Abstract
The light response of starburst amacrine cells is initiated by glutamate released from bipolar cells. To identify the receptors that mediate this response, we used a combination of anatomical and physiological techniques. An in vivo, rabbit eyecup was preloaded with [3H]-choline, and the [3H]-acetylcholine (ACh) released into the superfusate was monitored. A photopic, 3 Hz flashing light increased ACh release, and the selective AMPA receptor antagonist, GYKI 53655, blocked this light-evoked response. Nonselective AMPA/kainate agonists increased the release of ACh, but the specific kainate receptor agonist, SYM 2081, did not increase ACh release. Selective AMPA receptor antagonists, GYKI 53655 or GYKI 52466, also blocked the responses to agonists. We conclude that the predominant excitatory input to starburst amacrine cells is mediated by AMPA receptors. We also labeled lightly fixed rabbit retinas with antisera to choline acetyltransferase (ChAT), AMPA receptor subunits GluR1, GluR2/3, or GluR4, and kainate receptor subunits GluR6/7 and KA2. Labeled puncta were observed in the inner plexiform layer with each of these antisera to glutamate receptors, but only GluR2/3-IR puncta and GluR4-IR puncta were found on the ChAT-IR processes. The same was true of starburst cells injected intracellularly with Neurobiotin, and these AMPA receptor subunits were localized to two populations of puncta. The AMPA receptors are expected to desensitize rapidly, enhancing the sensitivity of starburst amacrine cells to moving or other rapidly changing stimuli.
Indexing terms: glutamate, ionotropic receptors, localization, cholinergic, interneuron, confocal microscopy
Starburst amacrine cells are one of the most common types of amacrine cells in rabbit retina (MacNeil et al., 1999), and there are two subtypes. Type a cells have perikarya in the inner nuclear layer, processes ramifying in the outer half of the inner plexiform layer (IPL) in stratum 2 (S2) and OFF responses to light. Type b cells have perikarya displaced to the ganglion cell layer, processes ramifying in stratum 4 (S4) of the IPL and ON responses to light (Famiglietti, 1983; Tauchi and Masland, 1984; Vaney, 1984; Bloomfield and Miller, 1986). In rabbits, starburst amacrine cells are known to receive synaptic inputs from cone bipolar cells and amacrine cells, including other starburst cells (Brandon, 1987; Millar and Morgan, 1987; Famiglietti, 1991).
Starburst amacrine cells synthesize and release both γ-aminobutyric acid (GABA) and acetylcholine (ACh) when depolarized (Masland and Livingstone, 1976; Massey and Neal, 1979; O’Malley and Masland, 1989). ACh release from the rabbit retina increases at both light onset and light offset and is maximally stimulated by large stimuli at temporal frequencies of 1–4 Hz (Massey and Neal, 1979; Massey and Redburn, 1985; O’Malley and Masland, 1993). The majority of the synaptic output from the starburst cells is onto ganglion cell dendrites (Brandon, 1987; Millar and Morgan, 1987; Famiglietti, 1991). Starburst amacrine cells are known to provide a major synaptic input to directionally selective ganglion cells, but it is uncertain what role they play in establishing the selectivity of their responses (reviewed in Vaney and Taylor, 2002).
The excitatory input to starburst cells is from bipolar cells, which release glutamate. At least two types of ionotropic glutamate receptors stimulate ACh release from the rabbit retina. In magnesium-free medium, NMDA increases ACh release from the rabbit retina, but the observation that NMDA antagonists do not block light-evoked release of ACh suggests that NMDA receptors are not essential for the responses of starburst amacrine cells to light (Linn and Massey, 1991). The agonist α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) is more potent than kainate or quisqualate (QQ) at stimulating ACh release from rabbit retina, and kynurenic acid and 6,7-dinitroquinoxaline-2,3-dione (DNQX) block the light-evoked release of ACh (Linn et al., 1991; Linn and Massey, 1991). Electrophysiological studies of starburst amacrine cells yield similar results (Zhou and Fain, 1995; Peters and Masland, 1996). However, it is uncertain whether these responses are mediated by AMPA or kainate type receptors. This is important because AMPA receptors recover from desensitization 10 times more rapidly than kainate receptors (Dingledine et al., 1999), and this would allow starburst amacrine cells to respond more effectively to rapidly changing stimuli.
Our goal was to further characterize the ionotropic receptors mediating the light-evoked responses of starburst amacrine cells using a combination of pharmacological methods and immunolabeling. Recently, specific ligands have become available that allow AMPA and kainate receptors to be more clearly differentiated. The 2,3-benodiazepine derivatives GYKI 524466 and GYKI 53655 (LY300168) are potent and selective noncompetitive AMPA receptor antagonists (Bleakman et al., 1996) and SYM 2081 (methylglutamate) is a potent and selective kainate receptor agonist (Donevan et al., 1998). Using these drugs, we show that light-evoked ACh release is mediated by AMPA receptors. AMPA receptors are composed of GluR1-4 subunits and kainate receptors are composed of GluR5-7 and KA1-2 subunits, forming tetrameric or pentameric receptors (reviewed in Dingledine et al., 1999). Antibodies are available to many of these AMPA and kainate receptor subunits, and we used them to characterize the ionotropic glutamate receptors present on the starburst amacrine cells. We showed that there are two populations of puncta containing AMPA receptors on the dendrites of starburst amacrine cells, one composed of GluR4 subunits and the other composed of GluR2 or GluR3 subunits.
MATERIALS AND METHODS
Animals
New Zealand white rabbits (3–4 kg) were deeply anesthetized with intraperitoneal (IP) urethane (Sigma-Aldrich, St. Louis, MO), and the orbit was infused with 2% lidocaine (Burns Veterinary Supply, Rockville, NY) in accordance with the institutional Animal Welfare Committee approved protocol (HSC-AWC-00-011). The anesthesia began with an IP loading dose of urethane (40% w/v, 10 ml), followed by smaller doses of urethane every 30 minutes until the toe-pinch withdrawal reflex was no longer present. Next the rabbit was intubated and its body temperature was maintained by keeping it on a heated water blanket. After completing the experiment, the rabbit was euthanized with an overdose of urethane, and the other eye was used for anatomy. Most of these eyes were he-misected, the vitreous was removed, and the posterior eyecup was immersion-fixed with 4% paraformaldehyde in 0.1M sodium phosphate buffer (PB, pH = 7.4) for 15 minutes. Some retinas were also isolated and incubated in oxygenated Ames medium (Ames and Nesbett, 1981) for injections with Neurobiotin (see below).
Physiology
The preparation for the continuous superfusion of the rabbit retina in vivo has been described in detail previously (Massey and Redburn, 1982). Briefly, a support ring was attached to the eye so that the cornea, lens, and vitreous could be removed, leaving the intact retina lining the eyecup. The retina was then loaded with [3H]-choline by incubating the retina for 30 minutes with 20 μCi of [3H]-choline (NET-109, Perkin Elmer Life Sciences, Boston, MA) that had been evaporated to dryness and reconstituted in sufficient choline-free Ames medium to fill the eyecup (0.5–0.75 ml). During this incubation, the retina was stimulated with a photopic, 3 Hz flashing white light (Grass PS22 Photic Stimulator with flash intensity 4 and 60 mS delay) to maximize choline uptake (Masland and Livingstone, 1976). After loading the [3H]-choline into the retina, the Ames medium was modified by including 30 μM eserine sulfate (Sigma-Aldrich). The eyecup was then superfused at 1.5 ml/min with this Ames medium heated to 37°C by an in-line heater (SF-28, Warner Instrument Corp., Hamden, CT). All experiments began with 60 minutes of superfusion under dim red light to ensure the background levels of radioactivity were relatively stable and to allow the rabbit to recover from surgery. Then 1-minute fractions were collected, combined with 4 ml of Ultima Gold scintillation fluid (Packard, Meriden, CT), and counted using a liquid scintillation counter (1900CA Tri-Carb, Packard).
The same light used for loading the [3H]-choline was also used for the 4-minute stimuli. Drugs were freshly prepared in the superfusion solution, and the solution was switched using a Hamilton four-way distribution valve (HM86738, Varian, Palo Alto, CA). The retina was exposed to glutamate receptor agonists for 1 minute to minimize their toxicity. The antagonists were always introduced for 1 minute prior and during the treatment or for an equivalent time when given alone. Drugs used included: AMPA/kainate receptor agonists, kainic acid (Sigma-Aldrich), and bromowillardiine (Tocris Cookson, Ellisville, MO); a specific kainate receptor agonist, SYM 2081 (Tocris Cookson); and specific AMPA receptor antagonists GYKI 53655 (a gift from Dr. S. Solyom, IVAX Drug Research Institute, Budapest, Hungary) and GYKI 52466 (a gift from Dr. I. Tarnawa, Gedeon Richer, Budapest, Hungary).
Data from the liquid scintillation counter was collected in the form of CPMs for all samples, and a blank (1.5 ml of Ames medium with 4 ml of scintillation fluid) value was subtracted from all raw CPM counts. The data were used only if responses to 4 minutes of 3 Hz flashing light were greater than 160% of the basal efflux of tritium. The fractional release was then plotted as a function of time, which was calculated using the following formula: CPM for the 1 minute fraction/(total CPM − CPMs from previous fractions). In order to obtain the total radioactivity, the retina was isolated from the eyecup and incubated in 0.5 ml of 1% SDS and 1 mM EDTA in water until the retina disintegrated (Mitchell and Redburn, 1991). Then the retinal mixture was made up to 1.5 ml with Ames medium and then counted in 4 ml of scintillation fluid. Fractional release was calculated as a function of time and the area under the curve was calculated using SAS (v. 8.1, Cary, NC). The response was defined as an average of the fractional release values during the stimulus and the 6 minutes that followed. The baseline was defined as an average of the fractional release values from the 5-minute fractions prior to and 5 minutes following the response period. The results are given as the mean ± standard error of the mean (SEM, n = 4 rabbits) and ANOVAs with repeated measurements were used for statistical analysis.
Counting the radioactivity in the superfusate from [3H]-choline loaded retina provides an estimate of the change in rate of ACh release. Previous studies indicate that greater than 90% of the radioactivity released in response to glutamate agonists is [3H]-ACh (Linn et al., 1991) and that virtually all the radioactivity released in response to light is [3H]-ACh (Masland and Livingstone, 1976; Massey and Redburn, 1982). When comparisons are made to previous studies, peak to base ratios are calculated as the peak CPM value during or after the stimuli divided by the average of the CPM values 4 minutes prior to the stimulus. This is an underestimate of ACh release because only 50% of the basal efflux of radioactivity is due to [3H]-choline (Linn et al., 1991).
Starburst amacrine cell injections with Neurobiotin
The intracellular injection procedure has been described previously (Li et al., 2002). Briefly, isolated pieces of retina prelabeled with DAPI were visualized on an upright microscope with a fixed stage and epifluorescent illumination (Olympus BX-50WI, Tokyo, Japan). Cells were impaled under visual control using a pipette filled with 4% Neurobiotin (Vector Laboratories, Burlingame, CA) and 1% Lucifer Yellow-CH (Molecular Probes, Eugene, OR) and backfilled with 3M LiCl. The electrodes had resistances of ~150 MΩ. The impaled cells were then injected with a biphasic current (+1.0 nA for 100 msec and −1.0 nA for 100 msec) for 10 –15 minutes. After the last injection, pieces of retina were fixed in 4% paraformaldehyde in 0.1M sodium phosphate buffer (pH = 7.4) for 15 minutes.
Immunolabeling
After rinsing the tissue in PBS, the retina was isolated as a whole mount. Retinas were preincubated in 2–4% normal donkey serum with 0.5% Triton X-100 for 2 hours or overnight at 4°C and then incubated in the primary antibody with 0.5% Triton X-100 and 2% normal donkey serum in PBS with 0.3% sodium azide (PBSA) for 7–10 days at 4°C. For double and triple labeling, the primary antibodies were applied together. Primary antibodies included: affinity-purified rabbit anti-GluR 1 (AB1504, Chemicon, Temecula, CA) 1:100, goat anti-GluR2 (SC7610, Santa Cruz Biotechnology, Santa Cruz, CA) 1:50, affinity-purified rabbit anti-GluR2/3 (AB1506, Chemicon) 1:100, affinity-purified rabbit anti-GluR4 (AB1508, Chemicon) 1:100, affinity purified goat anticho-line acetyltransferase (AB144P, Chemicon) 1:200, monoclonal mouse kinesin II (clone K2.4, MMS-198P, Covance, Princeton, NJ) 1:50, rabbit anti GluR6/7 (Upstate Biotechnology, Lake Placid, NY) 1:500, and rabbit antikainate receptor 2 (Upstate Biotechnology) 1:400.
After rinsing the retinas for at least a day in PBS, affinity-purified secondary antibodies were applied together in PBS at 4°C overnight. These secondary antibodies, which were raised in donkeys and conjugated to different fluorophores, included: Cy-3, indodicarbocyanine (Cy-5, Jackson Immunoresearch Laboratories, Westgrove, PA) at 1:100, Alexa 488 (Molecular Probes) at 1:500. The injected cells were labeled with streptavidin-Alexa 488 1:500 (Molecular Probes). The whole mounts and pieces of retina were rinsed several times in PBS over at least 4 hours and mounted in Vector Shield (Vector Laboratories).
Images were acquired using a confocal microscope (Zeiss LSM410, Thornwood, NY) with a krypton-argon laser and an oil immersion lens (63×, numerical aperture 1.4). Excitation was at 568 nm for Cy-3, 647 nm for Cy-5, and 488 nm for Alexa 488 with emission filters of 590 –610 nm, 670 –810 nm, and 515–540 nm, respectively. These digital images were processed in Adobe PhotoShop (v. 5.5, Adobe Systems, San Jose, CA) to enhance the color and contrast. For statistical analysis, the puncta on swellings, spine-like structures, or branch points were counted. Then the puncta on the same structures were counted after rotating the channel containing the glutamate receptor label 90° to give an estimate of the number of such appositions expected by chance. These analyses were done on single optical sections (0.5 μm), and the numbers were combined for each stack of optical sections. The original and the rotated images were compared using a one-way analysis of variance with repeated measurements. The computation was performed using the GENMOD procedure in SAS v. 8.1. All results are expressed as mean ± SEM, and for all statistical measures, significance was defined as P < 0.05. The digital images were also analyzed using the signal averaging techniques described by Li et al. (2002). Briefly, 1.5 μm squares were centered on GluR-IR puncta that were within 1 μm of ChAT-IR amacrine cell processes. The squares were then aligned and averaged to produce 2D plots of signal intensity vs. position for each channel. Two associated peaks indicate a correlation between the labeled structures and a caldera in one channel associated with a peak in another indicates an anti-correlation.
RESULTS
Physiology
As previously reported, 3 Hz photopic light flashes for 4 minutes significantly increased the release of [3H]-ACh from the rabbit retina in vivo. This physiologically evoked release was blocked by the specific AMPA antagonist GYKI 53655 at 20 μM. The light-evoked response was reduced by 91.6 ± 7.4% (Fig. 1A,B). GYKI 53655 alone caused no significant change in the release rate except for a small artifact associated with solution changes. Because this antagonist has been shown to act selectively at AMPA receptors, with negligible activity at other glutamate receptors, we concluded that the increase in ACh release due to the 3 Hz light stimulus is mediated by AMPA receptors. Kainate was used as a nonspecific AMPA/kainate receptor agonist because this drug had been used in previous experiments using the same in vivo rabbit eyecup preparation (Linn et al., 1991). The same submaximal dose of kainate, 15 μM, caused a massive efflux of [3H]-ACh and comparable peak-to-base ratios were obtained (a typical example is illustrated in Fig. 1A). The kainate-induced response was completely blocked by 20 μM GYKI 53655, reduced by 101.8 ± 10.1% (Fig. 1C). This indicates that the excitatory effect of kainate is also mediated by AMPA receptors.
Fig. 1.
A: [3H]-acetylcholine (ACh) released from a single superfused rabbit retina. These are responses to either photopic 3 Hz flashing light (*) for 4 minutes or kainate (KA) 15 μM for 1 minutes, with peak-to-base ratios of 2.8 and 15.9, respectively. These responses were blocked by the selective AMPA antagonist GYKI 53655 20 μM (GY53655) given for 1 minute prior and during the stimulus. B: Photopic 3 Hz light stimuli increased [3H]-ACh release (P = 0.0085). The change in [3H]-ACh release following flashing light (n = 4 rabbits) was significantly suppressed by GYKI 53655 (P = 0.0013), to [3H]-ACh release not significantly different from applying GYKI 53655 alone (P = 0.74). C: Kainate 15 μM significantly increased [3H]-ACh release (P = 0.025). The change in [3H]-ACh release following kainate 15 μM was significantly suppressed GYKI 53655 (P = 0.0045) to [3H]-ACh release not significantly different from GYKI 53655 alone (P = 0.99).
We used another nonselective AMPA/kainate receptor agonist, bromowillardiine, which has the advantage of causing less desensitization than AMPA (Patneau et al., 1992). Several doses were tested to establish the effective dosage range of bromowillardiine and a submaximal dose of 5 μM was selected (Fig. 2A). Bromowillardiine at 5 μM significantly increased [3H]-ACh release. This increase in [3H]-ACh release was blocked by GYKI 52466 at 20 μM, reduced by 87.0 ± 7.6% (Fig. 2B). In contrast to the mixed AMPA/kainate agonists, the specific kainate receptor agonist SYM 2081 did not increase ACh release at doses effective for kainate receptors: 1, 10 (results not shown), or 100 μM (Fig. 2B). These results also suggest that AMPA receptors rather than kainate receptors mediate ACh release from the rabbit retina.
Fig. 2.
A: Change in [3H]-ACh release in response to bromowillardiine 0.5, 1, 2, 5, 10, and 20 μM (n = 3 rabbits, except 0.5 and 20 μM where n = 2). B: Bromowillardiine 5 μM significantly increased [3H]-ACh (P = 0.04). The change in [3H]-ACh release following bromowillardiine 5 μM (n = 4 rabbits) was significantly suppressed by a specific AMPA antagonist, GYKI 52466, 20 μM (P = 0.014) to [3H]-ACh release not significantly different from GYKI 52466 20 μM given alone (P = 0.76). SYM 2081 did not significantly change [3H]-ACh release at 100 μM (P = 0.14, n = 4 rabbits).
Composition of AMPA subunits on ChAT-immunoreactive amacrine cells
A second technique, double immunofluorescent labeling, was used to determine whether AMPA receptors are present on starburst amacrine cells and, if so, to determine which subtypes. GluR4-like immunoreactive (−IR) puncta were found on both the ON and OFF subtypes of the ChAT-IR amacrine cells. The GluR4-IR puncta were most commonly located in areas where many ChAT-IR dendrites were cofasiculated (Fig. 3). Some GluR2/3-IR puncta were also present on the ChAT-IR plexus in the same areas (Fig. 4). There did not appear to be any differences between the ON and OFF sublaminae in the distribution of GluR4-IR or GluR2/3-IR puncta. There were clearly many GluR4-IR Glur2/3-IR puncta in these layers that were not found on the ChAT-IR processes, presumably the sites of excitatory synapses onto ganglion cells or unlabeled amacrine cells. The retinas were also labeled with antibodies to kinesin II, a protein present at the synaptic ribbons of bipolar cells (Muresan et al., 1999; Li et al., 2001). Virtually all of the GluR4-IR (Fig. 3C) and GluR2/3-IR (Fig. 4C) puncta were associated kinesin II-IR ribbons, and this provided a control for the specificity of glutamate receptor labeling. The signal averaging techniques were also applied to the datasets illustrated in these two figures. There is close correspondence between the GluR2/3 and GluR4-IR peaks and the kinesin 2-IR peaks, which indicates close association at a ribbon synapse. The GluR2/3 and GluR4-IR peaks were also coincident with a broader peak in the ChAT-IR channel, which indicates correlation with a larger structure, the dendrite (Figs. 3E–G, 4C–E). GluR1-IR puncta were much less common than the other AMPA subunits in the strata of the IPL containing ChAT-IR dendrites (Fig. 5A). The analysis using signal averaging showed that the labeling with antiserum to GluR1 was anticorrelated with immunoreactive ChAT (Fig. 5D,E). We concluded that a subset of AMPA receptors, including GluR2/3 and GluR4, but not GluR1, are located on the dendrites of starburst amacrine cells on bipolar cell ribbon synapses.
Fig. 3.
GluR4-IR puncta (red) are localized on the starburst amacrine cell dendrites labeled with antibodies to choline acetyltransferase (ChAT, blue). Labeling with the synaptic ribbon antibody kinesin II (KII, green) is included as a control. These optical sections are from sublamina b of the starburst amacrine cell plexus in peripheral whole-mounted retina. A: In this stack of optical sections (5 × 0.5 μm), many GluR4-IR puncta are localized on ChAT processes, while many other GluR4-IR puncta are not. The area within the box is shown at higher magnifications in B, C, D. B: A single optical section shows the triple label of GluR4, KII, and ChAT. C: In the same image as B, with the green channel hidden, some of the GluR4-IR puncta were found on the ChAT-IR processes (arrows). D: The same section as B, C is shown with the blue channel hidden. This demonstrates that most of the GluR4-IR puncta are associated with synaptic ribbons from bipolar cells (arrows). E–G: Signal averaging analysis shows the peak of GluR4 signal (E) correlated with a broader peak of ChAT signal (G). The KII signal (F) is deviated from the center of GluR4 peak (E), indicating the ribbons are adjacent, but not colocalized with GluR4. Scale bar = 5 μm in B (applies to B–D); 10 μm for A.
Fig. 4.
GluR2/3 glutamate receptor-IR puncta (red) are also localized on the ChAT-IR processes. As a control, bipolar cell synaptic ribbons are labeled with antibodies to kinesin II (green). These optical sections are from sublamina b of the starburst amacrine cell plexus in peripheral whole-mounted retina. A: GluR2/3-IR puncta are detected in the vicinity of the ChAT-IR processes. There are also GluR2/3-IR puncta that are not associated with the ChAT processes, but many GluR2/3-IR puncta are localized on ChAT-IR processes (arrows). B: The association of many of the GluR2/3-IR puncta with the kinesin-IR puncta suggests that most, if not all, of the GluR2/3-IR puncta are present at synaptic densities. Scale bars = 5 μm. C–E: Signal averaging analysis shows the peak of GluR2/3 signal (C) correlated with a broader peak of ChAT signal (E). The KII signal (D) is deviated from the center of GluR2/3 peak, indicating the ribbons are adjacent, but not colocalized with GluR4.
Fig. 5.
A: The GluR1-IR puncta (red) are very rarely detected on the ChAT-IR starburst amacrine cell dendrites (blue) in this stack of two optical sections (0.5 μm). There are not sufficient numbers of GluR1-IR puncta seen on the starburst amacrine cell processes to differentiate these puncta from chance superposition of puncta from neighboring dendrites. B: GluR6/7-IR puncta are rare in the strata containing ChAT immunoreactivity and colocalized puncta were not detected. C: Kainate receptor subunits, labeled with antibodies to KA2, are detected in the vicinity of the ChAT-IR processes. However, there was no convincing colocalization. D,E: Signal averaging analysis shows the peak of GluR1 signal (D) correlated to the depression of ChAT signal (E), indicating that GluR1 is not colocalized with dendrites of starburst cells. Scale bars = 10 μm.
Antibodies to kainate receptor subunits also labeled puncta in the IPL. GluR6/7-IR puncta were rare in the strata containing the ChAT-IR plexus and were not found on ChAT-IR dendrites (Fig. 5B). KA2-IR puncta were commonly present in the same strata as the ChAT-IR dendrites (Fig. 5C). However, there were very few, if any, contacts because colocalized pixels were rarely seen in single optical sections. Thus, the anatomical results were consistent with the physiological data.
AMPA receptor subunits on injected starburst cells
The GluR4 and GluR2/3-IR subunits of AMPA receptors were commonly localized on the parts of the ChAT-IR plexus that were most densely labeled and we were unable to examine the distribution of these AMPA receptors on individual starburst cells using this method. Instead, amacrine cells with somata in the ganglion cell layer and labeled with DAPI were targeted, and either individual cells or 2–3 neighboring cells were injected intracellularly with Neurobiotin. The injected neurons were identified as starburst amacrine cells based on their characteristic morphology, and the proximal, intermediate, and distal dendritic zones were defined according to Famiglietti (1991).
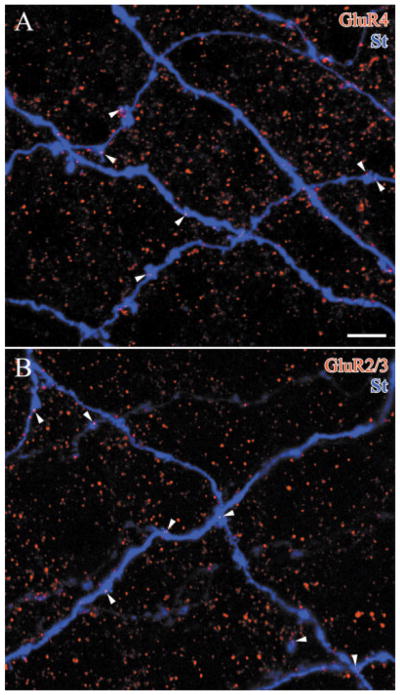
Both GluR4-IR and GluR2/3-IR puncta were localized on the dendrites of starburst cells and both types of AMPA receptor subunits appeared to have similar distributions along the dendrites. On the intermediate dendrites, where immunoreactive puncta were most common, both the GluR4 and GluR2/3-IR puncta were usually found on swellings, branch points, or spine-like structures on the starburst amacrine cell dendrites (arrows, Fig. 6). When the GluR4-IR puncta were counted, 71.8 ± 3.6% were detected on these structures. This was significantly different from the number of associations expected by chance, 49.9 ± 3.5%, as determined by rotating the GluR4 immunoreactivity in each optical section 90° and repeating the analysis (seven confocal stacks from n = 3 rabbits, P = 0.0004). A similar result was found with GluR2/3-IR puncta; 76.2 ± 5.7% of the puncta on the labeled starburst amacrine cells were found on swellings, branch points, or spine-like structures. When the channel containing GluR2/3-IR puncta was rotated 90°, only 46.4 ± 9.5% of the puncta on the filled dendrites were found on these structures (six confocal stacks from n = 3 rabbits, P = 0.0001). This analysis demonstrated that the associations of GluR2/3-IR and GluR4-IR puncta with starburst dendrites were correlated with specific structures known from electron microscopy to be associated with synaptic specializations (Famiglietti, 1991).
Fig. 6.
Overlapping dye-injected starburst amacrine cell dendrites (St, blue) are shown in each confocal image. Each of the glutamate receptor-IR puncta localized on the starburst dendrites (arrowheads) could also be demonstrated on a single optical section and was on a swelling, branch point, or spine-like structure. These pieces of rabbit retina are double-labeled and show: A: GluR4-IR puncta (red, 4 × 0.5 μm), and B: GluR2/3-IR puncta (red, 3 × 0.5 μm). Scale bar = 5 μm.
Because the distributions of the GluR4-IR and GluR2/3-IR puncta were very similar, it was possible these glutamate receptor subunits were expressed at the same synaptic densities. A goat GluR2 antibody (Santa Cruz Biotechnology) that is also known to label GluR3 receptors in mouse, rat, and human tissue was used for double-labeling experiments. There was no obvious difference between the distributions of GluR4-IR and GluR2/3-IR puncta on the Neurobiotin-injected starburst amacrine cells (Fig. 7). However, colocalization of the GluR4-IR puncta and GluR2/3-IR puncta was infrequent, a finding suggesting that there are at least two types of synaptic densities with glutamate receptors on star-burst amacrine cells.
Fig. 7.
A comparison of the distribution of GluR4-IR puncta (green) and GluR2 (3)-IR puncta (red) on a starburst amacrine cell dendrite (St, blue) in a confocal stack (3 × 0.5 μm, right). A: Examples of GluR2 puncta were marked by the white arrowheads and the clear arrowheads indicate the locations of GluR4 puncta in B. B: Examples of GluR4 puncta are marked by the clear arrowheads and the white arrowheads indicate the locations of GluR2 puncta in A. Scale bar = 5 μm.
DISCUSSION
AMPA rather than kainate receptors mediate ACH release
Photopic light flashes of 3 Hz significantly increased ACh release from the rabbit retina, and this response was blocked by the specific AMPA receptor antagonist GYKI 53655. ACh release was also significantly increased by the nonspecific AMPA/kainate receptor agonists bromowillardiine and kainate, and these responses were blocked by specific AMPA receptor antagonists, GYKI 53655 or GYKI 52466. The specific kainate receptor agonist SYM 2081 did not increase ACh release. These results are consistent with an earlier study using agmatine as a probe of excitation by glutamate and its analogs in rabbit retina. Star-burst amacrine cells were more sensitive to AMPA than to kainate and also highly sensitive to glutamate (Marc, 1999a,b). In slice preparations from rabbit retina, star-burst amacrine cells also show desensitizing responses to AMPA and sustained responses to kainate typical of AMPA receptors (Zhou and Fain, 1995). Taken together, these findings provide strong evidence that the excitatory inputs to starburst amacrine cells are mediated through AMPA receptors rather than kainate receptors. In this respect, starburst amacrine cells rabbits are similar to amacrine cells in other species (Shen et al., 1999; Tran et al., 1999).
AMPA receptors on starburst cells are composed of GluR2/3 and GluR4 subunits
Using immunofluorescence, our study shows punctate labeling with antisera to GluR2/3 and GluR4 subunits on starburst amacrine cell dendrites. Although the puncta are close to the limit of resolution of the light microscope, several lines of evidence suggest that they are associated with dendrites of starburst amacrine cells and not with other elements at the dyad synapses of cone bipolar cells. Using the same antibodies to GluRs as we did, Li et al. (2002) were conclusively able to assign the expression of several glutamate receptor subunits at rod bipolar cell dyads to either AII or S1/S2 amacrine cells. We assume that the postsynaptic processes at cone bipolar cells can also be resolved. This is supported by our results showing that the GluR2/3 and GluR4 were correlated with the ChAT signals, but the GluR1 signals were anticorrelated. It is unlikely that the labeled puncta are associated with the dendrites of direction-selective ganglion cells because their glutamatergic inputs are mediated by NMDA-type receptors (Kittila and Massey, 1997). They might originate from other types of ganglion cells or amacrine cells, however, and we would have greater confidence in our results had it been possible to label the other elements at the dyad synapses and demonstrate that they have a different set of glutamate receptors.
These results are generally consistent with previous studies of mammalian retinas. GluR1, GluR2, GluR2/3, and GluR4-IR puncta were seen throughout the IPL in the rat retina (Hack et al., 2002). In the macaque retina, GluR1-IR puncta were much less abundant than the other types (Grünert et al., 2002), a finding consistent with our observation that GluR1-IR puncta were not present on starburst amacrine cells in the rabbit retina. In macaques, there were also high densities of immunoreactive GluR2, GluR2/3, and GluR4-IR puncta in the strata where star-burst amacrine cell dendrites ramify, S2 and S4 (Ghosh et al., 2001; Grünert et al., 2002). We found that GluR2/3-IR and GluR4-IR subunits on starburst amacrine cell dendrites were associated with ribbon synapses. This is consistent with electron microscopic studies of macaque and cat retinas, in which GluR2/3 and GluR4 subunits are found on dendrites postsynaptic to cone bipolar cells (Qin and Pourcho, 1999a,b; Grünert et al., 2002). Under our conditions, the perikarya were not labeled with antisera to AMPA receptor subunits, but in rats perikarya of star-burst amacrine cells are GluR2/3-IR and GluR4-IR. However, in rats starburst amacrine cell perikarya were also GluR1-IR (Grunder et al., 2000).
Other types of amacrine cells express the same combination of GluR2/3 and GluR4 receptor subunits that we found on rabbit starburst amacrine cells. Amacrine cells cultured from chick retina express higher levels of GluR4 and GluR2/3 subunits than GluR1 subunits (Carvalho et al., 2002). AII amacrine cells express both GluR2/3 and GluR4 receptor subunits but not GluR1 receptor subunits in rabbit (Li et al., 2002), cat (Qin and Pourcho, 1999a,b) and macaque (Ghosh et al., 2001; Grünert et al., 2002) retinas. In rats, punctate immunoreactive AMPA receptor binding protein and GluR2/3 were detected on AII amacrine cells and dopaminergic amacrine cells. However, in contrast to our results in rabbit retina, there were very few, if any, GluR2/3-IR puncta on starburst amacrine cells (Gabriel et al., 2002).
AMPA receptor subunits are localized to two distinct populations of puncta
GluR2/3 and GluR4 receptor subunits have similar distributions along the starburst amacrine cell dendrites. In macaque retina, GluR2/3 and GluR4-IR puncta also contain PSD-95, a finding suggesting that all three markers are colocalized in postsynaptic specializations (Grünert et al., 2002). In the rabbit retina, however, we show that GluR2/3 and GluR4 are seldom colocalized on starburst amacrine cell dendrites. This finding suggests that there are at least two populations of puncta containing AMPA receptors. One population contains GluR4 subunits and the other population may contain GluR2 subunits, GluR3 subunits, or a combination of the two.
It is possible that these two populations of puncta are associated with distinct types of bipolar cells in S2 and S4. At least two types of cone bipolar cells terminate in each stratum (Brown and Masland, 1999; Famiglietti, 1981, 2002; Mills and Massey, 1992; McGillem and Dacheux, 2001). Two of the bipolar cells ending in S2 have been studied by electron microscopy and both were presynaptic to amacrine cell processes (Merighi et al., 1996). Further studies using intracellular injection or specific markers will be required to identify the bipolar cells presynaptic to starburst amacrine cells and determine whether specific bipolar cell types utilize different combinations of glutamate receptor subunits.
AMPA receptors on starburst cells formed from GluR2/3 subunits may be functionally different from those formed by GluR4 subunits. Excitatory postsynaptic currents (EPSCs) in whole-cell patch-clamp recordings from star-burst cells in rabbit retina have a wide range of amplitudes and one possible explanation for this variability is the differences in the sensitivity of their glutamate receptors (Peters and Masland, 1996). Based on studies of thalamic neurons, the larger EPSCs would be expected to originate from GluR4 subunits (Golshani et al., 2001), but additional experiments will be required to determine precisely how each contributes to the light responses of star-burst cells.
Acknowledgments
The authors thank Dr. Alice Chuang for assistance with the statistical analysis.
Grant sponsor: Fight for Sight, Inc.; Grant number: PD01032; Grant sponsor: the Helen C. and Robert Kleberg Jr. Foundation; Grant sponsor: the National Eye Institute; Grant number: EY06472; Grant number: EY06515; Grant number: EY10608.
LITERATURE CITED
- Ames A, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981;37:867–877. doi: 10.1111/j.1471-4159.1981.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, et al. Activity of 2,3-benzodiezepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A functional organization of ON and OFF pathways in the rabbit retina. J Neurosci. 1986;6:1–13. doi: 10.1523/JNEUROSCI.06-01-00001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res. 1987;426:119–130. doi: 10.1016/0006-8993(87)90431-8. [DOI] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Costratification of a population of bipolar cells with direction-selective circuitry of the rabbit retina. J Comp Neurol. 1999;408:97–106. [PubMed] [Google Scholar]
- Carvalho AL, Correia S, Faro CJ, Duarte CB, Carvalho AP. Phosphorylation of GluR4 AMPA-type glutamate receptor subunit by protein kinase C in cultured retina amacrine neurons. Eur J Neurosci. 2002;15:465–474. doi: 10.1046/j.0953-816x.2001.01881.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Donevan SD, Beg A, Gunther JM, Twyman RE. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther. 1998;285:539–545. [PubMed] [Google Scholar]
- Famiglietti EV. Functional architecture of cone bipolar cells in mammalian retina. Vision Res. 1981;21:1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. ’Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells in the rabbit retina. Brain Res. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Synaptic organization of starburst amacrine cells in the rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991;309:40–70. doi: 10.1002/cne.903090105. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. A structural basis for omnidirectional connections between starburst amacrine cells and directionally selective ganglion cells in rabbit retina, with associated bipolar cells. Visual Neurosci. 2002;19:145–162. doi: 10.1017/s0952523802191139. [DOI] [PubMed] [Google Scholar]
- Gabriel R, de Souza S, Ziff EB, Witkovsky P. Association of the AMPA receptor-related postsynaptic density proteins GRIP and ABP with subsets of glutamate-sensitive neurons in the rat retina. J Comp Neurol. 2002;449:129–140. doi: 10.1002/cne.10280. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Haverkamp S, Wässle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4-subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci USA. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder T, Kohler K, Guenther E. Distribution and developmental regulation of AMPA receptor subunit proteins in rat retina. Invest Ophthalmol Visual Sci. 2000;141:3600–3606. [PubMed] [Google Scholar]
- Grünert U, Haverkamp S, Fletcher EL, Wässle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Hack I, Koulen P, Peichl L, Brandstatter JH. Development of glutamatergic synapses in the rat retina: the postnatal expression of ionotropic glutamate receptor subunits. Visual Neurosci. 2002;19:1–13. doi: 10.1017/s0952523801191017. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. The pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997;77:675–689. doi: 10.1152/jn.1997.77.2.675. [DOI] [PubMed] [Google Scholar]
- Li W, Trexler EB, Massey SC. Distribution of Ribbon Synapses in the Rabbit Retina. Invest Ophthalmol Visual Sci. 2001;42(3606):S670. [Google Scholar]
- Li W, Trexler B, Massey SC. Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol. 2002;448:230–248. doi: 10.1002/cne.10189. [DOI] [PubMed] [Google Scholar]
- Linn DM, Massey SC. Acetylcholine release from the rabbit retina mediated by NMDA receptors. J Neurosci. 1991;11:123–133. doi: 10.1523/JNEUROSCI.11-01-00123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn DM, Blazynski C, Redburn DA, Massey SC. Acetylcholine release from the rabbit retina mediated by kainate receptors. J Neurosci. 1991;11:111–122. doi: 10.1523/JNEUROSCI.11-01-00111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- Marc RE. Mapping glutamatergic drive in the vertebrate retina with channel-permeant organic cation. J Comp Neurol. 1999a;407:47–64. doi: 10.1002/(sici)1096-9861(19990428)407:1<47::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Marc RE. Kainate activation of horizontal, bipolar, amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1999b;407:65–76. [PubMed] [Google Scholar]
- Masland RH, Livingstone CJ. Effect of stimulation with light on synthesis and release of acetylcholine by an isolated mammalian retina. J Neurophysiol. 1976;39:1210–1219. doi: 10.1152/jn.1976.39.6.1210. [DOI] [PubMed] [Google Scholar]
- Massey SC, Neal MJ. The light evoked release of acetycholine from the rabbit retina in vivo and its inhibition by γ-aminobutyric acid. J Neurochem. 1979;32:1327–1329. doi: 10.1111/j.1471-4159.1979.tb11062.x. [DOI] [PubMed] [Google Scholar]
- Massey SC, Redburn DA. A tonic gamma-amminobutyric acid-mediated inhibition of cholinergic amacrine cells in rabbit retina. J Neurosci. 1982;2:1633–1643. doi: 10.1523/JNEUROSCI.02-11-01633.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Redburn DA. Light evoked release of acetylcholine in response to a single flash: cholinergic amacrine cells receive ON and OFF input. Brain Res. 1985;328:374–377. doi: 10.1016/0006-8993(85)91052-2. [DOI] [PubMed] [Google Scholar]
- McGillem GS, Dacheux RF. Rabbit cone bipolar cells: correlation of their morphologies with whole-cell recordings. Visual Neurosci. 2001;18:675–685. doi: 10.1017/s0952523801185019. [DOI] [PubMed] [Google Scholar]
- Merighi A, Raviola E, Dacheux RF. Connections of two types of flat cone bipolars in the rabbit retina. J Comp Neurol. 1996;371:164–178. doi: 10.1002/(SICI)1096-9861(19960715)371:1<164::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Millar TJ, Morgan IG. Cholinergic amacrine cells in the rabbit retina synapse onto other cholinergic amacrine cells. Neurosci Lett. 1987;74:281–285. doi: 10.1016/0304-3940(87)90310-7. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Morphology of bipolar cells labeled by DAPI in the rabbit retina. J Comp Neurol. 1992;321:133–149. doi: 10.1002/cne.903210112. [DOI] [PubMed] [Google Scholar]
- Mitchell CK, Redburn DA. Melatonin inhibits ACh release from the rabbit retina. Visual Neurosci. 1991;7:479–486. doi: 10.1017/s0952523800009767. [DOI] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Masland RH. Co-release of acetylcholine and γ-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci USA. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Masland RH. Responses of the starburst amacrine cells to moving stimuli. J Neurophysiol. 1993;69:730–738. doi: 10.1152/jn.1993.69.3.730. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML, Jane DE, Watkins JC. Activation and desensitization of AMPA/kainate receptors by novel derivatives of Willardiine. J Neurosci. 1992;12:595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BN, Masland RH. Responses to light of starburst amacrine cells. J Neurophysiol. 1996;75:469–480. doi: 10.1152/jn.1996.75.1.469. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Distribution of AMPA-selective glutamate receptor subunits in the cat retina. Brain Res. 1996;710:303–307. doi: 10.1016/0006-8993(95)01476-4. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron-microscopic study. Visual Neurosci. 1999a;16:169–177. doi: 10.1017/s0952523899161121. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. AMPA-selective glutamate receptor subunits GluR2 and GluR4 in the cat retina: an immunocytochemical study. Visual Neurosci. 1999b;16:1105–1114. doi: 10.1017/s0952523899166100. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhou Y, Yang X-L. Characterization of AMPA receptors on isolated amacrine-like cells in carp retina. Eur J Neurosci. 1999;11:4233–4240. doi: 10.1046/j.1460-9568.1999.00851.x. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B. 1984;223:101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Tran MN, Higgs MH, Lukasiewicz PD. AMPA receptor kinetics limit retinal amacrine cell excitatory synaptic responses. Visual Neurosci. 1999;16:835–842. doi: 10.1017/s0952523899165039. [DOI] [PubMed] [Google Scholar]
- Vaney DI. ‘Coronate’ amacrine cells in the rabbit retina have the ’starburst’ dendritic morphology. Proc R Soc Lond B. 1984;220:501–508. doi: 10.1098/rspb.1984.0016. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Curr Opin Neurobiol. 2002;12:405–410. doi: 10.1016/s0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci. 1995;15:5334–5345. doi: 10.1523/JNEUROSCI.15-07-05334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]