Abstract
Background
Neuroprotective effects of lithium (Li) have been well documented in tissue cultures and animal models, whereas human data continue to be limited. Previous studies investigating the association between Li treatment and brain N-acetylaspartate (NAA), a putative neuronal marker, showed mixed results because of methodological heterogeneity.
Methods
To investigate the effects of Li on prefrontal cortex NAA levels, we compared patients with bipolar disorder from specialized Li clinics in Berlin and Halifax with at least 2 years of ongoing Li treatment (Li group), patients with lifetime Li exposure of less than 3 months more than 2 years ago (non-Li group) and healthy controls. Participants in both patient groups had at least 10 years of illness and 5 episodes. We measured left prefrontal NAA levels using 1.5-T magnetic resonance spectroscopy.
Results
We enrolled 27 participants in the Li, 16 in the non-Li and 21 in the healthy control groups. The non-Li group had lower prefrontal NAA levels than the Li group (t41 = −3.44, corrected p < 0.01) or control participants (t35 = −2.91, corrected p < 0.05), who did not differ from the Li group (t46 = −0.14, p = 0.89). The same pattern of prefrontal NAA differences was replicated in both sites. In addition, there was a negative correlation between prefrontal NAA and duration of illness in the non-Li group (r = −0.60, p = 0.019) but not in the Li group (r = 0.07, p = 0.74).
Limitations
Study limitations include the cross-sectional design and exposure to other medications.
Conclusion
Whereas patients with bipolar disorder, substantial illness burden and limited lifetime Li exposure had significantly lower prefrontal NAA levels than controls, Li-treated patients with similar illness burden showed prefrontal NAA levels comparable to those of healthy controls. These findings provide indirect support for neuroprotective effects of Li and for negative effects of illness burden on prefrontal NAA levels in patients with bipolar disorder.
Introduction
Neuroprotective effects of lithium (Li) have been well documented in tissue cultures and animal models.1,2 It has been suggested that Li has neuroprotective effects in humans,3,4 but the data are still limited. Proton magnetic resonance spectroscopy (MRS) allows for in vivo, noninvasive measurement of concentrations of certain chemicals, including N-acetylaspartate (NAA). N-acetylaspartate is the most dominant proton MRS peak and one of the most abundant amino acids in the brain, where it is predominantly localized in neurons and absent in mature glia. As such, NAA is considered a putative neuronal marker, which is decreased in conditions characterized by loss of neurons or axons, e.g., stroke, Alzheimer dementia, amyotrophic lateral sclerosis, multiple sclerosis, traumatic brain injury and epilepsy. In addition, NAA synthesis is dependent on energy metabolism. Decreased levels of NAA have been used as an indirect marker of neuronal/axonal loss or compromised neuronal metabolism.5–7
The spectroscopic evidence for the neuroprotective effects of Li in clinical populations is much less consistent than in the preclinical data. Prospective studies have predominantly shown no changes in brain NAA levels during Li treatment.8–11 A single prospective study reported a small NAA increase after 4 weeks of Li treatment,3 whereas another investigation found an NAA decrease after 6 weeks of Li exposure.12 Cross-sectional studies have also been inconsistent, with reports of greater13,14 as well as comparable15–17 brain NAA levels in Li-treated versus control participants.
Previous studies have been instrumental in identifying methodological issues, which likely underlie the heterogeneous results. For example, short treatment duration, low doses, lack of Li monitoring in the Li-treated group, and past or recent exposure to Li in the comparison group may diminish the effect size and contribute to false-negative findings. In addition, neurochemical brain changes, which are absent in participants at genetic risk for bipolar disorder,18,19 start appearing early in the course of illness20–22 and tend to be associated with duration of illness.23,24 As a result, studying the effects of Li on NAA would ideally require controlling for and maximizing the illness burden.
We designed this study to investigate the neuroprotective effects of Li. By using strict inclusion criteria, we attempted to control for the potential sources of heterogeneity, including duration of Li exposure, past and recent exposure to Li, burden of illness and symptomatic status. Our a priori hypotheses were that prefrontal NAA levels would be comparable between Li-treated patients and controls, with lower pre-frontal NAA concentrations in non-Li patients relative to both Li-treated patients and controls.
Methods
This study was specifically designed to test the effects of long-term Li exposure on prefrontal NAA, while controlling for illness burden. To this end, we recruited patients with bipolar disorder with substantial illness burden and varied the exposure to Li by using strict inclusion criteria. In particular, we studied 3 groups:
participants with bipolar disorder, substantial ongoing exposure to Li and a marked illness burden (Li group),
participants with bipolar disorder, limited or no lifetime exposure to Li and a marked illness burden (non-Li group), and
age- and sex-matched controls.
This investigation was performed by the International Group for Study of Lithium Treated Patients (IGSLi). Participants for the spectroscopic part of the study were recruited in Halifax, Canada, and Berlin, Germany. The study was approved by the Research Ethics Boards at each site. After complete description of the study all included patients provided written, informed consent.
Settings
Halifax
Participants with bipolar disorder were recruited among patients registered in a tertiary care mood disorders program at Dalhousie University. The program provides consultation services to family physicians and community psychiatrists and follows patients with bipolar disorder. The diagnosis of bipolar disorder was made in all patients by psychiatrists using the Structured Clinical Interview for DSM-IV (SCID).25 Patients had regular follow-up visits at the clinic, including monitoring of Li levels at least twice per year. Recruitment of Li-treated patients from a specialized clinic ensured that Li levels fell within the therapeutic range. This prevented sub-therapeutic levels, which could be insufficient to elicit neuroprotective changes, or levels above the therapeutic range, which could lead to neurotoxicity. We also recruited age- and sex-matched controls among hospital employees. Controls underwent a SCID interview and were included if they had no lifetime history of Axis I psychiatric disorders.
Berlin
Participants with bipolar disorder were recruited via a specialized outpatient bipolar disorder clinic at the Department of Psychiatry and Psychotherapy of the Charité University Medicine Berlin, Campus Mitte, a tertiary care clinic comparable to the one in Halifax. We also recruited age- and sex-matched controls via advertisement and among hospital employees. Controls underwent a SCID interview and were included if they had no lifetime history of Axis I psychiatric disorders.
Inclusion criteria
Participants with bipolar disorder (both Li and non-Li groups) were required to have
a diagnosis of bipolar I or II disorder made by a psychiatrist using the SCID;
at least 10 years of illness;
at least 5 episodes of illness (including manic, depressive or mixed episodes);
current Hamilton Rating Scale for Depression, 17-item version (HAM-D-17),26 score less than 7;
current Young Mania Rating Scale (YMRS)27 score less than 5;
current Clinical Global Impressions Scale bipolar (CGI-BP)28 score less than 3; and
at least 4 months of euthymia.
In addition, the non-Li group had to have less than 3 months of lifetime Li treatment and no Li exposure within 2 years prior to scanning. The Li group had to have a current Li treatment lasting a minimum of 2 years.
Exclusion criteria
Participants from any of the 3 groups were excluded if they met any of the exclusion criteria for MRI use (pacemaker, metal implants) or had any serious medical illness (e.g., brain injury, Cushing disease, conditions treated with corticosteroids).
Patients with bipolar disorder were excluded if they had
more than 1 lifetime course of electroconvulsive therapy,
comorbid Axis I or Axis II psychiatric disorders,
active substance abuse in the last 12 months,
change in psychiatric medication in the last 3 months, or
current psychotic features or acute suicidality.
Patients from the non-Li group were excluded if they had
Li exposure less than 2 years before the scanning, or
lifetime Li exposure of more than 3 months.
The healthy controls were excluded if they had a personal or family history of psychiatric disorders.
Magnetic resonance imaging methods
Halifax
All magnetic resonance (MR) acquisitions were obtained with a 1.5-T General Electric Signa scanner and a standard quadrature head coil. After a localizer scan, a T1-weighted spoiled gradient recalled (SPGR) scan was prescribed with the following parameters: flip angle 40°, echo time (TE) 5 ms, repetition time (TR) 25 ms, field of view (FOV) 24 cm × 18 cm, matrix 256 × 160 pixels, no interslice gap, 124 images, 1.5 mm thick.
One single volume (20 × 20 × 20 mm) 1H-MRS acquisition was obtained using PROBE point-resolved spectroscopy (PRESS) sequence and the whole-gradient mode. This sequence acquires the unsuppressed water and suppressed spectra from the same location. The unsuppressed water signal was used for eddy current compensation and also for metabolite quantification. Parameters for the MRS acquisition were TE 30 ms, TR 2000 ms, 320 acquisitions, 2500-Hz spectral bandwidth, 2048 data points.
Berlin
All MR acquisitions were obtained with a 1.5-T Siemens scanner and a standard quadrature head coil. After a localizer scan, a T1-weighted 3-dimensional (3-D) magnetization-prepared rapid acquisition with gradient echo (3-D MPRAGE) scan was prescribed with the following parameters: flip angle 15°, TE 3.93 ms, TR 2280 ms, matrix 256 × 256 pixels, no interslice gap, 160 images, 1 mm thick.
One single volume (20 × 20 × 20 mm) 1H-MRS acquisition was obtained with a stimulated-echo acquisition mode sequence (STEAM) with and without a chemical shift selective water peak suppression prepulse (CHESS, bandwidth 35 Hz). Parameters for the MRS acquisition were as follows: TR 1300 ms, TE 20 ms, TM 10 ms, 256 acquisitions, 1000-Hz spectral bandwidth, 1024 data points.
Voxel placement
In both sites, the spectroscopic region of interest (ROI) was prescribed blindly to participant status in the left prefrontal cortex (Fig. 1). The posterior borders of the ROI were aligned to the slice where the anterior-most point of the corpus callo-sum first appeared. The inferior borders were aligned with the upper limit of the horn of the lateral ventricles. In cases where the superior border of the aligned ROI extended outside of the frontal lobe, the voxel was lowered until it was fully located inside the frontal lobe. The voxels were placed as medially as possible while avoiding pockets of cerebrospinal fluid (CSF). Outer-volume suppression bands were manually placed close to each ROI as a further protection against the signals coming from the pericranial region or from orbital lipids.
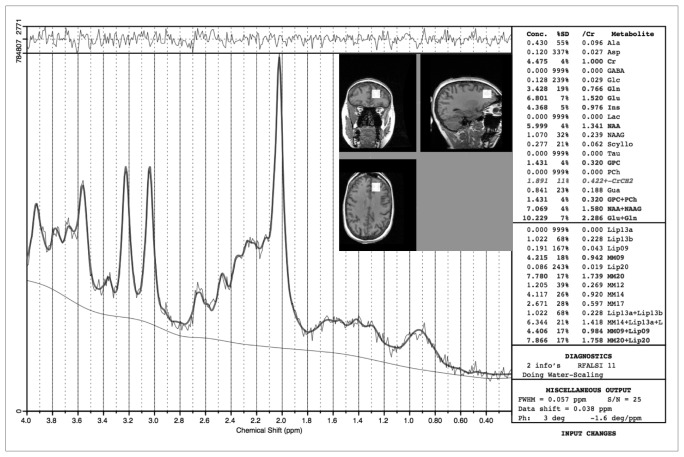
Fig. 1.
An example of a proton magnetic resonance spectrum and the spectroscopic region of interest placement on a T1-weighted anatomic magnetic resonance scan.
Tissue type parcellation of the ROIs
The concentration of 1H-MRS visible neurochemicals varies systematically with brain tissue type,29 which could lead to partial volume effects in the estimated concentration of neurochemicals. To assess the partial volumes of grey matter, white matter and CSF within each ROI, we performed a tissue type segmentation of the whole brain using the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). Subsequently, we created a mask of the spectroscopic ROI on the original T1-weighted MR images, using the 3-D coordinates defined during 1H-MRS acquisitions and obtained the numbers of voxels belonging to grey matter, white matter and CSF within this mask.
Spectral analysis
Metabolite concentrations of NAA were quantified using a linear combination model of in vitro spectra (LCModel) version 6.1, a commercially available, automatic (user-independent) frequency-domain fitting routine.30 The method employs a basis set of concentration-calibrated model spectra of individual metabolites to estimate absolute concentrations of similar brain metabolites from in vivo spectral data, correcting for residual eddy current effects and actual coil loading by using the transmitter reference amplitude.31 Each concentration is reported with a confidence measurement (% standard deviation [%SD]) reflecting maximum likelihood estimates and their uncertainties (Cramer–Rao lower bounds).30 The window of frequency domain data analyzed was left with the default settings: 0.2–4.0 ppm. An example of LCModel output is provided in Figure 1.
The quality of the output data was determined based on the criteria outlined in the literature.32 In summary, spectral profiles were considered to have acceptable quality when the full-width at half-maximum (FWHM) was smaller than 0.08, as measured by the LCModel program; the signal-to-noise ratio was 6 or greater along with randomly distributed noise; and there was an absence of artifacts in the spectral profile or of doublets in the peaks. Among spectra that were judged to have overall acceptable quality, only the estimated neurochemical concentrations with a %SD lower than 15% were retained. Exclusion of spectra based on the previously mentioned criteria was performed blind to participant group assignment. All spectral profiles from Halifax and 62% of spectral profiles acquired in Berlin met the quality criteria. There were no significant differences between the groups in proportion of spectral profiles that did not meet a particular quality criterion. The excluded participants did not differ from the included ones in age, sex or diagnosis of bipolar I versus bipolar II disorders.
The neurochemical quantifications are reported in terms of institutional units. Values relative to water peak rather than ratios to other metabolites were used, as this makes the interpretation of results clearer. The use of ratios to other metabolites has previously been questioned.33 A similar approach was used in our previous study,19 as well as in other studies of patients with bipolar disorder.34,35
Statistical analysis
To be able to combine spectroscopic data between sites and remove scanner-specific scaling, we converted the results in each site to a z score, using the means and SDs within each site. We performed a 1-way analysis of variance (ANOVA) with NAA z scores per site as the dependent variable and group (Li, non-Li, control) as the grouping variable. This was followed by post hoc pairwise Student t tests with Bonferoni correction for multiple comparisons. To obtain estimates of effect size, we calculated Cohen d (Cohen d = M1 – M2/s pooled, where s pooled = [(s12+ s22)/2], M = mean and s = standard deviation) for pairwise comparisons (i.e., Li v. non-Li, Li v. control, non-Li v. control groups). To check for within-study replication, we also calculated separate ANOVAs with raw NAA levels in each site as the dependent variable and group (Li, non-Li, controls) as the grouping variable.
To investigate the association between the duration of illness or the duration of Li treatment and NAA levels, we used the z scores and calculated the Spearman rank correlation coefficient.
Results
Demographic and clinical data
Overall we analyzed data from 27 Li-treated patients with bipolar disorder, 16 non-Li patients with bipolar disorder and 21 controls. The Li, non-Li and control groups did not differ in age, sex, proportion of grey matter or CSF in the spectroscopic ROI, total brain grey matter or white matter, or the quality of MRS data (Table 1).
Table 1.
Comparison of demographic and neuroimaging variables among patients (non-lithium, lithium groups) and controls whose spectroscopic data met the quality criteria
| Group; mean (SD)* | ||||
|---|---|---|---|---|
| Measure | Non-lithium, n = 16 | Lithium, n = 27 | Control, n = 21 | p value |
| Female sex, no. (%) | 8 (50.0) | 17 (63.0) | 12 (57.1) | 0.70 |
| Age, yr | 46.6 (10.6) | 48.0 (11.3) | 44.1 (9.3) | 0.46 |
| Total brain grey matter volume, mm3 | 541 441.37 (93599.97) | 580 253.67 (76981.77) | 604 590.38 (66678.79) | 0.06 |
| Total brain white matter volume, mm3 | 517 829.93 (99602.84) | 550 502.70 (86897.99) | 583 394.33 (90604.90) | 0.10 |
| Grey matter in the spectroscopic ROI, % | 33.0 (4.2) | 35.2 (3.7) | 34.5 (3.5) | 0.18 |
| CSF in the spectroscopic ROI, % | 11.2 (4.0) | 8.6 (3.2) | 10.0 (4.5) | 0.11 |
| Quality of the spectral profiles | ||||
| Signal-to-noise ratio | 14.62 (6.22) | 16.96 (7.33) | 14.10 (6.41) | 0.30 |
| N-acetylaspartate, %SD | 6.56 (2.78) | 5.19 (2.51) | 6.24 (1.81) | 0.14 |
| FWHM | 0.061 (0.012) | 0.061 (0.015) | 0.064 (0.011) | 0.63 |
| NAA | ||||
| Halifax, institutional units | 5.60 (0.86) | 6.21 (0.44) | 6.22 (0.60) | 0.029 |
| Berlin, institutional units | 4.62 (0.87) | 5.64 (0.61) | 5.69 (0.85) | 0.06 |
| Combined NAA z scores | −0.68 (1.16) | 0.26 (0.64) | 0.29 (0.88) | 0.002 |
%SD = standard deviations expressed in percent of the estimated concentrations; CSF = cerebrospinal fluid; FWHM = full-width at half-maximum; NAA = N-acetylaspartate; ROI = region of interest; SD = standard deviation.
Unless otherwise indicated.
Both patient groups had a marked burden of illness, but without statistically significant differences between the Li and the non-Li groups in the duration of illness, numbers of episodes, proportion of patients with bipolar I versus bipolar II disorders, HAM-D scores, YMRS scores, or exposure to antidepressant or antipsychotic medications. More patients in the non-Li group than the Li group were treated with anticonvulsants. The average duration of Li treatment in the Li group was 11.3 (SD 6.5) years (Table 2).
Table 2.
Comparison of clinical variables between participants (non-lithium, lithium groups) whose spectroscopic data met the quality criteria
| Group; mean (SD)* | |||
|---|---|---|---|
| Measure | Non-lithium, n = 16 | Lithium, n = 27 | p value |
| Diagnosis of bipolar disorder I/II | 10/6 | 18/9 | 0.78 |
| Duration of illness, yr† | 24.0 (9.9) | 26.0 (8.9) | 0.51 |
| Total no. of episodes§ | 13.7 (13.8) | 12.8 (7.9) | 0.80 |
| YMRS total score‡ | 1.3 (1.5) | 0.96 (1.3) | 0.43 |
| HAM-D-17 total score‡ | 2.7 (2.6) | 2.0 (1.8) | 0.38 |
| At time of scan: | |||
| Duration of lithium treatment, yr† | NA | 11.3 (6.5) | NA |
| Lithium levels, mmol/L‡ | NA | 0.71 (0.16) | NA |
| Anticonvulsants, no. (%) | 11 (68.8) | 4 (14.8) | < 0.001 |
| Antidepressants, no. (%) | 7 (43.8) | 8 (29.6) | 0.35 |
| Antipsychotics, no. (%) | 4 (25.0) | 4 (14.8) | 0.41 |
MRS results
The 3 groups differed significantly in concentrations of NAA (F2,61 = 7.23, p = 0.002). This difference was driven by significantly lower NAA levels in the non-Li than the Li (t41 = −3.44, p = 0.001, corrected p < 0.01, d = −1.08) or the control groups (t35 = −2.91, p = 0.006, corrected p < 0.05, d = −0.96). The control group did not differ from the Li group (t46 = −0.14, p = 0.89, d = −0.04). The same pattern of differences, with the lowest NAA levels in the non-Li group and comparable levels between the control and Li groups, was found in each of the 2 sites (Table 1).
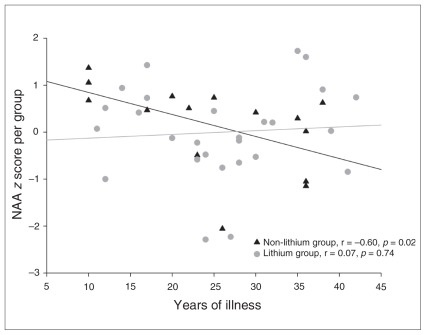
There was a negative correlation between NAA and duration of illness in the non-Li group (r = −0.60, p = 0.019), whereas no such association was seen in the Li group (r = 0.07, p = 0.74; Fig. 2). There was no association between the duration of Li treatment and NAA levels.
Fig. 2.
Association between the duration of illness and prefrontal N-acetylaspartate (NAA) levels in the lithium and non-lithium groups of patients with bipolar disorder.
Discussion
We found that participants with bipolar disorder, substantial burden of illness and limited lifetime Li exposure had lower prefrontal NAA levels than controls or Li-treated patients with comparable illness burden. The effect sizes for these differences were large. In addition, Li-treated patients had pre-frontal NAA levels comparable to those of controls despite substantial burden of bipolar disorder (average of 26.0 [SD 8.9] years of illness and 12.8 [SD 7.9] episodes). The same pattern of differences was replicated in each of the 2 sites.
These findings are in keeping with those of previous cross-sectional studies, which reported greater brain NAA levels in Li-treated patients with bipolar disorder versus those with-out current Li treatment13,14 or comparable brain NAA levels between Li-treated patients and controls.15–17 Contrary to our findings, a single study in patients with shorter duration of illness showed no differences in brain NAA levels between Li-treated and untreated patients.15 Two additional studies reported greater brain NAA levels among Li-treated patients than controls.13,14 One of these investigations was a small post hoc comparison of 6 Li-treated patients.14 In the second study, the observed effects may not have been due to Li, as most of the patients were on doses of Li at the lower end of the therapeutic range.13
Our results may reflect the effects of Li or patient heterogeneity. There may be differences between patients with bipolar disorder who respond to Li and those who do not. Perhaps, contrary to Li responders, Li nonresponders have a neurodegenerative type of the illness or an illness that stems from pre-existing neuroanatomical/NAA changes. This interpretation is, however, unlikely. Our study did not specifically select unambiguous Li responders and nonresponders. By using strict inclusion and exclusion criteria, we ensured that the Li and non-Li groups were comparable in relevant clinical and demographic variables. Patient-related factors were thus unlikely to underlie the observed differences in prefrontal NAA levels. In addition, brain NAA levels comparable to those of controls were found in unaffected or young affected offspring of parents with bipolar disorder,18,19 making it less likely that brain NAA changes predispose to bipolar disorder.
A more parsimonious explanation is that our results provide indirect evidence for the neuroprotective/neurotrophic effects of Li. This is further supported by the negative association between NAA and duration of illness that was present in the non-Li, but not in the Li group. It is difficult to distinguish whether these effects were caused by direct neurochemical effects of Li on the pathways involved in apoptosis and neuroprotection, or whether this was an indirect clinical effect related to prevention of further illness burden. A prospective study would be better suited to distinguish between these options. It is of note that the non-Li group had lower pre-frontal NAA levels than the Li group, despite having comparable duration of illness and number of episodes. In addition, recent studies have shown association between levels of Li and NAA in the brain,36 as well as increases in the brain-derived neurotrophic factor in Li-treated patients.37 Even more interestingly, Li slowed the decline in cognitive performance and decreased the levels of phosphorylated tau in patients with amnestic mild cognitive impairment.38 Together with the wealth of preclinical data, these studies appear to argue for a direct neuroprotective/neurotrophic effect of Li.
It is of note that we saw a large decrease in NAA among patients with limited exposure to Li who were, however, treated with other medications with putative neuroprotective effects (i.e., antidepressants, antipsychotics and anticonvulsants). This is in keeping with other studies that showed lower brain NAA levels relative to controls in groups of patients who were mostly treated with mood stabilizers other than Li39,40 and with the lack of effect of valproate treatment on NAA in most clinical studies.13,18,41 Similarly, no effect of atypical antipsychotics on NAA levels has been reported in a prospective study,34 and lower levels of NAA have been found in patients treated with a combination of selective serotonin reuptake inhibitors and atypical antipsychotics.42 A single study reported greater NAA levels in patients with bipolar disorder treated with valproate relative to medication-free patients.43 This difference could have been related to the fact that 75% of the valproate-treated patients had previously been exposed to Li.
Limitations
This study has several limitations. A prospective design would be better suited to establish causality of Li effect on prefrontal NAA levels. We obtained MRS data on 2 scanners. Instead of combining the raw data from different scanners, we combined z scores to remove scanner-related differences in absolute values of metabolites. We used a 1.5-T scanner. A stronger magnetic field strength would have allowed us to measure other metabolites, such as glutamate/glutamine. For measures of NAA, 1.5-T is sufficient and has been used in most previous studies. The sample size in the non-Li group was modest, since it was difficult to find patients who met our strict criteria. Nevertheless, with 64 participants, to our knowledge this is currently the largest study investigating the effect of Li on NAA concentrations. In addition, the effect size was sufficient to show statistical significance. Exposure to other potentially neuroprotective medications was allowed in both treatment groups. This was motivated by the fact that requiring Li monotherapy would further decrease feasibility and would select for more Li-responsive patients, who may differ from other patients with bipolar disorder in their neurobiology. Contrasting the Li-treated patients with a medication-naive group would not be optimal either, as medication-naive patients are typically at the early stages of the illness, where NAA changes are unlikely to be present. Exposure to other neuroprotective medications was unlikely to have confounded the results, as the groups were comparable regarding exposure to antidepressants or antipsychotics. The only difference was in exposure to anticonvulsants, for which clinical evidence for neuroprotective effects is predominantly negative. As noted in Table 2, owing to the closure of the Berlin clinic, some clinical data points were missing in the Berlin sample. The strict inclusion and exclusion criteria used in our study limit the generalizability of the findings. Previous studies have assessed less selected, more generalizable populations; however, this may make the interpretation and replicability of findings difficult owing to presence of uncontrolled confounders. In the present study, our main goal was to test specific questions and to allow for a clean interpretation of the data, which required a more selected sample. This cross-sectional study was not designed to test whether normalization of NAA is necessary for clinical response. A prospective investigation or inclusion of patients who did not stabilize on Li or other mood stabilizers would be better suited to test this question. However, it is of note that all patients with bipolar disorder in both groups were euthymic, irrespective of their NAA levels. Similarly, a prospective study would be better able to investigate whether Li is neuroprotective (i.e., prevents further damage to neurons) or neurotrophic (i.e., restores structural damage to neurons).
This study has the following strengths. It was specifically designed to test the neurochemical effects of exposure to Li. Patients were carefully, prospectively monitored for Li treatment at specialized clinics, thus ensuring that neither too-low (subtherapeutic) nor too-high (potentially neurotoxic) levels could have confounded the results. To our knowledge, we used more stringent criteria for exposure as well as lack of exposure to Li than any other previous study. Unlike previous investigations, we also used inclusion criteria to maximize and control the illness burden. The multicentre nature of this study allowed us to check for within-study replications to safeguard against false-positive findings.
Regarding the spectroscopic methods, we used strict quality criteria for inclusion of spectral profiles to ensure high validity of the results. Our data acquisition allowed us to claim institutional units rather than rely on ratios to other metabolites. This is important since bipolar disorder, as well as Li exposure, can alter the levels of creatine, which had been used as internal standard in previous studies (for a more detailed discussion, see Silverstone and colleagues13). Also, we controlled for partial volume averaging effect and found no differences between the groups in proportions of grey matter within the spectroscopic voxel. This is important, as concentrations of MRS metabolites differ between grey and white matter.29 Treatment with Li can increase grey matter volumes and could thus artifactually increase the NAA concentrations (partial volume effect). N-acetylaspartate is involved in maintaining cellular osmosis,7 which may be affected by treatment with Li. The changes in NAA were unlikely to reflect changes in water content, as we did not see overall differences in total brain or ROI grey matter or white matter volumes between the groups.
Conclusion
Patients with bipolar disorder, substantial burden of illness and limited lifetime Li exposure had significantly lower pre-frontal NAA levels than controls or Li-treated patients, who showed comparable prefrontal NAA levels to those of healthy controls despite substantial burden of bipolar disorder. Since the groups did not differ in relevant demographic and clinical variables, these results provide indirect in vivo support for neuroprotective effects of Li and for negative effects of illness burden on prefrontal NAA levels in individuals with bipolar disorder once lifetime exposure to Li is limited.
Acknowledgements
This study was supported by funding from the Canadian Institutes of Health Research, the Nova Scotia Health Research Foundation and the Dalhousie Clinical Research Scholarship to T. Hajek.
Footnotes
Contributors: M. Bauer, A. Pfennig, R. Klingebiel, L.T. Young and M. Alda designed the study. T. Hajek, M. Bauer, A. Pfennig, J. Ploch, C. O’Donovan, G. Bohner, R. Klingebiel and M. Alda acquired the data. T. Hajek, M. Bauer, J. Cullis, G. MacQueen and M. Alda analyzed the data. T. Hajek wrote the article. All authors reviewed the article and approved its publication.
Competing interests: None declared for J. Cullis, C. O’Donovan, J. Ploch and M. Alda. As above for T. Hajek. M. Bauer declares having received institutional grant support from The Stanley Medical Research Institute and NARSAD; consultancy fees and travel support from AstraZeneca, Lilly, Servier, Janssen-Cilag, Lundbeck, BMS/ Otsuka, Pfizer and GlaxoSmithKline; lecture fees and travel support from AstraZeneca, GlaxoSmithKline, Lundbeck, BMS/Otsuka and Pfizer; and board membership with AstraZeneca, Lilly, Servier, Janssen-Cilag, Lundbeck and BMS/Otsuka. A. Pfennig declares having received grant support from GlaxoSmithKline, and speaker fees from AstraZeneca and Eli Lilly. G. Bohner declares having received consultancy fees from Wyeth, BMS and Novartis. L.T. Young declares having received speaker fees from Eli Lilly and AstraZeneca. G. MacQueen declares having received consultancy and speaker fees from Lilly, AstraZeneca, BMS, Pfizer and Lundbeck; a grant from AstraZeneca; and payment for developing educational presentations for the Canadian Psychiatric Association and Lundbeck.
References
- 1.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59:1006–20. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Gould TD, Picchini AM, Einat H, et al. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7:1399–409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- 3.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2’s neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 4.Moore GJ, Bebchuk JM, Wilds IB, et al. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–2. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 5.Schuff N, Meyerhoff DJ, Mueller S, et al. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–62. doi: 10.1007/0-387-30172-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffett JR, Namboodiri AM. Expression of N-acetylaspartate and N-acetylaspartylglutamate in the nervous system. Adv Exp Med Biol. 2006;576:7–26. doi: 10.1007/0-387-30172-0_2. [DOI] [PubMed] [Google Scholar]
- 7.Moffett JR, Ross B, Arun P, et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambilla P, Stanley JA, Sassi RB, et al. 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–24. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SD, Dager SR, Parow A, et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004;56:340–8. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–69. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein DP, Towbin KE, van der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19:61–73. doi: 10.1089/cap.2008.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel NC, DelBello MP, Cecil KM, et al. Temporal change in N-acetyl-aspartate concentrations in adolescents with bipolar depression treated with lithium. J Child Adolesc Psychopharmacol. 2008;18:132–9. doi: 10.1089/cap.2007.0088. [DOI] [PubMed] [Google Scholar]
- 13.Silverstone PH, Wu RH, O’Donnell T, et al. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–9. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86:61–7. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Hamakawa H, Shioiri T, et al. Choline-containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. J Psychiatry Neurosci. 1996;21:248–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral JA, Tamada RS, Issler CK, et al. A 1HMRS study of the anterior cingulate gyrus in euthymic bipolar patients. Hum Psychopharmacol. 2006;21:215–20. doi: 10.1002/hup.761. [DOI] [PubMed] [Google Scholar]
- 17.Colla M, Schubert F, Bubner M, et al. Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol Psychiatry. 2009;14:696–704. 647. doi: 10.1038/mp.2008.26. [DOI] [PubMed] [Google Scholar]
- 18.Gallelli KA, Wagner CM, Karchemskiy A, et al. N-acetylaspartate levels in bipolar offspring with and at high-risk for bipolar disorder. Bipolar Disord. 2005;7:589–97. doi: 10.1111/j.1399-5618.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 19.Hajek T, Bernier D, Slaney C, et al. A comparison of affected and un-affected relatives of patients with bipolar disorder using proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2008;33:531–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Olvera RL, Caetano SC, Fonseca M, et al. Low levels of N-acetyl aspartate in the left dorsolateral prefrontal cortex of pediatric bipolar patients. J Child Adolesc Psychopharmacol. 2007;17:461–73. doi: 10.1089/cap.2007.0102. [DOI] [PubMed] [Google Scholar]
- 21.Caetano SC, Olvera RL, Hatch JP, et al. Lower N-acetyl-aspartate levels in prefrontal cortices in pediatric bipolar disorder: a (1)H magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 2011;50:85–94. doi: 10.1016/j.jaac.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Sassi RB, Stanley JA, Axelson D, et al. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. Am J Psychiatry. 2005;162:2109–15. doi: 10.1176/appi.ajp.162.11.2109. [DOI] [PubMed] [Google Scholar]
- 23.Winsberg ME, Sachs N, Tate DL, et al. Decreased dorsolateral pre-frontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–81. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 24.Ohara K, Isoda H, Suzuki Y, et al. Proton magnetic resonance spectroscopy of the lenticular nuclei in bipolar I affective disorder. Psychiatry Res. 1998;84:55–60. doi: 10.1016/s0925-4927(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. B J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 28.Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–71. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 29.McLean MA, Woermann FG, Barker GJ, et al. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magn Reson Med. 2000;44:401–11. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 31.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 32.Kreiss R. Quantitation and quality assessment in clinical spectroscopy. International Society for Magnetic Resonance in Medicine, Twelfth Scientific Meeting; 2004 May 15–21; Kyoto, Japan. [Google Scholar]
- 33.Gruber S, Frey R, Mlynarik V, et al. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest Radiol. 2003;38:403–8. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 34.DelBello MP, Cecil KM, Adler CM, et al. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31:1264–73. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 35.Davanzo P, Yue K, Thomas MA, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–52. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- 36.Forester BP, Finn CT, Berlow YA, et al. Brain lithium, N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy study. Bipolar Disord. 2008;10:691–700. doi: 10.1111/j.1399-5618.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494:54–6. doi: 10.1016/j.neulet.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 38.Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198:351–6. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 39.Cecil KM, DelBello MP, Morey R, et al. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–65. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- 40.Blasi G, Bertolino A, Brudaglio F, et al. Hippocampal neurochemical pathology in patients at first episode of affective psychosis: a proton magnetic resonance spectroscopic imaging study. Psychiatry Res. 2004;131:95–105. doi: 10.1016/j.pscychresns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Garcia M, Huppertz HJ, Ziyeh S, et al. Valproate-induced metabolic changes in patients with epilepsy: assessment with H-MRS. Epilepsia. 2009;50:486–92. doi: 10.1111/j.1528-1167.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 42.Sumitani S, Harada M, Kubo H, et al. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Atmaca M, Yildirim H, Ozdemir H, et al. Hippocampal 1H MRS in first-episode bipolar I patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1235–9. doi: 10.1016/j.pnpbp.2006.03.032. [DOI] [PubMed] [Google Scholar]