Abstract
Recent studies have shown the involvement of cyclin-dependent kinase 5 (Cdk5) in cell cycle regulation in postmitotic neurons. In this study, we demonstrate that Cdk5 and its co-activator p35 were detected in the nuclear fraction in neurons and Cdk5/p35 phosphorylated retinoblastoma (Rb) protein, a key protein controlling cell cycle re-entry. Cdk5/p35 phosphorylates Rb at the sites similar to those phosphorylated by Cdk4 and Cdk2. Furthermore, increased Cdk5 activity elevates activity of E2F transcription factor, which can trigger cell cycle re-entry, leading to neuronal cell death. A normal Cdk5 activity in neurons did not induce E2F activation, suggesting that Cdk5 does not induce cell cycle re-entry under normal conditions. Taken together, these results indicate that Cdk5 can regulate cell cycle by its ability to phosphorylate Rb. Most importantly, increased Cdk5 activity induces cell cycle re-entry, which is especially detrimental for survival of postmitotic neurons.
Keywords: Cdk5, cell cycle, cell death, E2F transcription factor, neurons, phosphorylation, retinoblastoma protein
Introduction
Cyclin-dependent kinases (Cdks) are serine/threonine kinases that play essential roles in proper control of cell cycle progression. They exert their functions by phosphorylating and modulating the functions of target proteins that are pivotal to each step of cell cycle progression. In mammals, more than ten Cdks, which are structurally related with one another, have been identified, and most Cdks associate with corresponding co-factors, designated as cyclins (Cyc), that are necessary for the kinase activity. Cdks can be categorized into three major groups according to their functions. The first group (G1/S phase Cdks), consisting of Cdk4/CycD and Cdk2/CycE, is required for G1/S phase transition and they act by phosphorylating retinoblastoma (Rb) protein at multiple sites. Unphosphorylated or hypo-phosphorylated Rb suppresses G1 advance by binding to E2F transcription factors (inactive E2Fs), but once hyper-phosphorylated, they dissociate from E2Fs (active E2Fs), which drive the expression of genes required for G1/S phase transition and initiation of DNA synthesis.1,2 The second group (S/G2 phase Cdks), consisting of Cdk2/CycA and Cdk1/CycA inhibits DNA over-replication to ensure that DNA is replicated exactly once in a single cell cycle.3,4 Their substrates include DNA licensing factors such as Cdt1, Cdc6 or Mcm4, and phosphorylation of these factors by Cdks inactivates them either by degradation or exclusion from the nucleus. The third group (M phase Cdk), Cdk1/CycB, phosphorylates proteins such as lamins, condensin, separase, among others, which are required for mitosis.5
Cdk5 is a unique member of Cdk family that plays a role in various neuronal functions, including neuronal migration,6 neurite extension,7 synaptic plasticity,8 neuronal differentiation,9 neuronal cell death10-12 and pain signaling,13-15 and it has been generally considered that Cdk5 is not involved in cell cycle regulation because it is mainly active in postmitotic neurons in which cell cycling activity is normally absent. Furthermore, unlike other Cdks that are mainly localized in the nucleus, it has been reported that primary intracellular localization of Cdk5 in neurons is in the cytoplasm and membrane. Instead of cyclins, p35 and p39 are required for Cdk5-kinase-activity. A number of substrates of Cdk5 have been reported, which potentially explain how Cdk5 deficiency results in defective neuronal migration leading to abnormal cortical layer patterning,6 impaired differentiation of neurons9 and impaired neuronal survival.10
While many Cdk5 substrates localize in the cytoplasm or on the membrane, there are also nuclear substrates of Cdk5.16-21 Recent studies have also shown involvement of Cdk5 in cell cycle regulation. Lee’s group reported that Cdk5 and p35 might be involved in centrosome-mediated cell cycle events.22-24 Herrup and colleagues examined the role of Cdk5 in the control of cell cycle in neurons, showing that Cdk5 negatively controls the cell cycle in neurons25-27 and its pathological deregulation leads to aberrant cell cycle re-entry and cell death.28,29 Tsai’s group reported that over-activation of Cdk5 by p25, a calpain-mediated cleavage product of p35, resulted in cell cycle activity in neurons along with DNA damage response leading to neuronal cell death.30-32 It is also reported that β amyloid protein and Cdk5 activity synergize to induce neuronal cell death33 and cell cycle re-entry underlies this phenomena.34
It is now becoming more evident that cell cycle re-entry and cell death of neurons are closely linked to each other although the mechanism is not clear.35 In human neurodegenerative diseases including Alzheimer disease (AD) and Parkinson disease (PD), ectopic expression of cell cycle-related proteins is reported.36,37 Although it needs to be further addressed whether cell cycle activity in neurons causally induces cell death, it is very likely to be so because cell cycle re-entry precedes apoptosis in neurodegenerative diseases and cell cycle activity is not usually observed in postmitotic neurons in normal conditions. Since a possible link between Cdk5 activity and pathogenesis of AD has been reported,38 it is important to elucidate the mechanism underlying involvement of Cdk5 in neuronal cell death.
In this study, we report that Cdk5 is capable of phosphorylating Rb protein at multiple sites in the nucleus, and over-activation of Cdk5 increases E2F transcription factor activity, whereas normal Cdk5 activity does not. Because E2F activation is a key event triggering cell cycle re-entry, our results raise the possibility that cell cycle re-entry by over-activated Cdk5-Rb-E2F pathway serves as one of the mechanisms underlying neuronal cell death in neurodegenerative diseases.
Results
Presence of Cdk5/p35 in the nuclear fraction in mouse brain
Unlike other members of the Cdk family, Cdk5 was thought to be localized mainly in cytoplasm and membrane but not in the nucleus, which is a discrepancy with recent reports showing the involvement of Cdk5 in cell cycle regulation.9,21,23,27 Thus we first reexamined the intracellular localization of Cdk5 by biochemical fractionation of mouse brain into cytoplasmic, nuclear, membrane and cytoskeletal fractions. We examined brain samples from 1 y old mice in which the neurons are completely differentiated and also from embryonic 17 d old (E17) mice in which the majority of the neurons are post-mitotic and Cdk5/p35 is becoming more dominant than Cdk4/CyclinD. Although the majority of Cdk5 was detected in cytoplasm and membrane, there was also a detectable amount in nucleus both in adult and in E17 mouse brain (Fig. 1A). P35, a co-activator of Cdk5, was also detected in nuclear fraction (Fig. 1A). Cdk4, which is not active in differentiated neurons, was undetectable in nuclear fraction of mouse brain at E17 and barely present at 1 y old (Fig. 1A). Next we sought to find out whether Cdk5/p35 in the nucleus has any functional activities. Cdk5 was immunoprecipitated from cytoplasmic, nuclear and membrane fractions of E17 and adult mouse brains, and was subjected to kinase assay in vitro in which the Cdk5 activity was evaluated by its ability to phosphorylate Histone H1. Cdk5 was active in all three fractions (Fig. 1B). Notably, the relative activity of Cdk5 (Cdk5-kinase activity compared with Cdk5 amount) was higher in the nuclear fraction compared with the cytoplasmic fraction both in adult cerebrum and in E17 whole brain. Rather high expression of p35 in the nuclear fraction may explain this phenomenon. Altogether, these results show that Cdk5/p35 complex is present and active in the nuclear fraction of mouse brain.
Figure 1. Cdk5/p35 is present and active in the nuclear fraction of mouse brain. (A) Intracellular localization of Cdk5, p35, Cdk4 and E2F1 was detected by western blotting in cytoplasmic (CP), nuclear (N), membrane (M) and cytoskeletal (CS) fractions of whole brain extracts of E17 mice and of cerebrum of adult mice. GAPDH, NeuN and PCMA are specific markers of cytoplasmic (CP), nuclear (N) and membrane (M) fractions, respectively. Note Cdk5 was found to be also present in the nuclear fraction. (B) Cdk5 kinase activity was detected at cytoplasmic (CP), nuclear (N) and membrane (M) fractions of E17 mice whole brain and adult mice cerebrum. Cdk5 and p35 in the immunoprecipitated samples either by Cdk5-antibody or control IgG were detected by western blotting. The relative activity of Cdk5 and the amount of p35 was higher in the nuclear fraction compared with the cytoplasmic fraction.
Cdk5 phosphorylates the cell-cycle regulator Rb protein
During development, neurons differentiate from proliferative progenitors to terminally differentiated post-mitotic mature neurons. In neuronal progenitors, Cdk4 regulates cell cycle.39 Since we found that Cdk5 but not Cdk4 is present in the nuclear fraction of mouse brain at E17 (Fig. 1A), we next sought to find out whether Cdk5 phosphorylates Rb protein which is the major substrate of Cdk4 in proliferative cells. We took advantage of Cdk5 knockout mice (Cdk5−/−) generated previously in our laboratory6 and analyzed the phosphorylation status of Rb protein using an antibody that recognizes Rb protein phosphorylated at Ser780.40 As Cdk5−/− mice die at birth, brain samples from E17 mouse embryos were used. As shown in Figure 2 A, the phosphorylation of Rb at Ser780 was significantly decreased in Cdk5−/− mice as compared with wild-type (Cdk5+/+) or heterozygote (Cdk5+/−) mouse brains. When the Cdk5−/− and Cdk5+/+ brain samples were fractionated into cytoplasmic and nuclear fractions, Rb phosphorylated at Ser780 was detected only in the nuclear fraction from Cdk5+/+ brain and was absent in Cdk5−/− brain samples (Fig. 2B). These results indicated that Cdk5 significantly affects the phosphorylation status of Rb.
Figure 2. Phosphorylation of Rb protein is decreased in Cdk5−/− mouse brain Phosphorylation of Rb at Ser780 in whole lysate (A) or in cytoplasmic or nuclear fractions (B) of Cdk5−/− mice was determined by western blotting. The intensity of the phospho-Rb blots shown on the panel together with other independent experiments (+/+: n = 7, +/−: n = 5, −/−: n = 7) were quantified and normalized to total Rb (A). Note Rb phosphorylation was decreased in Cdk5−/− mice.
Next we sought to find out whether Cdk5 directly phosphorylates Rb protein. For this purpose, we used purified Rb and Cdks proteins. As most of the key phosphorylation sites of Rb are in the C-terminus,2,41,42 we first used a synthesized Rb-C terminus fragment (aa 773–928) and performed in vitro phosphorylation with active forms of Cdks (Cdk5/p35, Cdk5/p25, Cdk2/CyclinE and Cdk4/CyclinD) to assess the phosphorylation status of Rb at Ser780, Ser795 and Ser807/811.43 Cdk4/CyclinD showed a low kinase activity (Fig. 3A) probably reflecting its function as spearhead that triggers Cdk2/CyclinE activity. In contrast, the ability of Cdk5, either co-activated by p35 or p25, to phosphorylate Rb protein was comparable to Cdk2/CylinE (Fig. 3A). This was further confirmed by using a full length Rb (aa 1–928) in presence of active Cdks (Cdk5/p35 and Cdk2/CyclinE). Here again, direct phosphorylation of Rb by Cdk5 was comparable to that of Cdk2 (Fig. 3B). In this in vitro assay, all the Rb-protein was phosphorylated as shown by the complete mobility shift of Rb (Fig. 3A and B), while Rb in brain sample showed a single band (Fig. 2A, 110 kD) without a mobility shift, suggesting that only a small portion of Rb is phosphorylated in vivo.
Figure 3. Cdk5 directly phosphorylates Rb in vitro. Synthesized peptide of Rb-C terminus (as 773–928) (A) or a full length Rb (B) were reacted with active Cdk complexes (Cdk5/p35, Cdk5/p25, Cdk2/CyclinE and Cdk4/CyclinD) and the phosphorylation at Ser780, Ser795 and Ser807/811 were determined by western blotting. Cdk5 was as potent as Cdk2 in phosphorylating Rb.
As commercially available phospho-specific antibodies of Rb were limited to Ser780, Ser795 and Ser807/811, we subjected the phosphorylation reaction products of Rb (aa 773–928) and active Cdks to mass spectrometry analysis to examine other phosphorylation sites. There are 16 Ser/Thr sites on Rb-protein that are potential Cdk targets.42 Among them seven are on the C-terminus (aa773–928) (Table 1, upper).42 Mass spectrometry analysis showed all seven C-terminus sites were phosphorylated by Cdk5. The overall result, except for a few differences (Table 1, * and #), was similar to the western blot in Figure 3, which further confirmed that Cdk5 can phosphorylate Rb-C-terminus as potent as Cdk4 and Cdk2.
Table 1. Mass spectrometry analysis of Rb-Cdks reaction products.
| 780 788 795 807 811 821 826 strpptlSpiphiprSpykfpsSplripggniyiSplkSpykiseglpTptkmTprsrilvsigesfgtsekfqkinqmvcnsdrv lkrsaegsnppkplkklrfdiegsdeadgskhlpgeskfqqklaemtstrtrmqkqkmndsmdtsnkeek |
| |
Phosphorylation sites on C-Rb |
||||||
|---|---|---|---|---|---|---|---|
| Kinase | S780 | S788 | S795 | S807 | S811 | T821 | T826 |
| Cdk5/p35 |
O |
O |
O |
O |
O |
O |
O |
| Cdk5/p25 |
O |
O |
O |
O |
O |
O |
O |
| Cdk2/CyclinE |
* |
O |
O |
O |
O |
O |
O |
| Cdk4/CyclinD | * | O | O | O# | O# | O | O |
Reactions products of synthesized Rb-C terminus (aa 773–928) and active Cdks (Cdk5/p35, Cdk5/p25, Cdk2/CyclinE and Cdk4/CyclinD) were subjected to mass spectrometry analysis. All the seven phosphorylation sites of Rb-C-terminus (sequence in upper) was phosphorylated by Cdk5. O, phosphorylation sites detected by mass spectrometry; *, phosphorylation was detected by western blotting; #, phosphorylation was not detected by western blotting.
Cdk5 is the main kinase of Rb in vivo
It was earlier shown that Cdk5 phosphorylates Rb protein in vitro, but this could be merely due to its structural similarity with Cdk2 or Cdk4, and does not necessarily mean that Cdk5 physiologically serve as the main kinase of Rb in vivo. To address this question, we performed phosphorylation reaction of a synthesized human-Rb full length protein (aa 1–928) with brain extracts from either Cdk5+/+ and Cdk5−/− (E17) or p35−/− (adult) mice. Cdk5−/− mice die before or at birth but p35−/− mice survive and have significantly reduced Cdk5 activity.44 To distinguish from intrinsic Rb-phosphorylation, a human specific antibody for phospho-Rb (Ser 807/811) was used for detection.
Although phosphorylated Rb-protein at Ser780 was detected only in the nucleus in vivo (Fig. 2B), the ability to phosphorylate Rb-protein at Ser807/811 for Cdk5 was also found in cytoplasm, nucleus and membrane fractions of E17 mouse brain, and it was rather dominant in the nucleus of the wild type adult mouse brain (Fig. 4). In a striking contrast, phosphorylation of Rb was not detected in Cdk5 and p35-deficient mice (Fig. 4), which strongly suggested that the phosphorylation of Rb in wild-type mice, at E17 and 1 y old was mainly dependent on the Cdk5 activity.
Figure 4. Cdk5 is the main kinase that phosphorylates Rb in vivo A full length Rb protein was reacted with fractionated protein extracts (CP, cytoplasmic; N, nuclear; M, membrane) from Cdk5−/− (for E17) / p35−/− (for adult) or wild type mouse brain, and the phosphorylation of Rb was determined by human-specific anti-phospho Ser807/811 Rb antibody. Cdk5 and p35 deficient mouse brain extract did not phosphorylate Rb protein.
Cdk5 regulates Rb-E2F pathway
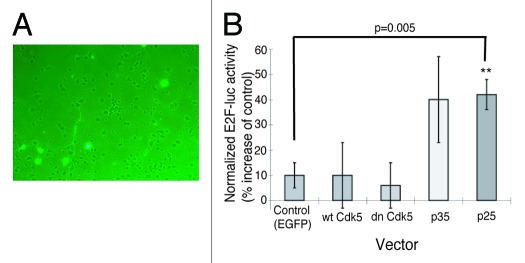
The physiological role of Rb protein is to regulate cell cycle by switching “ON” and “OFF” the transcription factor E2F that is present in the nuclear fractions of the brains from E17 and adult mice (Fig. 1A). Dephosphorylated Rb protein binds to E2F and turns it “OFF,” while phosphorylated Rb protein dissociates from E2F and turns it “ON.” Our next question was whether Cdk5-dependent Rb-phosphorylation activates E2F. To address this question, we designed an assay using a probe that codes firefly luciferase at the downstream of E2F target sequence (E2F-luc) (Fig. 5). E2F-luc was transfected into primary cultured rat cortical neurons together with vectors coding either wild type Cdk5, dominant negative Cdk5, p35, p25 or control EGFP. Overexpression of p35 or p25, which increases Cdk5 activity, induced significant activation of E2F. Interestingly, expression of dominant negative Cdk5, which suppresses Cdk5-activity (Fig. S1), did not affect E2F activity (Fig. 5). This suggests that at a normal level of Cdk5 kinase activity, E2F activity is at a basal level that does not activate the downstream signaling, and thus it remains unaltered by suppression of Cdk5 activity.
Figure 5. Increased activation of Cdk5 significantly induces E2F activity. (A) E2F-luc was transfected into primary cultured rat cortical neurons together with vectors coding wild type Cdk5, dominant negative Cdk5, p35, p25 or control EGFP. Fluorescent cells indicate transfected neurons. (B) Activity of E2F was assessed by luciferase assay. Overexpression of p35 and p25 induced significant activation of E2F. Dominant negative Cdk5 did not affect E2F activity. n = 4–8. **p < 0.05, T-Test.
Altogether, these results indicated that increased Cdk5 activity activates E2F, while in a normal condition Cdk5 does not affect the activity of E2F even though it phosphorylates Rb (Fig. 6).
Figure 6. A proposed model for regulation of E2F activity by Cdk5 Dephosphorylated Rb binds to E2F (top). When Cdk5 is active at normal level, it partially phosphorylates Rb but E2F is not released from Rb (middle). When Cdk5 is hyperactive, it completely phosphorylates Rb and E2F is released, which turns E2F “ON” and leads to cell cycle re-entry and neuronal cell death (bottom).
Discussion
This is the first study to assess the causal link between E2F transcription factor activity and Cdk5-mediated Rb phosphorylation. We have shown that Cdk5 phosphorylates Rb protein and when Cdk5 is overactivated the Rb phosphorylation reaches to the level that triggers E2F activity.
Cdk5 in pathological condition
The neuronal network of the brain consists of mature neurons that are post-mitotic and terminally differentiated. Several lines of evidence suggest that if mature neurons happen to re-enter the cell cycle, they are subjected to cell death.36,37 Once mature neurons die, they are not replaceable by newly generated neurons. Thus re-entry of the cells into the cell cycle is a huge disadvantage in the brain. Indeed, cell cycle re-entry of mature neurons seems to lead to neurodegenerative diseases such as AD and PD.36,37 Cdk5 is specifically expressed in mature neurons and its involvement in Rb-mediated cell cycle regulation has been suggested by Hamdane et al., in which they used p25-inducible neuroblastoma cell line.18,45 P25 is a proteolytic product of p35 by calpain, which exists only under a pathological condition. Here in our study, we demonstrated that even a physiological form of active Cdk5 (Cdk5/p35) phosphorylates Rb in normal mouse brain. Also using E2F-luciferase assay, we have shown that over-activation of Cdk5 leads to the activation of Rb-E2F pathway in primary cultured neurons. Intriguingly, E2F remained “OFF” with physiological level of Cdk5 activity even though Rb protein was phosphorylated. Accordingly, downregulation of Cdk5 did not affect the downstream of E2F. This suggests that there is a state in which Rb protein is phosphorylated but E2F is inactive, and there seems to be a threshold of Rb-phosphorylation-level to activate E2F (Fig. 6). The proposed model in Figure 6 implies the relation between the degree of Rb-phosphorylation and its binding with E2F, in terms of Cdk5-activity. Previous studies have shown that the activity of E2F is positively correlated with the degree of Rb-phosphorylation.46 Our data have shown that (1) Cdk5 phosphorylates Rb-protein (Figs. 2, 3 and 4) even under normal condition (Fig. 2) and (2) E2F is not activated under normal condition (Fig. 5B; wtCdk5) but is activated when the activator is overexpressed (Fig. 5B; p35) or hyperactive (Fig. 5B; p25). The possible implication is that the degree of Rb-phosphorylation could be higher in pathological condition than in normal condition. And although one of our experiments showed that Cdk5/p35 is able to phosphorylate Rb protein, probably only pathologically activated Cdk5/p25 reaches to the level that turns Rb-E2F pathway “ON” to affect cell cycle regulation.
Cdk5 in normal condition
We also have shown that Cdk5 is the main kinase that phosphorylates Rb protein in normal brain. As mentioned above, Cdk5-mediated Rb-phosphorylation can lead to neuronal cell cycle re-entry underlying neurodegenerative diseases. However it is unlikely that Cdk5 is an absolute harm in post-mitotic neurons, of which the only role is to promote cell cycle re-entry. In fact, its neuro-protective effect has also been revealed. Recent study has shown that Cdk5 suppresses cell cycle re-entry in a kinase-activity-independent manner, thereby protecting neurons from cell death.9,27,47 On the other hand, our data showed that neuro-degenerative effect of Cdk5 is brought by its kinase activity (i.e., phosphorylation of Rb-protein). Taken together, the role of Cdk5 in neuronal survival is bi-functional.48
Is Cdk5 different from other Cdks?
Despite previous studies showing the functional distinction of Cdk5 from other Cdk-family members, here we have shown that Cdk5 shares common features with Cdk2 and Cdk4, at least regarding Rb phosphorylation. It is possible that Cdk5 shares common substrates besides Rb with other Cdks. Some Cdks also function as cell cycle suppressors under certain circumstances.49 Thus Cdk5 may also serve as a cell cycle suppressor under normal condition, by sharing common substrates with the Cdks.
In summary, we have shown that Rb-E2F pathway can be activated by Cdk5. In this study we have not directly shown the link of Cdk5-Rb-E2F. Instead our data have shown the links of Cdk5-Rb and Cdk5-E2F. Together with the results from previous studies showing a degree-dependent positive correlation of Rb-phosphorylation with E2F-activity,46 our data indicates that when Cdk5 is hyper active (Cdk5/overexpressed-p35 or Cdk5/p25) Rb is phosphorylated to the degree that activates E2F, which explains at least one of the mechanisms of neurodegenerative diseases.
Materials and Methods
Animals
Cdk5 knockout mice6 and p35 knockout mice44 were generated as previously reported. All of the animal procedures were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Western blot analysis
Protein extracts of mouse brain were obtained by homogenizing dissected whole brain (E17) or cerebrum (1 y old) in lysis buffer [T-PER (Thermo Scientific, Waltham, MA) supplemented with Complete Mini protease inhibitor cocktail and PhosSTOP phosphatase inhibitor cocktail (Roche Applied Science, Indianapolis, IN)]. In the indicated experiments, the samples were fractionated into cytoplasmic, nuclear, membrane and cytoskeletal fractions using Compartment Protein Extraction kit (Millipore, Billerica, MA) following manufacturer’s protocol. The samples were then separated by SDS-PAGE and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). The membrane was blocked with 5% skim milk and 0.05% Tween 20 in PBS for 1 h at RT and incubated with a primary antibody overnight at 4°C. Primary antibodies used were as follows: anti-Cdk5 (C-8), anti-Cdk4 (C-22), anti-p35 (C-19), anti-Rb (C-15), anti-PMCA (H-300) (1:250–1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), anti-E2F1, phospho-specific anti-Rb (Ser780), phospho-specific anti-Rb (Ser795), phospho-specific anti-Rb (Ser807/811) (1:1000 dilution; Cell Signaling Technology, Danvers, MA), anti-αTubulin (DM1A) (1:20000 dilution; Sigma, St. Louis, MO), anti-GAPDH (ab9485) (1:2500 dilution, Abcam, Cambridge, MA), and anti-NeuN (A60) (1:500 dilution, Millipore, Billerica, MA). After washes, the membrane was incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at RT. The blots were then detected by Super Signal West Pico reagents (Pierce, Rockford, IL) on Scientific Imaging Film (Kodak). For quantification, the intensity of the blots was measured with ImageJ software and statistically analyzed with Student’s t-test.
Cdk5 kinase assay
Fifty micrograms of protein samples were immunoprecipitated with either 2 μg of anti-Cdk5 (C-8) or control rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) by overnight incubation with 25 μl of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) at 4°C and washed 4 times with cold PBS buffer and once with reaction buffer (20 mM Tris-Cl pH7.4, 10 mM MgCl2, 1 mM EDTA, 100 nM ATP, 1 mM DTT) followed by incubation with the substrate (10 μg of Histone H1) and 0.5 mM (5 μCi) of [γ−32P] ATP (DuPont NEN, Boston, MA) in reaction buffer for 30 min at 30°C. The reaction products were then separated by SDS-PAGE followed by detection of 32P-labeled Histone H1 by autoradiography on Scientific Imaging Film.
Rb phosphorylation assay
One microgram of Human Rb-C terminus peptide (aa 773–928, Millipore, Billerica, MA) or Human Rb full length purified protein (Abcam, Cambridge, MA) were incubated with either 0.05 μg of Cdk5/p35, Cdk5/p25, Cdk2/CyclinE (Millipore, Billerica, MA), Cdk4/CyclinD (Cell Signaling Technology, Danvers, MA) or 30 μg of fractionated proteins from mouse brain in phosphorylation reaction buffer (20 mM Tris-Cl pH 7.4, 10 mM MgCl2, 1 mM EDTA, 5 mM ATP, 0.5 mM DTT) at 30°C for 1–3 h.
Mass spectrometry analysis
The reaction products of Rb-C terminus peptide (aa 773–928) with either Cdk5/p35, Cdk5/p25, Cdk2/CyclinE or Cdk4/CyclinD were subjected to mass spectrometry analysis as described earlier.50
E2F luciferase assay
Cortical neurons dissociated from E18 rat and cultured for 4 d were co-transfected with either E2F responsive firefly luciferase (E2F-luc) or non-inducible firefly luciferase (negative control) reporter plasmids (E2F Cignal Reporter Assay Kit, QIAGEN, Germantown, MD), and plasmids encoding either Cdk5, dominant-negative Cdk5 (Fig. S1), p35, p25 or control EGFP using Lipofectamine 2000 transfection reagent (Invitrogen). Briefly, 1 × 106 cells in 35mm dish were transfected with 1 μg of luciferase vector and 1 μg of Cdk5 or p35 expressing vector, and 48 h post-transfection, reporter activity was measured by Dual reporter luciferase assay kit (Promega, Madison, WI) following manufacturer’s protocol. The measurements were normalized intra- and inter-cellularly with renilla luciferase activity and negative control, respectively.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgments
We would like to thank Dr. Sushil Rane for critical reading of the manuscript, Ms. Shelagh Powers for expert editorial assistance and Dr. Yoko Futatsugi for help in manuscript preparation. These studies were supported by the Intramural Divisions of the National Institute of Dental and Craniofacial Research and the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Glossary
Abbreviations:
- Cdk
cyclin-dependent kinase
- Cyc
cyclin
- Rb
retinoblastoma protein
- AD
Alzheimer disease
- PD
Parkinson disease
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20009
References
- 1.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–9. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 2.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–69. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 3.Nishitani H, Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–34. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 4.Hook SS, Lin JJ, Dutta A. Mechanisms to control rereplication and implications for cancer. Curr Opin Cell Biol. 2007;19:663–71. doi: 10.1016/j.ceb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–8. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3:453–9. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 8.Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A. 2001;98:12742–7. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–68. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi S, Ohshima T, Hirasawa M, Pareek TK, Bugge TH, Morozov A, et al. Conditional deletion of neuronal cyclin-dependent kinase 5 in developing forebrain results in microglial activation and neurodegeneration. Am J Pathol. 2010;176:320–9. doi: 10.2353/ajpath.2010.081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkkoetter PT, Pippin JW, Shankland SJ. Cyclin I-Cdk5 governs survival in post-mitotic cells. Cell Cycle. 2010;9:1729–31. doi: 10.4161/cc.9.9.11471. [DOI] [PubMed] [Google Scholar]
- 12.Trunova S, Giniger E. Absence of the Cdk5 activator p35 causes adult-onset neurodegeneration in the central brain of Drosophila. Dis Model Mech. 2012;5:210–9. doi: 10.1242/dmm.008847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, et al. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci U S A. 2006;103:791–6. doi: 10.1073/pnas.0510405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, et al. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc Natl Acad Sci U S A. 2007;104:660–5. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, et al. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem. 2009;284:2275–84. doi: 10.1074/jbc.M805052200. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K-Y, Helbing CC, Choi K-S, Johnston RN, Wang JH. Neuronal Cdc2-like kinase (Nclk) binds and phosphorylates the retinoblastoma protein. J Biol Chem. 1997;272:5622–6. doi: 10.1074/jbc.272.9.5622. [DOI] [PubMed] [Google Scholar]
- 17.Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, et al. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/S0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 18.Hamdane M, Bretteville A, Sambo A-V, Schindowski K, Bégard S, Delacourte A, et al. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–8. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- 19.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 20.Tian B, Yang Q, Mao Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat Cell Biol. 2009;11:211–8. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang E, Qu D, Park DS. Cdk5: Links to DNA damage. Cell Cycle. 2010;9:3142–3. doi: 10.4161/cc.9.16.12955. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Mummery A, Park J, Tariq H, Rosales JL. Localization of CDK5 in the midbody and increased aneuploidy in CDK5-/- cells. Cell Cycle. 2010;9:3629–30. doi: 10.4161/cc.9.17.12722. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Law V, Rosales JL, Lee KY. Cdk5 variant 1 (cdk5-v1), but not full-length cdk5, is a centrosomal protein. Cell Cycle. 2010;9:2251–3. doi: 10.4161/cc.9.11.11652. [DOI] [PubMed] [Google Scholar]
- 24.Rosales JL, Rattner JB, Lee KY. Cdk5 in the centriolar appendages mediates cenexin1 localization and primary cilia formation. Cell Cycle. 2010;9:2037–9. doi: 10.4161/cc.9.10.11600. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle. 2011;10:1208–14. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285:14052–61. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30:5219–28. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7:3487–90. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Cicero SA, Wang L, Romito-Digiacomo RR, Yang Y, Herrup K. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc Natl Acad Sci U S A. 2008;105:8772–7. doi: 10.1073/pnas.0711355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L-H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 31.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai L-H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–83. doi: 10.1016/S0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–17. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Utreras E, Maccioni R, González-Billault C. Cyclin-dependent kinase 5 activator p35 over-expression and amyloid beta synergism increase apoptosis in cultured neuronal cells. Neuroscience. 2009;161:978–87. doi: 10.1016/j.neuroscience.2009.04.002. b. [DOI] [PubMed] [Google Scholar]
- 34.Lopes JP, Oliveira CR, Agostinho P. Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-β and prion peptides. Cell Cycle. 2009;8:97–104. doi: 10.4161/cc.8.1.7506. [DOI] [PubMed] [Google Scholar]
- 35.Song B, Davis K, Liu XS, Lee HG, Smith M, Liu X. Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer’s disease. Aging (Albany NY) 2011;3:846–51. doi: 10.18632/aging.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801–7. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. Expression patterns of retinoblastoma protein in Parkinson disease. J Neuropathol Exp Neurol. 2003;62:68–74. doi: 10.1093/jnen/62.1.68. [DOI] [PubMed] [Google Scholar]
- 38.Tseng HC, Zhou Y, Shen Y, Tsai L-H. A survey of Cdk5 activator p35 and p25 levels in Alzheimer’s disease brains. FEBS Lett. 2002;523:58–62. doi: 10.1016/S0014-5793(02)02934-4. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson KL, Callaghan SM, O’Hare MJ, Park DS, Slack RS. The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J Biol Chem. 2000;275:33593–600. doi: 10.1074/jbc.M004879200. [DOI] [PubMed] [Google Scholar]
- 40.Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin SM, Gall AL, Zheng N, Pavletich NP. Structure of the Rb C-terminal domain bound to E2F1-DP1: a mechanism for phosphorylation-induced E2F release. Cell. 2005;123:1093–106. doi: 10.1016/j.cell.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 42.Gorges LL, Lents NH, Baldassare JJ. The extreme COOH terminus of the retinoblastoma tumor suppressor protein pRb is required for phosphorylation on Thr-373 and activation of E2F. Am J Physiol Cell Physiol. 2008;295:C1151–60. doi: 10.1152/ajpcell.00300.2008. [DOI] [PubMed] [Google Scholar]
- 43.Pan W, Cox S, Hoess RH, Grafström RH. A cyclin D1/cyclin-dependent kinase 4 binding site within the C domain of the retinoblastoma protein. Cancer Res. 2001;61:2885–91. [PubMed] [Google Scholar]
- 44.Ohshima T, Ogawa M, Veeranna, Hirasawa M, Longenecker G, Ishiguro K, et al. Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc Natl Acad Sci U S A. 2001;98:2764–9. doi: 10.1073/pnas.051628498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamdane M, Buée L. The complex p25/Cdk5 kinase in neurofibrillary degeneration and neuronal death: the missing link to cell cycle. Biotechnol J. 2007;2:967–77. doi: 10.1002/biot.200700059. [DOI] [PubMed] [Google Scholar]
- 46.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-F. [DOI] [PubMed] [Google Scholar]
- 47.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–78. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 48.Lopes JP, Agostinho P. Cdk5: multitasking between physiological and pathological conditions. Prog Neurobiol. 2011;94:49–63. doi: 10.1016/j.pneurobio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler LW, Lents NH, Baldassare JJ. Cyclin A-CDK activity during G1 phase impairs MCM chromatin loading and inhibits DNA synthesis in mammalian cells. Cell Cycle. 2008;7:2179–88. doi: 10.4161/cc.7.14.6270. [DOI] [PubMed] [Google Scholar]
- 50.Jaffe H, Vinade L, Dosemeci A. Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun. 2004;321:210–8. doi: 10.1016/j.bbrc.2004.06.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.