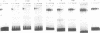Abstract
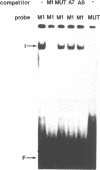
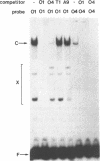
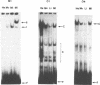
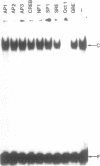
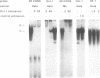
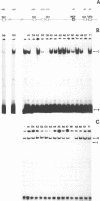
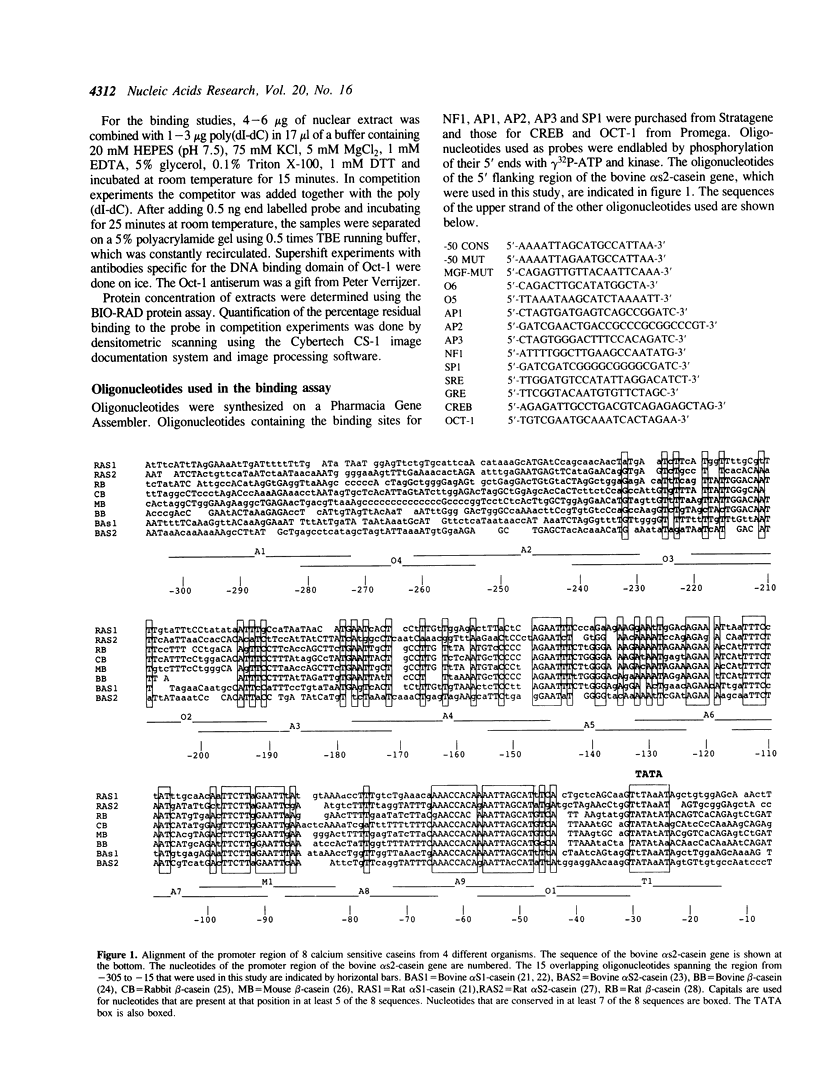
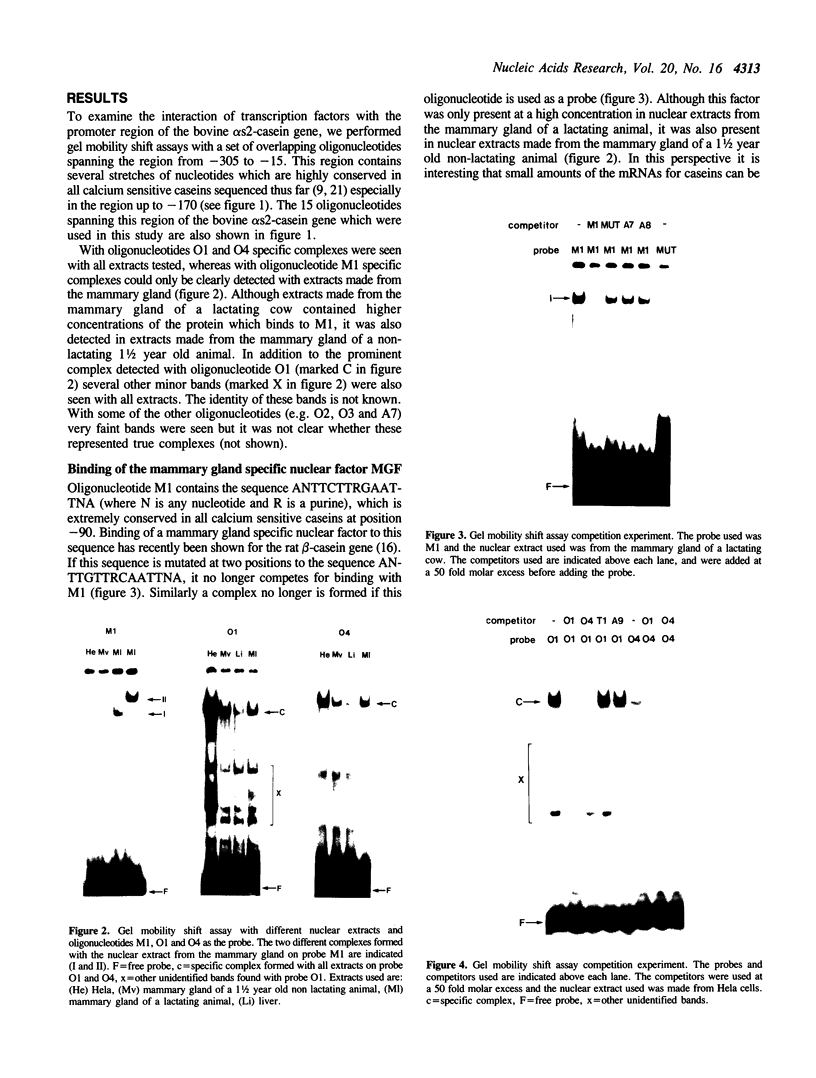
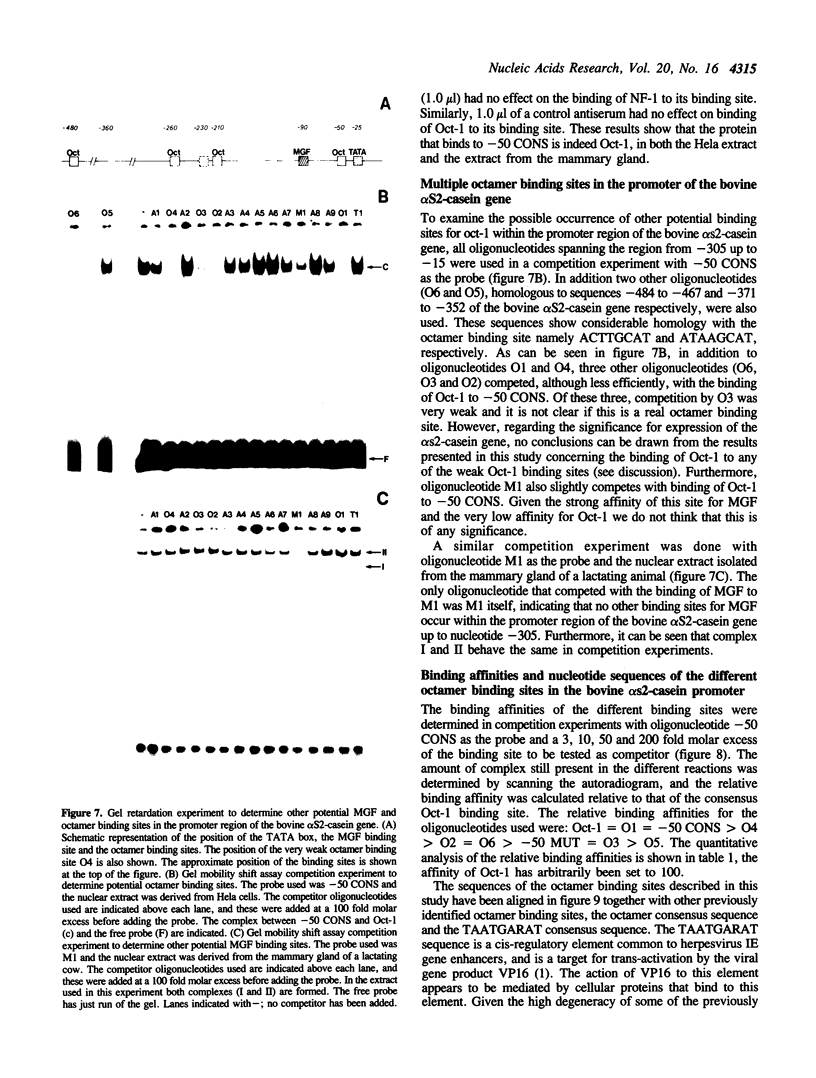
Using a set of overlapping oligonucleotides from the promoter region of the bovine alpha s2-casein gene we have identified two nuclear factors which probably are involved in expression of this gene and the related calcium sensitive alpha s1- and beta-casein genes. One of these factors which was present in extracts of all tissues that have been tested including Hela cells turned out to be the octamer binding protein OCT-1. Oct-1 binds with different affinity to 4 sites at positions centred around -480, -260, -210 and -50. The strongest of these 4 binding sites, the one around position -50, is highly conserved in all calcium sensitive caseins of mouse, rat, rabbit and cattle. The other nuclear factor (MGF, mammary gland factor) which is specifically expressed in the mammary gland, binds to a site around position -90. This binding site is also highly conserved in all calcium sensitive caseins of mouse, rat, rabbit and cattle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Park C. S., Bissell M. J. Regulation of milk protein and basement membrane gene expression: the influence of the extracellular matrix. J Dairy Sci. 1988 Oct;71(10):2830–2842. doi: 10.3168/jds.S0022-0302(88)79879-3. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumruker T., Sturm R., Herr W. OBP100 binds remarkably degenerate octamer motifs through specific interactions with flanking sequences. Genes Dev. 1988 Nov;2(11):1400–1413. doi: 10.1101/gad.2.11.1400. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Ferretti L., Leone P., Sgaramella V. Long range restriction analysis of the bovine casein genes. Nucleic Acids Res. 1990 Dec 11;18(23):6829–6833. doi: 10.1093/nar/18.23.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky S. I., Tkach T. M., Kapelinskaya T. V. Isolation and characterization of the Bos taurus beta-casein gene. Gene. 1988 Jun 15;66(1):87–96. doi: 10.1016/0378-1119(88)90227-2. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Robins A. J., Wells J. R. Independently evolving chicken histone H2B genes: identification of a ubiquitous H2B-specific 5' element. Nucleic Acids Res. 1982 Dec 11;10(23):7851–7863. doi: 10.1093/nar/10.23.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi N., Maguire K., Kralli A., Maldonado E., Reinberg D., Weinmann R. Direct interaction between adenovirus E1A protein and the TATA box binding transcription factor IID. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5124–5128. doi: 10.1073/pnas.88.12.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Koczan D., Hobom G., Seyfert H. M. Genomic organization of the bovine alpha-S1 casein gene. Nucleic Acids Res. 1991 Oct 25;19(20):5591–5596. doi: 10.1093/nar/19.20.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. F., Atiee S. H., Rosen J. M. Differential regulation of rat beta-casein-chloramphenicol acetyltransferase fusion gene expression in transgenic mice. Mol Cell Biol. 1989 Feb;9(2):560–565. doi: 10.1128/mcb.9.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. Transcription cell type specificity is conferred by an immunoglobulin VH gene promoter that includes a functional consensus sequence. Cell. 1985 Jun;41(2):479–487. doi: 10.1016/s0092-8674(85)80021-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Scafe C., Chao D., Lopes J., Hirsch J. P., Henry S., Young R. A. RNA polymerase II C-terminal repeat influences response to transcriptional enhancer signals. Nature. 1990 Oct 4;347(6292):491–494. doi: 10.1038/347491a0. [DOI] [PubMed] [Google Scholar]
- Schaffner W. How do different transcription factors binding the same DNA sequence sort out their jobs? Trends Genet. 1989 Feb;5(2):37–39. doi: 10.1016/0168-9525(89)90017-6. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R., Baumruker T., Franza B. R., Jr, Herr W. A 100-kD HeLa cell octamer binding protein (OBP100) interacts differently with two separate octamer-related sequences within the SV40 enhancer. Genes Dev. 1987 Dec;1(10):1147–1160. doi: 10.1101/gad.1.10.1147. [DOI] [PubMed] [Google Scholar]
- Threadgill D. W., Womack J. E. Genomic analysis of the major bovine milk protein genes. Nucleic Acids Res. 1990 Dec 11;18(23):6935–6942. doi: 10.1093/nar/18.23.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thépot D., Devinoy E., Fontaine M. L., Houdebine L. M. Structure of the gene encoding rabbit beta-casein. Gene. 1991 Jan 15;97(2):301–306. doi: 10.1016/0378-1119(91)90067-l. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B. K., Ziska S. E. Hormonal regulation of milk protein gene expression. Annu Rev Physiol. 1989;51:641–652. doi: 10.1146/annurev.ph.51.030189.003233. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Gordon K. E., Robertson M., Clark A. J. Interaction of DNA-binding proteins with a milk protein gene promoter in vitro: identification of a mammary gland-specific factor. Nucleic Acids Res. 1991 Dec 11;19(23):6603–6610. doi: 10.1093/nar/19.23.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Transfection of beta-casein chimeric gene and hormonal induction of its expression in primary murine mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3670–3674. doi: 10.1073/pnas.87.10.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Richter-Mann L., Couch C. H., Stewart A. F., Mackinlay A. G., Rosen J. M. Evolution of the casein multigene family: conserved sequences in the 5' flanking and exon regions. Nucleic Acids Res. 1986 Feb 25;14(4):1883–1902. doi: 10.1093/nar/14.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Rosen J. M. The rat casein multigene family. I. Fine structure of the gamma-casein gene. J Biol Chem. 1983 Sep 10;258(17):10794–10804. [PubMed] [Google Scholar]