, Yasuda O, Ikegami H, Rakugi H. Involvement of microsomal triglyceride transfer protein in nonalcoholic steatohepatitis in novel spontaneous mouse model. J Hepatol 52: 903– 912, 2010. . [DOI] [PubMed] [Google Scholar]
- 42. Shlomo SB, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 54: 1214– 1223, 2011. . [DOI] [PubMed] [Google Scholar]
- 43. Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis 30: 378– 390, 2010. . [DOI] [PubMed] [Google Scholar]
- 44. Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA 95: 4061– 4065, 1998. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 295: G987– G995, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 5: 145– 171, 2010. . [DOI] [PubMed] [Google Scholar]
- 47. Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta 1345: 136– 150, 1997. . [DOI] [PubMed] [Google Scholar]
- 48. Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem 281: 4075– 4086, 2006. . [DOI] [PubMed] [Google Scholar]