Abstract
Cognitive impairment is common among individuals with heart failure (HF), but the exact nature of these impairments remains unclear. The current study examined 140 older adults with heart failure and sought to determine whether there are distinct cognitive profiles using a cluster analytic approach. Results indicated three unique profiles comprising of individuals who were cognitively intact, memory impaired, and globally impaired. Clusters differed on several important demographic and clinical characteristics. These findings suggest cognitive impairment in persons with HF is more heterogeneous than commonly believed and have important implications for treatment recommendations.
Keywords: heart failure, cognitive function, cognitive profiles, older adults, cluster analysis
Heart failure (HF) affects nearly 6 million Americans and an estimated 670,000 new cases develop each year (American Heart Association, 2010). In addition to the common symptoms of fatigue, lethargy, and shortness of breath, (Watson & Gibbs, 2000), cognitive impairment is also common in HF and is associated with increased mortality and disability (Zuccala, et al., 2003; Zuccala et al., 2001). The estimated prevalence of cognitive impairment in HF typically ranges from approximately 30–50%, though impairment has been found in up to 80% of this population (Bennett & Sauvé, 2003). The risk of cognitive impairment in persons with HF is 4-times that of matched controls without HF (Sauvé, Lewis, Blankenbiller, Rickabaugh, & Pressler, 2009), and impairment is found in multiple domains including memory, attention, executive function, psychomotor speed, and language (Almeida & Flicker, 2001; Vogels, Scheltens, Schroeder-Tanka, & Weinstein 2007a; Vogels, et al., 2007b; Bennett & Sauvé, 2003; Pressler et al., 2010).
The exact mechanisms linking HF to cognitive dysfunction are still being clarified, though a growing number of contributors have been identified. Individuals with HF show structural brain changes including greater cerebral atrophy and infarcts (Schmidt, Fazekas, Offenbacher, Dusleag, & Lechner, 1991; Vogels, 2007c) as well as white matter hyperintensities (WMH) (Vogels, 2007c; Almeida et al., 2005). In addition, a reduction in gray matter volume is seen in areas such as the parahippocampal gyrus, cingulate gyrus, and frontal cortex (Woo, Macey, Fonarow, Hamilton, & Harper, 2003). HF patients also exhibit functional brain changes, demonstrating a 19–30% decrease in cerebral perfusion (Choi et al., 2006; Gruhn, et al., 2001), with notable reductions seen in the frontal, temporal, and parietal lobes (Alves, et al., 2005; Burra et al., 2002; Vogels, et al., 2008). In addition, there is evidence to suggest that even transient periods of reduced cerebral blood flow can have a negative effect on cognition. This is highlighted by the short-term cognitive dysfunction seen in some individuals undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. A recent review found cognitive decline in 4–33% of patients seven days after surgery, with decline see in the domains of attention, processing speed, and memory (Selnes & Gottesman, 2010). Moreover, acute events associated with HF (e.g., cardiac arrest) may also play a contributory role in reduced cognition. Recent work examining structural brain changes following cardiac arrest (with successful resuscitation) and subsequent global cerebral ischemia found reduced gray matter volume in several areas of the brain, and such atrophy was correlated with memory impairment (Horstmann et al, 2010).
It is well established that various vascular risk factors are associated with cognitive decline, including VaD and AD (Duron & Hanon, 2008). Vascular cognitive impairment (VCI) is a broad term used to describe the pattern of cognitive decline generally associated with vascular diseases and is typified by deficits in executive function, attention, and processing speed, (O'Brien, 2006). However, VCI is heterogeneous and the observed cognitive deficits vary based on the affected brain regions and the extent of damage (O’Brien, 2006; Moorhouse & Rockwood, 2008). Vogels and colleagues (2007b) examined clinical impairments in persons with HF and deficits were broadly consistent with VCI, but also with memory and language impairments (Vogels et al., 2007b) However, it is possible that because the participants were examined as a group, important individual differences that could categorize participants into subgroups were missed.
Using a cluster analytic approach, the aims of the current study were to determine if there are distinct cognitive profiles among individuals with HF and if so, to elucidate the patterns of strengths and weaknesses and examine potential demographic and medical differences among these profiles. In addition, we aimed to more precisely characterize any significant medical differences among profiles by examining the differential rate of occurrence and the ability to correctly classify individuals based on medical diagnoses.
Method
Participants
A total of 140 older adults who are enrolled in a longitudinal study examining the neurocognitive aspects of HF were included in the current study. Participants from the parent study were recruited from outpatient cardiology clinics in the Akron, Ohio area and were eligible for participation if they were between 50–85 years of age, English-speaking, and had a diagnosis of HF. Exclusion criteria included a history of neurological disorder (e.g., stoke, AD, severe head injury), history of significant psychological problems (e.g., schizophrenia, bipolar disorder, substance abuse), or developmental disability (e.g., mental retardation). Participants were 35.7% female and had a mean age of 68.94 ± 9.31 years. See Table 1 for complete demographic and clinical characteristics.
Table 1.
Demographic and Clinical Characteristics of 140 Older Adults with HF.
| Demographic Characteristics | Mean (SD) | Range |
| Age (years) | 68.94(9.31) | 50–85 |
| Female (%) | 35.7 | |
| Clinical Characteristics | Mean(SD)/% | Range |
| 3MS | 92.40(5.62) | 74–100 |
| Estimated IQ | 110.43(10.72) | 83.91–130.50 |
| Education (years) | 13.35(2.68) | 4–23 |
| Memory | 46.73(9.26) | 13.00–70.25 |
| Naming | 51.22(10.71) | 14.40–75.75 |
| Attention/Executive Function | 47.39(10.04) | 12.94–64.22 |
| Systolic Blood Pressure (mmHg)a | 118.29(16.41) | 83.67–167.33 |
| Diastolic Blood Pressure(mmHg)a | 65.93(9.85) | 48.67–100.33 |
| Heart Ratea | 65.18(11.63) | 33.00–104.67 |
| 2-Minute Step Test | 60.60(24.49) | 0–135 |
| Hypertension | 74.3 | |
| Myocardial Infarction | 59.3 | |
| Diabetes | 35.7 | |
| Sleep Apnea | 22.9 |
Note. Scores for Memory, Naming, and Attention/Executive Function are standardized T-scores.
average of three consecutive sitting reads.
Measures
Neuropsychological Test Battery
Participants completed a battery of well established neuropsychological measures that assessed multiple domains. Specifically, global cognitive functioning and cognitive performance in the domains of memory, naming and attention/executive function were examined. An estimate of premorbid intelligence was also calculated. Participants completed the following measures:
Global Cognitive Functioning
Modified Mini Mental Status Examination (3MS; Teng & Chui, 1987)
This test is a brief screening measure of global cognitive function and is an extension of the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975). Much like the MMSE, the 3MS is comprised of several short tasks, including orientation, animal fluency, learning and brief recall of a short list of target words, and a copy of a simple geometric figure. However, the 3MS also includes a delayed free recall of target words, additional orientation questions, and a measure of executive function (i.e., similarities). Previous work has found the 3MS to be better at identifying cognitive impairment and dementia among elderly individuals when compared to the MMSE (McDowell, Newell, Hill, & Hébert, 1997; Bland & Newman, 2001).
Memory
California Verbal Learning Test-Second Edition (CVLT; Delis, Kramer, Kaplan, & Ober, 2000)
Individuals are asked to learn, recall, and recognize a 16-item word list. Specifically, indices of learning (Sum of Trials 1–5), Immediate Recall, Delayed Recall, and Recognition were examined.
Naming
Animal Naming (Eslinger, Damasio, & Benton, 1984)
This test is a measure of semantic verbal fluency. Participants are asked to name as many different kinds of animals as they can in 60 seconds.
Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983)
This test is a measure of confrontation naming and language abilities. Participants are shown pictures and asked to name the depicted item. Items difficulty increases from high-frequency objects (e.g., bed) and lower-frequency objects (e.g., trellis).
Attention/Executive Function
Trail Making Test A and B (Reitan, 1958)
In the Trail Making A task, participants are asked to connect a series of 25 numbered dots in ascending order as quickly as they can (e.g., 1-2-3, etc.). Trail Making B adds a set-shifting component and requires participants to alternate between numbers and letters in ascending order (e.g., 1-A-2-B, etc.).
Frontal Assessment Battery (Dubois, Slachevsky, Litvan, & Pillon, 2000)
This test employs several short tasks to assess frontal system executive function. More specifically, participants are asked to identify similarities among two words (e.g., table, chair), name as many words as they can that start with a target letter (e.g., words that begin with ‘S’), complete frontal-motor hand movements, and tap patterns with their dominant hand.
Letter Number Sequencing (Wechsler, 1997)
This test is a measure of complex attention and working memory. Participants are read strings of numbers and letters of increasing length, and asked to reorganize the numbers and letters according to predetermined rules.
Stroop Test (Golden, 1978)
This test measures selective attention and mental flexibility. Participants are asked to first read columns of words spelling out colors printed in black ink (word subtest), they are then asked to identify the color a series of X’s is printed in (color subtest) and finally to indicate the color of the ink of a word (which spells out a color) is printed in, regardless of the verbal content (color-word subtest). An interference score was calculated based on word and color subtest performances to determine expected performance on the color-word subtest; this was then compared to actual color-word test performance.
Estimated Premorbid Intelligence
North American Adult Reading Test (NAART; Blair & Spreen 1989)
Individuals are asked to read a list of irregularly pronounced words. This test provides a reliable estimate of IQ in medical populations.
Cardiovascular Measures
Cardiovascular Fitness
Cardiovascular endurance was assessed with a 2-minute step test (Rikli & Jones, 2001) and was used as a proxy for HF severity. Participants were asked to march in place for two minutes bringing each knee up to a marked target on the wall set at each individual’s own midpoint between their hip and knee. The number of times the right knee met this point was counted. Participants were asked to stand at the start of the task and asked to put forth their best effort in completing the task. They were informed that they could take breaks as needed, but that the timer would run for the full 2 minutes and they were to complete as many steps as possible during that time. Participants of the parent study who were physically unable to complete this task (e.g., wheelchair bound) were not included in the current study.
Cardiac Measures
Resting heart rate and systolic and diastolic blood pressure were assessed using an automated oscillometric blood pressure device (Accutor Plus Oscillometric BP Monitor, Datascope Corp, Mahwah, NH). Participants completed a total of seven resting, seated blood pressure and heart rate reads over the course of 10 minutes. The average of the three consecutive reads taken at minutes four through six were averaged.
Procedure
This study was approved the Summa Health System and Kent State University institutional review boards. All participants provided informed consent prior to beginning study procedures and were monetarily compensated for their time and effort. The neuropsychological battery and cardiovascular measures were completed in one visit, totaling approximately three to four hours.
Analyses
Raw test scores were converted into T-scores using normative data based on age, and when possible, education and gender, prior to running analyses to facilitate interpretation. A composite score was then created for each cognitive domain by averaging the scores of each domain's subtests. Missing data was excluded listwise.
Cluster analysis was completed in two parts. First, hierarchical agglomerative cluster analysis was conducted to estimate a starting value for the k-mean algorithm (Norušis, 2011). Specifically, a hierarchical cluster analysis using Ward's method and squared Euclidean distance as the similarity measure was conducted using SPSS version 17.0 to cluster the 140 participants within the cognitive domains of memory, naming, and attention/executive function. The number of clusters was determined through examination of the inverse scree plot for the last 24 steps of the analysis and the dendrogram of the last 25 branches. Using the inverse scree plot, the number of clusters is determined by identifying the first large change in slope; the "step" at which this change occurs is subtracted from the number of observations to determine the number of clusters. The dendrogram generated by SPSS was examined and an additional dendrogram using Stata version 11.2 and the same analysis procedures was also created to facilitate interpretation. A K-mean cluster analysis was then used to determine the final cluster solution. The resulting cluster groups were then examined for potential differences in demographics, clinical and medical characteristics, and cognitive performance using MANOVA (with Bonferroni-corrected post-tests) and/or chi-square analyses with all possible group comparisons examined (i.e., Intact-Memory Impaired, Intact-Globally Impaired, and Memory Impaired-Globally Impaired). Significant (i.e., p < .05) group differences for medical variables were further examined using multinominal logistic regression. Specifically, the presence/absence of medical conditions was examined to more precisely quantify group differences and to determine if status of these conditions could accurately predict cluster membership. Individual analyses were conducted for each medical condition for which group differences emerged. Cluster membership served as the dependent variable and the medical variable, in isolation, was the factor.
Results
Determining Number of Clusters
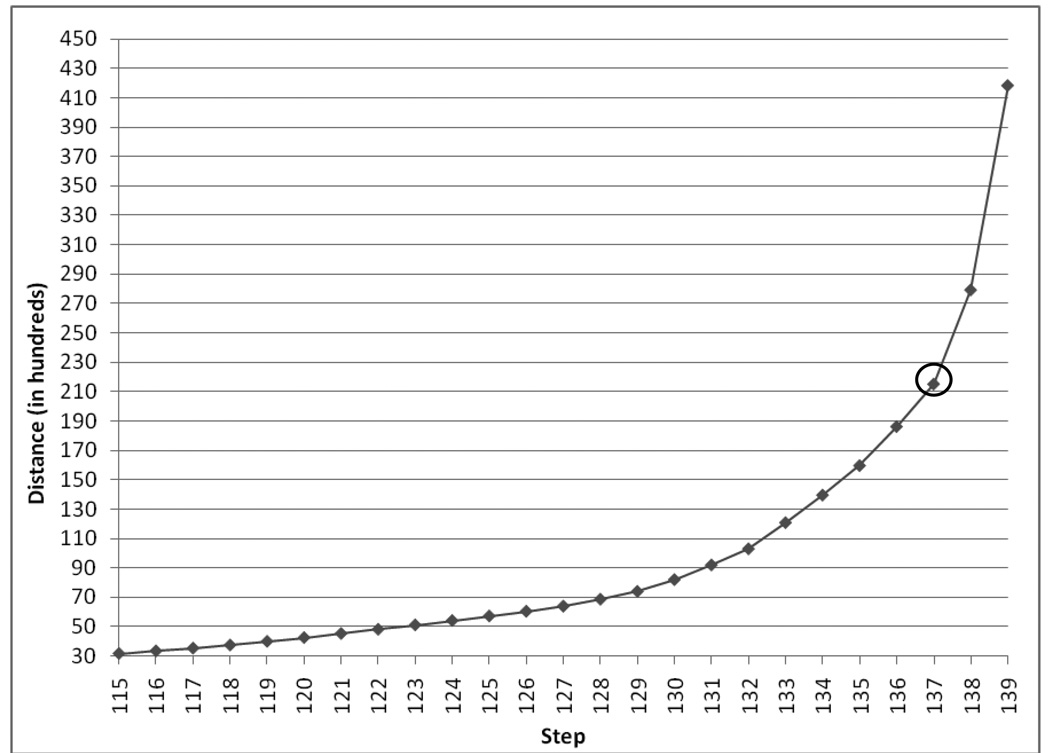
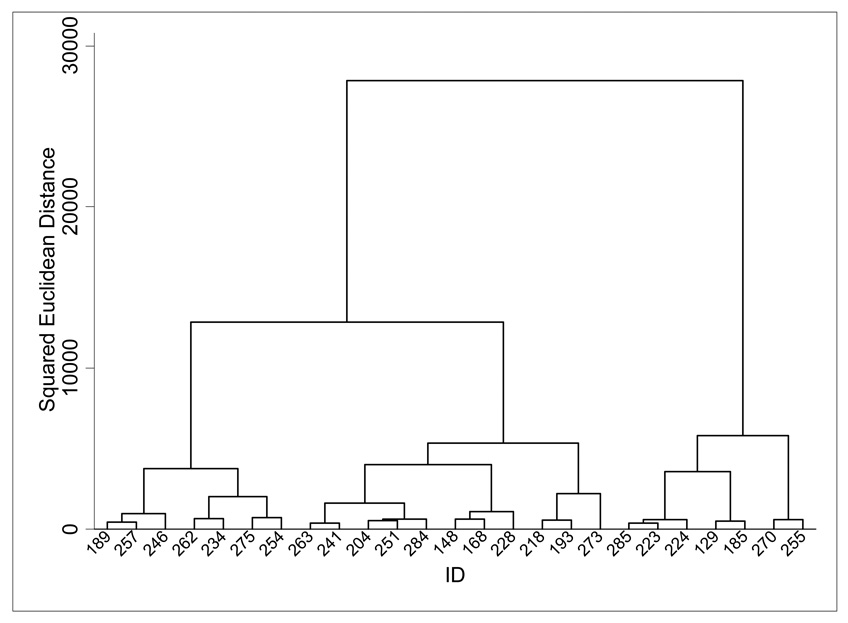
Upon completing the hierarchical cluster analysis, both the inverse scree plot (see Figure 1) and dendrogram (see Figure 2) suggested a three cluster solution. A K-mean cluster analysis was then implemented using an iterative procedure and specifying three clusters.
Figure 1.
Inverse Scree Plot from Hierarchical Cluster Analysis.
Figure 2.
Dendrogram of Last 25 Branches of Hierarchical Cluster Analysis.
Note. Dendrogram created using Stata version 11.2.
Clusters of Cognitive Test Performance in Persons with HF
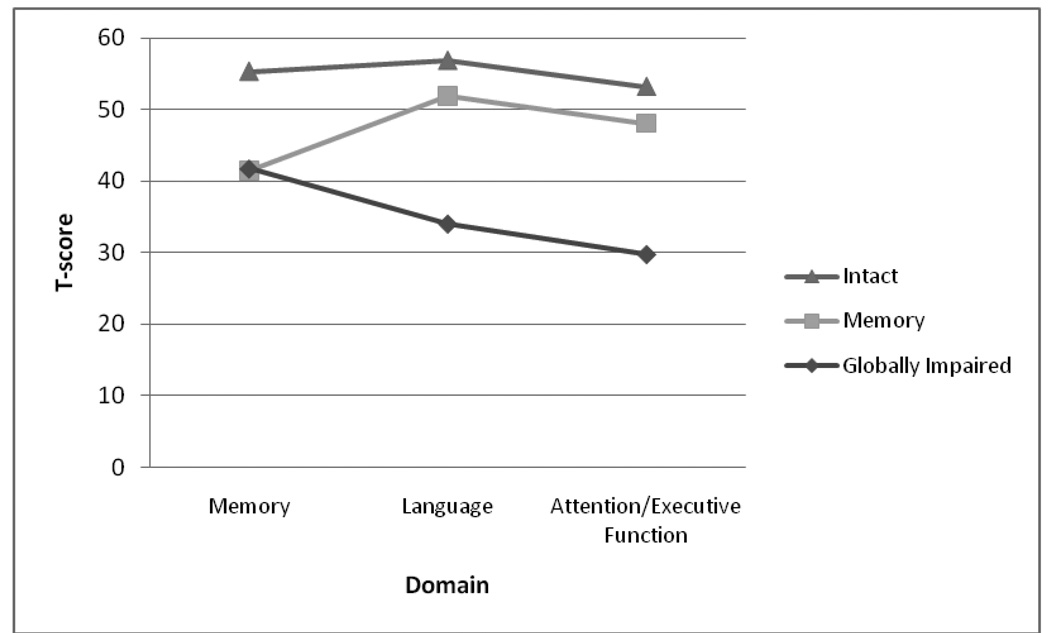
Cluster 1 consisted of participants with intact performance on all tasks (Intact, n = 53; Memory = 55.33, Naming = 56.87; Attention/Executive Function = 53.19) Cluster 2 consisted of participants with reduced memory performance (Memory, n = 67; Memory = 41.41, Naming = 51.89; Attention/Executive Function = 48.08). Cluster 3 consisted of participants with reduced memory performance and impaired performance on naming and attention/executive function (Globally Impaired, n = 20; Memory = 41.75, Naming = 34.01; Attention/Executive Function = 29.71) (See Figure 2).
Cluster Differences in Demographic and Clinical Characteristics
Clusters differed on several continuous, λ = .46, F(16,260) = 7.71, p < .001, ηp2 = .32, and dichotomous demographic and clinical variables Specifically, clusters significantly differed in age, gender, global cognitive function, estimated premorbid IQ, years of education, presence of hypertension, systolic blood pressure, cardiovascular fitness level, and history of sleep apnea.
Post tests indicated the Intact cluster had higher levels of global cognitive function and estimated premorbid IQs than the other clusters, significantly more education than the Globally Impaired cluster, and a trend for more education than the Memory cluster. The Memory cluster had higher levels of global cognitive function and estimated premorbid IQs than the Globally Impaired cluster, and were significantly older than the Intact cluster. The Globally Impaired cluster had significantly lower levels of cardiovascular fitness, were more likely to have sleep apnea and be female, and demonstrated a trend for higher systolic blood pressure, than the other clusters. These individuals were also more likely to have hypertension than those in the Intact cluster and demonstrated a trend for an increased likelihood of hypertension when compared to the Memory Impaired cluster (See Table 2).
Table 2.
Differences in Demographic and Clinical Characteristics by Cluster Membership.
| Variable |
Intact (n = 53) |
Memory (n = 67) |
Globally Impaired (n =20) |
Omnibus Test Statistic (F or χ2) |
p |
|---|---|---|---|---|---|
| Age (years)a | 66.79(10.07) | 71.13(8.37) | 67.30(8.95) | 3.73 | .03 |
| Female (%)b,c | 33.96 | 29.85 | 60.00 | 6.21 | .05 |
| 3MSd | 95.91(3.28) | 91.88(4.64) | 84.85(5.60) | 48.04 | < .001 |
| Estimated IQd | 115.38(9.63) | 110.03(9.11) | 98.66(9.17) | 23.50 | < .001 |
| Education (years)e,f† | 14.16(2.90) | 13.09(2.63) | 12.10(1.37) | 5.12 | .01 |
| Systolic Blood Pressure (mmHg)†b,c | 116.64(16.48) | 117.08(14.72) | 126.70(19.64) | 3.17 | .05 |
| Diastolic Blood Pressure (mmHg) | 65.79(9.37) | 65.05(9.29) | 69.27(12.46) | 1.43 | .24 |
| Heart Rate | 64.26(11.59) | 65.27(10.46) | 67.27(15.30) | 0.49 | .62 |
| 2-Minute Step Teste,g | 64.57(20.00) | 63.36(26.72) | 40.85(18.22) | 8.44 | < .001 |
| Hypertension (%)b,c† | 64.15 | 76.12 | 95.00 | 7.46 | .02 |
| Myocardial Infarction (%) | 58.49 | 61.19 | 55.00 | 0.27 | .88 |
| Diabetes (%) | 33.96 | 31.34 | 55.00 | 3.87 | .15 |
| Sleep Apnea (%)b,c | 18.87 | 19.40 | 45.00 | 6.49 | .04 |
Memory > Intact;
Globally Impaired > Intact;
Globally Impaired > Memory;
Intact > Memory > Globally Impaired;
Intact > Memory;
Intact > Globally Impaired;
Memory > Globally Impaired;
indicates p < .10.
Differences in Individual Cognitive Test Performance between Clusters
Clusters differed on individual cognitive variable performance, λ = .14, F(22,254) = 18.96, p < .001, ηp2 = .62. Bonferroni-corrected post-tests indicated clusters significantly differed in consistent patterns across tests for the domains of memory and naming, while a variable pattern of differences emerged for attention/executive function variables. Specifically, for all variables in the domain of memory the Intact cluster performed better than both the Memory and Globally Impaired clusters. For the variables in the naming domain, the pattern of performance was: Intact > Memory > Globally Impaired cluster. Lastly, for the variables comprising the attention/executive function domain, clusters demonstrated a more variable pattern of performance (See Table 3).
Table 3.
Standardized T-Scores of Individual Neuropsychological Test Performance by Cluster.
| Cognitive Test |
Intact (n = 53) |
Memory (n = 67) |
Globally Impaired (n =20) |
|---|---|---|---|
| Memory | |||
| CVLT-Learning Totala,b | 56.89(8.23) | 43.39(8.10) | 42.50(7.16) |
| CVLT-Short Delaya,b | 57.17(7.17) | 42.09(7.44) | 41.00(9.40) |
| CVLT-Long Delaya,b | 56.60(7.52) | 41.12(7.48) | 40.25(8.02) |
| CVLT-Recognitiona,b | 50.66(8.49) | 39.03(12.44) | 43.25(16.08) |
| Naming | |||
| Boston Naming Testc | 55.65(8.42) | 50.05(9.91) | 23.41(19.01) |
| Animal Namingc | 58.10(10.70) | 53.73(9.88) | 44.61(6.24) |
| Attention/Executive Function | |||
| TMT-Ab,d | 53.70(8.12) | 50.99(9.32) | 34.76(13.82) |
| TMT-Bb,d | 50.17(10.11) | 45.59(10.70) | 14.67(26.97) |
| Frontal Assessment Batteryc | 53.74(12.22) | 43.63(16.20) | 11.07(33.19) |
| Letter Number Sequencingc | 55.02(7.90) | 49.60(7.95) | 40.95(7.32) |
| Stroop Interferenceb | 53.34(7.62) | 50.60(6.69) | 47.10(7.49) |
Note.
Intact > Memory;
Intact > Globally Impaired;
Intact > Memory > Globally Impaired;
Memory > Globally Impaired.
Medical Characteristics and Predictors of Cluster Membership
Hypertension
Multinomial logistic regression indicated that individuals in the Globally Impaired cluster were over 10× more likely to have hypertension that those in the Intact cluster (χ2(1) = 4.92, p = .03). However, cluster classification based on presence/absence of hypertension was no better than chance, as only 50% of individuals were accurately classified (See Table 4).
Table 4.
Multinomial Logistic Regression between Cluster Membership and Medical Variables.
| Reference/Comparison Group | Hypertension | Sleep Apnea | |
|---|---|---|---|
| Intact/Memory | β | 0.58 | 0.04 |
| Standard Error | 0.41 | 0.47 | |
| Exp (B) | 1.78 | 1.04 | |
| Intact/Globally Impaired | β | 2.36 | 1.26 |
| Standard Error | 1.07 | 0.57 | |
| Exp (B) | 10.62* | 3.52* | |
| Memory/Globally Impaired | β | 1.79 | 1.22 |
| Standard Error | 1.07 | 0.55 | |
| Exp (B) | 5.96 | 3.40* | |
Note.
p < 0.05.
Sleep Apnea
Individuals in the Globally Impaired cluster were over 3× more likely to have sleep apnea when compared to both the Intact cluster (χ2(1) = 4.87, p = .03) and the Memory Impaired cluster (χ2(1) = 5.03, p = .03). However, only 47.9% of individuals were accurately classified based on presence/absence of sleep apnea.
Discussion
The current study used a cluster analytic approach to determine the cognitive profiles present in a sample of older adults with HF. Cluster analysis identified distinct groups, specifically those with intact cognitive performance, impaired memory performance, and globally impaired cognitive performance. These clusters differed on several demographic and clinical characteristics. Several aspects of these findings warrant further discussion.
In the current sample, 62% demonstrated some degree of cognitive impairment and this prevalence is consistent with past studies (Bennett & Sauvé, 2003). The Intact cluster demonstrated higher levels of estimated premorbid intelligence and education which suggests the possibility of cognitive reserve serving as a buffer against cognitive decline. Cognitive reserve models propose that individual differences in cognitive performance may emerge as a function of the brain actively trying to cope with insult by relying on pre-existing factors, including increased intellectual ability and educational attainment (Stern, 2009). Despite a limited understanding of how cognitive reserve may influence cognitive decline, cognitive reserve has been found to play a protective role in AD (Stern, 2009), frontotemporal dementia (Borroni et al., 2009), and traumatic brain injury (Kesler, Adams, Blasey, & Bigler, 2003). In addition, cognitive reserve has specifically been found to influence cognition in disease involving white matter insult including stroke (Elkins et al., 2006) and multiple sclerosis (Benedict et al., 2010). Such findings are particularly relevant given the increased incidence of infarcts and WMH among individuals with HF (Vogels, 2007c; Almeida et al., 2005) and their potential contribution to the cognitive decline seen in HF. Moreover, recent work from our group has also demonstrated a similar moderating effect of cognitive reserve on cognition in HF (Alosco et al., in press).
Another contributor to the observed variability in cognitive function in this sample might be disease burden. Persons in the Intact group had better cardiovascular fitness than the Globally Impaired group. Past work has shown the risk for cognitive impairment is higher with greater HF severity (Vogels, et al., 2007b; Pressler et al., 2010). Taken together, it appears that cognitive reserve, as estimated by educational attainment and premorbid intellectual functioning, and cardiovascular fitness are factors contributing to the preserved cognition in the Intact cluster and likely have a synergistic effect. However, while these individuals were intact at the time of the assessment, prospective studies are necessary to better characterize their cognitive profile over time. Given their vascular risk factors, it is possible these individuals may exhibit cognitive decline consistent with VCI if monitored longitudinally.
The majority of the individuals showing cognitive impairment were categorized into the Memory cluster, specifically exhibiting reduced performance on memory tasks though with preserved performance in other cognitive domains. Such performance raises the possibility that this group is at particularly high risk for mild cognitive impairment (MCI). MCI is generally considered a descriptive term for cognitive changes that occur prior to fully developing symptoms of dementia. These symptoms can be more fully differentiated, and prognosis anticipated, by determining the etiology of these symptoms (e.g., degenerative processes, vascular; Petersen, 2007). Considered within this context, these individuals in the Memory cluster may be at risk for an amnestic MCI with a degenerative etiology (e.g., Alzheimer's disease pathology). Given that vascular risk factors promote MCI to AD conversion (Li et al., 2011) and individuals with HF show a generalized increased risk of AD and dementia (Qiu, 2006), these individuals may be at increased risk for AD or a mixed dementia over time. In addition, individuals in the Memory cluster were significantly older than the other two clusters, despite the use of age-corrected test score, which further suggests these individuals may be at increased risk for developing AD, as age is the single greatest risk factor in the development of AD (Castellani, Rolston, & Smith 2010). However, it is also possible that these individuals are at risk for developing MCI of a vascular etiology as it is well established that the deficits observed in VCI depend on the regions of the brain affected and the extent of damage (O’Brien, 2006; Moorhouse & Rockwood, 2008). Regardless of etiology, the reduced memory performance among these individuals suggests they may be vulnerable to further cognitive decline. Longitudinal work, that includes clinical evaluations to establish diagnoses, is needed to clarify this possibility.
The Globally Impaired cluster demonstrated decreased performance in all cognitive domains, with marked impairment on tasks of attention/executive function and naming. This pattern of performance appears consistent with what would be expected in a mixed dementia where both AD pathology and vascular impairment and pathology are present (Jellinger, 2007). In addition to reduced memory performance, decreased performance in language also emerges in the early stages of AD, with notable deficits in semantic fluency (e.g., animal naming) and confrontation naming (Lezak, Howieson, & Loring, 2004), a deficit present in the Globally Impaired cluster. In addition to demonstrating deficits broadly consistent with AD (Braaten, Parsons, McCue, Sellers, Burns, 2006; Castellani, 2010; Lezak et al., 2004), the Globally Impaired cluster also exhibited a pattern of reduced performance on attention/executive function tasks generally consistent with vascular disease (O'Brien, 2006). Moreover, this group demonstrated the lowest level of cardiovascular fitness within the sample which suggests greater HF severity, and in turn a greater risk for cognitive impairment (Vogels, et al., 2007b; Pressler et al., 2010).
Finding the Globally Impaired cluster differed from other clusters on other demographic and clinical characteristics may also provide etiological clues For example, individuals in the Globally Impaired cluster were more likely to be female, have sleep apnea, and exhibited a trend toward higher systolic blood pressure. While both sleep apnea and hypertension are common among individuals with HF (Herrscher, Akre, Øverland, Sandvik, Westheim, 2011; Metra et al., 2011) and have been independently linked to cognitive dysfunction (Aloia, Arndett, Davis, Riggs, & Byrd, 2004; Paglieri et al., 2008), the link to gender remains less clear. Gender differences in the epidemiology, prognosis, and etiology of HF have been observed (Regitz-Zagrosek, Oertelt-Prigione, Seeland, & Hetzer, 2011) and has been posited as a potential covariate for the cognitive deficits seen in HF (Bennett, Sauvé, & Shaw, 2005). However, as discussed by Sauvé and colleagues (2009), the findings examining the relationship between cognitive outcomes and demographic variables have been inconsistent. Further work is much needed to clarify any potential gender differences.
Although the present study is the first to examine distinct cognitive profiles in HF, the findings are limited in several ways. The cross-sectional nature of the study does not allow for examination of the potential impact of disease duration on cognition, perhaps obscuring an important disease by time interaction. Prospective studies including time since diagnosis and multiple measures of HF severity (e.g., New York Heart Association Class criteria, ejection fraction) are needed to fully elucidate this possibility and to determine the longitudinal patterns of change. Moreover, examination of acute incidences within the context of HF need to be considered, as these may have exacerbated pre-existing cognitive deficits. Given that previous work has shown that the risk for cognitive impairment is higher among individuals with greater HF severity (Vogels, et al., 2007b; Pressler et al., 2010) future studies considering these factors would likely provide more precise classification. In addition, while medical differences were found between groups, the presence/absence of these variables were considered in isolation, possibly missing an important interactive effect. Future work is necessary to fully clarify this possibility. While a comprehensive neuropsychological battery was used in the current study, future studies should also include a clinical measure of overall functioning that also considers functional changes (e.g., Clinical Dementia Rating scale). Taken together, these measures would likely serve to better guide treatment recommendations. In addition, a larger and more diverse sample, including cardiac and healthy controls, is necessary in order to fully distinguish the unique impact of HF on cognition from the contributions of other cardiovascular risk factors. Lastly, more detailed information regarding medical, psychiatric (e.g., depression, anxiety), and family histories, genetic testing, and neuroimaging would provide additional useful information. Inclusion of neuroimaging, both structural and functional, in future studies would be particularly informative given the well established adverse brain changes associated with HF but the limited research linking such changes to cognition. For example, it is well established the HF is associated with several adverse structural brain changes (Schmidt, Fazekas, Offenbacher, Dusleag, & Lechner, 1991; Vogels, 2007c; Almeida et al., 2005) but the limited literature linking these changes to cognition have produced mixed findings (e.g., Beer, et al., 2009; Vogels, et al., 2007d). Similarly, individuals with HF are known to have reduced levels of cerebral perfusion (Choi et al., 2006; Gruhn, et al., 2001) and the work examining associated with cognition is limited and have produced inconsistent results (e.g., Jesus et al., 2006; Vogels et al., 2008).
Given the risk for adverse outcomes associated with cognitive decline in HF, determining precise profiles is important in effectively implementing the most appropriate treatment recommendations. Non-compliance of the therapeutic regime represents a significant problem in this population (van der Wal, Jaarsma, & van Veldhuisen, 2005). For example, Moser and colleagues (2005) found that only 34% of HF patients were taking all medications as prescribed, only 16% could name two or more symptoms of worsening HF, and just 9% actually monitored symptoms of worsening HF. Another study examined knowledge of prescribed medication and found that only 55% could correctly name their medication and only 36% could indicate when they were to take their medication (Cline, Björck-Linné, Israelsson, Willenheimer, & Erhardt, 1999). This poor compliance likely extends into the monitoring and treatment compliance of other medical conditions in this population (e.g., sleep apnea, hypertension) which increases the likelihood of further medical complications. For example, sleep apnea places individuals with HF at an increased risk for disease progression (Bradley & Floras2003a,b) and a recent study found excessive daytime sleepiness, a common sleep apnea symptom, is associated with poor medication adherence in HF, regardless of cognitive status (Riegel et al., 2011). Adherence strategies based on specific cognitive profiles, targeting particular strengths and weaknesses, may increase compliance and ultimately increase quality of life among these individuals. Increased adherence may also reduce the associated economic burden of this disease, as HF costs an estimated $40 billion annually with more than half of these costs attributable to direct hospital costs (American Heart Association, 2010).
In sum, a substantial amount of variance in cognitive performance was found within this sample of older adults with HF. Three distinct cognitive profiles that differed on several demographic and clinical variables emerged, namely Intact, Memory, and Globally Impaired. Such a pattern suggests that the cognitive profiles of HF are indeed more complicated than originally expected and cannot be simplified to a single VCI profile. Replication and extension in future studies are necessary to further clarify the cognitive profiles and their clinical and therapeutic implications in this population.
Figure 3.
Composite Cognitive Performance of Clusters in 140 Individuals with HF.
Acknowledgments
This research was supported by National Institutes of Health HL089311 and DK075119.
References
- Almeida JRC, Alves TCTF, Wajngarten M, Rays J, Castro CC, Cordeiro Q. Late-life depression, heart failure, and frontal white matter hyperintensity: a structural magnetic resonance imaging study. Brazilian Journal of Medical and Biological Research. 2005;38:431–436. doi: 10.1590/s0100-879x2005000300014. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Internal Medicine Journal. 2001;31:290–295. doi: 10.1046/j.1445-5994.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Arndett JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hyponea syndrome: a critical review. Journal of the International Neuropsychological Society. 2004;10:772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Naftali R, Cohen R, Sweet LH, van Dulmen M. Cognitive reserve moderates the association between heart failure and cognitive impairment. Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803395.2011.614596. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves TCTF, Rays J, Fraguas R, Wajngarten M, Meneghetti JC, Prando S. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. Journal of Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- American Heart Association. Heart disease and stroke statistics 2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Beer C, Ebenezer E, Fenner S, Lautenschlager NT, Arnolda L, Flicker L, et al. Contributors to cognitive impairment in congestive heart failure: a pilot case-control study. Internal Medicine Journal. 2009;39:600–605. doi: 10.1111/j.1445-5994.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, Schretlen DJ. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. Journal of the International Neuropsychological Society. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Sauvé MJ, Shaw RM. A conceptual model of cognitive deficits in chronic heart failure. Journal of Nursing Scholarship. 2005;37:222–228. doi: 10.1111/j.1547-5069.2005.00039.x. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Sauvé MJ. Cognitive deficits in patients with heart failure: a review of the literature. Journal of Cardiovascular Nursing. 2003;18:219–242. doi: 10.1097/00005082-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State Examination (3MS) as a screen for dementia. Canadian Journal of Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- Borroni B, Premi E, Agosti C, Alberici A, Garibotto V, Bellelli G. Revisiting brain reserve hypothesis in frontotemporal dementia: evidence from a brain perfusion study. Dementia and Geriatric Cognitive Disorders. 2009;28:130–135. doi: 10.1159/000235575. [DOI] [PubMed] [Google Scholar]
- Braaten AJ, Parsons TD, McCue R, Sellers A, Burns WJ. Neurocognitive differential diagnosis of dementing diseases: Alzheimer's dementia, vascular dementia, frontotemporal dementia, and major depressive disorder. International Journal of Neuroscience. 2006;116:1271–1293. doi: 10.1080/00207450600920928. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Sleep apnea and heart fFailure: part I: obstructive sleep apnea. Circulation. 2003a;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Sleep apnea and heart fFailure: part II: central sleep apnea. Circulation. 2003b;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- Burra P, Senzolo M, Pizzolato G, Tursi V, Livi U, Chierichetti F. Frontal cerebral blood flow is impaired in patients with heart transplantation. Transplant International. 2002;15:459–462. doi: 10.1007/s00147-002-0448-3. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Disease-a-Month. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B-R, Kim JS, Yang, Park YJ, Lee K-M, Kim CW. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. American Journal of Cardiology. 2006;97:1365–1369. doi: 10.1016/j.amjcard.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Cline CMJ, Björck-Linné AK, Israelsson BYA, Willenheimer RB, Erhardt LR. Non-compliance and knowledge of prescribed medication in elderly patients with heart failure. The European Journal of Heart Failure. 1999;1:145–149. doi: 10.1016/s1388-9842(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition: Adult Version. Manual. Psychological Corporation. 2000 [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasular Health and Risk Management. 2008;4:363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins JS, Longstreth WT, Jr, Manolio TA, Newman AB, Bhadelia RA, Johnston SC. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology. 2006;67:435–440. doi: 10.1212/01.wnl.0000228246.89109.98. [DOI] [PubMed] [Google Scholar]
- Eslinger P, Damasio A, Benton A. The Iowa Screening Battery for Mental Decline. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golden JC. Stroop Color and Word Test. Chicago, IL: Stoelting Co.; 1978. [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. Journal of Cardiac Failure. 2011;17:420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Frisch S, Jentzsch RT, Müller K, Villringer A, Schroeter ML. Resuscitating the heart but losing the brain: brain atrophy in the aftermath of cardiac arrest. Neurology. 2010;74:306–312. doi: 10.1212/WNL.0b013e3181cbcd6f. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The enigma of mixed dementia. Alzheimer's and Dementia. 2007;3:40–53. doi: 10.1016/j.jalz.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: An investigation of the cognitive reserve hypothesis. Applied Neuropsychology. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Yan JC. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: The Mini-Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. Journal of Clinical Epidemiology. 1997;50:377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Metra M, Zazà V, Parati G, Agostoni P, Bonadies M, Ciccone M. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. Journal of Cardiovascular Medicine. 2011;12:76–84. doi: 10.2459/JCM.0b013e32834058d1. [DOI] [PubMed] [Google Scholar]
- Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurology. 2008;7:246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. American Heart Journal. 2005;150:984.e7–984.e13. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Norušis MJ. IBM SPSS statistics 19 statistical procedures companion. Pearson Education; 2011. [Google Scholar]
- O'Brien JT. Vascular cognitive impairment. American Journal of Geriatric Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- Paglieri C, Bisbocci D, Caserta M, Rabbia F, Bertello C, Canadè A. Hypertension and cognitive function. Clinical and Experimental Hypertension. 2008;30:701–710. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: current research and clinical implications. Seminars in Neurology. 2007;27:22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauvé MJ. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratoglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Archives of Internal Medicine. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Oertelt-Prigione S, Seeland U, Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circulation. 2010;74:1265–1273. doi: 10.1253/circj.cj-10-0196. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Riegel B, Moelter ST, Ratcliffe SJ, Pressler SJ, De Geest S, Potashnik S. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. Journal of Cardiac Failure. 2011;17:340–348. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Senior Fitness Test Manual. Champaign IL: Human Kinetics; 2001. [Google Scholar]
- Sauvé MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. Journal of Cardiac Failure. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychological evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Gottesman RF. Neuropsychological outcomes after coronary artery bypass grafting. Journal of the International Neuropsychological Society. 2010;16:221–226. doi: 10.1017/S1355617709991196. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive Reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Chui H. The modified mini-mental (3MS) examination. The Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- van der Wal MHL, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? The European Journal of Heart Failure. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P. Transcranial doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congestive Heart Failure. 2008;14:61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dementia and Geriatric Cognitive Disorders. 2007d;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM. Profile of cognitive impairment in chronic heart failure. Journal of the American Geriatric Society. 2007b;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. The European Journal of Heart Failure. 2007a;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM. Brain magnetic resonance imaging abnormalities in patients with heart failure. The European Journal of Heart Failure. 2007c;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Watson RDS, Gibbs CR. ABC of heart failure: clinical features and complications. British Medical Journal. 2000;320:236–239. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. Journal of Applied Physiology. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Cocchi A, Carosella L, Cattel C. Cognitive dysfunction as a major determinant of disability with heart failure: results from a multicentre survey. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:109–112. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. The American Journal of Medicine. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]